This study demonstrates that low oxygen (O2) levels induce the embryonic protein Nodal. This finding is significant, as low O2 levels characterize the microenvironments associated with both early development and tumor progression, and Nodal has been shown to promote tumorigenicity and to govern stem cell fate.
Abstract
Low oxygen (O2) levels characterize the microenvironment of both stem cells and rapidly growing tumors. Moreover, hypoxia is associated with the maintenance of stem cell–like phenotypes and increased invasion, angiogenesis and metastasis in cancer patients. Metastatic cancers, such as breast cancer and melanoma, aberrantly express the embryonic morphogen Nodal, and the presence of this protein is correlated with metastatic disease. In this paper, we demonstrate that hypoxia induces Nodal expression in melanoma and breast cancer cells concomitant with increased cellular invasion and angiogenic phenotypes. Of note, Nodal expression remains up-regulated up to 48 h following reoxygenation. The oxygen-mediated regulation of Nodal expression occurs via a combinatorial mechanism. Within the first 24 h of exposure to low O2, there is an increase in protein stability. This increase in stability is accompanied by an induction of transcription, mediated by the HIF-1α–dependent activation of Notch-responsive elements in the node-specific enhancer of the Nodal gene locus. Finally, Nodal expression is maintained upon reoxygenation by a canonical SMAD-dependent feed-forward mechanism. This work provides insight into the O2-mediated regulation of Nodal, a key stem cell–associated factor, and reveals that Nodal may be a target for the treatment and prevention of hypoxia-induced tumor progression.
INTRODUCTION
Metastatic cancer cells often express stem cell–associated proteins that support increased tumorigenesis, drug resistance, and metastasis (Hendrix et al., 2007). Nodal, an embryonic morphogen belonging to the transforming growth factor β (TGF-β) superfamily, is an example of a stem cell protein associated with cancer. Nodal expression is normally restricted to embryonic lineages, and in addition to patterning the embryo, this protein maintains the pluripotency of embryonic stem cells (ESCs; Hendrix et al., 2007; Schier, 2009). Recent studies have shown that Nodal is aberrantly expressed in melanoma, glioma, prostate cancer, and breast cancer, where it correlates with invasive, metastatic disease (Topczewska et al., 2006; Strizzi et al., 2008; Hueng et al., 2010; Lee et al., 2010; McAllister et al., 2010; Yu et al., 2010; Lawrence et al., 2011). For example, Nodal is expressed in vertical growth phase and metastatic melanomas, whereas normal skin and melanoma in situ show lower levels of Nodal expression (Topczewska et al., 2006; Yu et al., 2010). Similarly, Nodal expression is high in metastatic cancer cell lines, such as C8161 melanoma and MDA-MB-231 breast cancer cells, and low in poorly metastatic lines, such as C81-61 melanoma and T47D breast cancer cell lines (Topczewska et al., 2006; Postovit et al., 2008b). Since Nodal is absent in adult tissue and ectopically expressed in the later stages of cancer, the induction of Nodal may be due to the changes that occur in the microenvironment during early tumor progression.
An important microenvironmental characteristic of solid tumors is low O2 (hypoxia; Semenza, 2003). Hypoxia is a biophysical consequence of accelerated tumor growth accompanied by insufficient, structurally disorganized vascularization (Semenza, 2003; Le et al., 2004). In tumors, low O2 levels induce a gene expression profile associated with increased angiogenesis, invasion, and metastatic potential (Le et al., 2004; Chaudary and Hill, 2007). Hypoxia also contributes to the maintenance of cellular plasticity and pluripotency (Ezashi et al., 2005; Mazumdar et al., 2009). Low O2 levels prevent the differentiation of ESCs by inducing pluripotency-associated transcriptional mediators, such as Oct-4 and Notch, and hypoxia promotes a dedifferentiated phenotype in a variety of cancer cells, concomitant with the acquisition of ESC proteins, such as Nanog (Helczynska et al., 2003; Jogi et al., 2003; Mathieu et al., 2011).
The mechanisms by which cells sense O2 and modify gene expression in response to hypoxia are complex, involving transcriptional regulation, preferential translation, protein stabilization, and epigenetic modifications. One major mode of O2-mediated gene regulation involves transcription factors, such as the hypoxia-inducible factors (HIFs; Keith and Simon, 2007; Semenza, 2007). HIFs are heterodimeric transcription factors, each composed of an α and a β subunit. The HIF β subunit is constitutively expressed, while the α subunit is tightly regulated through protein stability and transactivational activity (Semenza, 2007). Following a reduction in pO2, there is a marked increase in HIF DNA binding to hypoxia-responsive elements (HREs), which increases the transcription of target genes (Semenza, 2007). There are three known HIF-α proteins (HIF-1, HIF-2, and HIF-3). HIF-1α and HIF-2α display high sequence similarity, and both heterodimerize with HIF-β and bind to HREs (Keith and Simon, 2007; Semenza, 2007). However, HIF-1 and HIF-2 regulate divergent gene expression patterns (Keith and Simon, 2007).
In addition to canonical mechanisms of activation, HIF-1 can regulate gene expression by enhancing Notch signaling (Gustafsson et al., 2005). There are four known mammalian Notch receptors (Notch1–4) and five ligands (Jagged1,2 and Delta1–4). In addition to their normal expression in progenitor cells, these receptors and ligands are widely expressed in breast cancer and melanoma cell types (Koch and Radtke, 2010; Liu et al., 2010). Notch receptors are activated by binding ligands expressed on adjacent cells. On activation, the Notch ectodomain is cleaved and the intracellular domain (NICD) is subsequently released as a consequence of γ-secretase–mediated cleavage. The NICD translocates to the nucleus, where it interacts with CSL proteins, leading to transcriptional activation of target genes (Postovit et al., 2007; Strizzi et al., 2009). A recent study demonstrated that HIF-1α can stabilize NICDs and that it is recruited to Notch-responsive elements, where it promotes the expression of Notch-induced genes (Gustafsson et al., 2005). Of note, studies in mice and in human melanoma cells have demonstrated that Nodal expression is regulated by Notch at a node-specific enhancer (NDE) region located ∼10 kb upstream from the transcriptional start site (TSS; Krebs et al., 2003; Raya et al., 2003; Strizzi et al., 2009; Hardy et al., 2010). However, the interplay between HIF and Notch at this site has not been examined.
In this study, we show for the first time that the embryonic protein Nodal is up-regulated by hypoxia in human breast cancer and melanoma cells via a combinatorial mechanism involving protein stabilization and the HIF-dependent activation of the Nodal NDE. Furthermore, this hypoxia-induced Nodal expression is associated with increased invasiveness and angiogenic potential.
RESULTS
Nodal expression is induced by hypoxia
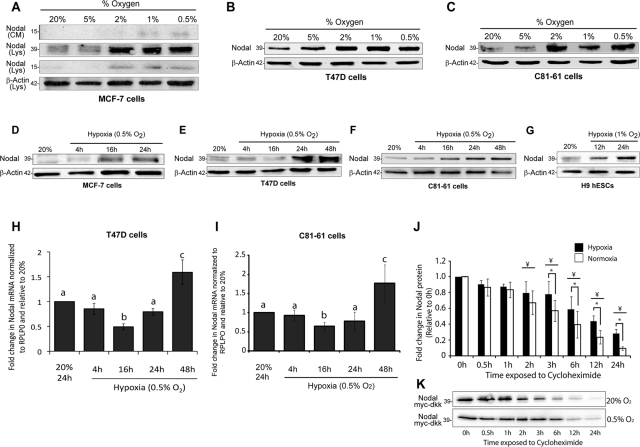
To determine the role of O2 availability in the regulation of Nodal protein expression, we exposed poorly metastatic breast cancer (MCF-7, T47D) and melanoma (C81-61) cells to varying levels of O2 for 24 h. Previous work has shown that basal Nodal protein levels are low in MCF-7, T47D, and C81-61 cells compared with highly metastatic cell lines (Topczewska et al., 2006; Postovit et al., 2008b). Thus, by using cells that express lower levels of Nodal, we were able to examine whether hypoxia can induce Nodal expression and promote a switch that supports aggressive phenotypes. Exposure of MCF-7, T47D, and C81-61 cells to successively decreasing O2 concentrations (20–0.5% O2) for 24 h resulted in an up-regulation of Nodal protein at concentrations ≤ 2% O2 (Figure 1, A–C). Moreover, Nodal protein levels were increased in cell lysates and in conditioned medium, and both pro-Nodal and cleaved Nodal were up-regulated in response to hypoxia (Figure 1A). We chose to present cell-associated pro-Nodal for all subsequent experiments, as it was in the greatest abundance and showed the same trends as the cleaved species. For all subsequent experiments, we also used 1 or 0.5% O2 as our hypoxic conditions, as these concentrations were equally effective at inducing Nodal expression. To better understand the kinetics of hypoxia-induced Nodal expression, time-course studies were conducted in which cells were exposed to 0.5% O2 for 4–48 h. Between 16 and 24 h, Nodal protein expression started to increase in MCF-7-7, T47D, and C81-61 cells, reaching a maximum after 48 h (Figure 1, D–F). This increase was not restricted to transformed cell types or to cells that express low levels of Nodal, as hESCs (H9), which have a normal karyotype and express high endogenous amounts of Nodal, experienced a similar increase in Nodal protein expression (Figure 1G). In contrast, Nodal mRNA levels did not experience a linear up-regulation. Rather, in T47D and C81-61 cells, mRNA levels decreased in 0.5% O2 after 4 and 16 h, and only began to increase after 24 h. After 48 h, Nodal mRNA levels increased significantly compared with control (20% O2) cells (Figure 1, H and I). Given this surprising result, we measured lysyl oxidase (LOX) mRNA levels in the same samples as a control for canonical transcriptionally regulated gene expression (Erler et al., 2006; Postovit et al., 2008a). In accordance with previous studies, we observed a positive linear up-regulation of LOX mRNA levels in T47D cells with increasing exposure time to hypoxia (Supplemental Figure S1A). These results suggested that the hypoxia-induced up-regulation of Nodal expression might involve both transcriptional and posttranscriptional alterations. One of the best examples of such a phenomenon is the hypoxia-induced stabilization of HIF protein (Semenza, 2007). To determine the effects of hypoxia on Nodal protein stability, we performed cycloheximide-based stability assays using T47D breast cancer cells transfected with a myc-dkk–tagged Nodal expression vector. T47D cells were treated with cycloheximide (0.1 mg/ml) for up to 24 h under either hypoxic (0.5% O2) or normoxic (20% O2) conditions, and protein expression was measured for each time point, using Western blotting. To ensure equal loading for these experiments, membranes were stained with Amido black following protein transfer (Figure S1B). We determined that cells cultured in 0.5% O2 experienced a significant increase in Nodal protein stability compared with cells cultured in 20% O2 (Figure 1, J and K). Collectively, these results suggest that Nodal is induced in cells by an increase in protein stability and enhanced transcription.
