The impact of mechanical forces on kinetochore motility was investigated using laser microsurgery and fluorescent speckle microscopy on kinetochores and associated microtubules during anaphase in crane fly spermatocytes. Kinetochores detached from their chromosomes moved at twice their normal speed, entering a motile state identified as “park.”
Abstract
The impact of mechanical forces on kinetochore motility was investigated using laser microsurgery to detach kinetochores with associated chromatin (K fragment) from meiotic chromosomes in spermatocytes from the crane fly Nephrotoma suturalis. In spermatocytes, elastic tethers connect telomeres of homologues during anaphase A of meiosis I, thus preventing complete disjunction until mid- to late anaphase A. K fragments liberated from tethered arms moved at twice the normal velocity toward their connected poles. To assess functional states of detached and control kinetochores, we loaded cells with fluorescently labeled tubulin for fluorescent speckle microscopy on kinetochore microtubules. Control kinetochores added fluorescent speckles at the kinetochore during anaphase A, whereas kinetochores of K fragments generally did not. In cases in which speckles reappeared in K-fragment K fibers, speckles and K fragments moved poleward at similar velocities. Thus detached kinetochores convert from their normal polymerization (reverse pac-man) state to a different state, in which polymerization is not evident. We suggest that the converted state is “park,” in which kinetochores are anchored to plus ends of kinetochore microtubules that shorten exclusively at their polar ends.
INTRODUCTION
Characterization of the mitotic spindle as a “flux machine” stems from recognition of the role played by the bulk flow of microtubule polymer, or poleward flux (Mitchison, 1989), in spindle-related motility (LaFountain et al., 2001). In many types of mitotic cells, flux exhibited by kinetochore microtubules accounts for a significant fraction of poleward chromosome movement during anaphase (reviewed by Rogers et al., 2005), and anaphase is entirely flux-based in certain insect spermatocytes (LaFountain et al., 2004). Poleward force imparted by the flux machine can also bend chromosome arms toward poles (Adames and Forer, 1996), and its action accounts for movement of chromatin fragments, as well as other objects (LaFountain et al., 2001), and sliding of isolated spindles in relation to skewering needles (Gatlin et al., 2010).
It has been proposed that flux within kinetochore microtubules plays a role in regulating the function of kinetochores. In a model based on direct observation of microtubule flux in Xenopus extract spindles, Maddox and coworkers (2003; see also Mitchison, 2005) proposed that the polymerization state exhibited by kinetochores engaged in microtubule flux is due to applied mechanical tension. Their hypothesis is that kinetochore attachment sites exert molecular friction at the kinetochore interface. Such friction would resist sliding of microtubules through the interface, resulting in poleward force generation, the so-called slip-clutch. In other systems (e.g., Saccharomyces cerevisiae and Caenorhabditis elegans), low-affinity binding of kinetochore proteins like Ndc80 to the microtubule lattice explains why kinetochores remain attached to microtubules under conditions of high tension (Cheeseman et al., 2004; Powers et al., 2009). At metaphase, when sister kinetochores are stably positioned at the spindle equator and under mechanical tension, balanced plus-end addition and minus-end loss would be evident. At the onset of anaphase, kinetochores would then shift to a depolymerization state (pac-man motility) as a consequence of disjunction of sisters and the consequential drop in tension on kinetochores. The present study was undertaken to expand our understanding of this interplay of kinetochore function with spindle mechanics.
Our impetus came from two earlier findings, the first of which emerged from our study of poleward microtubule flux in spermatocytes of the crane fly Nephrotoma suturalis (LaFountain et al., 2004). Fluorescent speckle microscopy (FSM) in conjunction with microinjection of spermatocytes with rhodamine-conjugated tubulin revealed kinetochores that persisted in a polymerization (reverse pac-man) state during anaphase. This finding is quite out of the ordinary in comparison with most mitotic systems studied to date, where kinetochores typically switch to a depolymerization (pac-man) state upon entering anaphase. A possible explanation, however, resides in the outcome of another earlier study (LaFountain et al., 2002), from which we learned that telomeres of trailing arms of half-bivalents are connected by elastic tethers that appear to exert a significant backward resistance force, which would impose mechanical tension on kinetochores during at least the initial part of anaphase A. Hence, when such tethers are in place, they enable resistance of complete disjunction and impose tension on kinetochores due to the backward resistance the tethers supply. Kinetochores under such tension could then possibly continue to exhibit polymerization properties similar to those seen under bipolar tension at metaphase, in full agreement with the spindle mechanics model put forth by Maddox et al. (2003).
To test the influence of mechanical tension on kinetochore motility, we used laser microsurgery to detach a small kinetochore-containing chromosome fragment (K fragment) from one of the half-bivalents from a dichiasmic bivalent at the onset of anaphase A. K fragments moved poleward immediately upon detachment, with velocities that were faster than those exhibited by kinetochores of the other chromosomes in the same cell. Additionally, based on FSM of their kinetochore microtubules, kinetochores of K fragments appeared to have converted to a different functional state.
