Abstract
Large-scale patterns of current species geographic range-size variation reflect historical dynamics of dispersal and provide insights into future consequences under changing environments. Evidence suggests that climate warming exerts major damage on high latitude and elevation organisms, where changes are more severe and available space to disperse tracking historical niches is more limited. Species with longer generations (slower adaptive responses), such as vertebrates, and with restricted distributions (lower genetic diversity, higher inbreeding) in these environments are expected to be particularly threatened by warming crises. However, a well-known macroecological generalization (Rapoport's rule) predicts that species range-sizes increase with increasing latitude-elevation, thus counterbalancing the impact of climate change. Here, I investigate geographic range-size variation across an extreme environmental gradient and as a function of body size, in the prominent Liolaemus lizard adaptive radiation. Conventional and phylogenetic analyses revealed that latitudinal (but not elevational) ranges significantly decrease with increasing latitude-elevation, while body size was unrelated to range-size. Evolutionarily, these results are insightful as they suggest a link between spatial environmental gradients and range-size evolution. However, ecologically, these results suggest that Liolaemus might be increasingly threatened if, as predicted by theory, ranges retract and contract continuously under persisting climate warming, potentially increasing extinction risks at high latitudes and elevations.
Introduction
The dynamics of species geographic range-size evolution are mediated by ecological, physiological and physical factors that set the boundaries for viable dispersal [1], [2], [3]. As a result, most species have ranges restricted to particular areas of the planet, and most are restricted to particular environmental spots where even local habitat fragmentations prevent dynamic migration between them [4]. Multiple hypotheses have attempted to elucidate the causes, and hence the predictability, of current patterns of range-sizes in nature under the context of different organismal and environmental factors [5]. However, despite decades of research, the search for general explanations underlying range-size variation remains a challenging endeavour [1], [5].
Research on the ecological and evolutionary dynamics of range limits has become increasingly important with the observation that species distributions are rapidly altered by human-induced climate change. In recent years, numerous reports have shown ongoing range alterations across diverse organisms consistent with climate change predictions [1], [3], [6], [7], [8], [9], [10], [11], [12], [13]. For example, climate change-driven range alterations have been shown in groups as diverse as butterflies [9], [13], frogs [14], [15], and birds [16]. As predicted, these range alterations have been involved in population declines or in actual extinctions in species where adaptive responses to environmental changes or dispersal into new areas have been obstructed by genetic or physical barriers [6], [8], [9], [10], [17], [18]. The potential for threatened species to escape extinction following rapid climatic shifts depends on multiple biological features. For example, rapid adaptive responses to climate change are more likely in species with short generation times, such as insects [19], [20], but appear less likely in longer-generation organisms, such as vertebrates [17], [21], [22]. Also, species distributed at high latitudes and elevations are expected to experience threats as range alterations caused by upward and poleward advances of warming climate may cause range contractions while available space to disperse tracking historical niches progressively declines, such as on mountaintops [8],[10],[12],[23],[24],[25]. Subsequently, range contractions and fragmentations may compromise population persistence via reduced genetic diversity [4]. Warming will also promote dispersal of species from warm areas that may compete with resident species from historically cold areas, intensifying environmental stress, population damage, and extinction ([6], [22], [23], but see [26]). Therefore, species with longer generations from high latitudes and elevations and with restricted range-sizes are expected to become particularly threatened under persisting climate warming.
In this context, the macroecological study of spatial variation of geographic range-sizes across environmental gradients is of primary interest to infer factors involved in the evolution of their boundaries, and hence, to reinforce predictions about potential large-scale responses to changing climates. Particularly, although cold climate species with restricted ranges are expected to be more threatened by climate warming, a widely known macroecological generalization (known as Rapoport's rule) posits that species range-sizes tend to increase with decreasing climatic temperatures along biogeographical gradients [27], [28], [29]. Therefore, in lineages where this trend holds, larger ranges toward higher latitudes-elevations may contribute to counterbalance the impact of climate warming, potentially retarding range contractions and extinctions. However, empirical support to this rule is equivocal, being increasingly discredited as a generality [29].
The use of prominent adaptive radiations offers excellent conditions to investigate within the phylogenetic boundaries of a given lineage (where evolutionary events are related and comparable) the impact of factors expected to affect the trajectories of ecological and evolutionary processes, such as range-size variation. Here, I investigate the questions whether range-sizes among species of the prominent Liolaemus (family Liolaemidae) lizard adaptive radiation vary predictably across one of the most extreme environmental gradients known for a single lizard genus, and whether this variation would most likely enhance or counterbalance potential threats under persistent climate change (i.e. whether range-sizes increase or decrease with latitude-elevation). In addition, I investigate whether range-sizes are influenced by interspecific differences in body size, as suggested by previous studies. However, the direction of these relationships is inconsistent. While some studies reveal that larger ranges result from higher ecological tolerance and competitiveness of larger species [1], [30], [31], [32], [33], [34], others show both positive or negative covariations [35], or even triangular relationships [1]. The Liolaemus radiation provides an ideal model organism to address these questions. Consisting of 220+ species, these lizards have extensively radiated throughout central and southern South America and have colonized a unique variety of environments [36], occurring from the extreme Desert of Atacama to Patagonian areas that include the southernmost place inhabited by lizards, and from sea level to over 5000 m of elevation [37], [38], [39], [40]. Across this environmental gradient, Liolaemus species have evolved a diversity of range-sizes, life histories and thermal adaptations [38], [41], [42], [43], [44] that offer the ideal evolutionary scenario to conduct large-scale comparative analyses within a single radiation.