FIGURE 1:
Nodal is regulated by hypoxia in breast cancer, melanoma, and human embryonic stem cell lines. (A) Immunoblot analyses demonstrating that Nodal protein is up-regulated in MCF-7 breast cancer cells cultured for 24 h in 2, 1, or 0.5% O2, as compared with cells cultured in 20 or 5% O2. Cell-associated pro-Nodal (∼39 kDa) and cleaved Nodal (∼15 kDa) are increased in response to decreasing O2 levels, as determined by immunoblotting of cell lysates (Lys). The cleaved and secreted 15-kDa species is also increased in the conditioned media (CM) of MCF-7 cells in response to decreased O2 levels. β-Actin is used as a loading control. (B and C) Immunoblot analyses demonstrating that Nodal is up-regulated in response to decreasing O2 levels in (B) T47D breast cancer cells and (C) C81-61 melanoma cells. Cells were cultured in the designated O2 concentration for 24 h. The pro-Nodal (∼39 kDa) band is presented and β-actin is used as a loading control. (D to G) Immunoblot analyses demonstrating that Nodal expression increases over time in hypoxia (0.5% O2) in (D) MCF-7 cells, (E) T47D cells, (F) C81-61 cells, and (G) H9 human embryonic stem cells (hESCs). The pro-Nodal (∼39 kDa) band is presented and β-actin is used as a loading control. (H and I) Real-time RT-PCR analysis of Nodal mRNA in (H) T47D cells and (I) C81-61 cells exposed to 0.5% O2 for 0–48 h. Nodal drops with initial exposure to hypoxia and then increases over time. Expression levels are normalized to large ribosomal protein (RPLPO). Bars represent mean gene expression ± SD relative to the 20% O2 control. Different letters indicate significantly different gene expression (n = 4; p < 0.05). (J) Quantification of Nodal protein stability in cycloheximide-treated T47D cells cultured for 24 h in normoxia (20% O2) or hypoxia (0.5% O2). T47D cells were transfected with a myc-dkk–tagged Nodal expression vector prior to culture in order to measure stability at 20% O2 vs. 0.5% O2. Protein abundance was assayed by immunoblotting for myc-dkk, which was followed by densitometry. Results show Nodal protein levels begin to decrease after 2 h in both normoxia and hypoxia (compared with respective 0 h controls), and that Nodal protein stability is significantly higher in hypoxia at all time points after 3 h (compared with normoxia for each respective time point). Bars represent mean protein expression ± SEM relative to the 0 h values. *, significant difference between normoxia and hypoxia at a given time point (n = 6; p < 0.05); ¥, significant difference compared with corresponding treatment at 0 h (n = 6; p < 0.05). (K) Representative immunoblot corresponding to (J).
Hypoxia-induced nodal expression is mediated via HIF-1–dependent activation of the NDE
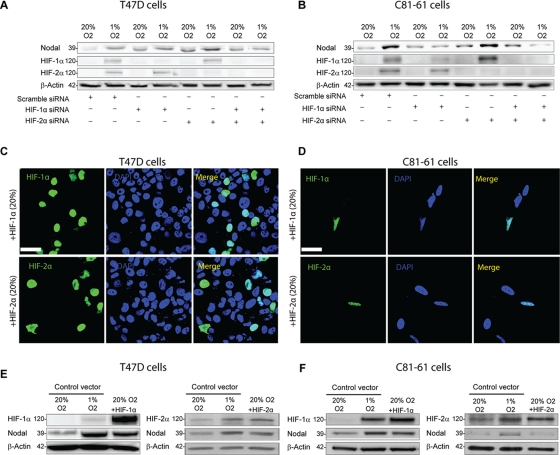
Given their widespread function in the regulation of hypoxia-induced gene expression, we next sought to determine the roles of HIF-1 and HIF-2 in the O2-mediated regulation of Nodal. We first examined the consequences of knocking down HIF-1α and/or HIF-2α in C81-61 and T47D cells. Western blot analyses confirmed that HIF-1α and HIF-2α protein levels are greatly induced following exposure to 1% O2 for 24 h (Figure 2, A and B). Furthermore, immunofluorescence localization revealed that HIF-1α and HIF-2α translocated to the nucleus in response to 0.5% O2 (Figure S1, C–E). The up-regulation in HIF-1α and HIF-2α expression that occurred in response to 1% O2 was abrogated by treatment with small interfering RNAs (siRNAs) specific to each protein (Figure 2, A and B). We discovered that inhibition of HIF-1α, but not HIF-2α, expression prevented the hypoxic up-regulation of Nodal protein in T47D and C81-61 cells cultured for 24 h in 1% versus 20% O2 (Figure 2, A and B). However, when combined, siRNAs directed against HIF-1α and HIF-2α were more effective at blunting the hypoxic up-regulation of Nodal than siRNA to HIF-1α alone (Figure 2, A and B). Of note, HIF-1α and HIF-2α siRNAs appeared to increase Nodal protein levels in T47D cells cultured in 20% O2 and baseline Nodal expression varied in this cell line. To further test the role of HIF-1α and HIF-2α in the regulation of Nodal, we next transfected T47D and C81-61 cells with a HIF-1α or HIF-2α expression vector. Immunofluorescence localization and Western blot analyses confirmed that transfection with the HIF-1α and HIF-2α expression vectors resulted in an induction of HIF-1α or HIF-2α, respectively, and that HIF-1α and HIF-2α accumulated in the nuclei of both T47D and C81-61 cells, even in 20% O2, when overexpressed (Figure 2, C–F). Overexpression of HIF-1α up-regulated Nodal protein levels in T47D and C81-61 cells to levels similar to those observed with culture at 1% O2 (Figure 2, E and F). Overexpression of HIF-2α to levels observed in response to hypoxia did not affect Nodal protein levels in C81-61 cells and weakly induced Nodal protein in T47D cells (Figure 2, E and F). Collectively, these results suggest that the hypoxia-induced up-regulation of Nodal is mediated predominantly via an HIF-1α–dependent pathway in the cell lines tested.
FIGURE 2:
Hypoxia-induced Nodal expression is mediated via HIF-1α. (A and B) Immunoblot analyses of (A) T47D cells and (B) C81-61 cells exposed to normoxic (20% O2) or hypoxic (0.5% O2) conditions for 24 h following transfection with Scramble (control) siRNA, HIF-1α siRNA, HIF-2α siRNA, or HIF-1α siRNA + HIF-2α siRNA. In both cell lines, HIF-1α and HIF-2α protein levels are elevated in hypoxia, and this increase is abolished by HIF-1α siRNA and/or HIF-2α siRNA, respectively. Nodal protein expression increases under hypoxic conditions, and this hypoxia-induced up-regulation in Nodal expression is abrogated with HIF-1α siRNA and with HIF-1α siRNA + HIF-2α siRNA, but is not affected by HIF-2α siRNA alone. The pro-Nodal (∼39 kDa) band is presented, and β-actin is used as a loading control. (C and D) Immunofluorescence validating overexpression and nuclear localization of both HIF-1α and HIF-2α proteins following transfection of HIF-1α or HIF-2α expression constructs, respectively, into (C) T47D cells and (D) C81-61 cells under normoxic (20% O2) conditions. Scale bar: 10 μm. (E and F) Immunoblot analyses of Nodal protein expression in (E) T47D cells and (F) C81-61 cells transfected with a control vector, an HIF-1α expression construct, or an HIF-2α expression construct. In normoxic conditions, Nodal expression in HIF-1α–transfected cells is higher than controls at 20% O2 and similar to controls at 1% O2. However, Nodal expression is weakly induced (E) or unchanged (F) in HIF-2α–transfected cells. The pro-Nodal (∼39 kDa) band is presented, and β-actin is used as a loading control.