RESULTS
Generating K fragments
Dichiasmic bivalents were most suitable for kinetochore-detachment operations (see Materials and Methods for details about chromosomes and bivalents), because sister kinetochores of each homologue are at the tips of their pole-directed portions, offering a target sufficiently thin to be cut by our laser microbeam. The approach was to take aim on the kinetochore-containing region of one of the homologues at metaphase and then, upon loss of cohesion between homologues at the onset of anaphase A, to use the laser microbeam to detach a small kinetochore-containing chromosome fragment—K fragment—from its four chromosome arms. The objective of these kinetochore-detachment operations was to compare the poleward motility of K fragments lacking both trailing arms and tethers with the anaphase motility of kinetochores on intact, uncut chromosomes. The alternative of ablating tethered sites on telomeres was not practical, because it would have required at least two operations for monochiasmic bivalents and four operations for dichiasmic constructs, and it could not confirm that the target had been fully ablated. The behavior of arms that were detached from K fragments (see next section), on the other hand, gave us confidence that complete disjunction had been achieved
K fragments move twice as fast as control chromosomes at anaphase onset
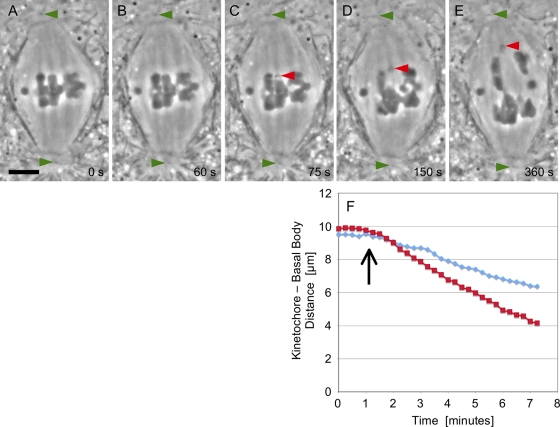
Figure 1, A–E, is a series from a time-lapse movie (Supplemental Movie S1) made of a detachment operation performed just after the onset of anaphase A and loss of cohesion (interval between Figure 1A and Figure 1B). The laser flash released a K fragment that immediately moved poleward, while the arms tethered to their partner homologues moved backward and across the equator, made contact with their partner's telomere, and then were dragged to the “wrong” pole (Figure 1, C–E), as was described previously (LaFountain et al., 2002). Arms lacking tethers after an operation either remained at the equator or drifted into one half-spindle or the other, again as referenced above. K fragments released at anaphase onset always completed anaphase A, as judged by their arrival at the edge of the centrosome well before the kinetochores of the intact half-bivalents.
FIGURE 1:
(A–E) Images were taken from a time-lapse movie (Movie S1) of a K fragment generated at anaphase onset. (A) At metaphase, the three autosomal bivalents are positioned at the spindle equator. The middle dichiasmic bivalent is a desirable target for laser cutting, because the chromosome arms are especially thin in the plane just under its kinetochore fiber–attachment sites. (B) At anaphase onset, cohesion between homologues is lost. Measurement of distance between kinetochores and the upper pole is readily achieved using the position of the polar basal bodies as a reference (green arrowhead). (C) Subsequent to the laser flash, the K fragment (red arrowhead) moves poleward, and its severed arms move backward to make contact with its partner homologue. (D and E) Anaphase A continues. The K fragment (red arrowheads) has a poleward velocity twice that of controls. Some collateral damage is evident in the arms of adjacent chromosomes, an unavoidable complication when using the laser beam configured as a line, as it was in this case. Scale bar: 5 μm. (F) Results of frame-by-frame analysis of images yielded these distance vs. time plots for the K fragment (red squares) following detachment (arrow) and for the control kinetochore (blue diamonds) to the left of the fragment in (C).
In Figure 1F, the distance versus time data obtained for the K fragment (Figure 1C) in Movie S1 are plotted with similarly obtained data for the control half-bivalent to the left of the K fragment in that cell. Although the kinetochore-to-basal body distance for the K fragment is greater than that of the control kinetochore at the onset of anaphase, within 1 min after the operation, the K fragment passes by and then moves at twice the velocity (∼1.0 μm/min) of that exhibited by the control (∼0.5 μm/min).
A summary of the data from the 25 spermatocytes on which such operations were performed is included in Table 1. On average, the velocity with which K fragments moved poleward (0.9 ± 0.2 μm/min) was about twice that of normal anaphase kinetochores (0.4 ± 0.2 μm/min) of uncut chromosomes that were moving to the same pole during the same time frame as a K fragment during anaphase A.
TABLE 1:
Poleward velocities of K fragments versus uncut controls.
| Velocity (μm/min) | ||
|---|---|---|
| Type of kinetochore | Range | Averageb |
| On K fragments generated at anaphase onseta | 0.6–1.3 | 0.9 ± 0.2, n = 25 |
| On control, uncut chromosomes moving to the same pole as the K fragmenta | 0.2–0.9 | 0.4 ± 0.2, n = 35 |
aDuring first 3 min after detachment.
bn refers to the number of K fragments or number of chromosomes analyzed. In some cases, both uncut half-bivalents in the K-fragment's half-spindle could be analyzed.
Partner homologues, that is, those that remained tethered to the cutoff arms of K fragments and moved to the opposite pole, also continued through anaphase A subsequent to the operation. Their velocities (0.6 ± 0.2 μm/min; Table 2) were not significantly different (Student's t test, p = 0.4) from the velocities of uncut controls (0.5 ± 0.2 μm/min; Table 2) in the same half-spindle over the same time frame. Velocity similarities among partner homologues and uncut controls are also evident upon viewing the movies (Movie S1).
TABLE 2:
Poleward velocities of partner homologues versus uncut controls.
| Velocity (μm/min) | ||
|---|---|---|
| Type of kinetochore | Range | Averageb |
| Kinetochores of partner homologues of K fragmenta | 0.3–1.1 | 0.6 ± 0.2, n = 13 |
| On control, uncut chromosomes moving to the same pole as the partner homologuea | 0.3–1.0 | 0.5 ± 0.2, n = 19 |
aDuring first 3 min after detachment of K fragment.
bn refers to the number of chromosomes that were analyzed. In some cases, both uncut half-bivalents in the partner homologue's half-spindle could be analyzed.
