Abstract
Background
Flowering reversion can be induced in soybean (Glycine max L. Merr.), a typical short-day (SD) dicot, by switching from SD to long-day (LD) photoperiods. This process may involve florigen, putatively encoded by FLOWERING LOCUS T (FT) in Arabidopsis thaliana. However, little is known about the potential function of soybean FT homologs in flowering reversion.
Methods
A photoperiod-responsive FT homologue GmFT (renamed as GmFT2a hereafter) was cloned from the photoperiod-sensitive cultivar Zigongdongdou. GmFT2a gene expression under different photoperiods was analyzed by real-time quantitative PCR. In situ hybridization showed direct evidence for its expression during flowering-related processes. GmFT2a was shown to promote flowering using transgenic studies in Arabidopsis and soybean. The effects of photoperiod and temperature on GmFT2a expression were also analyzed in two cultivars with different photoperiod-sensitivities.
Results
GmFT2a expression is regulated by photoperiod. Analyses of GmFT2a transcripts revealed a strong correlation between GmFT2a expression and flowering maintenance. GmFT2a transcripts were observed continuously within the vascular tissue up to the shoot apex during flowering. By contrast, transcripts decreased to undetectable levels during flowering reversion. In grafting experiments, the early-flowering, photoperiod-insensitive stock Heihe27 promotes the appearance of GmFT2a transcripts in the shoot apex of scion Zigongdongdou under noninductive LD conditions. The photothermal effects of GmFT2a expression diversity in cultivars with different photoperiod-sensitivities and a hypothesis is proposed.
Conclusion
GmFT2a expression is associated with flowering induction and maintenance. Therefore, GmFT2a is a potential target gene for soybean breeding, with the aim of increasing geographic adaptation of this crop.
Introduction
The timely transition to flowering is crucial for successful plant reproduction. As such, this process is controlled by both endogenous and environmental signals. Arabidopsis thaliana contains at least four flowering pathways that are responsive to these cues including the gibberellin, autonomous, photoperiod and vernalization flowering pathways [1]–[4]. Therefore, a complex flowering regulatory network exists that includes many known genes such as FLOWERING LOCUS T (FT), CONSTANS (CO), FLOWERING LOCUS C (FLC), APETALA1 (AP1), and SUPPRESSOR OF OVEREXPRESSION OF CO 1 (SOC1) [5]–[7]. FT, the putative florigen gene, is central to this network. FT functions by integrating signals from various pathways to regulate flowering time [8], [9]. FT and its homologues, belonging to the FT-like subfamily of the PEBP (phosphatidylethanolamine binding domain) family, promote flowering [10], [11]. Members of the FT-like subfamily are most highly expressed in leaves in response to inductive photoperiods. In Arabidopsis, an inductive LD photoperiod activates FT expression to regulate flowering [12], [13] and there is a similar case for Hd3a in rice in inductive SD photoperiods [14]. From evidence of grafting experiments, FT protein can be transported from the leaves to the shoot apex to induce flowering [15]–[18]. It is therefore believed that either FT protein is itself florigen, or else it is a potential component of florigen [11], [18]–[20]. Li et al. [21] found that FT mRNA can mediate long-distance trafficking of heterologous RNAs, indicating that FT mRNA may also function in florigen transportation. However, the role it plays in doing so is still a conundrum. Therefore, FT plays an important role in flowering transition, but more details are needed to elucidate the mechanism of florigen transportation.
Flowering transition can be reversed. In some species, flowering reversion occurs after specific treatments [22]–[25]. Soybean is one such species, in which flowering will be reversed in response to LD. It is expected that FT should be involved in flowering reversion, and Molinero-Rosales et al. [26] demonstrated that the tomato FT mutant sft displays a flowering-reversion phenotype with some sepals converted into leaves in the first floral whorl. Nevertheless, details are quite absent and additional studies will be necessary to elucidate if and how FT functions in flowering reversion.
In addition to photoperiod, temperature is also an important environmental cue for successful reproduction. FT is also likely involved in this response. High temperatures promote Arabidopsis flowering via the autonomous pathway [27] and this effect is related to FT expression [28]–[31]. In Arabidopsis, SHORT VEGETATIVE PHASE (SVP) mediates the response to ambient temperatures through the negative regulation of FT expression [31], [32]. In Satsuma mandarin, low temperature specifically affected CiFT transcripts in adult plants but not in juveniles [33]. The Arabidopsis phyB mutant exhibits a temperature-sensitive precocious-flowering phenotype [29]. It is suggested that flowering is controlled by interactions between photoperiod and temperature, although the underlying mechanism remains unclear.
Soybean, an SD dicot crop of economic and agricultural importance, includes many cultivars with diverse photoperiod-sensitivities. The photoperiod-sensitive late-flowering cultivar Zigongdongdou (from Zigong, Sichuan Province in south China) does not flower during long days or in northern regions [34]. The photoperiod-insensitive early-flowering cultivar Heihe27 (from Heihe, Heilongjiang Province in northeast China) fails to produce high yields in southern regions due to the high temperatures that occur there [35]. Following a shift from SD to LD, flowering reversion will occur in Zigongdongdou, but not in Heihe27 [24], [25], [36]. With Zigongdongdou as a model plant, Wu et al. [25] established an effective experimental system to study flowering mechanisms in both forward and reverse directions. Therefore, these two cultivars are very suitable models for addressing questions about the mechanisms involved in flowering transition and maintenance.
To date, based upon the soybean genome draft sequence [37], two reports have been made concerning FT-like genes in the soybean genome [10], [38]. Thakare et al. [38] found nine FT-like genes: Glyma16g26660, Glyma16g26690, Glyma08g47810, Glyma08g47820, Glyma18g53690, Glyma18g53680, Glyma16g04830, Glyma19g28400 and Glyma19g28390. Two of these genes, Glyma16g26660 and Glyma16g04830, can be detected by real-time quantitative PCR and their expression is inhibited by LD photoperiods. Kong et al. [10] identified an additional FT-like gene (Glyma16g04840), cloned six of the ten FT-like genes, and confirmed that transgenic overexpression of GmFT2a (Glyma16g26660) and GmFT5a (Glyma16g04830) can promote precocious flowering in Arabidopsis. GmFT2a and GmFT5a respond differently to photoperiod, and PHYA may be involved [10]. However, the relationship between these two genes and the flowering process need to be further elucidated.
This study reports the cloning of a new soybean FT homologue GmFT (later renamed to GmFT2a as Kong et al. first designated [10]) and the characterization of its expression using real-time quantitative PCR and in situ hybridization during flowering and flowering reversion. The ability of GmFT2a to promote flowering in Arabidopsis and soybean was analyzed. Additionally, GmFT2a expression in Zigongdongdou and Heihe27 grown under different photoperiods and temperatures was examined. The results indicate that GmFT2a may play an important role, not only in flowering transition, but also in flowering maintenance. Furthermore, the impact of temperature on GmFT2a is dependent on photoperiod sensitivity.