Results
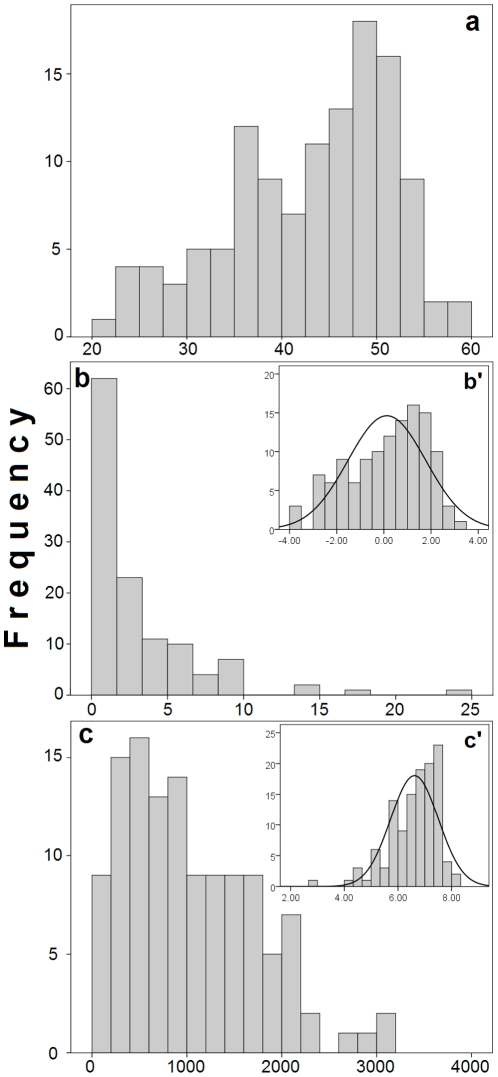
The range-size frequency-distributions on arithmetic scales in Liolaemus are consistently right-skewed in both latitudinal and elevational ranges (Kolmogorov-Smirnov test, latitudinal range: D(121) = 0.22, P<0.001; elevational range: D(121) = 0.11, P = 0.002; Fig. 1b, c), and hence, the tendency within the genus is towards geographically restricted species, with some examples of extreme historical dispersal ability. In contrast, the frequency-distribution of the ALM is left-skewed (Fig. 1a). As shown by these frequency-distribution plots, both latitudinal (range mean = 02°58′S±3.8 SD, range = 0°01′S–23°33′S, mode = 0°06′S) and elevational ranges (range mean = 1011±691 m, range = 20–3153 m, mode = 300 m) show considerable interspecific variation. Logarithmic transformations (ln) of arithmetic frequency distributions of latitudinal and elevational ranges reduced skewness, but failed to reach normality (latitudinal range: D(121) = 0.12, P<0.001; elevational range: D(121) = 0.09, P = 0.01; Fig. 1b', c').
Figure 1. Frequency distributions of geographic locations of Liolaemus species expressed as a combination of latitude and elevation under the adjusted latitudinal midpoint (ALM, a), and of their latitudinal (expressed in degrees of latitude, b) and elevational (expressed in metres of elevation, c) geographical range sizes expressed in arithmetic scales, and in their corresponding logarithmic scales (b' for latitude, c' for elevation).
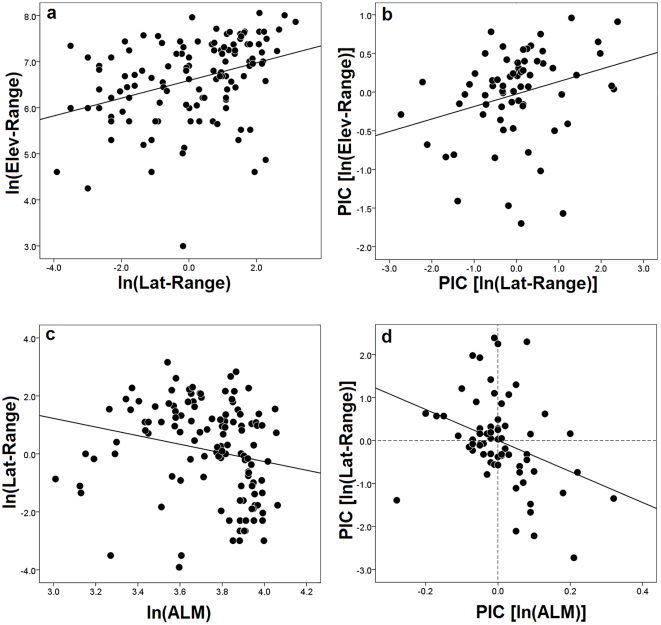
Quantitative analyses of range-size variation revealed qualitatively identical results when employing both conventional statistics and phylogenetic comparative methods (Table 1), which suggests a substantial consistency between predictors and range-size independent of the analytical approach employed. This finding contrasts with a previous similar study in Liolaemus lizards based on a smaller sample [42] where results from both conventional and phylogenetic analyses differed significantly. The test of the primary question whether range-sizes vary predictably across an environmental and geographical gradient revealed that latitudinal range-sizes decrease predictably with increasing latitude-elevation (ALM), and hence, with decreasing climatic temperatures (Table 1; Fig. 2c,d). Also, figure 2c shows that the magnitude of residuals below the fit line is greater than above it, and hence, latitudinal range-sizes deviate more strongly towards smaller ranges than expected than the deviations towards larger ranges than expected. However, a weak, non-significant, relationship was detected between ALM and elevational range-size (Table 1; figure not shown), despite the significant positive correlation between latitudinal and elevational range-sizes (Table 1; Fig. 2a,b). Analyses involving body size showed that the historical dispersal ability of Liolaemus species appears to be unrelated to average species size, as no predictable covariation was observed (Table 1). When differences in body size between the sexes were accounted for, similar relationships were observed between body size and range-size variation (Table 1). These results are also entirely consistent between conventional and phylogenetic analyses (Table 1).
Table 1. Conventional (non-phylogenetic, abbreviated as NP) and phylogenetic (based on phylogenetic independent contrasts, abbreviated as PIC) analyses of large-scale patterns of latitudinal (Lat) and elevational (Elev) range size variation as a function of geographical distribution (adjusted latitudinal midpoint, ALM) and body size (SVL; for two of these tests the effect of sexual size dimorphism, SSD, is controlled for) in the lizard genus Liolaemus.
| Analysis | Test | N | r | R2 | F (df) | P |
| Range (Elev) on Range (Lat) | NP | 121 | 0.36 | – | – | <0.001 |
| PIC | 68 | 0.29 | – | – | 0.02 | |
| Range (Lat) on ALM | NP | 121 | −0.21 | 0.04 | 5.42 (1,119) | 0.02 |
| PIC | 68 | −0.35 | 0.12 | 9.02 (1,66) | <0.01 | |
| Range (Elev) on ALM | NP | 121 | 0.15 | 0.02 | 2.80 (1,119) | 0.1 |
| PIC | 68 | 0.17 | 0.03 | 1.97 (1,66) | 0.17 | |
| Range (Lat) on SVL | NP | 115 | −0.04 | 0.002 | 0.18 (1,113) | 0.67 |
| PIC | 65 | 0.05 | 0.002 | 0.13 (1,63) | 0.72 | |
| Range (Elev) on SVL | NP | 115 | 0.08 | 0.01 | 0.68 (1,113) | 0.41 |
| PIC | 65 | 0.2 | 0.04 | 2.57(1,63) | 0.11 | |
| Range (Lat) on SVL (SSD) | NP | 115 | −0.07 | 0.01 | 0.31 (2,112) | 0.74 |
| PIC | 65 | 0.2 | 0.04 | 1.13 (2,62) | 0.33 | |
| Range (Elev) on SVL (SSD) | NP | 115 | 0.08 | 0.01 | 0.34 (2,112) | 0.71 |
| PIC | 65 | 0.2 | 0.04 | 1.27 (2,62) | 0.29 |
Relationships between latitudinal and elevational ranges are analysed using correlations as no causal weight can be attributed to any of these variables. See methods section for additional details.