Combining FSM with FRAP
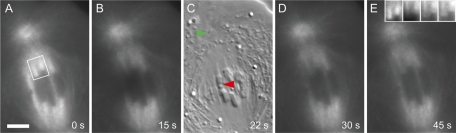
We developed a variation of FSM in conjunction with fluorescence recovery after photobleaching (FRAP) to assess the status of K-fragment kinetochores. Cells were injected iontophoretically with rhodamine-conjugated tubulin (Rh-tubulin; LaFountain et al., 2004). FSM before the laser operation established the functional state of kinetochores up to the moment of anaphase onset. During the laser operation, speckles in the kinetochore fiber of the newly generated K fragment were bleached, and new speckles appearing during FRAP could be observed with high contrast. For the experiments described here, injections of Rh-tubulin used volumes greater than that used for conventional FSM to account for the loss of fluorescently labeled tubulin due to laser photobleaching. As a consequence, initial speckle density within K fibers following injection was in many cases much higher than with conventional FSM, as is evident in the strikingly fluorescent K fibers depicted in Figure 2A.
FIGURE 2:
Control experiment. Images from a time-lapse, alternating-frame FSM-DIC movie after a spot ablation that drilled a hole in a chromosome arm but did not detach the arm from its kinetochore (Movie S2). (A) With FSM following injection of Rh-tubulin, kinetochore fibers become strikingly fluorescent. Before the laser flash, locations of plus-end attachment to bivalents are clearly evident. (B) At the onset of anaphase, photobleaching is evident, as the laser beam in spot-flash mode was used to drill a hole in one of the trailing arms. (C) With DIC, red arrowhead locates the hole in the trailing arm; green arrowhead locates polar basal body. (D and E) Recovery of fluorescence at the plus ends of kinetochore microtubules is evident as plus-end addition of Rh-tubulin monomers—due to the polymerization state of early anaphase kinetochores—continues after the operation. (E) Inset at top right shows the fluorescence of the same two K fibers identified by white frame in (A) and imaged in (A), (B), (D), and (E), respectively. The inset is contrast-enhanced to clearly show the recovery of fluorescent speckles in K fibers after photobleaching by the laser pulse. Scale bar: 5 μm.
With injected cells, laser flashes of 0.5–1.0 s required for kinetochore detachment caused considerable fluorophore bleaching that was particularly prominent within 3–4 μm from the target site. Immediately after the flash, all structured fluorescence within a bleached zone was lost (Figure 2B), and only very weak diffuse background fluorescence was evident, such that the interface between chromosomes and adjacent spindle appeared in low contrast.
FSM and FRAP in control operations
We tested the combination of FSM and FRAP by performing control operations on injected cells with the microbeam aimed at either 1) a point within one of the half-spindles near kinetochores but between K fibers, or 2) a chromosome arm, with the intent of drilling a hole in the arm without actually detaching it from its kinetochore (Figure 2C). Similar to kinetochore-detachment operations considered in the next section, such control operations were performed at anaphase onset, soon after separation of homologues had occurred. It was evident that FRAP proceeded rapidly, because in just 2 min after the flash, distinction between the bleached zone and the rest of the spindle had completely disappeared (Figure 2B). Owing to the high concentration of microtubule plus ends at kinetochores, FRAP showed that they were speckle-labeled within only 15 s after the flash (i.e., in our alternating frame regimen of image capture, that was the first fluorescence image of FRAP; Figure 2D). On the basis of such controls, we concluded that FSM-FRAP microscopy provided an effective way to confirm the polymerization state of kinetochores during anaphase in the material we studied.
Additionally, as FRAP progressed, incorporation of fluorescent speckles at kinetochores continued over time. Based on such poleward movement of speckles, combined with speckles recovering interstitially along the length of K fibers, speckle-labeling of the entire K fiber was restored within 1 or 2 min after photobleaching (Figure 2, D and E). Such poleward movement of speckles (Movie S2) provided confirmation that the flux machine was indeed functioning during FRAP to move newly assembled polymer from kinetochores to the poles.
FSM and FRAP in detachment operations
In applying the FSM-FRAP approach to kinetochore-detachment operations, we sought first to determine whether kinetochores of K fragments were also in a polymerization state, and second to ascertain the relationship between poleward movement of K fragments and poleward flux, as evidenced by poleward movement of fluorescent speckles. Both goals were achieved, as summarized in the following paragraph.
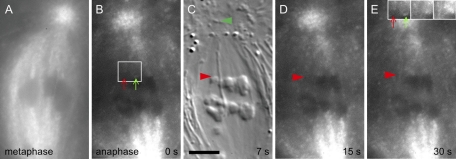
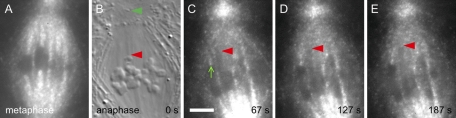
Our time-lapse movies (Movies S3 and S4) revealed that initial fluorescence at the plus ends of kinetochore microtubules of K fragments was obviously different from that seen at kinetochores of the uncut chromosomes in the same cell. While the latter exhibited FRAP similar to that seen in control irradiations described in the preceding section, there was no detectable FRAP at kinetochores of K fragments. The images presented in Figures 3, A–E, and 4, A–E, illustrate this result. With an alternating (differential interference contrast microscopy [DIC]-FSM) frame regimen for image acquisition, DIC images (Figures 3C and 4B) were used to locate K fragments, and their x,y pixel locations were then marked on the next-acquired fluorescence image (Figures 3D and 4C). It is important to bear in mind, however, that 7.5 s elapsed in the interval between those two images. Nevertheless, in the first three time-lapse images after detachment, there was no detectable FRAP at the K-fragment's kinetochore, whereas FRAP was clearly evident at the control kinetochores. This failure to exhibit FRAP at K-fragment kinetochores—in cells where FRAP was evident at control kinetochores—was confirmed in a total of eight cells. Thus, based on initial FRAP, kinetochores of K fragments do not appear to be in a polymerization state.
