Cbk1 kinase was previously implicated in regulating polarized morphogenesis, gene expression, and cell integrity. This study reveals that Cbk1 regulates heat shock signaling and stress adaptation by modulating Mpk1 activity and MAPK phosphatase localization. A model for Cbk1 and its putative substrate for these functions is presented.
Abstract
Saccharomyces cerevisiae Cbk1 kinase is a LATS/NDR tumor suppressor orthologue and component of the Regulation of Ace2 and Morphogenesis signaling network. Cbk1 was previously implicated in regulating polarized morphogenesis, gene expression, and cell integrity. Here we establish that Cbk1 is critical for heat shock and cell wall stress signaling via Bck2, a protein associated with the Pkc1-Mpk1 cell integrity pathway. We demonstrate that cbk1 and bck2 loss-of-function mutations prevent Mpk1 kinase activation and Mpk1-dependent gene expression but do not disrupt Mpk1 Thr-190/Tyr-192 phosphorylation. Bck2 overexpression partially restores Mpk1-dependent Rlm1 transcription factor activity in cbk1 mutants, suggesting that Bck2 functions downstream of Cbk1. We demonstrate that Bck2 precisely colocalizes with the mitogen-activated protein kinase (MAPK) phosphatase Sdp1. During heat shock, Bck2 and Sdp1 transiently redistribute from nuclei and the cytosol to mitochondria and other cytoplasmic puncta before returning to their pre-stressed localization patterns. Significantly, Cbk1 inhibition delays the return of Bck2 and Sdp1 to their pre-stressed localization patterns and delays Mpk1 Thr-190/Tyr-192 dephosphorylation upon heat shock adaptation. We conclude that Cbk1 and Bck2 are required for Mpk1 activation during heat shock and cell wall stress and for Mpk1 dephosphorylation during heat shock adaptation. These data provide the first evidence that Cbk1 kinase regulates MAPK-dependent stress signaling and provide mechanistic insight into Sdp1 phosphatase regulation.
INTRODUCTION
Stress response and cell growth must be precisely coordinated to ensure cell survival during adverse environmental conditions (Brauer et al., 2008). Typically, stress signaling promotes adaptive mechanisms by influencing the pattern of gene expression (Mager and De Kruijff, 1995). Adverse environmental conditions frequently lead to cell cycle delays, reflecting a link between stress and growth signaling (Pearce and Humphrey, 2001; Clotet and Posas, 2007). Similarly, aberrant cell growth can reduce the effectiveness of stress response pathways, leading to decreased cellular resistance to environmental stress.
Two Saccharomyces cerevisiae signaling pathways, the Cell Wall Integrity (CWI) and the Regulation of Ace2 and Morphogenesis (RAM) signaling networks, provide insight into the mechanisms that coordinate cell growth control and stress signaling (Nelson et al., 2003; Levin, 2005; Chen and Thorner, 2007; Fuchs and Mylonakis, 2009). The S. cerevisiae CWI pathway is a protein kinase C–dependent and mitogen-activated protein kinase (MAPK) stress-signaling pathway that is activated in response to heat shock and cell wall damage (Martin et al., 2000; Harrison et al., 2004; Levin, 2005; Fuchs and Mylonakis, 2009). Heat shock and cell wall stress cause protein kinase C (Pkc1) to activate a downstream MAP kinase cascade comprising three protein kinases, MAPKKK (Bck1), MAPKK (Mkk1/2), and MAPK (Mpk1/Slt2). The terminal CWI kinase Mpk1 induces a variety of cellular responses by influencing gene expression and is subject to negative regulation by MAPK kinase phosphatases (Mattison et al., 1999; Martin et al., 2000, 2005; Collister et al., 2002; Hahn and Thiele, 2002; Flandez et al., 2004; Harrison et al., 2004; Chen and Thorner, 2007). A key substrate for Mpk1 during heat shock and cell wall stress is Rlm1, a MADS-box transcription factor that promotes the expression of cell wall maintenance proteins (Watanabe et al., 1995; Dodou and Treisman, 1997; Heinisch et al., 1999; Jung et al., 2002; Garcia et al., 2004; Fuchs and Mylonakis, 2009). Mpk1 also regulates the Swi4–Swi6 transcriptional complex, which controls G1-specific gene expression (Madden et al., 1997; Baetz et al., 2001; Truman et al., 2009). In addition, both Pkc1 and Mpk1 were shown to influence cytoskeleton function during polarized growth (Mazzoni et al., 1993; Zarzov et al., 1996; Harrison et al., 2001; Torres et al., 2002).