Figure 2. Analyses of range size variation in Liolaemus lizards, showing correlations between latitudinal and elevational ranges based on conventional (a) and phylogenetic analyses (b), and regression analyses of latitudinal range variation as a function of adjusted latitudinal midpoint (ALM) in both raw (c) and phylogenetically controlled data (d).
Abbreviations include latitude (Lat), elevation (Elev), and phylogenetic independent contrasts (PIC).
Discussion
This study provides evidence that latitudinal range-sizes in Liolaemus lizards decrease predictably with increasing latitude-elevation across an extreme environmental gradient. Hence, these observations entirely reverse the pattern predicted by Rapoport's rule [28], [29], while no effects of distribution were observed on elevational range-sizes. The phylogenetic analyses revealed the same relationship. These results contrast with a previous study on a smaller sample of Liolaemus species, where non-historical analyses revealed a positive relationship between latitudinal range-size and species latitudinal and elevational distributions, while phylogenetic tests showed no association between these variables [42]. In addition, I found that body size appears not to influence range-size variation in these lizards. Collectively, these results suggest an historical connection between the radiation of Liolaemus lizards into cold-climate environments and their dispersal potential, and that the evolutionary outcome of decreasing ranges with increasing latitude-elevation may result in higher levels of population vulnerability and potentially extinction in colder climate species, as a result of range contractions if upward and poleward climate warming persists.
Evolutionary inference and ecological expectations of range-size dynamics
The observed relationships between range-size and environmental gradients can be interpreted from, first, an evolutionary perspective, and second, an ecological perspective involving potential consequences of climate warming. Evolutionarily, these results suggest that the historical dynamics of latitudinal range limits have been influenced significantly by the environmental conditions encountered by Liolaemus during their radiations into high latitudes and elevations, where the increasingly colder and unstable climatic conditions stand as primary candidate factors. However, given that only latitudinal ranges predictably decrease as a function of increasing ALM, in contrast to elevational ranges (Table 1), range limits are unlikely to be restricted by thermophysiological demands of colder climates alone. This inference is supported by a previous study where thermal tolerance was shown to increase with increasing latitudes-elevations across Liolaemus species [42], in agreement with theory [45] and additional empirical evidence coming from other ectotherms [46], [47]. If range dispersal relied exclusively on thermophysiology, it would be expected that given greater thermal tolerance in colder climates, latitudinal ranges would not be restricted by declining climatic temperatures (as observed in elevational ranges), in contrast to the results of this paper. Therefore, this suggests that additional factors associated with higher ALMs play an important role in shaping range-size variation in these lizards (e.g., [1], [48]). In the case of cold climate Liolaemus species, range boundaries are known to be influenced by the irregular topography of the Andes, characterized by multiple mountain peaks spread across thousands of kilometres of latitude, and where an important part of this evolutionary radiation has taken place [38], [43], [49]. This topographical scenario then imposes severe physical barriers for latitudinal dispersal, while elevational dispersal would not be equally restricted within mountains, which would be further facilitated by greater thermal tolerance. However, the fact that latitudinal ranges are affected by Andean topography necessarily indicates that elevational dispersal is possible only within certain limits. Otherwise, there would be no limits to latitudinal distribution. Indeed, several Andean Liolaemus species are restricted to ‘elevational islands’ (as observed in other mountain lizards; e.g. [22]) represented by high elevation zones isolated by lower elevation valleys and cliffs from similar high elevation peaks where related species occur (e.g., [50], [51]). Therefore, species dispersal between high elevation areas through lower elevation corridors appears, in fact, to be impeded.
Despite greater thermal tolerance of cold-climate Liolaemus, mountain restrictions may be explained by at least three evolutionary scenarios that potentially apply for cold-climate lizards in general. First, the evolution of increasing thermal tolerance in colder climate species might only be possible within a narrow range (e.g., [41]), allowing elevational dispersal within similarly narrow thermal limits. Second, dispersal can be impeded, independent of thermal selection, if phylogenetic niche conservatism precludes lizard emigrations from elevationally restricted environmental patches even in the absence of geographical barriers for range expansion (e.g., [2]). For example, isolated vegetational areas determined by climatic conditions [52], [53] associated to particular geological formations, such as rocky outcrops, sustain different Liolaemus species and assemblages in different areas of the Andes (see also [22], [51], [54]). Third, independent of climatic constraints on thermoregulation for ecological and reproductive activities, the evolution of viviparity in cold climates can hamper lizard dispersal along elevational gradients. The detrimental effects that cold and unstable environments exert on externally incubating eggs has forced cold climate lizards in general [55], including Liolaemus [43], to evolve viviparity. Given that viviparity is tremendously costly in warm environments, and hence mostly viable only in cold climates [55], and almost entirely impeded to re-evolve into oviparity [55], [56], the evolution of viviparity can be regarded as a major factor precluding expansion of cold climate lizards into warmer environments, such as downward dispersal in mountains to access lower elevation corridors. In accordance with this alternative, almost all known cases of viviparous Liolaemus species are restricted to high latitudes-elevations [36], [43].