Results
Molecular Cloning and bioinformatic analysis of GmFT
GmFT (Genbank accession number EU287455), a soybean homolog of FT, was cloned, based on an EST isolated from a Zigongdongdou SSH library. GmFT contains 888 base pairs (bp) and encodes a protein of 176 amino acids (shown in Supporting Information S1). GmFT includes a conserved PEBP domain and belongs to the FT-like subfamily of the PEBP family. To date, the soybean genome data is available at http://www.phytozome.net/soybean.php. This genome contains at minimum nine, and possibly ten, putative soybean FT-like genes [10], [38]. By aligning GmFT with Glyma16g26660 (shown in Supporting Information S1), GmFT was shown to fully contain the Glyma16g26660 putative transcript, i.e., GmFT2a as described by Kong et al. [10], therefore, GmFT was renamed to GmFT2a hereafter although GmFT contains 5′ and 3′ UTR sequences which are absent from GmFT2a [10]. Shown in Supporting Information S1, GmFT2a has a conserved gene structure with other FTs. GmFT2a has greater sequence similarity to FT than to TFL1, especially in the external loop related to the FT/TFL activity [39]. A key amino acid residue, Tyr85 (FT)/His88 (TFL1), differentiates the activities of FT and TFL1 [40]. GmFT2a contains the same Tyr85 as FT. Therefore, it is reasonable to hypothesize that GmFT2a can promote flowering as FT does.
GmFT2a is primarily expressed in leaves and regulated by photoperiods
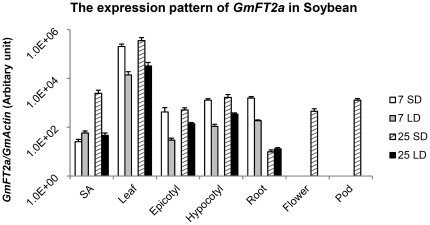
Seeds of the cultivar Zigongdongdou were planted and maintained under LD conditions until the unifoliates were fully expanded. One set of plants were then grown under LD conditions and another set under SD conditions. In the course of the experiment, the plants grown under SD flowered at the 25th day of this condition, but the plants under LD did not flower. GmFT2a expression was profiled with real-time quantitative PCR using GmActin as a reference. GmFT2a expression in the leaves was higher than in other tissues or organs. Moreover, inductive SD photoperiods significantly increased GmFT2a expression with the exception of 7-day-treated shoot apices or 25-day-treated roots (Figure 1). These results are consistent with FT [41], GmFT2a and GmFT5a [10]. Therefore, photoperiod may regulate GmFT2a expression to control the soybean flowering process. Notably, GmFT2a was detected in both flowers and pods (Figure 1), suggesting that GmFT2a may function in reproductive development.
Figure 1. The expression pattern of GmFT2a in soybean.
The expression level of GmFT2a in different tissues or organs was detected by real-time quantitative PCR at 7 or 25 days after the first pair of unifoliate leaves fully expanded under short days (SD) or long days (LD). GmACTIN was used as a control gene. The data of flower and pod were not available for 7 SD, 7 LD or 25 LD, as cultivar Zigongdongdou did not flower at that time. SA, shoot apex (not including any manually removable leaves). For each real-time quantitative PCR point, the averages and standard errors are the result of three replications.
LD treatments postpone the diurnal peak of GmFT2a gene expression
From two cultivars, late-flowering photoperiod-sensitive Zigongdongdou and early-flowering photoperiod-insensitive Heihe27, unifoliate leaves were sampled every 3 hours since 1 hour after dawn. We isolated RNA from these leaves and analyzed the diurnal circadian rhythm of GmFT2a gene expression by quantitative real-time PCR (Figure 2). In Zigongdongdou, SD induced the expression of GmFT2a, consistently with previous results. In Heihe27, the gene expression level was higher than in Zigongdongdou under both photoperiods, which could explain the fact that Heihe27 is early-flowering and photoperiod-insensitive. For these two cultivars, GmFT2a exhibited a diurnal circadian rhythm both under SD and LD conditions. Under SD conditions, GmFT2a gene expression reached a peak 4 h and 7 h after dawn for Zigongdongdou and Heihe27 respectively, which was similar with the results of Kong et al. [10]. Under LD conditions, the expression peak was postponed for about 3 hours for both cultivars. It was suggested that GmFT2a gene expression might be regulated by circadian clock.
Figure 2. The diurnal circadian rhythm of GmFT2a gene expression under SD and LD conditions.
Unifoliate leaves were sampled every 3 hours since 1 hour after dawn. White bars at the bottom represent light phases, and black bars dark phases. ZG SD, Zigongdongdou under SD conditions; ZG LD, Zigongdongdou under LD conditions; HH SD, Heihe27 under SD conditions; and HH LD, Heihe27 under LD conditions.
GmFT2a accompanies flower development
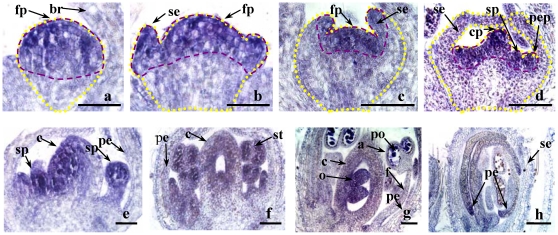
GmFT2a expression during flowering was analyzed using in situ hybridization. GmFT2a transcripts initially appeared in the upper portion of the global floral primordia (Figure 3a) and then spread to the sepals and sepal primordia (Figure 3b). During sepal development, GmFT2a expression gradually decreased from the base to the apex of young sepals (Figure 3c). When the primordia of petals, stamens and carpels appeared, the GmFT2a levels of these primordia were robust (Figure 3d) while the GmFT2a levels in the young petals were attenuated (Figure 3e). GmFT2a transcripts at the stamen apex were detected throughout stamen development. During the development of the carpel, the GmFT2a transcripts gradually accumulated in the inner carpel but attenuated at the carpel periphery (Figure 3e,f). GmFT2a expression at the ovary wall disappeared gradually but increased in the new-borne ovules (Figure 3f,g). GmFT2a transcripts were also present at the edge of the petal (Figure 3h). These results indicate that during flower development, GmFT2a transcripts are continuously present in the primordia of flower organs where cell division is vigorous. These data also suggest that GmFT2a plays important roles in flower development.
Figure 3. GmFT2a expression during flower development.
(a) Global floral primordia. (b) Floral primordia with emerging sepals. (c) Floral primordia with sepals. (d) Flower structure initially formed with primordia of petal, stamen and pistil. (e) Developing carpel and stamen. (f) Initially formed ovary and stamen. (g) Ovary with ovules and developed stamen with pollen. (h) Flower bud. The profile of the flower primordia is enclosed with yellow dashes, and the GmFT2a signals are contained mainly in the region enclosed with red dashes. a, anther; br, bract; c, carpel; cp, carpel primordium; f, filament; fp, flower primordium; p, pistil; pe, petal; po, pollen; se, sepal; sp, stamen primordium; st, stamen. Bar, 250 µM.