CWI-mediated stress response is influenced by two associated proteins, Bck2 and Knr4. Bck2 is a serine- and threonine-rich protein that was originally identified as a dosage suppressor for pkc1 and CWI deletion mutants, including mpk1Δ (Lee et al., 1993). The role of Bck2 in Mpk1-dependent Rlm1 activation is not known; however, recent data suggest that Bck2 is involved in G1 gene expression via the Swi4–Swi6 transcriptional complex (Di Como et al., 1995; Ferrezuelo et al., 2009). Knr4 is a Bck2-associated protein and a putative scaffold protein that is required for Mpk1-dependent Rlm1 activation (Lee et al., 1993; Martin-Yken et al., 2002; Martin-Yken et al., 2003). In the absence of Knr4, Mpk1 is phosphorylated during heat shock and cell wall stress but fails to robustly activate Rlm1 transcription factor (Martin-Yken et al., 2003). These data suggest that Bck2 and Knr4 are critical downstream components of the CWI pathway. Nevertheless, their precise molecular functions remain unknown.
The evolutionarily conserved S. cerevisiae RAM network is also critical for maintenance of cell integrity (Du and Novick, 2002; Jorgensen et al., 2002; Kurischko et al., 2005, 2008). The RAM signaling network is implicated in regulating a variety of processes, including late M/early G1 gene expression, polarized growth, Golgi function, polarized secretion, and cell wall biosynthesis. The equivalent pathway in other fungi also regulates cell polarity and cell integrity (Verde et al., 1998; Durrenberger and Kronstad, 1999; Hirata et al., 2002; Hou et al., 2003; Kanai et al., 2005; Mendoza et al., 2005; Walton et al., 2006; Song et al., 2008). The RAM signaling network comprises two kinases and four associated proteins that control polarized growth, differential gene expression, and maintenance of cell integrity (Weiss et al., 2002; Nelson et al., 2003; Kurischko et al., 2005). Cbk1 kinase, the terminal protein kinase in the RAM network, is an AGK family kinase and LATS/NDR tumor suppressor orthologue (Nelson et al., 2003; Hergovich et al., 2006). Conditional cbk1 mutants exhibit severe cell morphology defects and die by cellular lysis (Kurischko et al., 2008).
Recent data indicate that Cbk1 kinase influences cell integrity by modulating the function and localization of the mRNA-binding protein Ssd1 (Jansen et al., 2009; Kurischko et al., 2011a, 2011b). Ssd1 associates with a subset of mRNAs, many of which encode cell wall biosynthesis proteins (Hogan et al., 2008; Jansen et al., 2009; Ohyama et al., 2010; Kurischko et al., 2011a). In the absence of Cbk1 phosphorylation, Ssd1 and its associated mRNAs constitutively localize to mRNA-processing bodies (P-bodies) and stress granules, which are known to repress translation during cellular stress (Kurischko et al., 2011a). These data suggest that conditional cbk1 mutants die by lysis because the cell wall proteins encoded by Ssd1-associated mRNAs are translationally repressed. In support, several dosage suppressors of conditional cbk1 mutants encode Ssd1-associated mRNAs of cell wall biosynthesis proteins, including the mannoproteins Sim1, Srl1, and Ccw12 (Kurischko et al., 2005, 2011a; Hogan et al., 2008). These data suggest that Cbk1 regulates cell integrity during polarized growth and stress response via Ssd1 by modulating the expression of a subset of cell wall proteins (Jansen et al., 2009; Kurischko et al., 2011a).
It is intriguing that not all cbk1 dosage suppressors encode Ssd1-associated mRNAs, suggesting that Cbk1 also influences cell integrity via Ssd1-independent mechanisms (Kurischko et al., 2008). Although the relationship between the RAM and CWI signaling networks has not been established, it seemed plausible that these two signaling networks are functionally linked. Here we provide molecular and genetic evidence that Cbk1 kinase regulates Mpk1 activation and Mpk1-dependent transcription during heat shock and cell wall stress via the CWI pathway–associated Bck2. We establish that Cbk1 and Bck2 are required for Mpk1 inactivation during heat shock adaptation via the MAP kinase phosphatase Sdp1. Our experiments provide the first evidence that Cbk1 and MAP kinase signaling networks are functionally linked. Given the conservation of RAM and CWI signaling proteins among eukaryotes, this work may anticipate conserved mechanisms for LATS/NDR tumor suppressor kinases in regulating cell growth and stress signaling via MAPK pathways.