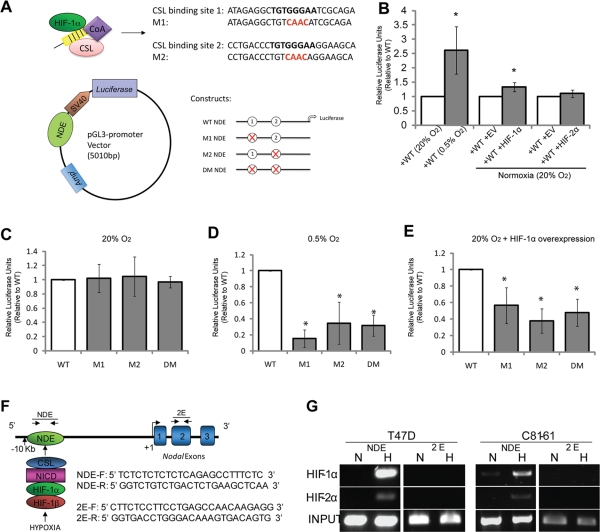
To elucidate the mechanisms by which HIF-1 regulates the transcriptional activation of Nodal, we examined the effects of hypoxia and HIF-1 on the activity of the NDE. The NDE contains two Notch-responsive CSL-binding sites that share 100% conservation between humans and mice (Krebs et al., 2003; Raya et al., 2003; Hardy et al., 2010). Developmental studies using mouse models have demonstrated that the NDE induces Nodal expression around the Node in a Notch-dependent manner to initiate left–right asymmetry (Krebs et al., 2003; Raya et al., 2003). Similarly, Notch signaling activates the NDE and positively regulates Nodal expression in human melanoma cells (Hardy et al., 2010). Moreover, studies have reported that Notch receptors and ligands are expressed in breast cancer and melanoma cell lines, including T47D, MCF-7, and C81-61 (Liu et al., 2006; Chen et al., 2010b; Hardy et al., 2010). Given the ability of HIF-1α to enhance Notch signaling, and the role of Notch in the regulation of Nodal, we sought to determine whether hypoxia-induced Nodal expression was associated with an increase in Notch-dependent NDE activity. Luciferase enhancer constructs containing either the wild-type NDE sequence or NDE sequences with mutations in one or both CSL-binding site(s) were transfected into T47D cells (Figure 3A). Following 24 h of culture in 20% or 0.5% O2, luciferase activity was assayed. When exposed to 0.5% O2 for 24 h, the wild-type NDE construct (containing both intact CSL-binding sites) exhibited a significant induction compared with wild-type activity in normoxia (Figure 3B). Wild-type NDE activity was also increased when HIF-1α was overexpressed; however, HIF-2α overexpression did not significantly increase activity (Figure 3B). To ascertain the importance of the Notch-responsive CSL-binding sites in the hypoxic and HIF-1α–mediated induction of NDE activity, we next examined whether mutating the CSL-binding sites could abrogate the increased activity observed under these conditions. Interestingly, reporter activity did not alter significantly from levels of wild-type NDE activity when CSL sites were mutated in cells cultured in normoxia (Figure 3C). However, when compared with the activity of the wild-type NDE, mutations in either one or both of the CSL-binding sites significantly decreased reporter activity in T47D cells cultured in 0.5% O2 (Figure 3D) or transfected with a HIF-1α overexpression vector (Figure 3E). As an extension to these findings, we performed a chromatin immunoprecipitation (ChIP) of the human NDE, to determine whether HIF-1α and/or HIF-2α were associated with the endogenous enhancer site in hypoxia (Figure 3F). In agreement with our reporter assays, we determined that HIF-1α associates with the human NDE under hypoxic (0.5% O2) conditions in T47D and C81-61 cells (Figure 3G). HIF-2α was also found to associate with the NDE in both cell lines, although the association of HIF-2α was relatively weak compared with the association with HIF-1α (Figure 3G). Importantly, neither HIF-1α, nor HIF-2α was found to associate with an area within the second exon of the Nodal gene, which served as a negative control. Collectively, these findings suggest that HIF-1α promotes Nodal expression in T47D breast cancer and C81-61 melanoma cells by associating with, and increasing the activity of, Notch-responsive elements in the NDE.
FIGURE 3:
HIF-1α interacts with the NDE to enhance Nodal expression. (A) Schematic depicting CSL-binding sites that are mutated in the pGL3 constructs used for luciferase assays in (B to E). CSL (pink), in conjunction with the NICD (yellow) and additional transcriptional coactivators (purple), as well as HIF-1α (green), are predicted to bind to two CSL-binding sites in the NDE, which is located ∼10-kb upstream from the Nodal TSS. Constructs containing either the two wild-type CSL-binding sites (WT), CSL-binding site 1 mutated (M1), CSL-binding site 2 mutated (M2), or both CSL-binding sites 1 and 2 mutated (DM) were assembled into a pGL3-promoter vector. (B) Graphical representation of luciferase reporter assays demonstrating the effects of hypoxia, HIF-1α expression, or HIF-2α expression on wild-type NDE activity in T47D cells. T47D cells were transfected with pGL3 vectors containing the NDE wild-type construct. Cells were allowed to recover overnight, and were then exposed to 20% or 0.5% O2 for 24 h. Alternatively, cells were transfected with an empty vector (EV), an HIF-1α expression vector, or an HIF-2α expression vector. Firefly luciferase reporter activity for each parameter was normalized to Renilla luciferase activity, which was cotransfected as an internal control. Results show that luciferase activity is significantly higher in hypoxia vs. normoxia (n = 4; p < 0.05). Furthermore, cotransfection with HIF-1α induces a significant up-regulation of luciferase activity in normoxia (n = 7; p < 0.05), whereas cotransfection with HIF-2α does not significantly alter activity (n = 4; p = 0.34). Data are presented as mean activity ± SEM relative to corresponding control values (open bars). *, significant difference compared with corresponding controls. (C to E) Luciferase reporter assays demonstrating Notch-dependent NDE activity in T47D cells cultured in (C) normoxia (20% O2) or (D) hypoxia (0.5% O2) and (E) in T47D cells expressing HIF-1α in normoxia (20% O2). T47D cells were transfected with pGL3 vectors containing either NDE wild-type or mutant constructs. Cells were allowed to recover overnight and were then exposed to 20% or 0.5% O2 for 24 h. Alternatively, cells were cotransfected with an empty vector (EV) or an HIF-1α expression vector. Firefly luciferase reporter activity for each parameter was normalized to Renilla luciferase activity, which was cotransfected as an internal control. Results indicated that (C) in 20% O2, mutations in Notch-responsive CSL-binding sites do not affect luciferase activity, suggesting that these sites are not active under basal conditions in T47D cells. In contrast, mutations in the CSL-binding sites abrogated luciferase activity (D) in cells cultured in hypoxia (0.5% O2) and (E) in cells overexpressing HIF-1α in normoxia, suggesting that the increased NDE activity observed under these conditions is dependent upon CSL-binding sites. Bars represent mean activity ± SEM relative to the wild-type control value. Asterisks (*) indicate a significant difference from enhancer activity in wild-type NDE cells (4 ≤ n ≤ 7; p < 0.05). (F) Schematic depicting proposed interaction between HIF proteins and the NDE upstream of the Nodal gene in hypoxia. The relative location and sequences of primers used to amplify the NDE and a region in the second exon of the Nodal gene (2E), used as a negative control, are also depicted. (G) ChIP followed by PCR showing association of HIF-1α and HIF-2α with the human Nodal NDE in T47D and C81-61 cells. Genomic DNA acquired via ChIP using HIF-1α or HIF-2α antibodies and respective IgG controls demonstrates that both HIF-1α and HIF-2α are associated with the endogenous NDE in T47D cells cultured in hypoxia. Note that HIF-2α is associated at a relatively lower level than HIF-1α. Neither HIF-1α nor HIF-2α was associated with 2E.