FIGURE 3:
Images from a time-lapse, alternating-frame FSM-DIC movie after generating a K fragment at anaphase onset (Movie S3). (A) FSM at metaphase. (B) FSM illustrating photobleaching at and around the flash site at 57 s after the first of a total of four laser flashes that were required to achieve complete detachment. Bleaching of fluorescence label within the K fiber of the K fragment, as well as within the two uncut, control half-bivalents to the upper pole, is clearly evident. Recovery of fluorescence at the plus ends of control K fibers has already begun in this first fluorescence frame after the laser flash. (C) DIC image of the released K fragment (red arrowhead) and polar basal body (green). (D and E) FSM of FRAP at the kinetochore regions of controls; such fluorescence is not evident at the K-fragment's kinetochore. When fluorescence is eventually recovered at the K fragment, both the fragment and its associated fluorescence move poleward at similar velocities. (E) Inset at top right shows the fluorescence after enhancement of contrast of the same K fibers (identified with red (K fragment) and green (uncut chromosome) arrows in the white frame in (B) imaged in (B), (D), and (E), respectively. Inset: Enhanced images clearly show the recovery of fluorescent speckles in the K fiber of the uncut chromosome after photobleaching by the laser pulse, whereas in the K fiber of the K fragment, no new speckles are visible. Scale bar: 5 μm.
FIGURE 4:
Images from a time-lapse, alternating-frame FSM-DIC movie after generating a K fragment at anaphase onset (Movie S4). (A) FSM at metaphase. (B) DIC image of the released K fragment (red arrowhead) and polar basal body (green). (C–E) FSM at intervals after detachment of the K fragment to illustrate the lack of fluorescence at the plus end of the fragment's K fiber (red arrowheads) in comparison with fluorescence emerging from the kinetochores of controls moving to the same pole. Scale bar: 5 μm.
During subsequent poleward movement of K fragments, analysis of movies showed that in some cases (six cells), failure to exhibit FRAP was evident throughout the course of the movie (6 min), whereas in other cases (three cells), speckles did appear at K-fragment kinetochores after a delay, but newly formed speckles then moved poleward at essentially the same velocity as that exhibited by the K fragment. In both scenarios, speckles distal to the kinetochore—interpreted as plus ends of microtubules within a K fiber but not attached to the kinetochore—could also be observed moving poleward at velocities similar to those of K fragments.
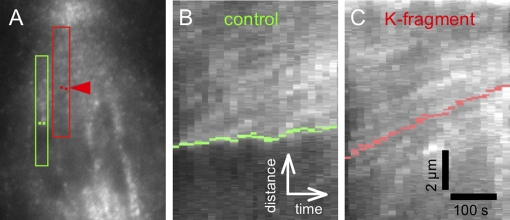
To address the relationship between kinetochores and speckles during their poleward movement, we made kymographs that displayed time-lapse movement of speckles in graphical form (see Materials and Methods). In kymographs, speckle movement appears as bright tracks, and the slope of a track expresses the velocity of the speckle. There were seven cells from which it was possible to obtain useful kymographs, and our analysis of speckle tracks in the regions between K-fragment kinetochores and their poles produced an average speckle velocity of ∼0.7 ± 0.2 μm/min from values ranging between 0.4 and 1.2 μm/min (n = 19 speckle tracks). Figure 5, B and C, gives a specific example of this approach. The kymograph (Figure 5C) of the region of interest (ROI) marked in red around the K-fragment's K fiber in Figure 5A shows speckles moving at velocities similar to that of the kinetochore, the position of which in each frame of the movie was located with red dots, as outlined above. In Figure 5C, the velocity of the K fragment was ∼0.7 μm/min, indeed similar to the velocities of speckles judged to be within its K fiber and also moving with velocities between 0.6 and 0.8 μm/min. Those results are in contrast to the kymograph (Figure 5B) made from the green ROI marked around the K fiber of the uncut half-bivalent on the left in Figure 5A, where speckles emerging from the kinetochore (marked with green dots) are evident. With controls (Figure 5B), speckles moved poleward with velocities greater than those of the kinetochore, and analysis of kymograph tracks from four control cells indicated speckle velocity data were similar to data for K fibers of K fragments (i.e., based on 25 speckle tracks: range: 0.4–1.3 μm/min; average: 0.8 ± 0.2 μm/min). In the specific case of the uncut chromosome referenced in Figure 5B, its poleward velocity was ∼0.2 μm/min, whereas its K-fiber's speckles had velocities ranging between 0.4 and 0.6 μm/min. On the basis of kymographic analysis, we conclude that K fragments appear to be moving poleward with a velocity similar to that of poleward flux, whereas uncut chromosomes have kinetochores in the expected polymerization state, moving poleward with velocities slower than the speckles that emerge from them.
FIGURE 5:
(A) Duplicate image of Figure 4D with ROIs used for making the kymographs: ROI for kymograph presented in (B) is outlined in green; ROI for kymograph presented in (C) is outlined in red. Green dots locate the edges of the kinetochore region of the control chromosome as judged by the K-fiber attachment site imaged by DIC; red dots locate the edges of the kinetochore region of the K fragment as imaged with DIC. (B) Kymograph of speckle movement in relation to the kinetochore (green dots); green dots are moving poleward at a velocity slower than speckles and also are showing at least one speckle track emanating from the kinetochore. (C) Kymograph of red ROI in (A) showing speckle tracks having poleward velocities similar to that of the K-fragment's kinetochore (located with red dots). Vertical bar: 2 μm; horizontal bar:100 s.