RESULTS
BCK2 is a dosage suppressor of conditional cbk1-8 mutant cells
Conditional cbk1-8 mutant cells display severe defects in cell integrity when shifted to restrictive temperature (Kurischko et al., 2008, 2011a). To gain insight into the role of Cbk1 in maintenance of cell integrity, we screened a yeast DNA library for dosage suppressors of the conditional lethality of cbk1-8 mutant cells (see Materials and Methods). We previously identified and described several cbk1 dosage suppressors that encode cell wall biosynthesis proteins whose expression is influenced by the Cbk1 substrate and mRNA-binding protein Ssd1 (Kurischko et al., 2008, 2011a). These findings are consistent with the model that Cbk1 modulates the synthesis of a subset of cell wall biosynthesis proteins via Ssd1 regulation (Jansen et al., 2009; Kurischko et al., 2011a).
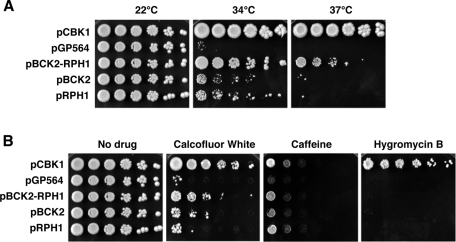
We also identified cbk1 dosage suppressors whose mRNAs are not known to interact with Ssd1 (Kurischko et al., 2008). One such dosage suppressor plasmid (pGP564-BCK2-RPH1) contained a chromosomal fragment encoding BCK2 and RPH1 (Figure 1A). Bck2 is a bypass suppressor of the protein kinase C– and MAPK-dependent CWI pathway and has been implicated in regulating gene expression (Lee et al., 1993; Di Como et al., 1995; Ferrezuelo et al., 2009). Rph1 is a histone demethylase that is involved in regulating gene expression and mediating DNA damage response and has not been previously implicated in regulating cell integrity or polarized growth (Jang et al., 1999; Kim et al., 2002; Liang et al., 2011). To determine which gene in the BCK2-RPH1 suppressor plasmid is responsible for cbk1 dosage suppression, we subcloned each gene into high-copy plasmids and assayed for complementation of the temperature sensitivity of cbk1-8 mutant cells. Surprisingly, both BCK2 and RPH1 plasmids suppressed the lethality of cbk1-8 cells at 34°C, although to a lesser degree than the original BCK2-RPH1 dosage suppressor plasmid (Figure 1A). Neither BCK2 nor RPH1 plasmid significantly suppressed the lethality of cbk1 cells at 37°C, as did the original BCK2-RPH1 plasmid. These data indicate that both BCK2 and RPH1 are cbk1 dosage suppressors and demonstrate that BCK2 and RPH1 dosage suppression activities are additive.
FIGURE 1:
Dosage suppression of cbk1-8 mutants. (A) High-copy BCK2 and RPH1 plasmids partially suppress the temperature sensitivity of cbk1-8 mutants. A 10-fold dilution series of cbk1-8 cells (FLY2884) expressing high-copy plasmids pGP564 (empty vector), pCBK1 (pGP564-CBK1), pBCK2 (pGP564-BCK2), pRPH1 (pGP564-RPH1), and pBCK2-RPH1 (pGP564-BCK2-RPH1) were grown at 22, 34, and 37°C. pBCK2 and pRPH1 partially suppress the conditional lethality of cbk1-8 cells at 34°C. Note that pBCK2-RPH1 is a more potent dosage suppressor of cbk1-8 than pBCK2 or pRPH1. (B) Dosage suppression of cbk1-8 drug sensitivity at 22°C. A 10-fold dilution series of cbk1-8 cells expressing the designated plasmids were grown on plates containing 100 μg/ml calcofluor white, 15 mM caffeine, and 50 μg/ml hygromycin B at 22°C. At 22°C, cbk1-8 cells are sensitive to calcofluor white, caffeine, and hygromycin B. pBCK2 and pBCK2-RPH1 plasmids suppress the calcofluor white sensitivity. pBCK2 and pRPH1 suppress the caffeine sensitivity of cbk1-8 cells to the same extent as pBCK2-RPH1. None of the plasmids suppresses the hygromycin B sensitivity of cbk1-8 cells.