Hypoxia-induced nodal expression persists following reoxygenation due to a positive feedback loop
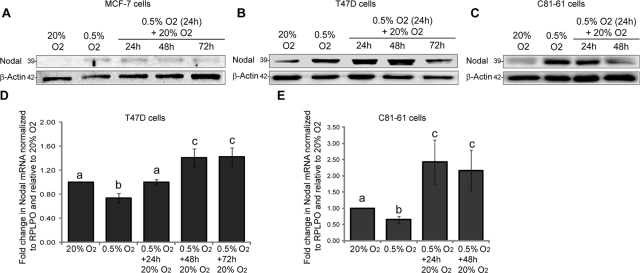
In addition to chronic hypoxia, tumor cells are frequently exposed to transient hypoxia followed by reoxygenation (Cairns et al., 2001). Hence we next sought to determine whether hypoxia-induced Nodal expression could be maintained if cells were returned to normoxic conditions. We measured Nodal protein levels in MCF-7, T47D, and C81-61 cells exposed to 0.5% O2 for 24 h, which was followed by exposure to 20% O2 for up to 3 d. To our surprise, we discovered that the hypoxia-induced increase in Nodal expression was neither transient nor dependent on prolonged exposure to hypoxia, as elevated Nodal expression persisted for up to 48 h after reoxygenation in 20% O2. (Figure 4, A–C). Nodal mRNA in T47D and C81-61 cells exposed to 0.5% O2 continued to increase after 24 h of reoxygenation and began to plateau after 48 and 72 h to levels significantly higher than those at 20% O2 (Figure 4, D and E). In the same T47D samples, LOX mRNA increased dramatically in response to hypoxia, and returned to normoxic control levels within 24 h of reoxygenation (Figure S1F), demonstrating sufficient hypoxia-induced transcriptional activation, as well as the ability to recover basal expression levels. Collectively, these findings suggest that once induced by hypoxia, Nodal expression is maintained for up to 48 h irrespective of the presence or absence of O2.
FIGURE 4:
Hypoxia-induced Nodal expression persists following up to 48 h of reoxygenation. (A to C) Immunoblot analyses of Nodal protein in (A) MCF-7, (B) T47D, and (C) C81-61 cells cultured in 20% O2 (24 h), 0.5% O2 (24 h), or 0.5% O2 (24 h), which was followed by reoxygenation in 20% O2 (24–72 h). Results indicate that hypoxia-induced Nodal expression is still elevated after up to 48 h of reoxygenation compared with 20% O2 controls. The pro-Nodal (∼39 kDa) band is presented, and β-actin is used as a loading control. (D and E) Real-time RT-PCR analyses of Nodal mRNA in (D) T47D and (E) C81-61 cells cultured in 20% O2, 0.5% O2, or 0.5% O2, which was followed by reoxygenation in 20% O2 (24–72 h). Nodal mRNA underwent an initial drop in hypoxia (24 h), which was followed by a significant up-regulation during reoxygenation. Gene expression was normalized to RPLPO, and bars represent mean gene expression ± SD relative to 20% O2 control. Different letters are significantly different (n = 6; p < 0.05).
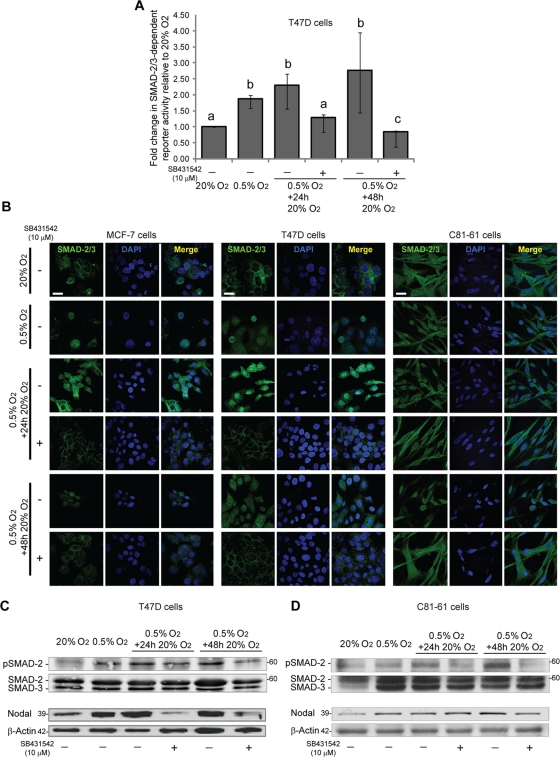
Studies in mice and in human melanoma cells have shown that Nodal is able to autoregulate itself via a positive feedback loop (Norris and Robertson, 1999; Saijoh et al., 2005; Topczewska et al., 2006). Nodal propagates its signal by binding to a receptor complex composed of Cripto and a heterodimer of type I (ALK 4/7) and type II (ActRIIB) activin-like kinase receptors. Activation of this complex results in the phosphorylation and nuclear translocation of a SMAD-2/3 complex that subsequently induces the transcription of target genes such as Lefty, Goosecoid, and Nodal (Schier, 2009). Importantly, Nodal autoregulates its transcription via the SMAD-2/3–mediated activation of the left side–specific enhancer (LSE) located 4 kb upstream from the TSS and the asymmetric enhancer (ASE) in the first intron (Norris and Robertson, 1999; Saijoh et al., 2005). Previous studies have shown that treatment of C81-61 cells with recombinant Nodal (rNodal) results in an induction of SMAD-2 phosphorylation (Topczewska et al., 2006), and we have determined that rNodal induces SMAD-2 phosphorylation in MCF-7 and T47D breast cancer cells (Figure S1G). Thus, although these cells do not normally express high levels of Nodal, they are capable of signaling in response to this ligand. Given that poorly metastatic cells can respond to Nodal, we hypothesized that hypoxia-induced Nodal protein expression initiates a positive feedback loop that can sustain Nodal expression upon reoxygenation. To test this hypothesis, T47D cells were exposed to 0.5% O2 and then treated with an ALK-4/5/7 inhibitor (SB431542; 10 μM) upon reoxygenation. Using a green fluorescent protein (GFP)-based transcription reporter assay, we determined that SMAD-2/3 transcriptional activity is significantly increased in response to hypoxia in T47D cells, and that this increase is maintained following up to 48 h of reoxygenation (Figure 5A). In addition, treatment with SB431542 abrogated the enhanced transcriptional activity observed upon reoxygenation. In agreement with the reporter assay, immunofluorescence localization of SMAD-2/3 in MCF-7, T47D, and C81-61 cells demonstrated that SMAD-2/3 translocates to the nucleus in response to 0.5% O2 and remains associated with the nucleus after 24 and 48 h of reoxygenation (Figure 5B). Moreover, treatment with SB431542 resulted in the redistribution of SMAD-2/3 to the cytoplasm (Figure 5B). Finally, we determined that SMAD-2 phosphorylation increases in response to hypoxia in T47D and C81-61 cells, and that this increase is maintained following reoxygenation (Figure 5, C and D). Also at 48 h, SB431542 brought phosphorylated SMAD-2 levels down to those observed at 20% O2 (Figure 5, C and D). Of note, preventing Nodal signaling with SB431542 abrogated hypoxia-induced Nodal expression upon reoxygenation in T47D cells (Figure 5C). A similar trend was observed in C81-61 cells, such that SB431542 prevented SMAD-2 phosphorylation concomitant with a reduction in Nodal expression after 48 h of reoxygenation (Figure 5D). These results suggest that the maintenance of Nodal following reoxygenation is dependent upon the activation of an ALK-4/5/7–dependent SMAD-2/3–mediated positive feedback loop.
FIGURE 5:
Hypoxia-induced Nodal expression persists via a SMAD-dependent positive feedback mechanism. (A) Graphical representation of SMAD-2/3 reporter activity in T47D cells cultured in 20% O2 (24 h), 0.5% O2 (24 h), or 0.5% O2 (24 h), which was followed by reoxygenation in 20% O2 (24 or 48 h). On reoxygenation, cells were cultured in the presence of a vehicle (−) or the ALK-4/5/7 inhibitor SB431542 (10 μM; +). SMAD-2/3 transcriptional activity was measured using flow cytometry in cells transfected with a SMAD-2/3–responsive GFP reporter construct. Exposure to hypoxia increased SMAD-2/3 transcriptional activity. This up-regulation in SMAD-2/3 activity was retained after 24 and 48 h of reoxygenation, and SMAD-2/3 activity was abrogated by SB431542. Bars represent the median fold change in SMAD-2/3 reporter activity ± interquartile range relative to control (20% O2) activity. Different letters are significantly different (n = 5; p < 0.05). (B) Immunofluorescence of SMAD-2/3 in MCF-7, T47D, or C81-61 cells cultured in 20% O2 (24 h), 0.5% O2 (24 h), or 0.5% O2 (24 h) followed by reoxygenation in 20% O2 (24 or 48 h). On reoxygenation, cells were cultured in the presence of a vehicle (−) or the ALK-4/5/7 inhibitor SB431542 (10 μM; +). In all cell lines, exposure to hypoxia resulted in the translocation of SMAD-2/3 to the nucleus. SMAD-2/3 was retained in the nucleus after 24 and 48 h of reoxygenation, and this nuclear localization of SMAD-2/3 was abrogated by SB431542. Scale bar: 10 μm. (C and D) Immunoblot analyses of phosphorylated SMAD-2, total SMAD-2/3, and Nodal in (C) T47D cells and (D) C81-61 cells cultured in 20% O2 (24 h), 0.5% O2 (48 h), or 0.5% O2 (24 h), which was followed by 20% O2 reoxygenation (24 or 48 h). On reoxygenation, cells were treated with either vehicle or an ALK 4/5/7 inhibitor (SB431542; 10 μM). SMAD-2 phosphorylation is elevated in hypoxia and persists during reoxygenation, and this up-regulation in phosphorylation is reduced with the addition of SB431542, with greater effects observed at 48 h than at 24 h. Relative to control (20% O2) levels, Nodal expression is elevated in hypoxia and during reoxygenation. In T47D cells (C), the elevated levels of Nodal protein observed during 24 and 48 h of reoxygenation are greatly reduced with the addition of SB431542. However, in C81-61 cells (D), SB431542 reduces Nodal expression only at 48 h of reoxygenation. The pro-Nodal (∼39 kDa) band is presented, and total SMAD-2/3 or β-actin are used as loading controls.