LD induces flowering reversion with GmFT2a expression decreasing
GmFT2a expression was analyzed by in situ hybridization in Zigongdongdou under different photoperiods. Under LD conditions, Zigongdongdou retains its vegetative growth [36]. Consistent with this, GmFT2a transcripts were barely detected in the apical or lateral meristems at the 1st or 13th LD (briefed as LD1 and LD13, respectively, Figure 4b,c). Under SD conditions, Zigongdongdou flowers approximately 29 days after cotyledon expansion [36]. Although GmFT2a transcripts were not detected at the 1st SD (abbreviated SD1, Figure 4d), they did appear slightly in the apical and lateral meristems at SD7. The lateral floral meristems also appeared at SD7, indicating that the flowering transition had already occurred (Figure 4e). At SD13, inflorescence differentiation was initiated at the shoot apices as indicated by the formation of small and slim bracts, the appearance of sepal primordia, and the enlargement of the floral meristems and primordia. Consistent with the timing of inflorescence differentiation, GmFT2a transcripts appeared in the primordia of the flowers, sepals and pistils and strengthened in the floral meristems (Figure 4f). At SD16 and SD19, the terminal inflorescences were still differentiating with the floral meristems, petals, pistils and stamens being produced. During this time, GmFT2a was strongly detected in these developing floral meristems, organs and primordial, but weakly in the inflorescence meristems (Figure 4g,h). At SD22, GmFT2a appeared in the floral primordia and organs but attenuated, even disappeared, in the stamens and petals of developed flowers at their inflorescence base (Figure 4i). It is notable that the GmFT2a transcripts were consistently detected in the vascular tissues (Figure 4d–i), suggesting that GmFT2a transcripts might be consecutively transcribed locally or transported through vascular system to meristems and primordia. Zigongdongdou flowering is reversed if plants are shifted to LD conditions after 13 days of SD [25]. As shown in Figure 4j–l, GmFT2a expression was intense in the floral meristems and floral organ primordia at the 3rd, 6th, and 9th LDs after SD13 (abbreviated SD13–LD3, SD13–LD6, and SD13–LD9, respectively), with terminal inflorescence differentiation still occurring until SD13–LD9. At SD13–LD9, the inflorescence meristems were reversed to vegetative meristems with small stipules and trifoliolates formed (Figure 4l). At SD13–LD12, the shoot apices were completely shifted into vegetative growth and one reversed flower was formed. However, no GmFT2a transcripts were detected (Figure 4m), suggesting that LD-induced flowering reversion is related to GmFT2a attenuation. At SD13–LD15 and SD13–LD27, GmFT2a levels were significantly decreased, even undetectable, in stem apices (Figure 4n,o). These results indicate that GmFT2a transcripts are correlated with the maintenance of flower differentiation.
Figure 4. The expression pattern of GmFT2a in soybean cultivar Zigongdongdou shoot apices under different photoperiod conditions by in situ hybridization.
(a) Shoot apex detected by the sense GmFT2a probe as a control. All other shoot apices were detected by the antisense GmFT2a probe shown in (b)–(o). (a) Shoot apex at the 22nd day of SD conditions (briefed as SD22). (b) and (c) Shoot apexes at LD1 and LD13, respectively. (d)–(i) Shoot apices at SD1, SD7, SD13, SD16, SD19, and SD22, respectively. (j)–(o) Shoot apices treated by LD for 3, 6, 9, 12, 15, and 27 days after SD13, respectively. am, apical meristem; axm, axillary meristem; br, bract; c, carpel; fm, floral meristem; fp, floral primordium; im, inflorescence meristem; ip, inflorescence primordium; le, leaf/trifoliolate leaf; p, pistil; pe, petal; pep, petal primordium; pp, pistil primordium; rf, reversed flower; s, stamen; se, sepal; sep, sepal primordium; sp, stamen primordium; tlp, trifoliolate leaf primordium. Bar, 250 µM.
Early-flowering stock Heihe27 promotes the appearance of GmFT2a transcripts in the late-flowering scion Zigongdongdou
Flowering occurs in self-grafted Heihe27 (HH/HH, scion/stock) under LD conditions. Floral differentiation began 3 days after grafting (3 DAG). Accordingly, GmFT2a transcripts were initially detected in flower primordia at 3 DAG with transcript abundance increasing at 6 DAG in concurrence with floral differentiation (Figure 5a,b). Under the experimental LD conditions of this study, self-grafted Zigongdongdou (ZG/ZG) did not flower. Consistently, ZG/ZG scions continued their vegetative growth with trifoliates produced continuously up to 18 DAG. In these plants, GmFT2a transcripts were rare in both apical and axillary meristems (Figure 5c). However, when the scion Zigongdongdou was grafted onto the stock Heihe27 (ZG/HH), flower differentiation occurred in the scion under LD. At 3 DAG, ZG/HH did not initiate floral meristems and GmFT2a transcripts were hardly detected in the scion apical meristems (Figure 5d). By 9 DAG, flower differentiation had been initiated and GmFT2a transcripts were apparent in flower meristems and primordia (Figure 5e). The amount of GmFT2a transcripts continued to increase up to 14 DAG (Figure 5f). These results suggest that the early-flowering stock Heihe27 is able to promote GmFT2a appearance in the late-flowering scion Zigongdongdou, prompting the scion to flower.
Figure 5. Early-flowering stock promotes the appearance of GmFT2a transcripts in late-flowering scion by grafting.
(a–b) Shoot apices of self-grafted Heihe27 (HH/HH, scion/stock) at the 3rd or 6th day after grafting (DAG), respectively. (c) Shoot apex of self-grafted Zigongdongdou (ZG/ZG) at 18 DAG. (d–f) Shoot apices of scion Zigongdongdou grafted on stock Heihe27 (ZG/HH) at 3, 9 or 14 DAG, respectively. am, apical meristem; axm, axillary meristem; br, bract; fm, floral meristem; fp, floral primordium; pp, pistil primordium; tlp, trifoliolate leaf primordium. Bar, 250 µM.
Transgenically overexpressed GmFT2a promotes flowering
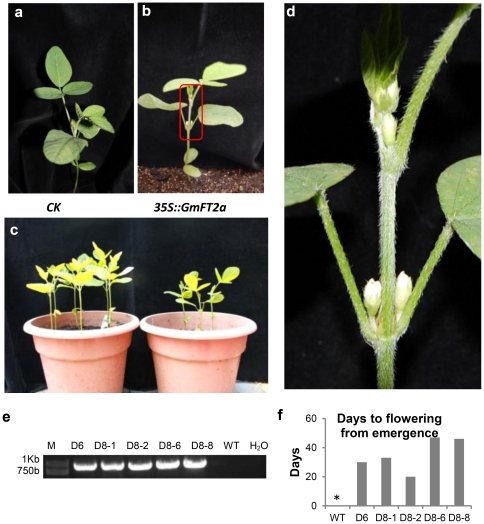
To confirm whether GmFT2a promotes flowering, GmFT2a driven by the 35S cauliflower mosaic virus (CaMV) promoter was transformed into Arabidopsis and its FT mutant ft10 [13]. Regardless of genetic backgrounds and photoperiods, plants overexpressing GmFT2a did promote flowering with few leaves (Figure 6a–d). Shown in Figure 6b,c, exogenously produced GmFT2a completely rescued, even over-compensated, ft10 [13]. Therefore, GmFT2a function may be similar to FT. Real-time quantitative PCR of the transgenic lines revealed that some flowering-related genes (FT, SOC1, and AP1) were induced while FLC was suppressed. As expected, GmFT2a was expressed only in the transgenic lines (Figure 6e). Furthermore, overexpressed GmFT2a also promoted the flowering of Zigongdongdou even under noninductive LD conditions (Figure 7). One transgenic line flowered approximately 20 days after emergence. By contrast, the early-flowering Heihe27 flowers approximately 27 days after emergence. At the first flowering, the transgenic line contained few leaves, only one pair of unifoliates and one trifoliate. In this line, the flowers were located at the axils of the unifoliates, a location where flowers are not typically produced in this and most other soybean cultivars (Figure 7b,d). These results demonstrate that GmFT2a overexpression can promote precocious flowering independent of the photoperiod.