The identification of BCK2 as a cbk1 dosage suppressor suggests that Cbk1 influences the function of the CWI pathway. If Cbk1 is required for CWI signaling, cbk1 mutants should display similar phenotypes as CWI mutants, such as hypersensitivity to heat shock, cell wall–disrupting agents, and drugs that impair Tor1 signaling (Levin, 2005). In agreement, cbk1-8 cells are temperature sensitive and hypersensitive to the chitin-binding drug calcofluor white (CW) (Kurischko et al., 2008). In addition, we discovered that cbk1-8 cells are hypersensitive to the chitin-binding drug and β-glucan synthase inhibitor Congo red (CR) and to the Tor1 inhibitors caffeine and rapamycin, which are all known to activate CWI signaling (Figure 1B and Supplemental Figure S1A).
To determine the extent of cbk1 dosage suppression, we assayed high-copy BCK2 or RPH1 plasmids for their ability to suppress the CWI-like phenotypes of cbk1-8 cells. Significantly, high-copy BCK2 plasmids partially rescued the CW, CR, caffeine, and rapamycin sensitivities of cbk1-8 mutants (Figure 1B and Supplemental Figure S1B). It is intriguing that high-copy RPH1 plasmids also partially rescued the caffeine, rapamycin, and CR sensitivities of cbk1-8 cells. We did not observe any additive suppressive effects for the BCK2-RPH1 plasmid. These data suggest that Bck2, and possibly Rph1, overexpression restores the viability of cbk1-8 mutants by stimulating CWI pathway signaling or by enhancing cell wall biogenesis.
Conditional cbk1 mutants are also hypersensitive to hygromycin B (Hyg B), indicative of a role in protein glycosylation (Dean, 1995; Kurischko et al., 2008). Of note, high-copy BCK2 and RPH1 plasmids do not suppress the Hyg B sensitivity of cbk1 mutants (Figure 1B). These data are consistent with the model that BCK2 and RPH1 overexpression suppresses the lethality of cbk1 mutants by enhancing CWI signaling and not by restoring Cbk1-dependent protein glycosylation.
Phenotypes of bck2Δ and rph1Δ cells
The phenotypes of bck2Δ and rph1Δ mutants with regard to cell integrity and CWI signaling have not been reported. We therefore assayed bck2Δ and rph1Δ mutants for phenotypes common among CWI pathway mutants, including sensitivity to CW, CR, caffeine, rapamycin, and Hyg B (Supplemental Figure S1A). As a control, we analyzed the phenotypes of cells lacking Mpk1/Slt2, the terminal MAP kinase in the CWI pathway. We discovered that bck2Δ cells were hypersensitive to CW, rapamycin, and Hyg B, as were cbk1-8 and mpk1Δ cells, but not to CR and caffeine. In contrast, rph1Δ cells were not hypersensitive to any of the drugs tested. These data suggest that Bck2, but not Rph1, is important for CWI pathway function. We therefore focused the remainder of this study on defining the roles of Cbk1 and Bck2 with respect to CWI signaling.