Although the independent or combined effect of the above factors may provide an explanation for the observed patterns of distributional range variation among Andean Liolaemus, it may not fully explain the occurrence of small ranges among several Patagonian species, where climates are cold given the high latitudes, but the Andes decrease considerably in elevation [57]. Therefore, in these cold latitudes, low temperatures are constant across extensive, flat, areas with considerably less topographic complexities and environmental fluctuations compared to the Andes. Yet, as in the Andes, some Patagonian Liolaemus are isolated in mesetas (trap basalts of up to 1,700 m of elevation), but these mesetas are unlikely to impose severe restrictions for dispersal as a generality. For example, while some Patagonian species are isolated on elevated mesetas (e.g. L. archeforus and L. silvanae), other species (e.g. L. lineomaculatus) are geographically widespread, and coexist in different areas of their distribution with other Liolaemus restricted to smaller ranges [39], [58]. However, despite these differences between Andean and Patagonian ecosystems, some of the three scenarios detailed above may at least in part account for the restricted distribution of lizards in Patagonia. For example, rock-specialist Liolaemus may be forced to remain in bouldery areas, as observed in Phymaturus lizards (sister genus to Liolaemus) in Patagonia [59], [60].
On the other hand, ecologically, these results may be of conservation concern as the observed negative relationship between latitudinal range and ALM suggests that cold climate Liolaemus may be at higher risk of population decline under persisting climate change via range retractions and contractions [3] and habitat fragmentation [61]. Empirical studies have repeatedly shown that climate warming exerts particularly severe negative impacts on species from high latitude-elevations [4], [7], [8], [24], [25], [62], [63], often characterized by hotspots of high endemism [12], [64]. Indeed, in a number of lineages, range-restricted species from high latitudes-elevations are currently experiencing dramatic fragmentation, range retractions and contractions that translate into rapid extinction rates [4], [8], [10], [15]. Liolaemus biodiversity may face increasing extinction risks through different processes linked to persisting climate warming. First, assuming some dispersal ability, as species move upward and poleward tracking their historical niches as a result of warming advances in the same direction, dispersal can be impeded by declines in the quality and quantity of available space [8], [10]. Although upward and poleward range expansions may counterbalance range retractions at the lowest latitudinal and elevational distributional limits (where range retractions take place), dispersal might be particularly hampered in species from high Andean elevations and extreme Patagonian latitudes, where mountaintops and coastlines set absolute limits on dispersal. Second, persisting range retractions are expected to increase habitat fragmentation, thus increasing risks of population declines caused by genetic crises with high fitness costs, for example via increased inbreeding rates, and hence, reduced heterozygosity and greater inbreeding depression [4], [65]. Third, warming advances toward historically cold areas are expected to facilitate invasions of species from warm areas, resulting in increasing intensity of competition through, for example, resource competition or predation [22], [23]. Finally, it has been shown that lizard extinctions may occur in structurally intact habitats when climate warming imposes alterations to thermoregulatory behaviour. Lizards prevent body overheating mostly by intermittent retreats into cooler shelters during hot days [21], [66]. With climate warming, lizards will be forced to spend longer periods retreated in these shelters, resulting in reduced opportunities for reproduction and foraging. Because the breeding season requires significant energy intakes to be allocated in reproduction, lizards experiencing climate warming are expected to suffer severe energetic shortfalls [22]. For viviparous species (see third evolutionary scenario above), mostly restricted to high latitudes-elevations [43], [55], this may incur in even greater fitness costs as the high energy requirements of pregnant females to fully develop embryos are accompanied by considerable foraging risks caused by the detrimental impact of the pregnancy burden on escaping efficiency [55], [67]. These factors are expected to interact in additional ways with some of the three evolutionary scenarios described above to functionally link historical dynamics of dispersal with future consequences under climate change. For example, if niche conservatism is important, habitat fragmentation may become an important factor behind depletion of genetic diversity in Liolaemus populations.
Alternatively, species facing climate warming can escape extinction through rapid genetic responses to the changing climate [4], [6], [23]. However, as stated above, rapid adaptations are likely to occur in short-generation organisms [19], but seem less likely in larger organisms like lizards [17], [22]. Therefore, under any of the warming-related scenarios described in the previous paragraph, Liolaemus biodiversity from high latitudes-elevations may become increasingly threatened under persisting climate change.
Body size and range-size variation
The influence of body size on most evolutionary and ecological processes has led to suggest that body size mediates differential dispersal ability among different sized species, and hence, that body size might predict range-size [1], [32], [34], [35]. However, Liolaemus body sizes are unrelated with interspecific variation in range-size, and no other pattern (e.g. triangular distributions of data points; see Gaston, 2003) is present. This finding is consistent with a recent study on Liolaemus range-size variation as a function of body size [42], and with previous observations that Liolaemus body sizes do not vary predictably with latitude-elevation gradients [38], [68], which, in contrast, are related with range-size variation (see above).
Despite the lack of predictable covariation between body size and range-size in Liolaemus, it seems unlikely that body size does not influence dispersal ability in these lizards. An important difficulty that may preclude the identification of a common effect of body size is that no general tendencies are always expected to exist as several factors are known to interact in different ways across different phylogenetic groups and environmental contexts. For example, Bowler & Benton [35] suggested that dispersal ability is context-dependent, and that depending on given selective regimes dispersal ability may or may not be related with body size. This is probably the case in Liolaemus. Since these lizards inhabit one of the widest environmental ranges known within reptiles, multiple selective contexts may operate across species to shape specific body size-dispersal ability relationships, potentially impeding the detection of a generalized mechanism from a generalized pattern involving the relationship between body size and range dimensions.
Materials and Methods
Data collection
Data were collected for a total sample of 121 Liolaemus species (Table S1) representing all major clades within the genus and occurring in a latitudinal and elevational range that represents its entire diversity (e.g., [36]). Therefore, this dataset covers the phylogenetic and ecological diversity that has resulted from the evolutionary radiation of this group. Data comprise information on geographical distribution and body size per species.