Regarding speckle analysis, we used the same criteria as we established in earlier work (LaFountain et al., 2004) for categorizing speckles as being within K fibers, which was that they appeared to be within the region of the image of the K fiber acquired with DIC. Accordingly, speckles not within such overlapping regions would be judged to be part of nonkinetochore microtubules. As an example from the present work, speckles that overlapped with the in-focus K fibers presented in Figure 4B (included in the ROIs in Figure 5A) would have been judged to be K-fiber speckles. In the cases of speckles that were observed to actually emerge from a kinetochore, there would have been no doubt. However, live-cell FSM has pitfalls, and we acknowledge that categorization of speckles that formed near a kinetochore and those that appeared to form interstitially and distal from a kinetochore as being kinetochore microtubule speckles cannot be made with absolute certainty. Attachment to kinetochores was not resolved with FSM for speckles that overlaid K fibers but were not observed to emerge from a kinetochore. Nevertheless, in all cases reported, we did operate within these constraints and think that the criteria we have applied are valid.
Additional functional properties of K-fragment kinetochores
A concern was that energy released by laser flashes not only detached K fragments but also caused loss of function by possibly “killing” kinetochores. This can be ruled out based on the following observations. First, incomplete detachment of a kinetochore (see Materials and Methods) following laser flashes of the duration, intensity, and distance to the kinetochore used to elicit complete detachment did not result in any detectable effect on its polymerization state. FRAP showed that such kinetochores were functioning in their normal reverse pac-man state.
Second, K-fragment kinetochores that could be tracked through meiosis II exhibited functional properties that were no different from normal meiosis II kinetochores. To elaborate, cell viability in live-cell spermatocyte preparations is about 4–5 h; therefore, anaphase I, interkinesis, and anaphase of meiosis II could be studied in individual cells in some of our prepared material. We found that K-fragment kinetochores in meiosis II assembled K fibers and congressed to the spindle equator, after which sisters separated and segregated to opposite poles. Thus functional capabilities of K-fragment kinetochores were not destroyed by laser flashes. Rather, damage inflicted by laser flashes must have been restricted to the chromatin underlying kinetochores.
Third, in a related study in an early stage of development, we are finding that when K fragments are generated in a manner just like that in this study—but during prometaphase—the kinetochores not only move rapidly poleward, but also reorient from syntelic to amphitelic orientation shortly thereafter and then quickly congress to the spindle equator to reach an equatorial position prior to anaphase onset. They then behave normally during meiosis II (as described above). All this would not be possible if K-fragment kinetochores had been killed as a consequence of nearby laser flashes.
DISCUSSION
Our earlier work had shown that kinetochores of half-bivalent chromosomes function in a polymerization—or reverse pac-man—state during at least the initial stages of anaphase A in crane fly spermatocytes (LaFountain et al., 2004). This was revealed through direct visualization of fluorescent speckles within kinetochore microtubules, which exhibited poleward microtubule flux during anaphase A at a velocity that was about twice the anaphase velocity of the chromosomes.
In the present study, we used laser microsurgery to detach small K fragments from anaphase half-bivalents and observed that released K fragments move poleward at about twice their normal velocity. Importantly, detachment of a K fragment facilitated complete disjunction of the kinetochores of the K fragment from the kinetochores of its partner homologue to the opposite pole. Such disjunction is not achieved normally during anaphase A, as long as resistive forces imposed by the chromosomes' arms and their tethered telomeres are in place.
With K fragments, their kinetochores were investigated with a combination of FSM and FRAP, and they were found not to have the polymerization capabilities evident in controls. Additionally, poleward velocity of K fragments was similar to that of poleward flux as visualized by FSM.
We therefore conclude that, upon detachment of their tethered arms, K-fragment kinetochores switched from reverse pac-man to “park”—a functional state in which kinetochores would be anchored to the plus ends of kinetochore microtubules that would shorten during anaphase only at their minus (poleward) ends (Skibbens et al., 1993). In the park state, microtubules at the kinetochores would exhibit neither polymerization nor depolymerization. Park provides a ready explanation for the failure of K-fragment kinetochores to exhibit FRAP in comparison with controls, as well as for results of fluorescent speckle analysis showing speckles and kinetochores moving poleward at similar velocities. In crane fly spermatocytes, K fibers normally function as traction fibers, with K-fiber shortening taking place only at the poles (LaFountain et al., 2001, 2004). Kinetochores functioning in park mode after having lost their normal polymerization function would be expected to exhibit more rapid than normal poleward movement—just as we observed.
It is relevant to reflect on data from our earlier study of poleward flux, which used FSM (LaFountain et al., 2004) to visualize the range (0.5–1.2 μm/min) and average (∼0.9 μm/min) speckle velocity within anaphase kinetochore microtubules (Figure 4 in LaFountain et al., 2004). Those data very closely match the range (0.6–1.3 μm/min) and average (0.9 μm/min) velocity of K fragments presented here (Table 1). This underscores the point that similar velocity profiles exhibited by poleward flux (2004 data) and K fragments (present data) support the conclusion that K-fragment kinetochores are in a park state.
Our finding that kinetochores move at the same velocity as flux is reminiscent of earlier work on anaphase in cell-free spindles made from Xenopus oocyte extracts (Murray et al., 1996; Desai et al., 1998). The interpretation of those Xenopus data in terms of kinetochores being in park mode stood only as long as it took to develop better methods for resolving spindle microtubule dynamics at the kinetochore interface. In what is notably the most detailed analysis of kinetochore function in relation to microtubule flux completed to date, Maddox et al. (2003) showed that what had appeared to be park was actually two functional states—pac-man and reverse pac-man—essentially operating together as short, sequential bursts during anaphase. In retrospect, it is understandable how such bistability of a kinetochore repeatedly moving faster and then slower than the flux velocity could be interpreted as park if observations were based only upon comparing average flux rate with average poleward chromosome movement.