Figure 6. Transgenic analysis of GmFT2a in Arabidopsis thaliana.
(a) Upper, Arabidopsis plants grown under long days (LD); Lower, Arabidopsis plants grown under short days (SD) when the transgenic plants flowered. From left to right, plants represent wildtype, transgenic line 17 and 18. (b) Transgenic overexpression of GmFT2a fully suppresses the late-flowering phenotype of a FT mutant ft10. From left to right, plants represent wild type, mutant ft10, and transgenic line 2 with mutant ft10 genetic background. (c) The days to flowering of Arabidopsis plants. (d) The number of cauline and rosette leaves at flowering. SR, rosette leaves under SD conditions. SC, cauline leaves under SD conditions. LR, rosette leaves under LD conditions. LC, cauline leaves under LD conditions. (e) Detection of GmFT, AtFT, AtAP1, AtSOC1, and AtFLC by real-time quantitative PCR. Asterisks show that the data was not available at flowering. For each real-time quantitative PCR point, averages and standard errors are the result of three replications.
Figure 7. Transgenic GmFT2a induces precocious flowering in soybean cultivar Zigongdongdou.
(a) A wild-type Zigongdongdou plant. (b) A transgenic Zigongdongdou plant showing precocious flowering at the axils of the unifoliate leaves. (c) The overview of wild-type (left) and transgenic (right) plants, including the plants shown in (a) and (b). (d) The close shot of the transgenic plant in (b) shows the precocious flowers at the axils of the unifoliate leaves. (e) Identification of transgenic GmFT2a plants by reverse transcription PCR with primers specific to 35S::GmFT. (f) Days to flowering from emergence of the transgenic plants and wild-type plants. The asterisk indicates that wild-type plants cannot flower at LD conditions during this experiment.
Temperature impacts GmFT2a expression in a genotype-dependent manner
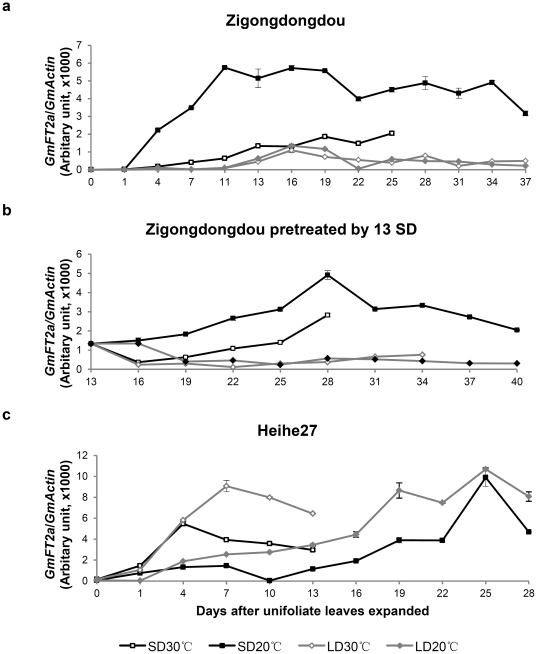
Characterization of the GmFT2a expression of cultivars grown under varying photoperiods and temperatures will help elucidate GmFT2a functions and ultimately benefit soybean breeding. The photoperiod-sensitive Zigongdongdou flowered approximately 24 days after the unifoliates fully expanded (24 DAUE) under SD conditions and a temperature of 30°C (SD30°C). Flowering occurred at approximately 36 DAUE under SD20°C. However, Zigongdongdou did not flower under LD. Under SD conditions, GmFT2a transcripts increased gradually with higher expression noted under SD20°C compared to SD30°CC Under LD conditions, GmFT2a transcripts were rarely detected and no significant differences were observed between LD30°C and LD20°C (Figure 8a). Similar results were obtained when Zigongdongdou was pretreated by 13 SD (Figure 8b). These results indicate that under inductive photoperiods, low temperature promotes GmFT2a expression in Zigongdongdou. Under noninductive photoperiods, temperature does not regulate GmFT2a expression (Figure 8a,b). The photoperiod-insensitive Heihe27 flowered approximately 15 DAUE under SD30°C, 38 DAUE under SD20°C, 18 DAUE under LD30°C, and 40 DAUE under LD20°C. GmFT2a also increased gradually, peaked, and then decreased slowly (Figure 8c). Notably, GmFT2a expression was higher under LD when compared to SD grown at the same temperature. GmFT2a expression was greater at high temperature compared to low temperature when grown under the same photoperiod (Figure 8c). Therefore, photoperiod is hypothesized to play a dominant role in GmFT2a expression in photoperiod-sensitive cultivars while temperature is suspected to play an important role in GmFT2a expression in photoperiod-insensitive cultivars.
Figure 8. GmFT2a expression under different combinations of photoperiod and temperature.
GmFT2a expression was detected in Zigongdongdou (a), Zigongdongdou pretreated by 13 SD (b), and Heihe27 (c) under short days at 30°C (SD30°C) or 20°C (SD20°C), and under long days 30°C (LD30°C) or 20°C (LD20°C). Zigongdongdou plants, including pretreated plants, did not flower throughout this experiment under LD conditions. For all other treatments, sampling ceased when the plants began blooming. For each real-time quantitative PCR point, averages and standard errors are the result of three replications.
Discussion
Soybean is not only a typical SD crop of high economic importance, but is also an excellent model to elucidate flowering mechanisms using both forward and reverse approaches. Using soybean as a model to study the role of FT in flowering reversion will lead to a greater understanding of the flowering mechanism.
GmFT2a promotes flowering
The FT homologue described in this paper contains 5′ and 3′ UTRs that are not present in the GmFT2a Kong et al. reported before [10]. An analysis of the role of the UTRs in the function of GmFT2a may provide interesting results as a PlantCARE search revealed that the 3′UTR contains a motif associated with the circadian mRNA accumulation [42]. Shown in Figure 2, for both Zigongdongdou and Heihe27, GmFT2a expression exhibits a diurnal circadian rhythm and LD can postpone the expression peak, suggesting that photoperiod might regulate the circadian peak time of GmFT2a expression to control flowering time. Consistent with previous studies, transgenic overexpression of GmFT2a not only promotes flowering of Arabidopsis and the soybean cultivar Zigongdongdou but also over-compensates the ft10 phenotype (Figure 6,7) [8]–[10], [43]. Thus, GmFT2a may encode florigen or a component of florigen in soybean. However, there are numerous other FT-like genes in the soybean genome, and at least one of them (Glyma16g04830, GmFT5a) has also been confirmed to promote flowering [10]. Therefore, why soybean contains so many FT-like genes and how they function and interact must be further studied before the soybean flowering mechanisms can be elucidated.