Hypoxia-induced nodal expression supports breast cancer angiogenesis, invasion, and migration in vitro
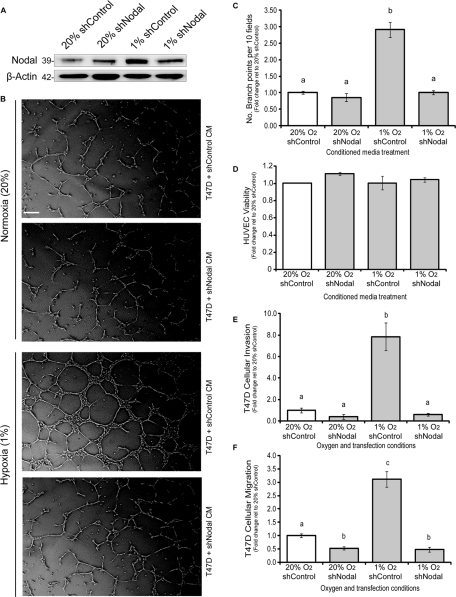
Low levels of O2 are known to induce expression of proangiogenic factors, such as vascular endothelial growth factor (VEGF), within growing tumors (Postovit et al., 2005). These factors signal to surrounding vasculature, causing blood vessel recruitment toward the growing tumor and generating new conduits for O2 and nutrient delivery concomitant with metastatic progression (Semenza, 2003; Postovit et al., 2005). Accordingly, we opted to determine whether Nodal supports hypoxia-induced endothelial cell tube formation in vitro. T47D cells were transfected with a short hairpin RNA (shRNA) targeting Nodal (shNodal) or a control shRNA (shControl) and incubated in normoxic (20% O2) or hypoxic (1% O2) conditions for 48 h, at which point conditioned media was collected. Western blot analysis confirmed an increase in Nodal expression in cells transfected with shControl under hypoxic conditions and an abrogation of this increase with shNodal treatment (Figure 6A). Human umbilical vein endothelial cells (HUVECs) were grown on Matrigel and treated with the aforementioned conditioned media for 6 h. We found that HUVECs treated with T47D-shControl– or T47D-shNodal–conditioned media did not exhibit a significant difference in tube formation under normoxic conditions. Conditioned media from T47D-EV cells did, however, induce a significant increase in HUVEC tube formation under hypoxic conditions. This hypoxia-induced increase in tube formation did not occur when HUVECs were treated with conditioned media from hypoxic T47D-shNodal cells (Figure 6, B and C). To eliminate the possibility that differential cell viability interfered with these results, HUVEC viability in response to each conditioned media treatment was measured, and showed no observable difference (Figure 6D). To our knowledge, these results are the first to implicate Nodal as a proangiogenic factor in hypoxia-induced tumor angiogenesis.
FIGURE 6:
Hypoxia-induced Nodal expression promotes metastatic phenotypes in breast cancer cells in vitro. (A) Immunoblot validation of Nodal expression in T47D cells transfected with a Nodal or control shRNA in hypoxia (1% O2) vs. normoxia (20% O2). Nodal protein levels are similar in T47D-shNodal and T47D-shControl cells in normoxic conditions; however, in hypoxia, Nodal protein expression is induced in T47D-shControl cells, and the hypoxic up-regulation of Nodal is abrogated by shRNA against Nodal. The pro-Nodal (∼39 kDa) band is presented, and β-actin is used as a loading control. (B) Representative phase-contrast micrographs of HUVECs grown on Matrigel in conditioned media from T47D cells transfected with a Nodal or control shRNA and grown in hypoxia (1% O2) or normoxia (20% O2) for 48 h. Scale bar: 50 μM. (C) Quantification of the branch points produced by HUVECs grown on Matrigel in conditioned media from T47D cells transfected with a Nodal or control shRNA and grown in hypoxia (1% O2) or normoxia (20% O2) for 48 h. HUVEC tube formation is significantly induced in response to conditioned media from T47D-shControl cells in hypoxic conditions; however, this effect is suppressed in response to conditioned media from T47D-shNodal cells in hypoxic conditions. Data is presented as mean number of branches ± SEM relative to HUVECs cultured in conditioned medium from 20% O2 control cells. (D) HUVEC viability in response to conditioned media from T47D cells transfected with a Nodal or control shRNA and grown in hypoxia (1% O2) or normoxia (20% O2) for 48 h. HUVEC viability is not altered by conditioned media treatments from T47D-shNodal and T47D-shControl cells in normoxia or hypoxia, indicating that differences in tube formation are not due to differences in cell viability. Data is presented as mean HUVEC viability ± SEM relative to cells cultured in conditioned medium from 20% O2 control cells. (E) In vitro invasion assay of T47D cells transfected with a Nodal or control shRNA and then allowed to invade through Matrigel for 48 h in 20% or 1% O2. T47D-shControl cells in normoxia display no significant difference in cellular invasion compared with T47D-shNodal cells in normoxia. Conversely, T47D-shControl cells in hypoxia display a significant induction of cellular invasion compared with T47D-shNodal cells in hypoxia. T47D-shNodal cells in hypoxia display invasion comparable with normoxic controls. Data are presented as mean invasion ± SEM relative to control cells in 20% O2. (F) In vitro migration assay of T47D cells transfected with a Nodal or control shRNA and then allowed to migrate for 48 h in 20% or 1% O2. T47D-shNodal cells in normoxia display a significant reduction in cellular migration compared with T47D-shControl cells in normoxia. T47D-shControl cells in hypoxia display a significant induction of cellular migration. This effect is abrogated in T47D-shNodal cells under hypoxic conditions. Data is presented as mean migration ± SEM relative to control cells in 20% O2. For (C to F), different letters indicate a significant difference (≥4; p < 0.05).
In addition to tumor angiogenesis, both hypoxia and Nodal signaling promote cellular invasion (Topczewska et al., 2006; Strizzi et al., 2008; Lee et al., 2010; Lawrence et al., 2011). Accordingly, we suspected that hypoxia could promote invasion and migration in poorly aggressive T47D breast cancer cells, in part through Nodal induction. T47D cells transfected with either shNodal or shControl were seeded in Transwell chambers with or without a Matrigel coating to assess invasion and migration, respectively, under both normoxic and hypoxic conditions. We found that, under normoxic conditions, shNodal had no effect on invasion and caused only a slight decrease in migration compared with controls. In hypoxia, invasion and migration of T47D-EV cells were significantly stimulated; however, shNodal transfection prevented these hypoxia-induced effects (Figure 6, E and F). The robust changes observed in these assays suggest that Nodal is a major regulator of hypoxia-induced breast cancer cell invasion and migration.
DISCUSSION
This study reveals, for the first time, that low O2 levels promote the expression of the embryonic morphogen Nodal in both cancer and embryonic stem cell types. This finding has significant implications, as decreased O2 levels characterize the microenvironments of solid tumors and the developing embryo, both of which are associated with the expression of Nodal (Yedwab et al., 1976; Genbacev et al., 1997; Keith and Simon, 2007). Low O2 and Nodal have been shown to promote invasion and metastasis in cancer, and Nodal and low O2 are required for normal embryonic patterning during development (Genbacev et al., 1997; Postovit et al., 2005; Hendrix et al., 2007; Keith and Simon, 2007). This study mechanistically links these phenomena, demonstrating that O2 is a common, perhaps universal, regulator of Nodal expression.
Our findings demonstrate that O2 regulates Nodal expression via a combinatorial mechanism involving transcriptional activation and increased protein stability (Figure 7). This complexity is not unique for hypoxia-induced proteins. For example, HIF-1α and HIF-2α can be regulated at the level of transcription, but are most robustly induced via the increased protein stability associated with reduced proteolytic degradation (Keith and Simon, 2007; Semenza, 2007). We have shown that Nodal protein stability is similarly increased in response to low O2. The mechanisms underlying this alteration in stability were not determined, but likely involve posttranslational modifications. Like many secreted proteins, Nodal is modified via glycosylation events that impose enhanced stability and signaling range in developmental models (Le Good et al., 2005). Interestingly, recent studies suggest that hypoxia promotes glycosylation (Shirato et al., 2011). Therefore it is possible that low O2 increases Nodal stability by promoting this posttranslational modification.