Geographical data consist of species-level information for spatial location and range-size in latitude and elevation. These data have been derived from a number of published sources [38], [39], [42], [43], [44], [58], [68], [69], [70], [71] and from a total record of ∼8,500 specimens from institutional collections (see Appendix S1; the use of the Liolaemus data for publication purposes has specifically being granted by all the listed institutions in this appendix) and field records. First, spatial location of species was estimated using a distributional midpoint approach, where a unique spatial point derived from the distributional data per species is used as a predictor of range-size [29], [72]. Given that this study's question focuses on range-size variation across an environmental gradient, I used the adjusted latitudinal midpoint (ALM) variable as an indicator of species distribution, which integrates in a single scale the climatic variation from decreasing environmental temperature in latitude and elevation [42], [73]. The ALM is calculated based on the assumption that temperatures in elevational transects decrease 0.65°C for each 100 m of increased elevation [42], [73]. To correct the dataset for latitudinal and elevational covariation in temperature, Cruz et al. [42] obtained a correction factor, computed for the latitudinal range occupied by Liolaemus lizards (based on the above 0.65°C for each 100 m) that consists in adding 1.752° (latitude) for every 200 m increases in elevation on altitudinal midpoint values higher than 699 m above sea level. Thus, Cruz et al. [42] derived a corrected latitudinal value for latitude and elevational thermal covariation with the formula y = 0.009x–6.2627, where x represents the altitudinal midpoint for each species, and y the corrected temperature for latitude, which is added to the latitudinal midpoint for each species. This results in ALM values for South American areas where Liolaemus occur [42]. The ALM scale is intuitively simple to interpret, as increasing ALM values represent the integrated effect of increasing latitude and elevation, and hence, a decrease in environmental temperature. Then, range-size variation was analyzed separately for latitudinal and elevational ranges as a function of ALM, where the minimum and maximum records of latitudinal and elevational distribution per species were taken as the limits of the range. Both variables were selected because they are expected to reflect the magnitude of species tolerance to different climatic and ecological conditions experienced by a single species.
Body size data were obtained from a total sample of 4,554 specimens (Table S1). I used snout-vent length (SVL) as a proxy for body size. SVL is the standard body size measure in lizards as it is simple to measure in living and preserved specimens, and covaries with ecological, life history and morphological traits [74], [75], [76], [77]. Given that lizards continue to grow after sexual maturity, it is difficult to estimate standard body size. Therefore, it has been suggested that intermediate percentiles between the mean body size and the largest recorded specimen (both extensively used) provide better estimates of adult size (e.g., [78]). Hence, SVL was obtained using means from the largest two-thirds of the adult samples (e.g., [38], [79]) to avoid under- or overestimations of body size. For analyses, a single SVL value per species was obtained by averaging male and female SVL averages. This approach is more appropriate than pooling all available adult specimens per species to calculate a single mean, as the average would be influenced by the number of males and females in the sample, and hence, by the overall frequency distribution of body size. I then calculated sexual size dimorphism (SSD) with the formula ln(male size/female size) [80]. SSD was then included in the regression models (as an additional predictor) where body size is the main predictor, in order to account for a potential effect of the magnitude of size differences between the sexes, which are in turn obscured by a single species SVL value.
Statistical analyses and phylogenetic control
A comparative approach based on species-level data was employed. Prior to statistical analyses, variables were ln-transformed to reduce skew and homogenize variances [81]. To investigate the questions whether range-size variation among species is a function of variation in midpoint geographical location (ALM) and of body size (SVL), regression analyses were performed. Given that trait expressions and environments recorded in closely related species can be influenced by their common phylogenetic history, data points from related species in a clade cannot be regarded as independent values for statistical analyses [82], [83]. Therefore, I conducted the same regressions employing, first, conventional (non-phylogenetic assuming a star phylogeny), and then phylogenetic statistical analyses to account for potential phylogenetic effects and infer correlated evolution between variables. Results from both analyses are reported to evaluate the consistency of expected effects of predictors (ALM and SVL, separately) on the response variable (range-size).
For phylogenetic analyses, I used a Liolaemus phylogeny (Fig. S1) containing 68 of the 121 species for which data were available (and which were included in non-phylogenetic analyses). For analyses of body size and range-size, SVL data were missing for three of the species in the phylogeny (Table S1), and hence, these phylogenetic analyses were reduced to 65 species. The phylogeny was derived from two previous phylogenetic hypotheses inferred by Espinoza et al. [49] and Abdala [84]. Phylogenetic studies of evolutionary relationships within Liolaemus have consistently revealed the existence of a major monophyletic clade nested within the genus, known as boulengeri complex (e.g., [43], [49], [84], [85]), which has recently been studied by Abdala [84]. Therefore, I used Espinoza et al.'s [49] tree as the basis for the Liolaemus phylogeny, but replaced the monophyletic boulengeri complex with that of Abdala [84] since this phylogenetic hypothesis contains a larger number of species sampled in my dataset. Since these two phylogenetic trees were inferred using combined molecular and morphological data [49], [84], branch lengths were set equal to 1.0, and a speciational Brownian motion model of evolutionary change was employed for phylogenetic analyses [49], [86], [87]. Then, Felsenstein's standardized phylogenetic independent contrasts (PIC) [82] were calculated from this phylogeny using the software COMPARE version 4.6b [88]. I obtained standardized PIC for all the variables involved in the analyses (given that this approach results in n-1 independent contrasts, phylogenetic regressions contain 67 and 64 values for the 68-species and 65-species trees, respectively). With PIC, the degree of covariation between variables reflects the potential (but not causation) for these variables to have been functionally related during evolutionary change (e.g. evolutionary dependence between two traits is inferred if large changes in the contrasts of one variable are paralleled by large changes in the contrasts of the other). Regressions based on PIC were forced through the origin [82], [83], [89].
Supporting Information
Phylogenetic relationships of Liolaemus lizard species inferred from combined molecular and morphological data (according to refs. [49], [84]). See main text for details.
(TIF)
Summary of Liolaemus species included in this study.
(DOC)
This appendix contains references to the institutions that kindly provided permission to study their Liolaemus collections. Important part of the data used in this study comes from these museum collections (see Materials and methods).