GmFT2a is related to flowering maintenance
In addition to promoting flowering transition, GmFT2a may play a role in subsequent flower development. In situ hybridization identified the continuous presence of GmFT2a transcripts in all primordia during flower development (Figure 3,4). Further indications arose from the results of flower reversion induced by LD treatment. As shown in Figure 4, LD treatment could neither terminate flower differentiation nor decrease GmFT2a transcripts immediately, possibly a consequence of the preceding SD treatment [34]. As LD treatment continued, GmFT2a transcripts attenuated gradually. Additionally, flower differentiation first slowed down and then terminated before leaf differentiation launched, meaning flowering reversion had occurred (Figure 4). Consistent with Molinero-Rosales et al. [26], these results suggest that GmFT2a is involved in flowering maintenance. On one hand, the maintenance of the identity of floral meristems may be dependent on the durative expression of GmFT. On the other hand, flowering reversion induced by LD treatment may decrease or degrade GmFT2a mRNA. Given that transgenically overexpressed GmFT2a promoted soybean flowering under LD conditions, it is more reasonable that GmFT2a maintains floral identity, although further investigation is needed. Furthermore, whole plant reversion occurs when soybean plants are transferred to LD conditions even after flowering under SD, indicating that soybean responds to photoperiods both before and after flowering [23], [24], [44], [45]. Further study of the role of GmFT2a in whole plant reversion will lead to a better understanding of florigen.
GmFT2a transcripts consecutively appear along vascular tissues to the primordia
As reviewed by Turck et al. [6], a discrepancy exists. That is, FT RNA is only expressed in vascular tissues [46], [47] and FT protein functions in meristems [41], [48]. With evidence from grafting studies, FT protein can be transported from the leaves to the shoot apex to induce flowering [15]–[18]. It is believed that the FT protein could be florigen or a component of florigen [11], [18]–[20]. To date, there is little direct evidence concerning the spatial distribution of FT expression using techniques like in situ hybridization. Because 10 FT-like genes exist in the soybean genome and six of these genes are transcribed [10], GmFT-specific probes are necessary. Based on sequence alignments, there are no significant similarities between the GmFT2a 3′ UTR and other genes (including the other nine FT-like genes) in the soybean genome (data not shown); therefore, probes recognizing the GmFT2a 3′ UTR are gene-specific. In situ hybridization using GmFT-specific probes revealed the presence of GmFT2a transcripts in vascular tissues as well as meristems and primordia (Figure 4). Therefore, GmFT2a is different to FT, Hd3a and SFT [46], [47], [49], [50]. There are at least two possibilities: GmFT2a could be consecutively transcribed locally, or GmFT2a could be transported along the vascular tissues to the primordia. These possibilities may exist given that soybean is an SD dicot, and thus, different from Arabidopsis (an LD dicot), rice (an SD monocot), and tomato (a day-neutral dicot). There may be differences in the mechanism of florigen transportation and activity between species. Recently, Mimida et al. found that in apple (Malus x domestica Borkh.), MdFT, a FT homolog, is expressed in the fruit-bearing shoot apex during the flower induction period and suggested that MdFT protein might not necessarily function as a transmissible floral inducer [51]. Their results seem to support our hypothesis. In some species, RNA may be the major, or most efficient form for florigen transportation; in other species, protein may be the major or most efficient form. In any case, more research is necessary before these florigen-related questions are answered.
By grafting, the stock Heihe27 promoted the appearance of GmFT2a in the shoot apices of the scion Zigongdongdou under LD conditions (Figure 5). However, GmFT2a expression was significantly inhibited by LD and nearly undetectable in the shoot apices of ungrafted and self-grafted Zigongdongdou grown under the same conditions. Given the rather high level of GmFT2a expression in Heihe27 under LD conditions (Figure 8), it is reasonable to hypothesize that the GmFT2a mRNA detected in the scion Zigongdongdou may be from stock Heihe27. As FT expression was induced in GmFT-overexpressing transgenic Arabidopsis (Figure 6), it is also possible that GmFT2a protein from the stock Heihe27 may activate the transcription of GmFT2a in the scion Zigongdongdou.
GmFT2a expression is dependent upon photoperiod
GmFT2a expression is much higher in leaves than in other organs, consistent with the expression of FT, and Hd3a [9], [10], [41], [50]. The expression of FTs is promoted by inductive photoperiods and inhibited by noninductive photoperiods in Arabidopsis [9], rice [43] and soybean [10], [38]. Accordingly, in the photoperiod-sensitive cultivar Zigongdongdou, GmFT2a was induced by SD and suppressed by LD (Figure 1). However, GmFT2a expression was different in the photoperiod-insensitive Heihe27. Under SD, GmFT2a expression peaked earlier in Heihe27 compared to Zigongdongdou (Figure 8), coinciding with earlier flowering in Heihe27 compared to Zigongdongdou. These results suggest that the time of flowering may be related to the peak of GmFT2a expression. Under LD conditions, Heihe27 flowered while Zigongdongdou did not. This result was consistent with GmFT2a expression, which was suppressed in Zigongdongdou but not in Heihe27. Notably, in Heihe27, GmFT2a expression was higher under LD than SD conditions. Considering that Heihe27 flowered under LD conditions at the same time as under SD conditions, it is hypothesized that more GmFT2a is helpful for Heihe27 to overcome a potential flowering barrier imposed by the intrinsic noninductive photoperiod LD. Therefore, GmFT2a may be involved in the diversity of photoperiod-sensitivity in soybean cultivars.
Photoperiod and temperature interactively regulate GmFT2a expression
Previous results indicated that photoperiod and temperature interact to control soybean development [52]; hence, it was hypothesized that they would also interact in the regulation of GmFT2a expression. In the photoperiod-sensitive cultivar Zigongdongdou grown under LD conditions (Figure 8), GmFT2a expression was suppressed significantly with temperature apparently playing no role. This result is consistent with the inability of Zigongdongdou to flower under LD but not SD conditions. High temperatures suppressed GmFT2a expression but promoted flowering of Zigongdongdou under SD. There are two potential reasons: high temperature may inhibit some flowering inhibitor(s), or high temperature may increase the transit efficiency of GmFT2a mRNA or protein from the leaves to the apical meristem. Other possibilities, however, cannot be discounted. For the photoperiod-insensitive Heihe27 grown under two temperature regimes (Figure 8), GmFT2a expression was always higher under LD in comparison to SD conditions possibly to overcome potential flowering barriers imposed by the noninductive LD. Furthermore, GmFT2a expression in Heihe27 was significantly greater under high temperatures rather than low temperatures when grown with the same photoperiod conditions, suggesting that high temperatures may promote GmFT2a expression in the photoperiod-insensitive cultivar. Hence, the following hypothesis was proposed: 1) for a photoperiod-insensitive cultivar, temperature plays a dominant role in GmFT2a expression and SD conditions downregulate GmFT2a expression; 2) for a photoperiod-sensitive cultivar, photoperiod plays a dominant role in GmFT2a expression and high temperature downregulates GmFT2a expression. Halliday et al. [29] reported that the Arabidopsis phytochrome phyB mutant has a temperature-sensitive precocious-flowering phenotype. Zhang et al. [53] suggested that the blue light receptor GmCRY1a plays an important role in photoperiodic flowering in soybean. Therefore, temperature and photoperiod interactively regulate GmFT2a expression, yet the underlying mechanism needs further investigation. Future studies utilizing more cultivars with diverse photoperiod sensitivities should prove useful in the unraveling of the relationship between temperature and photoperiod and their influence on flowering.