In light of the Xenopus story, we do not think such bistability is operative in our system, based on two counts. First, we found no evidence for periods of fast and slow movement of K fragments. Plots of poleward movement of K fragments were typically smooth over durations of 5–6 min, as presented in Figure 1F. Second, kymographs of K fragments and speckles within their K fibers during FRAP showed both moving with similar, essentially parallel profiles (Figure 5C). We did not find evidence for K fragments moving faster than speckles or “gobbling up” speckles, which would be required for pac-man to be invoked in this case.
We have presented strong evidence that kinetochores of K fragments shift to park. Our data fit with the idea that kinetochore function is largely regulated by spindle mechanics, and our view is therefore an extension of models proposed by others (Maddox et al., 2003; Mitchison, 2005). Kinetochores under mechanical tension, such as that imposed by tethers, would be in a polymerization (reverse pac-man) state. We propose kinetochores in spermatocytes convert to park on loss of tension that occurs either naturally during late anaphase (when tethers normally are lost) or due to laser microsurgery. While in park, poleward movement would be accounted for solely by the flux machine through microtubule shortening occurring exclusively at the poles. Where the motors that drive flux within spermatocyte spindles are located is a question that remains unanswered and open to debate (Fabian and Forer, 2005).
Within the model that is emerging from the present data, we envision the possibility that conversion to park, such as that suggested for K fragments, could be reversible. During their movement poleward, kinetochores could conceivably encounter pockets of resistance, such as “thickets” of nonkinetochore microtubules or minor obstructions due to elements of the spindle matrix (Fabian et al., 2007; reviewed by Johansen et al., 2011), that might prevent unimpeded movement to the pole. If such resistance were of sufficient magnitude, it could cause a momentary switch to a polymerization state that could cause the generation of a fluorescent speckle. Such a scenario could account for generation of speckles at kinetochores during the course of FRAP that, as discussed above, could also have their origins at plus ends of microtubules not even attached to kinetochores.
We think a shift from reverse pac-man to park (and accelerated velocity) could also occur if and when two partner half-bivalents lose their tether(s) during the course of normal anaphase (LaFountain et al., 2002). In this respect, accelerated velocity as a consequence of loss of load (i.e., the tether) is a concept that runs counter to the generally accepted view that velocity is load independent (Nicklas, 1965; reviewed by Maiato and Lince-Faria, 2010), Paradoxically, partner homologues—also separated via microsurgeries from the resistive action of tethers—did not accelerate but moved as slowly as control half-bivalents. To speculate, the additional load of the K-fragment's cutoff arms is a possible factor contributing to the slower velocity of a K-fragment's partner homologue. It will be of interest to apply our approach to other systems to determine whether this correlation between severing kinetochores from their arms and more rapid than normal velocity is more widely evident.
In conclusion, our finding that kinetochores can actually function in park mode provides evidence for a kinetochore state whose existence had been doubted. If our thinking about park is correct, its occurrence may be limited to systems that use a traction fiber (non-pac-man) mechanism for chromosome movement, and it may indeed be a transitory state, evident only when mechanical forces localized to the chromosomes and the spindle permit it.
MATERIALS AND METHODS
Live-cell spermatocyte preparations were made from testes obtained from a laboratory colony of crane fly (N. suturalis) larvae. For observation with inverted optics, spermatocytes were spread at the oil–glass interface of a well chamber made by sealing a coverglass to an aluminum microscope slide with a ¾-in. hole drilled through its center (Janicke and LaFountain, 1986); the well was filled with Voltalef 10s (Atofina, Colombes, France) oil before the cells were spread on the coverglass. Cells prepared in this way survive for hours, permitting observation of both meiotic divisions from start to finish.
The karyotype of N. suturalis males comprises three pairs of morphologically similar metacentric autosomes and two small telocentric sex chromosomes, X and Y. Bivalents formed from the autosomes may have a variety of shapes, depending on the number and location of their chiasmata. For the type of laser microsurgery operation performed here, dichiasmic bivalents in which chiasmata are positioned about halfway between centromere and telomeres were most desirable, and these were the type selected. In such constructs, the connection of each adjacent sister kinetochore of a homologue to its kinetochore fiber (K fiber) is clearly evident, as the underlying chromosome arms appear stretched poleward at metaphase. Such tension on kinetochores at the onset of anaphase appears to diminish, however, as an obvious change in the condensation state of the “fabric” of each half-bivalent occurs during its progression into anaphase A following loss of cohesion with its partner. The reality of the latter is clearly evident in operational terms, based on the finding that cutting operations capable of detaching kinetochore fragments (K fragments) before such tightening of the fabric occurs are essentially impossible within just a few minutes after anaphase onset. Ironically, such cutting failures had value, as they demonstrated that the laser pulses required to detach kinetochores did not affect the polymerization state of such “undetachable” kinetochores—that is, they yielded the same result as was obtained in reported control experiments in which the laser was used to drill a hole in a chromosome arm.