(DOCX)
Acknowledgments
I thank Jan Stipala and anonymous referees for thoughtful critical comments on earlier versions of this manuscript. I am also indebted to J.M. Cei (JMC-DC), M. Contreras, E. Solar and J. Artigas (MZUC), R. Gemel and H. Grillitsch (NHMV), N. Ibargüengoytía (UNComahue), I. Ineich (MNHNP), C. McCarthy (NHML), J. Navarro (DBGUCH), H. Nuñez (MNHN), M. Oliver-Roedel (ZMB), E. Pereyra (IBAUNC), J. Scolaro (CENPAT, JAS-DC), F. Videla (IADIZA), for providing unrestricted access to Liolaemus specimens, and to J.M. Cei for unpublished data on some species. Finally, I thank CRIDESAT (University of Atacama) for an honorary fellowship.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The author is indebted to the Leverhulme Trust for support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gaston KJ. The structure and dynamics of geographic ranges. Oxford: Oxford University Press; 2003. [Google Scholar]
- 2.Wiens JJ. Speciation and ecology revisited: phylogenetic niche conservatism and the origin of species. Evolution. 2004;58:193–197. doi: 10.1111/j.0014-3820.2004.tb01586.x. [DOI] [PubMed] [Google Scholar]
- 3.Thomas CD. Climate, climate change and range boundaries. Diversity and Distributions. 2010;16:488–495. [Google Scholar]
- 4.Hoglund J. Evolutionary conservation genetics. Oxford: Oxford University Press; 2009. [Google Scholar]
- 5.Gaston KJ. Geographic range limits: achieving synthesis. Proceedings of the Royal Society of London, Biological Sciences. 2009;276:1395–1406. doi: 10.1098/rspb.2008.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hewitt GM, Nichols RA. Genetic and evolutionary impacts of climate change. In: Lovejoy TE, Hannah L, editors. Climate change and biodiversity. New Haven & London: Yale University Press; 2005. pp. 176–192. [Google Scholar]
- 7.Parmesan C. Biotic responses: range and abundance changes. In: Lovejoy TE, Hannah L, editors. Climate change and biodiversity. New Haven & London: Yale University Press; 2005. pp. 41–55. [Google Scholar]
- 8.Parmesan C. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution and Systematics. 2006;37:637–669. [Google Scholar]
- 9.Parmesan C, Ryrholm N, Stefanescu C, Hillk JK, Thomas CD, et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature. 1999;399:579–583. [Google Scholar]
- 10.Thomas CD, Franco AMA, Hill JK. Range retractions and extinction in the face of climate warming. Trends in Ecology & Evolution. 2006;21:415–416. doi: 10.1016/j.tree.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Rosenzweig C, Casassa G, Karoly DJ, Imeson A, Liu C, et al. Assessment of observed changes and responses in natural and managed systems. In: Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE, editors. Climate Change 2007 Impacts, Adaptation and Vulnerability Working Group II Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press; 2007. pp. 79–131. [Google Scholar]
- 12.Williams SE, Bolitho EE, Fox S. Climate change in Australian tropical rainforests: an impending environmental catastrophe. Proceedings of the Royal Society of London, Biological Sciences. 2003;270:1887–1892. doi: 10.1098/rspb.2003.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson RJ, Gutierrez D, Martinez D, Agudo R, Monserrat VJ. Changes to the elevational limits and extent of species ranges associated with climate change. Ecology Letters. 2005;8:1138–1146. doi: 10.1111/j.1461-0248.2005.00824.x. [DOI] [PubMed] [Google Scholar]
- 14.Pounds JA, Bustamante MR, Coloma LA, Consuegra JA, Fogden MPL, et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature. 2006;439:161–167. doi: 10.1038/nature04246. [DOI] [PubMed] [Google Scholar]
- 15.Pounds JA, Fogden MPL, Campbell JH. Biological response to climate change on a tropical mountain. Nature. 1999;398:611–615. [Google Scholar]
- 16.Thomas CD, Lennon JJ. Birds extend their ranges northwards. Nature. 1999;399:213. [Google Scholar]
- 17.Chevin LM, Lande R, Mace GM. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biology. 2010;8:e1000357. doi: 10.1371/journal.pbio.1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massot M, Clobert J, Ferriere R. Climate warming, dispersal inhibition and extinction risk. Global Change Biology. 2008;14:461–469. [Google Scholar]
- 19.Bradshaw WE, Holzapfel CM. Evolutionary response to rapid climate change. Science. 2006;312:1477–1478. doi: 10.1126/science.1127000. [DOI] [PubMed] [Google Scholar]
- 20.Thomas CD, Bodsworth EJ, Wilson RJ, Simmons AD, Davies ZG, et al. Ecological and evolutionary processes at expanding range margins. Nature. 2001;411:577–581. doi: 10.1038/35079066. [DOI] [PubMed] [Google Scholar]
- 21.Huey RB, Losos JB, Moritz C. Are lizards toast? Science. 2010;328:832–833. doi: 10.1126/science.1190374. [DOI] [PubMed] [Google Scholar]
- 22.Sinervo B, Méndez-de-la-Cruz F, Miles DB, Heulin B, Bastiaans E, et al. Erosion of lizard diversity by climate change and altered thermal niches. Science. 2010;328:894–899. doi: 10.1126/science.1184695. [DOI] [PubMed] [Google Scholar]
- 23.Thomas CD. Recent evolutionary effects of climate change. In: Lovejoy TE, Hannah L, editors. Climate change and biodiversity. New Haven & London: Yale University Press; 2005. pp. 75–88. [Google Scholar]
- 24.Parmesan C. Influences of species, latitudes andmethodologies on estimates of phenological response to global warming. Global Change Biology. 2007;13:1860–1872. [Google Scholar]
- 25.La Sorte FA, Jetz W. Projected range contractions of montane biodiversity under global warming. Proceedings of the Royal Society of London, Biological Sciences. 2010;277:3401–3410. doi: 10.1098/rspb.2010.0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiens JJ. The causes of species richness patterns across space, time, and clades and the role of “ecological limits”. Quarterly Review of Biology. 2011;86:75–96. doi: 10.1086/659883. [DOI] [PubMed] [Google Scholar]
- 27.Rapoport EH. Areography. Geographical strategies of species. Oxford: Pergamon Press; 1982. [Google Scholar]
- 28.Stevens GC. The latitudinal gradient in geographical range: how so many species coexist in the tropics. American Naturalist. 1989;133:240–256. [Google Scholar]