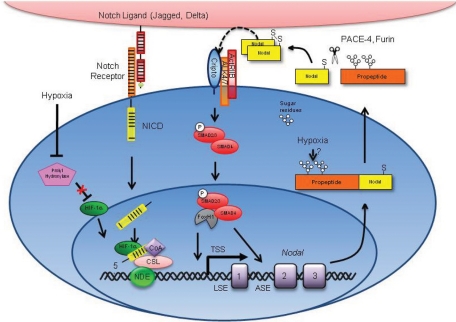
FIGURE 7:
Schematic model of the hypoxia-induced regulation of Nodal. The hypoxic regulation of Nodal involves alterations in protein stability and transcription. In response to decreased O2 levels, the NDE region is activated by the HIF-1α–mediated enhancement of Notch-dependent transcription. Specifically, HIF-1α stabilizes NICDs, thereby increasing Notch signaling, and leading to enhanced Nodal transcription driven by the NDE. Nodal protein is also stabilized in response to decreased O2 levels. Nodal is translated as a proprotein and is posttranslationally modified with two glycosylation sites. Glycosylation has been shown to enhance the stability of Nodal protein. Hence hypoxia may stabilize Nodal by promoting glycosylation. Once produced, Nodal protein positively regulates its own expression in a feed-forward manner. This process involves the cleavage of Nodal protein by the proprotein convertases Furin and Pace-4. Nodal then homodimerizes via an interchain disulfide bond and propagates its signal by binding to heterodimeric complexes between type I (ALK 4/7) and type II (ActRIIB) activin-like kinase receptors. This results in the phosphorylation and activation of ALK 4/7 by ActRIIB, followed by the ALK 4/7–mediated phosphorylation of SMAD2/3. Phosphorylated SMAD2/3 associates with SMAD4 and translocates into the nucleus, where it regulates gene expression through association with transcription factors, such as FOXH1. This transcriptional complex binds to the ASE and LSE regions in the Nodal locus to induce Nodal gene expression and perpetuate Nodal protein production. The absence of the endogenous Nodal inhibitor Lefty in cancer cells permits the continued production of Nodal upon reoxygenation.
The current study also determined that low O2 induces Nodal expression, in part through HIF-1α–dependent activation of the NDE. HIF-1α−activation of the NDE was dependent on Notch-responsive elements, such that mutation of these sites abrogated both the HIF-1α–induced and the hypoxia-induced activation of this enhancer. These results suggest that HIF-1α may indirectly induce Nodal transcription via Notch stabilization. Previous studies have shown that HIF-1α maintains stem cell pluripotency by stabilizing NICDs (Gustafsson et al., 2005). On the basis of our results and the ability of Nodal to promote pluripotency, we suggest that HIF-1α promotes stem cell phenotypes through NICD stabilization and subsequent induction of Nodal. Of note, we found that knockdown of HIF-2α does not significantly abrogate hypoxia-induced Nodal expression, and that overexpression of HIF-2α does not up-regulate Nodal to the same extent as HIF-1α, despite its documented ability to bind to NICDs (Chen et al., 2010a) and our finding that it can associate with the human NDE. These discrepancies suggest that HIF-2α may be less efficient than HIF-1α at promoting Notch-dependent transcription in the cell lines tested. The mechanisms underlying these differences were not studied here, but may involve a lower relative abundance of HIF-2α compared with HIF-1α or divergent cofactor recruitment.
In contrast to many hypoxia-induced genes, such as LOX (Le et al., 2004; Postovit et al., 2008a), we determined that hypoxia-induced Nodal expression is maintained up to 48 h following reoxygenation. In embryological systems, Nodal signaling is controlled by endogenous inhibitors such as Lefty-A, Lefty-B, and Cerberus (Schier, 2009). For example, elegant studies have shown the Lefty proteins (Lefty-A, Lefty-B), which are divergent members of the TGF-β superfamily, spatially and temporally antagonize Nodal during development (Schier, 2009). Of note, the Lefty genes are downstream targets of Nodal signaling and provide a powerful negative feedback loop for this pathway (Schier, 2009). Previous studies have determined that T47D, MCF-7, and C81-61 cells do not express Lefty (Postovit et al., 2008b). We confirmed these results and also found that Lefty could not be detected in these cell lines, even after hypoxia/reoxygenation. This suggests that cancer cells may undergo uncontrolled Nodal signaling through positive feedback regulation and circumvention of negative feedback control. Supporting this concept, we found that SMAD-2/3 was activated in response to hypoxia/reoxygenation and that Nodal expression upon reoxygenation could be abrogated with SB431542, a small molecule inhibitor that blocks Nodal signaling and autoregulation through binding ALK4/5/7. This finding has significant ramifications for solid tumors, where hypoxia is frequently observed at early stages. Indeed, it is possible that a single exposure to hypoxia in a localized lesion can promote Nodal expression and perpetuate a more aggressive phenotype in a manner that persists even after cancer cells escape the hypoxic environment. This may give cells a selective advantage during metastasis by sustaining tumorigenic phenotypes, such as high invasive capacity and angiogenic potential, regardless of O2 availability at the secondary site.
As indicated in Figure 6, hypoxia-induced Nodal expression is associated with an increase in angiogenic potential and cellular invasion. Preventing the hypoxic up-regulation of Nodal with shRNA abrogated the ability of T47D breast cancer cells to invade through Matrigel and to induce endothelial cell migration and tube formation. The mechanisms by which Nodal regulates these phenotypes were not investigated here but likely include the Nodal-mediated induction of proinvasive proteins, such as matrix metalloproteinases, and proangiogenic factors, such as VEGF. In agreement with this possibility, studies have shown that Nodal promotes MMP-2 and VEGF expression in glioma cells (Hueng et al., 2010; Lee et al., 2010).
Collectively, our studies demonstrate that low O2 levels induce Nodal expression via a combinatorial mechanism. These results highlight the complexities associated with the regulation of Nodal, and underscore the myriad mechanisms by which O2 can regulate gene expression. This work also provides insight into the regulation of stem cell proteins, such as Nodal, during cancer and development and reveals that Nodal may be a target for the treatment and prevention of hypoxia-induced tumor progression.
MATERIALS AND METHODS
Cell culture and treatments
Human breast carcinoma (MCF-7, T47D), cutaneous melanoma (C81-61), and H9 hESCs (WiCell, Madison, WI) were maintained as previously described (Postovit et al., 2008b). For experiments, hESCs were cultured on Matrigel (BD Biosciences, Mississauga, Ontario, Canada) in MTeSR (Stem Cell Technologies, Vancouver, BC) according to the manufacturer's instructions. Recombinant Nodal (R&D Systems, Minneapolis, MN) and SB431542 (Sigma-Aldrich, St. Louis, MO) were diluted according to the manufacturer's suggestions. O2 concentrations were regulated as previously described (Postovit et al., 2008a), using Pro-Ox Model 110 O2 regulators (BioSpherix, Redfield, NY). For Nodal knockdown experiments, cells were transfected with a HuSH 29mer shRNA construct (Id: GI311711) against Nodal (OriGene Technologies, Burlington, Ontario, Canada), targeted to the third exon, or an shRNA control via lipofectamine. shRNAs targeting four regions in the Nodal gene were tested for their ability to knock down Nodal protein expression, and the shRNA achieving the best knockdown was chosen. Hypoxia treatments for all functional experiments were performed for 48 h.
Immunoblotting
Protein lysates were prepared and quantified as previously described (Postovit et al., 2008b). Equal amounts of protein were separated by SDS–PAGE under reducing conditions, and resolved proteins were transferred onto Immobilon-P membranes (Millipore, Billerica, MA). Membranes were blocked, incubated with primary antibody (Supplemental Table S1), washed, and incubated with the appropriate horseradish peroxidase–labeled secondary antibody. Secondary antibodies were detected by enhanced chemiluminescence (Super Signal; Fisher Scientific, Toronto, Ontario, Canada). All images are representative of at least three experiments. For Nodal, up to three species were detected: a pro-Nodal band at ∼39 kDa, multiple processed (glycosylated, etc.) bands at ∼ 50 kDa, and a cleaved Nodal band at ∼15 kDa. The 50-kDa bands were highly variable due to differences in posttranslational modifications and protein lysate handling. The 15-kDa band was poorly abundant and most readily observed in conditioned media. For consistency and accuracy we used the 39-kDa band to assess Nodal expression in lysates and the 15-kDa band to assess Nodal levels in conditioned media.
