Abstract
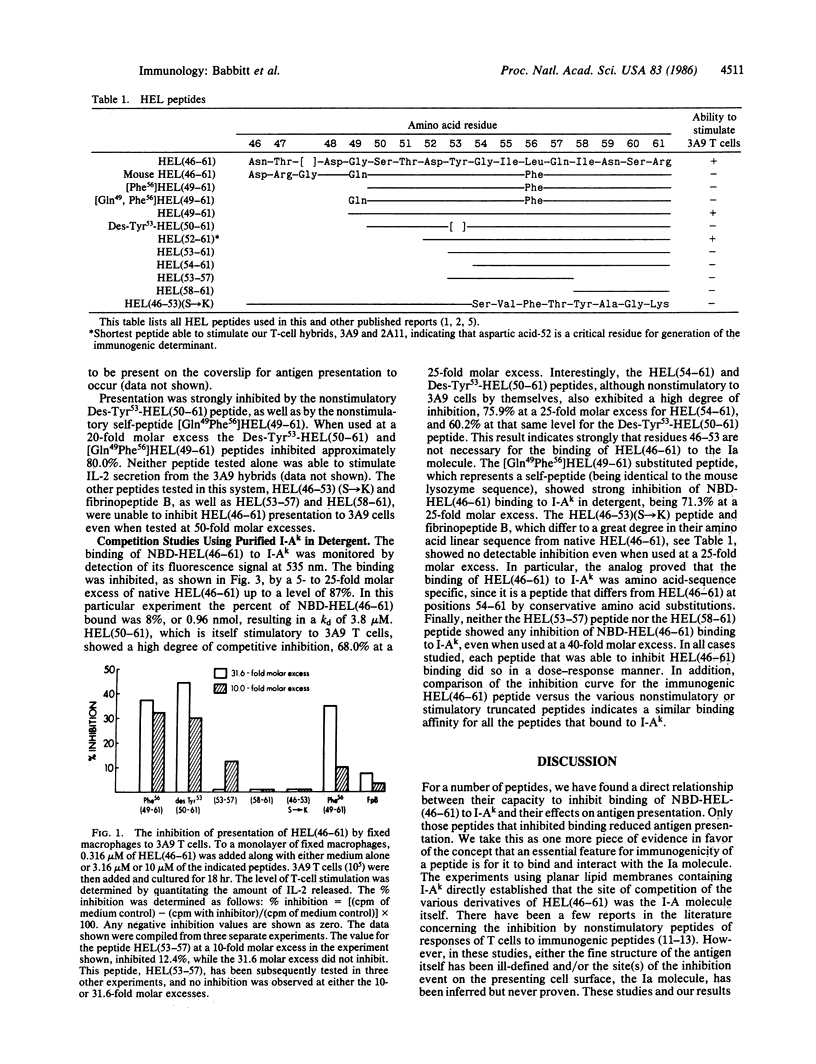
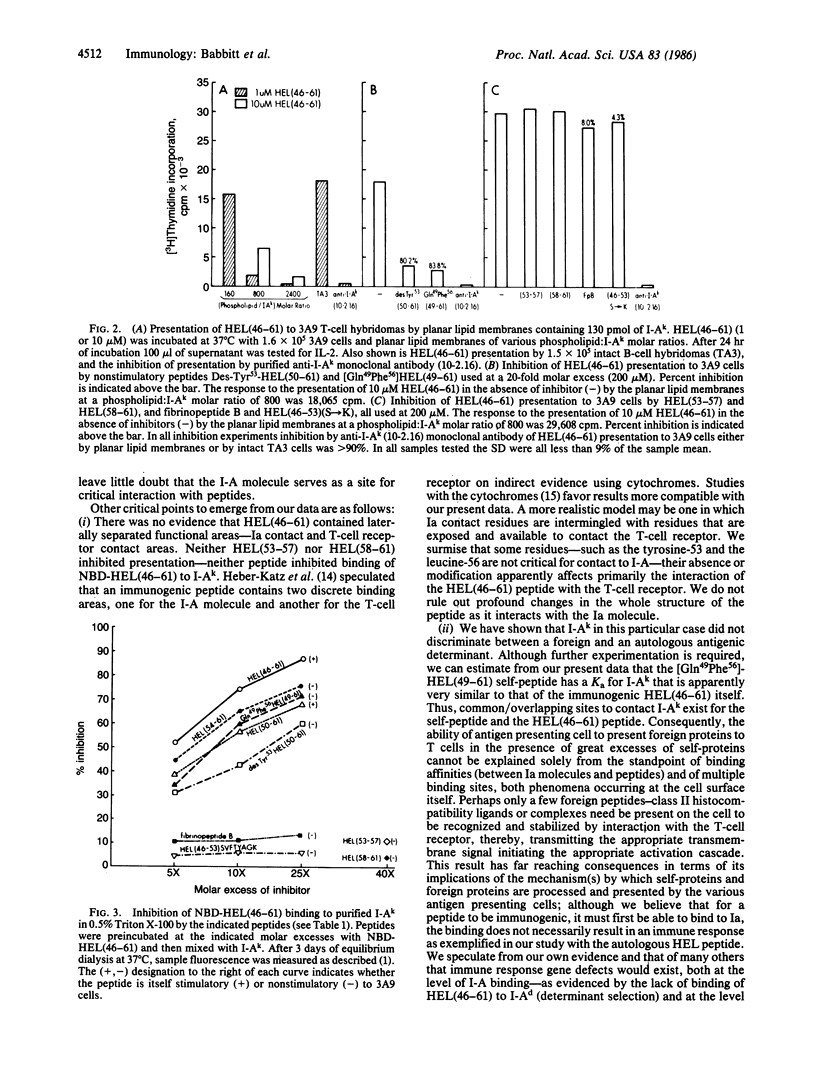
We examined the direct binding of a hen egg white lysozyme peptide, HEL(46-61), to membrane I-Ak (protein encoded in the A locus of the I region) molecules in the presence of detergent. A number of synthetic peptide derivatives, which did not stimulate our T-cell reactive hybridomas, competed for the binding of HEL(46-61) to I-Ak and also inhibited the functional presentation of HEL(46-61). Inhibitors included a peptide lacking a tyrosine at position 53 and a peptide corresponding to the autologous lysozyme peptide. Presentation was examined with cells or with supported planar phospholipid membranes bearing only I-Ak and HEL(46-61). Other peptides that did not compete for the binding did not inhibit functional presentation. We concluded that the binding of an immunogenic peptide to I-A is critical for presentation, that the I-A molecule does not discriminate between autologous and foreign related determinants but does recognize structurally different peptides. Our evidence suggests that our immunogenic peptide bears noncontiguous amino acids critical for contact I-A binding interspersed with amino acids critical for interaction with T cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboderin A. A., Boedefeld E., Luisi P. L. Reaction of chicken egg white lysozyme with 7-chloro-4-nitrobenz-2-oxa-1,3-diazole. Biochim Biophys Acta. 1973 Nov 11;328(1):20–30. doi: 10.1016/0005-2795(73)90325-5. [DOI] [PubMed] [Google Scholar]
- Allen P. M., Matsueda G. R., Haber E., Unanue E. R. Specificity of the T cell receptor: two different determinants are generated by the same peptide and the I-Ak molecule. J Immunol. 1985 Jul;135(1):368–373. [PubMed] [Google Scholar]
- Allen P. M., Strydom D. J., Unanue E. R. Processing of lysozyme by macrophages: identification of the determinant recognized by two T-cell hybridomas. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2489–2493. doi: 10.1073/pnas.81.8.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Hansburg D., Appella E. The sites of antigen-T cell and antigen-MHC interactions overlap. J Immunol. 1985 Dec;135(6):3712–3718. [PubMed] [Google Scholar]
- Heber-Katz E., Hansburg D., Schwartz R. H. The Ia molecule of the antigen-presenting cell plays a critical role in immune response gene regulation of T cell activation. J Mol Cell Immunol. 1983;1(1):3–18. [PubMed] [Google Scholar]