- 29.Gaston KJ, Blackburn TM, Spicer JI. Rapoport's rule: time for an epitaph? Trends in Ecology & Evolution. 1998;13:70–74. doi: 10.1016/s0169-5347(97)01236-6. [DOI] [PubMed] [Google Scholar]
- 30.Brown JH, Sibly RM. Life-history evolution under a production constraint. Proceedings of the National Academy of Sciences, USA. 2006;103:17595–17599. doi: 10.1073/pnas.0608522103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steven AJ. Body size, dispersal ability, and range size in North American species of Brachinus (Coleoptera: Carabidae). The Coleopterists Bulletin. 1983;37:232–238. [Google Scholar]
- 32.Brown JH, Maurer BA. Macroecology: the division of food and space among species on continents. Science. 1989;243:1145–1150. doi: 10.1126/science.243.4895.1145. [DOI] [PubMed] [Google Scholar]
- 33.Etienne RS, Olff H. How dispersal limitation shapes species-body size distributions in local communities. American Naturalist. 2004;163:69–83. doi: 10.1086/380582. [DOI] [PubMed] [Google Scholar]
- 34.Peters RH. The ecological implications of body size. Cambridge: Cambridge University Press; 1983. [Google Scholar]
- 35.Bowler DE, Benton TG. Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biological Reviews. 2005;80:205–225. doi: 10.1017/s1464793104006645. [DOI] [PubMed] [Google Scholar]
- 36.Pincheira-Donoso D, Scolaro JA, Sura P. A monographic catalogue on the systematics and phylogeny of the South American iguanian lizard family Liolaemidae (Squamata, Iguania). Zootaxa. 2008:1–85. [Google Scholar]
- 37.Pincheira-Donoso D, Hodgson DJ, Stipala J, Tregenza T. A phylogenetic analysis of sex-specific evolution of ecological morphology in Liolaemus lizards. Ecological Research. 2009;24:1223–1231. [Google Scholar]
- 38.Pincheira-Donoso D, Hodgson DJ, Tregenza T. The evolution of body size under environmental gradients in ectotherms: why should Bergmann's rule apply to lizards? BMC Evolutionary Biology. 2008;8:68. doi: 10.1186/1471-2148-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cei JM. Reptiles del centro, centro-oeste y sur de la Argentina. Herpetofauna de las zonas áridas y semiáridas. Torino: Museo Regionale di Scienze Naturali di Torino; 1986. 527 [Google Scholar]
- 40.Ibargüengoytía N, Medina SM, Fernández JB, Gutiérrez JA, Tappari F, et al. Thermal biology of the southernmost lizards in the world: Liolaemus sarmientoi and Liolaemus magellanicus from Patagonia, Argentina. Journal of Thermal Biology. 2010;35:21–27. [Google Scholar]
- 41.Labra A, Pienaar J, Hansen TF. Evolution of thermal physiology in Liolaemus lizards: adaptation, phylogenetic inertia, and niche tracking. American Naturalist. 2009;174:204–220. doi: 10.1086/600088. [DOI] [PubMed] [Google Scholar]
- 42.Cruz FB, Fitzgerald LA, Espinoza RE, Schulte JA. The importance of phylogenetic scale in tests of Bergmann's and Rapoport's rules: lessons from a clade of South American lizards. Journal of Evolutionary Biology. 2005;18:1559–1574. doi: 10.1111/j.1420-9101.2005.00936.x. [DOI] [PubMed] [Google Scholar]
- 43.Schulte JA, Macey JR, Espinoza RE, Larson A. Phylogenetic relationships in the iguanid lizard genus Liolaemus: multiple origins of viviparous reproduction and evidence for recurring Andean vicariance and dispersal. Biological Journal of the Linnean Society. 2000;69:75–102. [Google Scholar]
- 44.Pincheira-Donoso D, Tregenza T. Fecundity selection and the evolution of reproductive output and sex-specific body size in the Liolaemus lizard adaptive radiation. Evolutionary Biology. 2011;38:197–207. [Google Scholar]
- 45.Janzen DH. Why mountain passes are higher in the tropics? American Naturalist. 1967;101:233–249. [Google Scholar]
- 46.Addo-Bediako A, Chown SL, Gaston KJ. Thermal tolerance, climatic variability and latitude. Proceedings of the Royal Society of London, Biological Sciences. 2000;267:739–745. doi: 10.1098/rspb.2000.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zani PA, Swanson SET, Corbin D, Cohnstaedt LW, Agotsch MD, et al. Geographic variation in tolerance of transient thermal stress in the mosquito Wyeomyia smithii. Ecology. 2005;86:1206–1211. [Google Scholar]
- 48.Reed RN. Interspecific patterns of species richness, geographic range size, and body size among New World venomous snakes. Ecography. 2003;26:107–117. [Google Scholar]
- 49.Espinoza RE, Wiens JJ, Tracy CR. Recurrent evolution of herbivory in small, cold-climate lizards: breaking the ecophysiological rules of reptilian herbivory. Proceedings of the National Academy of Sciences, USA. 2004;101:16819–16824. doi: 10.1073/pnas.0401226101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuentes ER, Jaksic FM. Lizards and rodents: an explanation for their relative species diversity in Chile. Archivos de Biología y Medicina Experimental. 1979;12:179–190. [Google Scholar]
- 51.Jaksic FM. Ecología de los vertebrados de Chile. Santiago: Ediciones de la Universidad Católica de Chile; 1998. [Google Scholar]
- 52.Gomez-Molina E, Little AV. Geoecology of the Andes: the natural science basis for research planning. Mountain Research and Development. 1981;1:115–144. [Google Scholar]
- 53.Squeo FA, Veit H, Arancio G, Gutierrez JR, Arroyo MTK, et al. Spatial heterogeneity of high mountain vegetation in the Andean desert zone of Chile. Mountain Research and Development. 1993;13:203–209. [Google Scholar]
- 54.Carothers JH, Jaksic FM, Marquet PA. Altitudinal zonation among lizards of the genus Liolaemus: questions answered and unanswered questions. Revista Chilena de Historia Natural. 2001;74:313–316. [Google Scholar]
- 55.Shine R. Life-history evolution in reptiles. Annual Reviews of Ecology, Evolution and Systematics. 2005;36:23–46. [Google Scholar]
- 56.Lee MSY, Shine R. Reptilian viviparity and Dollo's Law. Evolution. 1998;52:1441–1450. doi: 10.1111/j.1558-5646.1998.tb02025.x. [DOI] [PubMed] [Google Scholar]
- 57.Nagy L, Grabherr G. The biology of alpine habitats. Oxford: Oxford University Press; 2009. [Google Scholar]
- 58.Scolaro JA. Reptiles patagónicos sur. Una guía de campo. Trelew: Editorial Universidad Nacional de la Patagonia; 2005. [Google Scholar]
- 59.Díaz-Gómez JM. Historical biogeography of Phymaturus (Iguania: Liolaemidae) from Andean and Patagonian South America. Zoologica Scripta. 2009;38:1–7. [Google Scholar]