RNA extraction and reverse transcriptase-PCR (RT-PCR)
RNA was extracted and genomic DNA was degraded with DNAse using the PerfectPure RNA Cell & Tissue kit (5 Prime, Gaithersburg, MD) according to the manufacturer's instructions. Reverse transcription of RNA was achieved using TaqMan Reverse Transcription Reagents (Applied Biosystems, Carslad, CA) with 2 μg of RNA according to the manufacturer's suggestion. qRT-PCR was performed using TaqMan Gold RT-PCR Kit (Applied Biosystems). TaqMan gene expression primer/probe sets used were: Nodal (Hs00250630), HIF-1α (Hs00153153), and LOX (Hs00184706) from Applied Biosystems. Expression was normalized to ribosomal protein, large, P0 (RPLP0 4326314E; Applied Biosystems). qRT-PCR was performed on the Bio-Rad CFX96 Real-Time System with 2 min at 50°C for uracil-N-glycosylase incubation and 10 min at 95°C for AmpliTaq Gold activation, which was followed by 40 subsequent cycles composed of a denaturing step for 15 s at 95°C and an annealing/extension step for 1 min at 58°C. Relative fold changes were normalized to the control gene RPLP0 and calculated by determining the ΔCT and ΔΔCT values.
Protein stability assays
T47D cells were transfected with a myc-dkk–tagged Nodal expression vector (OriGene Technologies, Burlington, Ontario, Canada) and selected using G418. Cells were treated with 0.1 mg/ml cycloheximide for 0 min, 30 min, 1 h, 2 h, 3 h, 6 h, 12 h, or 24 h under either hypoxic (0.5% O2) or normoxic (20% O2) conditions. Protein lysates were prepared and quantified as previously described (Postovit et al., 2008b), and protein abundance was determined with immunoblotting for myc-dkk.
Luciferase reporter assay
Reporter constructs containing sequences of the enhancer region upstream from the Nodal gene corresponding to wild-type NDE and mutant sites were assembled into pGL3-basic vectors (Promega, Madison, WI) as previously described (Takeuchi et al., 2007; Hardy et al., 2010). Vectors were kindly provided by Benoit Bruneau (Gladstone Institute of Cardiovascular Disease, San Francisco, CA). NDE construct DNA (0.2 μg) was transfected into T47D cells using a microporator according to the manufacturer's instructions. After overnight incubation, cells were exposed to hypoxia/normoxia for 24 h, and luciferase activity was measured using a Dual Luciferase Reporter Assay System (Promega, Madison, WI) and a dual-injector luminometer (FLUOstar Omega; Fisher Scientific, Toronto, Ontario, Canada). In all cases, cells were cotransfected with 0.2 μg of a pGL3-Renilla vector (Promega, Madison, WI) to control for variations in transfection efficiency. For HIF-1α and HIF-2α overexpression with NDE constructs, 0.2 μg of HIF-pCMV6 or 0.2 μg of the control pCMV6 empty vector was cotransfected with 0.2 μg of NDE constructs, and cells were allowed to recover overnight and were kept in 20% O2 for an additional 24 h, after which firefly and Renilla luciferase activities were measured.
HIF-1α and HIF-2α overexpression and knockdown
HIF-1α overexpression (pCMV6-HIF-1α), HIF-2α overexpression (pCMV6-HIF-1α), and control (pCMV6-XL5) (OriGene Technologies, Burlington, Ontario, Canada) DNA was transfected into T47D and C81-61 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Validated HIF-1α Silencer Select siRNA (ID #S6539), validated HIF-2α siRNA (Hs_EPAS1_5 HP; Qiagen, Toronto, Ontario, Canada) or Silencer Select negative control siRNA (4390846) were purchased from Ambion (Austin, TX). siRNA (100 nM) was transfected into T47D or C81-61 cells by microporation according to the manufacturer's instructions. Transiently transfected cells were allowed to recover overnight and were then exposed to hypoxia or normoxia for 24 h.
Immuonofluorescence
Cells were fixed with 4% paraformaldehyde; made permeable with 20 mM HEPES and 0.5% TritonX-100; and blocked with serum-free protein block (Dako, Carpinteria, CA). Primary antibodies were diluted in antibody dilutant (Dako) to the concentrations outlined in Table S1, and appropriate fluorochrome-conjugated secondary antibodies were used according to the manufacturer's recommendations. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; 0.1 mg/ml; Molecular Probes/Invitrogen, Eugene, OR), and images were obtained using confocal microscopy (Zeiss 510 META, Carl Zeiss, Jena, Germany).
Chromatin immunoprecipitation
Chromatin immunoprecipitation was performed on T47D and C81-61 cells using a ChIP-IT Express Chromatin Immunoprecipitation kit according to the manufacturer's instructions (Active Motif, Carlsbad, CA), using appropriate antibodies or immunoglobulin G (IgG) isotype controls (Table S1). Primers for the NDE were as follows: forward: 5′TCTCTCTCTCTCAGAGCCTTTCTC3′; reverse: 5′GGTCTGTCTGACTCTGAAGCTCAA3′. A region in the second exon of Nodal was amplified as a negative control. The primers for this region were: forward: 5′CTTCTCCTTCCTGAGCCAACAAGAGG3′; reverse: 5′GGTGACCTGGGACAAAGTGACAGTG3′. PCR was conducted using a Bio-Rad C1000 Thermal Cycler (Bio-Rad, Hercules, CA). PCR products were resolved on a 1% agarose gel and visualized with the Bio-Rad Molecular Imager Gel Doc XR+ and Quantity One Software. The identity of PCR products was confirmed with sequencing (Figure S2).
SMAD-2/3 activity assay
Reporter constructs from the CSignal SMAD GFP Reporter Kit (SA Biosciences/Qiagen, Valencia, CA) were transfected into T47D cells according to the manufacturer's instructions. Following exposure to various conditions, GFP levels were measured using flow cytometry according to the manufacturer's instructions.
In vitro tube formation
T47D cells were transfected with either a Nodal-targeted shRNA (shNodal) or a control shRNA (shControl). Endothelial cell medium 131 supplemented with 5% BSA was used to make conditioned media from these cells in normoxic (20% O2) or hypoxic (1% O2) conditions for 48 h. Endothelial cells were grown for 6 h on Matrigel with conditioned media from T47D-EV or T47D-shNodal cells grown at 20% or 1% O2. Tube formation was quantified based on an average number of branch points in 10 fields of view. HUVEC cell viability was assessed in each experimental condition using the LIVE/DEAD cell viability assay (Invitrogen) according to the manufacturer's protocol. Quantitative analysis of tube formation and cell viability were based on four experimental replicates. Images were taken from a representative replicate.
Invasion and migration
Poorly invasive T47D cells were transfected with either a Nodal-targeted shRNA (shNodal) or a control shRNA (shControl), and 100,000 cells were seeded onto Transwell chambers (24-well format; Cell Biolabs, San Diego, CA) coated with Matrigel. Each treatment was exposed to 20% O2 or 1% O2 for 48 h, and cellular invasion was assessed using DAPI staining (Invitrogen). To assess cellular migration, assay design was the same, except that Matrigel was excluded from the experiment.
Statistical analysis
One-way analysis of variance or Kruskal-Wallis tests with Dunn's, Student Newman-Keuls, or Holm-Sidak post hoc tests (either pairwise or vs. control) were used to assess the statistical significance using Sigma Stat software (San Jose, California). Parametric data are expressed as means and nonparametric data are expressed as medians. Differences with p < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by grants from the Canada Foundation for Innovation, the Canadian Institutes of Health Research, and the Cancer Research Society awarded to L.M.P. D.F.Q. is the recipient of a doctoral award from the Canadian Breast Cancer Foundation, and L.M.P. is the recipient of the premier new investigator award from the CIHR.
Abbreviations used:
- ASE
asymmetric enhancer
- ChIP
chromatin immunoprecipitation
- ESC
embryonic stem cell
- GFP
green fluorescent protein
- HIF
hypoxia-inducible factor
- HRE
hypoxia-responsive element
- HUVEC
human umbilical vein endothelial cell
- LOX
lysyl oxidase
- LSE
left side–specific enhancer
- NDE
node-specific enhancer
- NICD
Notch intracellular domain
- rNodal
recombinant Nodal
- RPLPO
large ribosomal protein
- RT-PCR
reverse transcriptase-PCR
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- TGF-β
transforming growth factor β
- TSS
transcriptional start site
- VEGF
vascular endothelial growth factor
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-03-0263) on October 26, 2011.
*These authors contributed equally to this work.