Materials and Methods
Plant materials
This study utilized two soybean cultivars: Zigongdongdou, a photoperiod-sensitive late-flowering cultivar and Heihe27, a photoperiod-insensitive early-flowering cultivar. Soybean seeds were planted in soil in 10-liter pots and grown under LD (16 h light/8 h dark) conditions using 25°C as a default temperature. After seedling emergence, similar pots were produced by removing some seedlings until each pot contained 10 uniform plants. These uniform plants were grown until the cotyledons opened or until the unifoliates fully expanded. The plants were then treated with different photoperiods (LD and SD, i.e., 12 h light/12 h dark) and temperatures (30°C, 25°C and 20°C). Additional details of plant growth and treatments were as reported by Wu et al. [25].
Arabidopsis thaliana ecotype Col and the mutant ft10 (a gift from Dr. Detlef Weigel, Max Planck Institute) were also used. Arabidopsis seeds were grown under LD or SD and grown at 22°C.
Grafting
Zigongdongdou and Heihe27 plants used as scions or stocks were raised under LD conditions until the 11th day after the cotyledons opened (at this time, the first trifoliate had fully expanded). A 5-cm scion was excised, beveled and defoliated with the exception of the newly expanded trifoliate. A stock was beveled at the first trifoliate node with the first trifoliate leaf reserved. The scion was fastened to the stock using Parafilm. To prevent dehydration during the first 5 days after grafting, the scion was covered with a transparent plastic bag and sealed with Parafilm.
RNA isolation and mRNA purification
Total RNA was isolated using TriZol reagent (Invitrogen). mRNA was purified using the Oligotex™ mRNA Purification Kit (QIAGEN).
Cloning of GmFT
A suppression subtractive hybridization (SSH) library of the Zigongdongdou cultivar was constructed using the PCR Select™-cDNA Subtraction Kit (Clontech). SD-treated leaves were used as the tester, and LD-treated leaves were included as a driver. An expressed sequence tag (EST), homologous to FT, was identified using the PCR-Select™ Differential Screening Kit (Clontech). The full-length GmFT2a was cloned into the pGEM-T easy vector (Promega) using the FirstChoice™ RLM-RACE Kit (Ambion).
Real-time quantitative PCR
Total RNA was digested with RQ1 RNase-Free DNase (Promega), and reverse-transcribed with Oligo dT (Sunbiotech, Beijing) using M-MLV Reverse Transcriptase (Promega). Target genes were detected with specific primers (listed in Supporting Information S1) according to the SYBR Premix Ex Taq™ manual (Takara). Primers for AtAP1 and AtSOC1 were those used by Chen et al. [54] and Liu et al. [55], respectively. GmACTIN or AtTUB was included as a reference for soybean or Arabidopsis genes, respectively [56].
In situ hybridization
To synthesize the antisense and sense GmFT2a RNA probes, a sense primer (5′-CGGGATCCCATTCAGAGGGAATCTGG-3′, with a BamHI restriction site) and an antisense primer (5′-GGAATTCTTCCAATTTACGTATATC-3′ with an EcoRI restriction site) were used to amplify the 3′-end GmFT2a gene-specific region. The product was subcloned into the pSPT18 vector and transcribed in vitro using the Digoxigenin (DIG) RNA Labeling kit (Roche Molecular Biochemicals). For each slide, 100 µL of probe was used to achieve a final concentration of 0.4 ng/µL. Tissue sections (10 µm thick) were collected as shown in Yu et al. [57]. In situ hybridization and immunological detection were performed as described by Xu et al. [58].
Transgenic analysis in Arabidopsis
Sense (5′-CGTCTAGAATGCCTAGTGGAAGTAG-3′, with a XbaI restriction site) and antisense (5′-ATGAGCTCTTAGTATAACCTCCTTC-3′, with a SacI restriction site) primers were used to amplify GmFT. The product was subcloned into the binary vector p3301m downstream of the 35 S cauliflower mosaic virus promoter. The resulting plasmid (p3301m-GmFT) was used to transform Arabidopsis plants by the floral dip method [59]. Transgenic lines were selected for on MS media supplemented with 10 mg/L glufosinate ammonium (Sigma), then grown to obtain T3 transgenic homozygous seeds for further investigation.
Transgenic analysis in soybean
Sense (5′-GGTCTAGAAAAATAATTCATAACAAAG-3′, with a XbaI restriction site) and antisense (5′-CCGAGCTCTCCAATTTACGTATATCAG-3′, with a SacI restriction site) primers were used to amplify GmFT. The product was subcloned into the vector pGFPGUSplus, replacing GFP [60]. From the resultant vector, a HindIII-EcoRI fragment containing the CaMV35S::GmFT::NOS cassette was isolated and inserted into the binary vector pTF101.1 [61]. This vector was designated pTF101.1-GmFT. pTF101.1-GmFT2a was used to transform Zigongdongdou plants, following the cotyledon-node method described by Flores et al. [62]. After selection on 8 and 4 mg/L glufosinate at the second shoot initiation and shoot elongation stages, respectively, primary transformants were planted and grown in the greenhouse. Identified by daubing leaves with 160 mg/L glufosinate (shown in Supporting Information S1), herbicide-resistant soybean lines and their progenies were subject to molecular and phenotypic analysis.
Supporting Information
Included are seven sections, that is, GmFT2a cDNA sequence (Genbank accession number EU287455), GmFT2a putative protein sequence, The alignment between GmFT2a and Glyma16g26660, Sequence analysis of GmFT2a, Sense probe controls for in situ hybridization, Transgenic soybean identified by daubing leaves with glufosinate, and Primers used in the research.