- 60.Scolaro JA, Pincheira-Donoso D. Lizards at the end of the world: two new species of Phymaturus of the patagonicus clade (Squamata, Liolaemidae) revealed in southern Patagonia of Argentina. Zootaxa. 2010;2393:17–32. [Google Scholar]
- 61.del Barrio G, Harrison PA, Berry PM, Butt N, Sanjuan ME, et al. Integrating multiple modelling approaches to predict the potential impacts of climate change on species' distributions in contrasting regions: comparison and implications for policy. Environmental Science & Policy. 2006;9:129–147. [Google Scholar]
- 62.IPCC. Climate change 2007. Impacts, adaptation and vulnerability. Intergovernmental Panel on Climate Change. Group II. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- 63.Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, et al. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- 64.Korner C. Mountain biodiversity, its causes and functions: an overview. In: Korner C, Spehn EM, editors. Mountain biodiversity A global assessment. London: Parthenon Publishing; 2002. pp. 3–20. [Google Scholar]
- 65.Hughes KA, Sawby R. Genetic variability and life-history evolution. In: Ferriere R, Dieckmann U, Couvet D, editors. Evolutionary conservation biology. Cambridge: Cambridge University Press; 2004. pp. 119–135. [Google Scholar]
- 66.Bauwens D, Hertz PE, Castilla AM. Thermoregulation in a lacertid lizard: the relative contributions of distinct behavioral mechanisms. Ecology. 1996;77:1818–1830. [Google Scholar]
- 67.Miles DB, Sinervo B, Anthony FW. Reproductive burden, locomotor performance, and the cost of reproduction in free ranging lizards. Evolution. 2000;54:1386–1395. doi: 10.1111/j.0014-3820.2000.tb00570.x. [DOI] [PubMed] [Google Scholar]
- 68.Pincheira-Donoso D, Tregenza T, Hodgson DJ. Body size evolution in South American Liolaemus lizards of the boulengeri clade: a contrasting reassessment. Journal of Evolutionary Biology. 2007;20:2067–2071. doi: 10.1111/j.1420-9101.2007.01394.x. [DOI] [PubMed] [Google Scholar]
- 69.Cei JM. Reptiles del noroeste, nordeste y este de la Argentina. Herpetofauna de las selvas subtropicales, puna y pampas. Torino: Museo Regionale di Scienze Naturali di Torino; 1993. 947 [Google Scholar]
- 70.Pincheira-Donoso D, Hodgson DJ, Tregenza T. Comparative evidence for strong phylogenetic inertia in precloacal signalling glands in a species-rich lizard clade. Evolutionary Ecology Research. 2008;10:11–28. [Google Scholar]
- 71.Scolaro JA. Reptiles patagónicos norte. Una guía de campo. Comodoro Rivadavia: Editorial Universidad Nacional de la Patagonia; 2006. 112 [Google Scholar]
- 72.Rohde K, Heap M, Heap D. Rapoport's rule does not apply to marine teleosts and cannot explain latitudinal gradients in species richness. American Naturalist. 1993;142:1–16. [Google Scholar]
- 73.Lutgens FK, Tarbuck EJ. The atmosphere. An introduction to meteorology. New Jersey: Prentice Hall; 1998. [Google Scholar]
- 74.Meiri S. Evolution and ecology of lizard body sizes. Global Ecology and Biogeography. 2008;17:724–734. [Google Scholar]
- 75.Meiri S. Length-weight allometries in lizards. Journal of Zoology. 2010;281:218–226. [Google Scholar]
- 76.Pincheira-Donoso D, Fox SF, Scolaro JA, Ibargüengoytía N, Acosta JC, et al. Body size dimensions in lizard ecological and evolutionary research: exploring the predictive power of mass estimation equations in two Liolaemidae radiations. Herpetological Journal. 2011;21:35–42. [Google Scholar]
- 77.Pough FH, Andrews RM, Cadle JE, Crump ML, Savitzky AH, et al. Herpetology. New Jersey: Pearson, Prentice Hall; 2004. [Google Scholar]
- 78.Brown RP, Znari M, El Mouden ELH, Harris P. Estimating asymptotic body size and testing geographic variation in Agama impalearis. Ecography. 1999;22:277–283. [Google Scholar]
- 79.Losos JB, Butler M, Schoener TW. Sexual dimorphism in body size and shape in relation to habitat use among species of Caribbean Anolis lizards. In: Fox SF, McCoy JK, Baird TA, editors. Lizard social behaviour. Baltimore and London: John Hopkins University Press; 2003. pp. 356–380. [Google Scholar]
- 80.Smith RJ. Statistics of sexual size dimorphism. Journal of Human Evolution. 1999;36:423–459. doi: 10.1006/jhev.1998.0281. [DOI] [PubMed] [Google Scholar]
- 81.Zar JH. Biostatistical analysis. New Jersey: Pearson International; 2009. [Google Scholar]
- 82.Felsenstein J. Phylogenies and the comparative method. American Naturalist. 1985;125:1–15. doi: 10.1086/703055. [DOI] [PubMed] [Google Scholar]
- 83.Harvey PH, Pagel MD. The comparative method in evolutionary biology. Oxford: Oxford University Press; 1991. [Google Scholar]
- 84.Abdala CS. Phylogeny of the boulengeri group (Iguania: Liolaemidae, Liolaemus) based on morphological and molecular characters. Zootaxa. 2007;1538:1–84. [Google Scholar]
- 85.Pincheira-Donoso D, Scolaro JA, Schulte JA. The limits of polymorphism in Liolaemus rothi: molecular and phenotypic evidence for a new species of the Liolaemus boulengeri clade (Iguanidae, Liolaemini) from boreal Patagonia of Chile. Zootaxa. 2007;1452:25–42. [Google Scholar]
- 86.Garland T, Dickerman AW, Janis CM, Jones JA. Phylogenetic analysis of covariance by computer simulation. Systematic Biology. 1993;42:265–292. [Google Scholar]
- 87.Martins EP, Garland T. Phylogenetic analyses of the correlated evolution of continuous characters: a simulation study. Evolution. 1991;45:534–557. doi: 10.1111/j.1558-5646.1991.tb04328.x. [DOI] [PubMed] [Google Scholar]
- 88.Martins EP. COMPARE, version 4.6b. 2004. Computer programs for the statistical analysis of comparative data. Distributed by the author at http://compare.bio.indiana.edu/. Indiana: Department of Biology, Indiana University.
- 89.Garland T, Harvey PH, Ives AR. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Systematic Biology. 1992;41:18–32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic relationships of Liolaemus lizard species inferred from combined molecular and morphological data (according to refs. [49], [84]). See main text for details.
(TIF)
Summary of Liolaemus species included in this study.
(DOC)
This appendix contains references to the institutions that kindly provided permission to study their Liolaemus collections. Important part of the data used in this study comes from these museum collections (see Materials and methods).
(DOCX)