Laser microsurgery was performed using a nanosecond-pulsed, solid-state laser (laser head SNG-03E-TB1, wavelength 532 nm, pulse energy 3 μJ, pulse duration 1 ns, repetition rate up to 10 kHz; Teem Photonics, Grenoble, France) directed to the specimen through the epi-port of an inverted microscope (Zeiss Axiovert 200M [Carl Zeiss Microimaging, Thornwood, NY] or Nikon Diaphot 300 [Nikon Instruments, Melville, NY]). The laser beam converged to the conjugate image plane near the epi-port and was brought to a diffraction-limited focus in the specimen by high-numerical-aperture objectives lenses (Plan Neofluar100×/1.4NA, or Plan Apo 63×/1.4NA or 100×/1.4NA) for optimal spatial resolution. The shape of the laser beam in the focal plane was chosen to be either a spot or a line. For spot ablation, the suitably expanded beam was focused into the conjugate image plane using a camera lens with 25-mm focal length. For line ablation, an additional cylindrical lens (focal length: 150 mm) was placed before the camera lens to expand the focal spot in one direction. The cylindrical lens was mounted on a carefully centered rotation stage for orienting the focal line in a direction required by the target geometry. When employing the line configuration, a single laser flash was often effective, as it cut like a knife (e.g., slicing off the poleward tip of the half-bivalent). Line cuts, however, occasionally resulted in some “collateral” damage to nontargeted chromosomes adjacent to the intended target. Nevertheless, such damage was usually minimal, and it did not adversely impact the results. When using the beam configured as a spot, there was usually no collateral damage, but with this tactic, multiple flashes (typically two to three) were required (e.g., to detach a K fragment from its arms). In such cases, the stage was moved between flashes to allow a cut to be made from one side of the chromosome/kinetochore target to the other.
The target inside the well slide was positioned relative to the laser microbeam either by using a piezo-electrically driven translation stage (M-663 with custom slide holder; Physik Instrumente, Auburn, MA) with a computer-interfaced controller (C-865; Physik Instrumente) or by a manual approach that entailed careful adjustment of the microscope stage. The laser pulses were triggered by a square wave (transistor–transistor logic specs) provided by a signal generator to the laser power supply. The number of transitions in the square wave determined the number of pulses applied to the target during one laser flash. Typically, a laser flash of 0.5–1.0 s in duration, with the signal generator operating at 2–5 kHz, was sufficient to achieve an intended ablation or cut. All instrument control was mediated with custom software enhancing the open-source imaging platform ImageJ (http://rsb.info.nih.gov/ij).
For distance versus time analysis, we used the positions of kinetochore and polar basal body as they appear in DIC images. The kinetochore position was defined as the poleward edge of the K fragment or chromosome (where kinetochores are located), and either one of the edges of the polar basal bodies or another distinctive structure within the centrosome served as a distance reference point at the spindle pole. Importantly, neither the centralized position of polar basal bodies in each centrosome nor the overall appearance of each centrosome changed detectably over the portion of anaphase A included in our velocity analysis. From frame-by-frame analysis, measurements of distance separating the poleward edge of a K fragment and the polar basal body toward which it was moving were made with Image J tools and then imported into Microsoft Excel software (Redmond, WA) for plotting. To maintain consistency in comparisons between K fragments or partner homologues with uncut control chromosomes, measurements were made only in the half-spindle of interest. In addition, the data presented in Tables 1 and 2 pertain to the initial 3 min following the operation. The reasoning behind this was that as time passed after the cut, it became impossible in all of the cells studied to keep both the released K fragment and the reference structures at the spindle pole in the same focal plane. In preparing spermatocytes for such operations, every effort was made to immobilize them to avoid such problems. Nevertheless, the eventuality is that one (or more) of the important features of a given experiment is when the microscope goes out of focus. The 3-min limit, then, represents a uniform background against which the velocity differences we detected can be compared. In favorable cells, accurate analysis could be extended beyond 3 min, as is presented in Figure 1.
Injection of Rh-tubulin (Cytoskeleton, Denver, CO) was accomplished using an iontophoretic approach (LaFountain et al., 2004), and acquisition of images of injected spermatocytes was done using a combination of DIC and fluorescence optics on a microinjection rig that was detailed in LaFountain et al. (2004). For most of the time-lapse movies made here, the interval between frames was 7.5 s, which converts to 15-s intervals for the DIC portion and 15-s intervals for the fluorescence portion of a combined DIC-FSM series.
Regarding photobleaching and FRAP, when the laser was used for detachment operations, each laser flash resulted in immediate photobleaching around the flash site. This was so even when we tried a fluorophore (HiLyteFluor 647 from Cytoskeleton.com) with an excitation maximum (e.g., 600–630 nm) much different from the 532-nm beam of the laser. On the basis of that photobleaching effect, we developed FSM in conjunction with FRAP to track speckles that appeared during recovery within the photobleached zone in which their contrast was enhanced. With this new approach, operations were performed with the microbeam configured as a spot, so as to localize photobleaching to the flash site and the radius peripheral to it. It was necessary to use multiple flashes and to move the specimen between flashes to detach a K fragment when the microbeam was in the spot configuration. Because of these requirements, the design of our microinjection/laser microsurgery/image acquisition rig did not permit acquisition of fluorescence images immediately prior to, or during, the laser operation. Rather, image acquisition began as soon as detachment of the K fragment had been confirmed visually in DIC mode, after which a movie was made in time lapse by alternating between fluorescence and DIC modes over the course of 6 min. Over this time course, our image acquisition regimen caused fluorescence of the entire spindle to become progressively bleached and made measurements lasting longer than 6 min impractical. This was so even when every precaution was taken to avoid fluorescence photobleaching prior to the operation. Also note that the first minute or so of K-fragment movement, as well as a similar segment of FRAP, was not recorded with this regimen.
Analysis of images acquired by FSM was done with IPLab software (Becton Dickinson, Franklin Lakes, NJ) using a customized Projection script that incorporated a number of IPLab commands, including the 3D Projector command, to create a projection stack in which the movement of fluorescent speckles from one frame to the next was displayed as a function of time within the resultant three-dimensional projection. Projections are similar to kymographs used to display time-dependent speckle movement as reported by others (Waterman-Storer et al., 1998).
Supplementary Material
Acknowledgments
Custom software for ImageJ was designed and implemented by Grant Harris of the MBL. This study was supported by a grant to R.O. from the National Institute of Biomedical Imaging and Bioengineering (R01EB002583-17).