REFERENCES
- Cairns RA, Kalliomaki T, Hill RP. Acute (cyclic) hypoxia enhances spontaneous metastasis of KHT murine tumors. Cancer Res. 2001;61:8903–8908. [PubMed] [Google Scholar]
- Chaudary N, Hill RP. Hypoxia and metastasis. Clin Cancer Res. 2007;13:1947–1949. doi: 10.1158/1078-0432.CCR-06-2971. [DOI] [PubMed] [Google Scholar]
- Chen H, Houshmand G, Mishra S, Fong GH, Gittes GK, Esni F. Impaired pancreatic development in Hif2-alpha deficient mice. Biochem Biophys Res Commun. 2010a;399:440–445. doi: 10.1016/j.bbrc.2010.07.111. [DOI] [PubMed] [Google Scholar]
- Chen J, Imanaka N, Chen J, Griffin JD. Hypoxia potentiates Notch signaling in breast cancer leading to decreased E-cadherin expression and increased cell migration and invasion. Br J Cancer. 2010b;102:351–360. doi: 10.1038/sj.bjc.6605486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci USA. 2005;102:4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Hardy KM, Kirschmann DA, Seftor EA, Margaryan NV, Postovit LM, Strizzi L, Hendrix MJ. Regulation of the embryonic morphogen Nodal by Notch4 facilitates manifestation of the aggressive melanoma phenotype. Cancer Res. 2010;70:10340–10350. doi: 10.1158/0008-5472.CAN-10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helczynska K, Kronblad A, Jogi A, Nilsson E, Beckman S, Landberg G, Pahlman S. Hypoxia promotes a dedifferentiated phenotype in ductal breast carcinoma in situ. Cancer Res. 2003;63:1441–1444. [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Seftor RE, Kasemeier-Kulesa J, Kulesa PM, Postovit LM. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat Rev Cancer. 2007;7:246–255. doi: 10.1038/nrc2108. [DOI] [PubMed] [Google Scholar]
- Hueng DY, et al. Inhibition of Nodal suppresses angiogenesis and growth of human gliomas. J Neurooncol. 2010;104:21–31. doi: 10.1007/s11060-010-0467-3. [DOI] [PubMed] [Google Scholar]
- Jogi A, Ora I, Nilsson H, Poellinger L, Axelson H, Pahlman S. Hypoxia-induced dedifferentiation in neuroblastoma cells. Cancer Lett. 2003;197:145–150. doi: 10.1016/s0304-3835(03)00095-8. [DOI] [PubMed] [Google Scholar]
- Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch U, Radtke F. Notch signaling in solid tumors. Curr Top Dev Biol. 2010;92:411–455. doi: 10.1016/S0070-2153(10)92013-9. [DOI] [PubMed] [Google Scholar]
- Krebs LT, Iwai N, Nonaka S, Welsh IC, Lan Y, Jiang R, Saijoh Y, O'Brien TP, Hamada H, Gridley T. Notch signaling regulates left-right asymmetry determination by inducing Nodal expression. Genes Dev. 2003;17:1207–1212. doi: 10.1101/gad.1084703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MG, et al. Reactivation of embryonic nodal signaling is associated with tumor progression and promotes the growth of prostate cancer cells. Prostate. 2011;71:1198–1209. doi: 10.1002/pros.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Good JA, Joubin K, Giraldez AJ, Ben-Haim N, Beck S, Chen Y, Schier AF, Constam DB. Nodal stability determines signaling range. Curr Biol. 2005;15:31–36. doi: 10.1016/j.cub.2004.12.062. [DOI] [PubMed] [Google Scholar]
- Le QT, Denko NC, Giaccia AJ. Hypoxic gene expression and metastasis. Cancer Metastasis Rev. 2004;23:293–310. doi: 10.1023/B:CANC.0000031768.89246.d7. [DOI] [PubMed] [Google Scholar]
- Lee CC, Jan HJ, Lai JH, Ma HI, Hueng DY, Gladys Lee YC, Cheng YY, Liu LW, Wei HW, Lee HM. Nodal promotes growth and invasion in human gliomas. Oncogene. 2010;29:3110–3123. doi: 10.1038/onc.2010.55. [DOI] [PubMed] [Google Scholar]
- Liu J, Sato C, Cerletti M, Wagers A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr Top Dev Biol. 2010;92:367–409. doi: 10.1016/S0070-2153(10)92012-7. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Xiao M, Balint K, Smalley KS, Brafford P, Qiu R, Pinnix CC, Li X, Herlyn M. Notch1 signaling promotes primary melanoma progression by activating mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt pathways and up-regulating N-cadherin expression. Cancer Res. 2006;66:4182–4190. doi: 10.1158/0008-5472.CAN-05-3589. [DOI] [PubMed] [Google Scholar]
- Mathieu J, et al. HIF induces human embryonic stem cell markers in cancer cells. Cancer Res. 2011;71:4640–4652. doi: 10.1158/0008-5472.CAN-10-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar J, Dondeti V, Simon MC. Hypoxia-inducible factors in stem cells and cancer. J Cell Mol Med. 2009;13:4319–4328. doi: 10.1111/j.1582-4934.2009.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister JC, Zhan Q, Weishaupt C, Hsu MY, Murphy GF. The embryonic morphogen, Nodal, is associated with channel-like structures in human malignant melanoma xenografts. J Cutan Pathol. 2010;37((suppl 1),):19–25. doi: 10.1111/j.1600-0560.2010.01503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris DP, Robertson EJ. Asymmetric and node-specific nodal expression patterns are controlled by two distinct cis-acting regulatory elements. Genes Dev. 1999;13:1575–1588. doi: 10.1101/gad.13.12.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postovit LM, Abbott DE, Payne SL, Wheaton WW, Margaryan NV, Sullivan R, Jansen MK, Csiszar K, Hendrix MJ, Kirschmann DA. Hypoxia/reoxygenation: a dynamic regulator of lysyl oxidase-facilitated breast cancer migration. J Cell Biochem. 2008a;103:1369–1378. doi: 10.1002/jcb.21517. [DOI] [PubMed] [Google Scholar]
- Postovit LM, Margaryan NV, Seftor EA, Kirschmann DA, Lipavsky A, Wheaton WW, Abbott DE, Seftor RE, Hendrix MJ. Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc Natl Acad Sci USA. 2008b;105:4329–4334. doi: 10.1073/pnas.0800467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postovit LM, Seftor EA, Seftor RE, Hendrix MJ. Targeting Nodal in malignant melanoma cells. Expert Opin Ther Targets. 2007;11:497–505. doi: 10.1517/14728222.11.4.497. [DOI] [PubMed] [Google Scholar]
- Postovit LM, Sullivan R, Adams MA, Graham CH. Nitric oxide signalling and cellular adaptations to changes in oxygenation. Toxicology. 2005;208:235–248. doi: 10.1016/j.tox.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Raya A, et al. Notch activity induces Nodal expression and mediates the establishment of left-right asymmetry in vertebrate embryos. Genes Dev. 2003;17:1213–1218. doi: 10.1101/gad.1084403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijoh Y, Oki S, Tanaka C, Nakamura T, Adachi H, Yan YT, Shen MM, Hamada H. Two nodal-responsive enhancers control left-right asymmetric expression of Nodal. Dev Dyn. 2005;232:1031–1036. doi: 10.1002/dvdy.20192. [DOI] [PubMed] [Google Scholar]
- Schier AF. Nodal morphogens. Cold Spring Harb Perspect Biol. 2009;1:a003459. doi: 10.1101/cshperspect.a003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007;2007:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- Shirato K, Nakajima K, Korekane H, Takamatsu S, Gao C, Angata T, Ohtsubo K, Taniguchi N. Hypoxic regulation of glycosylation via the N-acetylglucosamine cycle. J Clin Biochem Nutr. 2011;48:20–25. doi: 10.3164/jcbn.11-015FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strizzi L, Hardy KM, Seftor EA, Costa FF, Kirschmann DA, Seftor RE, Postovit LM, Hendrix MJ. Development and cancer: at the crossroads of Nodal and Notch signaling. Cancer Res. 2009;69:7131–7134. doi: 10.1158/0008-5472.CAN-09-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strizzi L, Postovit LM, Margaryan NV, Seftor EA, Abbott DE, Seftor RE, Salomon DS, Hendrix MJ. Emerging roles of Nodal and Cripto-1: from embryogenesis to breast cancer progression. Breast Dis. 2008;29:91–103. doi: 10.3233/bd-2008-29110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi JK, Lickert H, Bisgrove BW, Sun X, Yamamoto M, Chawengsaksophak K, Hamada H, Yost HJ, Rossant J, Bruneau BG. Baf60c is a nuclear Notch signaling component required for the establishment of left-right asymmetry. Proc Natl Acad Sci USA. 2007;104:846–851. doi: 10.1073/pnas.0608118104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, Nickoloff BJ, Topczewski J, Hendrix MJ. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12:925–932. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- Yedwab GA, Paz G, Homonnai TZ, David MP, Kraicer PF. The temperature, pH, and partial pressure of oxygen in the cervix and uterus of women and uterus of rats during the cycle. Fertil Steril. 1976;27:304–309. doi: 10.1016/s0015-0282(16)41722-x. [DOI] [PubMed] [Google Scholar]
- Yu L, Harms PW, Pouryazdanparast P, Kim DS, Ma L, Fullen DR. Expression of the embryonic morphogen Nodal in cutaneous melanocytic lesions. Mod Pathol. 2010;23:1209–1214. doi: 10.1038/modpathol.2010.101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.