(PDF)
Acknowledgments
We thank Dr. Detlef Weigel (Max Planck Institute) for providing Arabidopsis FT mutant ft10 seeds, Dr. Kan Wang (Iowa State University, USA) for giving us the vector pTF101.1 as a gift, and Weiwei Yao for her help in soybean transformation. We particularly thank Lisa Linstroth and Heather Owen (University of Wisconsin Milwaukee, USA) for their critical reading of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the National High Technology Research and Development Program of China (http://www.863.gov.cn; grant no. 2007AA10Z133), the State Key Basic Research and Development Plan of China (http://www.973.gov.cn; grant no. 2009CB118404), and the National Natural Science Foundation of China (http://www.nsfc.gov.cn; grant no. 30070456 and 30471054). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bäurle I, Dean C. The timing of developmental transitions in plants. Cell. 2006;125:655–664. doi: 10.1016/j.cell.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Bernier G, Périlleux C. A physiological overview of the genetics of flowering time control. Plant Biotechnol J. 2005;3:3–16. doi: 10.1111/j.1467-7652.2004.00114.x. [DOI] [PubMed] [Google Scholar]
- 3.Boss PK, Bastow RM, Mylne JS, Dean C. Multiple pathways in the decision to flower: enabling, promoting, and resetting. Plant Cell. 2004;16(Suppl):S18–S31. doi: 10.1105/tpc.015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson GG, Dean C. Arabidopsis, the Rosetta stone of flowering time? Science. 2002;296:285–289. doi: 10.1126/science.296.5566.285. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi Y, Weigel D. Move on up, it's time for change—mobile signals controlling photoperiod-dependent flowering. Genes Dev. 2007;21:2371–2384. doi: 10.1101/gad.1589007. [DOI] [PubMed] [Google Scholar]
- 6.Turck F, Fornara F, Coupland G. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu Rev Plant Biol. 2008;59:573–594. doi: 10.1146/annurev.arplant.59.032607.092755. [DOI] [PubMed] [Google Scholar]
- 7.Adrian J, Torti S, Turck F. From decision to commitment: the molecular memory of flowering. Mol Plant. 2009;2:628–642. doi: 10.1093/mp/ssp031. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. A pair of related genes with antagonistic roles in mediating flowering signals. Science. 1999;286:1960–1962. doi: 10.1126/science.286.5446.1960. [DOI] [PubMed] [Google Scholar]
- 9.Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, et al. Activation tagging of the floral inducer FT. Science. 1999;286:1962–1965. doi: 10.1126/science.286.5446.1962. [DOI] [PubMed] [Google Scholar]
- 10.Kong F, Liu B, Xia Z, Sato S, Kim B, et al. Two coordinately regulated homologs of FLOWERING LOCUS T are involved in the control of photoperiodic flowering in soybean. Plant Physiol. 2010:110–160796. doi: 10.1104/pp.110.160796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeevaart JA. Leaf-produced floral signals. Curr Opin Plant Biol. 2008;11:541–547. doi: 10.1016/j.pbi.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, et al. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science. 2004;303:1003–1006. doi: 10.1126/science.1091761. [DOI] [PubMed] [Google Scholar]
- 13.Yoo SK, Chung KS, Kim J, Lee JH, Hong SM, et al. CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiol. 2005;139:770–778. doi: 10.1104/pp.105.066928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SK, Yun CH, Lee JH, Jang YH, Park HY, et al. OsCO3, a CONSTANS-LIKE gene, controls flowering by negatively regulating the expression of FT-like genes under SD conditions in rice. Planta. 2008;228:355–365. doi: 10.1007/s00425-008-0742-0. [DOI] [PubMed] [Google Scholar]
- 15.Notaguchi M, Daimon Y, Abe M, Araki T. Graft-transmissible action of Arabidopsis FLOWERING LOCUS T protein to promote flowering. Plant Signal Behav. 2009;4:123–125. doi: 10.4161/psb.4.2.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Notaguchi M, Abe M, Kimura T, Daimon Y, Kobayashi T, et al. Long-distance, graft-transmissible action of Arabidopsis FLOWERING LOCUS T protein to promote flowering. Plant Cell Physiol. 2008;49:1645–1658. doi: 10.1093/pcp/pcn154. [DOI] [PubMed] [Google Scholar]
- 17.Lin MK, Belanger H, Lee YJ, Varkonyi-Gasic E, Taoka K, et al. FLOWERING LOCUS T protein may act as the long-distance florigenic signal in the cucurbits. Plant Cell. 2007;19:1488–1506. doi: 10.1105/tpc.107.051920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaeger KE, Wigge PA. FT protein acts as a long-range signal in Arabidopsis. Curr Biol. 2007;17:1050–1054. doi: 10.1016/j.cub.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007;316:1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- 20.Mathieu J, Warthmann N, Kuttner F, Schmid M. Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Curr Biol. 2007;17:1055–1060. doi: 10.1016/j.cub.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Li C, Zhang K, Zeng X, Jackson S, Zhou Y, et al. A cis element within Flowering Locus T mRNA determines its mobility and facilitates trafficking of heterologous viral RNA. J Virol. 2009;83:3540–3548. doi: 10.1128/JVI.02346-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Battey N, Lyndon R. Reversion of flowering. Bot Rev. 1990;56:162–189. [Google Scholar]
- 23.Washburn CF, Thomas JF. Reversion of flowering in Glycine max (Fabaceae). Am J Bot. 2000;87:1425–1438. [PubMed] [Google Scholar]
- 24.Han T, Gai J, Wang J, Zhou D. Discovery of flowering reversions in soybean plant. Acta Agron Sin. 1998;24:168–171. [Google Scholar]
- 25.Wu C, Ma Q, Yam KM, Cheung MY, Xu Y, et al. In situ expression of the GmNMH7 gene is photoperiod-dependent in a unique soybean (Glycine max [L.] Merr.) flowering reversion system. Planta. 2006;223:725–735. doi: 10.1007/s00425-005-0130-y. [DOI] [PubMed] [Google Scholar]
- 26.Molinero-Rosales N, Latorre A, Jamilena M, Lozano R. SINGLE FLOWER TRUSS regulates the transition and maintenance of flowering in tomato. Planta. 2004;218:427–434. doi: 10.1007/s00425-003-1109-1. [DOI] [PubMed] [Google Scholar]
- 27.Blázquez MA, Ahn JH, Weigel D. A thermosensory pathway controlling flowering time in Arabidopsis thaliana. Nat Genet. 2003;33:168–171. doi: 10.1038/ng1085. [DOI] [PubMed] [Google Scholar]
- 28.Cerdán PD, Chory J. Regulation of flowering time by light quality. Nature. 2003;423:881–885. doi: 10.1038/nature01636. [DOI] [PubMed] [Google Scholar]
- 29.Halliday KJ, Salter MG, Thingnaes E, Whitelam GC. Phytochrome control of flowering is temperature sensitive and correlates with expression of the floral integrator FT. Plant J. 2003;33:875–885. doi: 10.1046/j.1365-313x.2003.01674.x. [DOI] [PubMed] [Google Scholar]
- 30.Balasubramanian S, Sureshkumar S, Lempe J, Weigel D. Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS Genet. 2006;2:e106. doi: 10.1371/journal.pgen.0020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JH, Yoo SJ, Park SH, Hwang I, Lee JS, et al. Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes Dev. 2007;21:397–402. doi: 10.1101/gad.1518407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J, Lee J, Ahn J. Ambient temperature signaling in plants: An emerging field in the regulation of flowering time. J Plant Biol. 2008;51:321–326. [Google Scholar]