Abbreviations used:
- DIC
differential interference contrast microscopy
- FRAP
fluorescence recovery after photobleaching
- FSM
fluorescent speckle microscopy
- K fragment
kinetochore fragment
- Rh-tubulin
rhodamine-conjugated tubulin
- ROI
region of interest
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-06-0494) on October 26, 2011.
REFERENCES
- Adames KA, Forer A. Evidence for poleward forces on chromosome arms during anaphase. Cell Motil Cytoskeleton. 1996;34:13–25. doi: 10.1002/(SICI)1097-0169(1996)34:1<13::AID-CM2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Neissen S, Anderson S, Hyndman F, Yates JR III, Oegema K, Desai A. A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension. Genes Dev. 2004;18:2255–2268. doi: 10.1101/gad.1234104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A, Maddox PS, Mitchison TJ, Salmon ED. Anaphase A chromosome movement and poleward spindle microtubule flux occur at similar rates in Xenopus extract spindles. J Cell Biol. 1998;141:703–713. doi: 10.1083/jcb.141.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian L, Forer A. Redundant mechanisms for anaphase chromosome movements: crane-fly spermatocyte spindles normally use actin filaments but also can function without them. Protoplasma. 2005;225:169–184. doi: 10.1007/s00709-005-0094-6. [DOI] [PubMed] [Google Scholar]
- Fabian L, Xia X, Venkitaramani DV, Johansen KM, Johansen J, Andrew DJ, Forer A. Titin in insect spermatocyte spindle fibers associates with microtubules, actin, myosin and the matrix proteins skeletor, megator and chromator. J Cell Sci. 2007;120:2190–2204. doi: 10.1242/jcs.03465. [DOI] [PubMed] [Google Scholar]
- Gatlin JC, Matov A, Danuser G, Mitchison TJ, Salmon ED. Directly probing the mechanical properties of the spindle and its matrix. J Cell Biol. 2010;188:481–489. doi: 10.1083/jcb.200907110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke MA, LaFountain JR Jr. Bivalent orientation and behavior in crane-fly spermatocytes recovering from cold exposure. Cell Motil Cytoskeleton. 1986;6:492–501. doi: 10.1002/cm.970060508. [DOI] [PubMed] [Google Scholar]
- Johansen KM, Forer A, Yao C, Girton J, Johansen J. Do nuclear envelope and intranuclear proteins reorganize during mitosis to form an elastic, hydrogel-like spindle matrix. Chromosome Res. 2011;19:345–365. doi: 10.1007/s10577-011-9187-6. [DOI] [PubMed] [Google Scholar]
- LaFountain JR Jr, Oldenbourg R, Cole RW, Rieder CL. Microtubule flux mediates poleward motion of acentric chromosome fragments during meiosis in insect spermatocytes. Mol Biol Cell. 2001;12:4054–4065. doi: 10.1091/mbc.12.12.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFountain JR Jr, Cole RW, Rieder CL. Partner telomeres during anaphase in crane-fly spermatocytes are connected by an elastic tether that exerts a backward force and resists poleward motion. J Cell Sci. 2002;115:1541–1549. doi: 10.1242/jcs.115.7.1541. [DOI] [PubMed] [Google Scholar]
- LaFountain JR Jr, Cohan CS, Siegel AS, LaFountain DJ. Direct visualization of microtubule flux during metaphase and anaphase in crane-fly spermatocytes. Mol Biol Cell. 2004;15:5724–5732. doi: 10.1091/mbc.E04-08-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox P, Straight A, Coughlin P, Mitchison TJ, Salmon ED. Direct observation of microtubule dynamics at kinetochores in Xenopus extract spindles: implications for spindle mechanics. J Cell Biol. 2003;162:377–382. doi: 10.1083/jcb.200301088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiato H, Lince-Faria M. The perpetual movements of anaphase. Cell Mol Life Sci. 2010;67:2251–2269. doi: 10.1007/s00018-010-0327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ. Polewards flux in the mitotic spindle: evidence from photoactivation of fluorescence. J Cell Biol. 1989;109:637–652. doi: 10.1083/jcb.109.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ. Mechanism and function of poleward flux in Xenopus extract meiotic spindles. Phil Trans R Soc B. 2005;360:623–629. doi: 10.1098/rstb.2004.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AW, Desai AB, Salmon ED. Real time observation of anaphase in vitro. Proc Natl Acad Sci USA. 1996;93:12327–12332. doi: 10.1073/pnas.93.22.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas RB. Chromosome velocity during mitosis as a function of chromosome size and position. J Cell Biol. 1965;25:119–135. doi: 10.1083/jcb.25.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers AF, Franck AD, Gestaut DR, Cooper J, Gracyzk B, Wei RR, Wordeman L, Davis TN, Asbury CL. The Ndc80 kinetochore complex forms load-bearing attachments to dynamic microtubule tips via biased diffusion. Cell. 2009;136:865–875. doi: 10.1016/j.cell.2008.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GC, Rogers SL, Sharp DJ. Spindle microtubles in flux. J Cell Sci. 2005;118:1105–1116. doi: 10.1242/jcs.02284. [DOI] [PubMed] [Google Scholar]
- Skibbens RV, Skeen VP, Salmon ED. Directional instability of kinetochore motility during chromosome congression and segregation in mitotic newt lung cells: a push-pull mechanism. J Cell Biol. 1993;122:859–875. doi: 10.1083/jcb.122.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Desai A, Bulinski JC, Salmon ED. Fluorescent speckle microscopy, a method to visualize the dynamics of protein assemblies in living cells. Curr Biol. 1998;8:1227–1230. doi: 10.1016/s0960-9822(07)00515-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.