- Kurt-Jones E. A., Virgin H. W., 4th, Unanue E. R. Relationship of macrophage Ia and membrane IL 1 expression to antigen presentation. J Immunol. 1985 Dec;135(6):3652–3654. [PubMed] [Google Scholar]
- Matis L. A., Glimcher L. H., Paul W. E., Schwartz R. H. Magnitude of response of histocompatibility-restricted T-cell clones is a function of the product of the concentrations of antigen and Ia molecules. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6019–6023. doi: 10.1073/pnas.80.19.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield R. B., Vizioli L. D., Boman H. G. Synthesis of the antibacterial peptide cecropin A (1-33). Biochemistry. 1982 Sep 28;21(20):5020–5031. doi: 10.1021/bi00263a028. [DOI] [PubMed] [Google Scholar]
- Peterson L. B., Wilner G. D., Thomas D. W. Functional differentiation in the genetic control of murine T lymphocyte responses to human fibrinopeptide B. J Immunol. 1983 Feb;130(2):637–643. [PubMed] [Google Scholar]
- Rock K. L., Benacerraf B. Inhibition of antigen-specific T lymphocyte activation by structurally related Ir gene-controlled polymers. Evidence of specific competition for accessory cell antigen presentation. J Exp Med. 1983 May 1;157(5):1618–1634. doi: 10.1084/jem.157.5.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Benacerraf B. Inhibition of antigen-specific T lymphocyte activation by structurally related Ir gene-controlled polymers. II. Competitive inhibition of I-E-restricted, antigen-specific T cell responses. J Exp Med. 1984 Dec 1;160(6):1864–1879. doi: 10.1084/jem.160.6.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkewitz A. P., Sullivan C. P., Mescher M. F. Large-scale purification of murine I-Ak and I-Ek antigens and characterization of the purified proteins. Mol Immunol. 1983 Nov;20(11):1139–1147. doi: 10.1016/0161-5890(83)90137-2. [DOI] [PubMed] [Google Scholar]
- Watts T. H., Brian A. A., Kappler J. W., Marrack P., McConnell H. M. Antigen presentation by supported planar membranes containing affinity-purified I-Ad. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7564–7568. doi: 10.1073/pnas.81.23.7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdelin O. Chemically related antigens compete for presentation by accessory cells to T cells. J Immunol. 1982 Nov;129(5):1883–1891. [PubMed] [Google Scholar]