- 33.Nishikawa F, Endo T, Shimada T, Fujii H, Shimizu T, et al. Increased CiFT abundance in the stem correlates with floral induction by low temperature in Satsuma mandarin (Citrus unshiu Marc.). J Exp Bot. 2007;58:3915–3927. doi: 10.1093/jxb/erm246. [DOI] [PubMed] [Google Scholar]
- 34.Han T, Wang J. Studies on the post-flowering photoperiodic reversions in soybean plant. Acta Agron Sin. 1995:168–171. [Google Scholar]
- 35.Yan H, Zhang L, Lu W, Liang J, Liu Y, et al. New high quality and yield soybean variety Heihe No.27. Heilongjiang Agricultural Science. 2003:52. [Google Scholar]
- 36.Li X, Wu C, Ma Q, Zhang S, Li C, et al. Morphology and anatomy of the differentiation of flower bud and the process of flowering reversion in soybean cv.Zigongdongdou. Acta Agron Sin. 2005:1437–1442. [Google Scholar]
- 37.Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, et al. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463:178–183. doi: 10.1038/nature08670. [DOI] [PubMed] [Google Scholar]
- 38.Thakare D, Kumudini S, Dinkins RD. Expression of flowering-time genes in soybean E1 near-isogenic lines under short and long day conditions. PLANTA. 2010;231:951–963. doi: 10.1007/s00425-010-1100-6. [DOI] [PubMed] [Google Scholar]
- 39.Ahn JH, Miller D, Winter VJ, Banfield MJ, Lee JH, et al. A divergent external loop confers antagonistic activity on floral regulators FT and TFL1. EMBO J. 2006;25:605–614. doi: 10.1038/sj.emboj.7600950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanzawa Y, Money T, Bradley D. A single amino acid converts a repressor to an activator of flowering. Proc Natl Acad Sci U S A. 2005;102:7748–7753. doi: 10.1073/pnas.0500932102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, et al. Integration of spatial and temporal information during floral induction in Arabidopsis. Science. 2005;309:1056–1059. doi: 10.1126/science.1114358. [DOI] [PubMed] [Google Scholar]
- 42.Lescot M, Dehais P, Thijs G, Marchal K, Moreau Y, et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30:325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, et al. Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol. 2002;43:1096–1105. doi: 10.1093/pcp/pcf156. [DOI] [PubMed] [Google Scholar]
- 44.Han T, Wu C, Tong Z, Mentreddy RS, Tan K, et al. Postflowering photoperiod regulates vegetative growth and reproductive development of soybean. Environ Exp Bot. 2006;55:120–129. [Google Scholar]
- 45.Han T, Wang J, Zou J, Yang Q. The post-flowering responses of soybean to pre-flowering photoperiodic treatments. Soybean Sci. 1995;14:283–289. [Google Scholar]
- 46.An H, Roussot C, Suárez-López P, Corbesier L, Vincent C, et al. CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development. 2004;131:3615–3626. doi: 10.1242/dev.01231. [DOI] [PubMed] [Google Scholar]
- 47.Takada S, Goto K. TERMINAL FLOWER2, an Arabidopsis homolog of HETEROCHROMATIN PROTEIN1, counteracts the activation of FLOWERING LOCUS T by CONSTANS in the vascular tissues of leaves to regulate flowering time. Plant Cell. 2003;15:2856–2865. doi: 10.1105/tpc.016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, et al. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science. 2005;309:1052–1056. doi: 10.1126/science.1115983. [DOI] [PubMed] [Google Scholar]
- 49.Lifschitz E, Eshed Y. Universal florigenic signals triggered by FT homologues regulate growth and flowering cycles in perennial day-neutral tomato. J Exp Bot. 2006;57:3405–3414. doi: 10.1093/jxb/erl106. [DOI] [PubMed] [Google Scholar]
- 50.Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K. Hd3a protein is a mobile flowering signal in rice. Science. 2007;316:1033–1036. doi: 10.1126/science.1141753. [DOI] [PubMed] [Google Scholar]
- 51.Mimida N, Ureshino A, Tanaka N, Shigeta N, Sato N, et al. Expression patterns of several floral genes during flower initiation in the apical buds of apple (Malus x domestica Borkh.) revealed by in situ hybridization. Plant Cell Rep. 2011;30:1485–1492. doi: 10.1007/s00299-011-1057-3. [DOI] [PubMed] [Google Scholar]
- 52.Fei Z, Wu C, Sun H, Hou W, Zhang B, et al. Identification of photothermal responses in soybean by integrating photoperiod treatments with planting-date experiments. Acta Agron Sin. 2009:1525–1531. [Google Scholar]
- 53.Zhang Q, Li H, Li R, Hu R, Fan C, et al. Association of the circadian rhythmic expression of GmCRY1a with a latitudinal cline in photoperiodic flowering of soybean. Proc Natl Acad Sci U S A. 2008;105:21028–21033. doi: 10.1073/pnas.0810585105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen NZ, Zhang XQ, Wei PC, Chen QJ, Ren F, et al. AtHAP3b plays a crucial role in the regulation of flowering time in Arabidopsis during osmotic stress. J Biochem Mol Biol. 2007;40:1083–1089. doi: 10.5483/bmbrep.2007.40.6.1083. [DOI] [PubMed] [Google Scholar]
- 55.Liu C, Zhou J, Bracha-Drori K, Yalovsky S, Ito T, et al. Specification of Arabidopsis floral meristem identity by repression of flowering time genes. Development. 2007;134:1901–1910. doi: 10.1242/dev.003103. [DOI] [PubMed] [Google Scholar]
- 56.Jian B, Liu B, Bi Y, Hou W, Wu C, et al. Validation of internal control for gene expression study in soybean by quantitative real-time PCR. BMC Mol Biol. 2008;9 doi: 10.1186/1471-2199-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu H, Xu Y, Tan EL, Kumar PP. AGAMOUS-LIKE 24, a dosage-dependent mediator of the flowering signals. Proc Natl Acad Sci U S A. 2002;99:16336–16341. doi: 10.1073/pnas.212624599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu YY, Chong K, Xu ZH, Tan KH. The practical technique of in situ hybridization with RNA probe. Chin Bull Bot. 2002;19:234–238. [Google Scholar]
- 59.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 60.Vickers C, Schenk P, Li D, Mullineaux P, Gresshoff P. pGFPGUS Plus, a new binary vector for gene expression studies and optimising transformation systems in plants. Biotechnol Lett. 2007;29:1793–1796. doi: 10.1007/s10529-007-9467-6. [DOI] [PubMed] [Google Scholar]
- 61.Paz M, Shou H, Guo Z, Zhang Z, Banerjee A, et al. Assessment of conditions affecting Agrobacterium-mediated soybean transformation using the cotyledonary node explant. Euphytica. 2004;136:167–179. [Google Scholar]
- 62.Flores T, Karpova O, Su X, Zeng P, Bilyeu K, et al. Silencing of GmFAD3 gene by siRNA leads to low alpha-linolenic acids (18∶3) of fad3-mutant phenotype in soybean [Glycine max (Merr.)]. Transgenic Res. 2008;17:839–850. doi: 10.1007/s11248-008-9167-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Included are seven sections, that is, GmFT2a cDNA sequence (Genbank accession number EU287455), GmFT2a putative protein sequence, The alignment between GmFT2a and Glyma16g26660, Sequence analysis of GmFT2a, Sense probe controls for in situ hybridization, Transgenic soybean identified by daubing leaves with glufosinate, and Primers used in the research.
(PDF)