Cbk1 and Bck2 are required for Mpk1 kinase activity
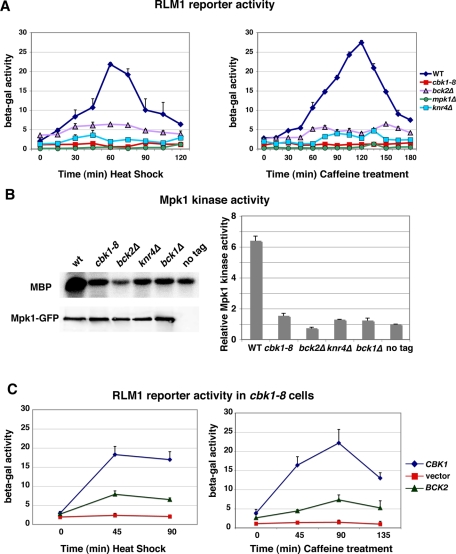
To investigate the role of Cbk1 and Bck2 in CWI signaling, we analyzed Rlm1 reporter activation in cbk1-8 and bck2Δ mutants (Figure 2A). Rlm1 is a MADS-family transcription factor that is phosphorylated and activated by Mpk1 during heat shock, cell wall stress, and caffeine treatment (Jung et al., 2002). Once activated, Rlm1 induces the expression of a subset of cell wall biosynthesis genes that help maintain cell wall integrity and ensure stress survival. We introduced a previously described reporter plasmid that bears both a lexA-RLM1 fusion and a lacZ reporter gene with lexA-binding sites and measured lacZ activity in cells after stress induction (Kirchrath et al., 2000). As a control, we conducted parallel experiments with wild-type, mpk1Δ, and knr4Δ cells, the latter of which lacks a Bck2-associated protein and a putative scaffold protein required for Mpk1-dependent Rlm1 activation (Lee et al., 1993; Martin-Yken et al., 2002, 2003). In wild-type cells, heat shock and caffeine treatment induce the Mpk1-dependent phosphorylation of Rlm1 and hence the activation of the Rlm1 reporter (Watanabe et al., 1995; Dodou and Treisman, 1997; Jung et al., 2002). Peak reporter activity occurs in wild-type cells at 60 min upon heat shock and at 120 min upon caffeine treatment (Figure 2A). In contrast, Rlm1 reporter activation was completely inhibited in cbk1 mutant cells, indicating that Cbk1 kinase is essential for Rlm1 activation during CWI stress signaling. Rlm1 reporter activity was also diminished by ∼6- to 7-fold in bck2Δ and ∼10-fold in knr4Δ mutants relative to peak reporter activities in stressed wild-type cells (Figure 2A). In contrast, RPH1 deletion only modestly diminished peak Rlm1 activity (<10% reduction; Supplemental Figure S2A). These data indicate that Cbk1, Bck2, and Knr4 are critical for Mpk1-dependent Rlm1 activation during heat shock and cell wall stress.
FIGURE 2:
Cbk1 and Bck2 are required for Mpk1 kinase activity and Rlm1 reporter activation. (A) An RLM1-lacZ reporter plasmid was introduced into yeast cells, and reporter activity was measured at the designated time points following heat shock (left) or caffeine treatment (right). Rlm1-dependent lacZ expression was quantified as nanomoles per minute per milligram of o-nitrophenyl phosphate produced from o-nitrophenyl β-d-galactoside substrate. (B) In vitro kinase assays were performed with immunoprecipitated Mpk1-GFP and myelin basic protein (MBP) as substrate. Top, an autoradiogram of phosphorylated MBP; bottom, an immunoblot of immunoprecipitated Mpk1 (probed with anti-GFP) from wild-type (WT), cbk1-8, bck2Δ, knr4Δ, bck1Δ, and untagged wild-type cells (lacking Mpk1-GFP). The graph shows the relative phosphorylated MBP levels quantified from two independent experiments. The relative MBP phosphorylation levels were normalized to the negative control (untagged cells). (C) Bck2 overexpression via high-copy pGP564-BCK2 partially restores the Rlm1 reporter activity in heat -shocked (left) and caffeine-treated (right) cbk1-8 cells. Parallel experiments were done with pCBK1 (YGPM11e20) and empty vector (pGP564) for positive and negative controls. The yeast strains used in these experiments were FLY1300, FLY2884, FLY3270, FLY3271, FLY3276, and FLY3277.
Low Rlm1 reporter activities could reflect impaired Mpk1 kinase activity or impaired Rlm1-dependent gene expression. Thus, as an independent method to determine whether Cbk1 and Bck2 influence Mpk1 kinase activity during stress signaling, we immunoprecipitated Mpk1-GFP from heat-shocked cells and conducted in vitro kinase assays using myelin basic protein (MBP) as substrate. As expected, immunoprecipitated Mpk1 from wild-type cells had significant kinase activity toward MBP (Figure 2B). In contrast, the Mpk1 activity from cbk1-8, bck2Δ, and knr4Δ cells was as low as that of Mpk1 from bck1Δ cells, which lack the activating MAPKKK (Figure 2B). These data establish that Cbk1, Bck2, and Knr4 are essential for Mpk1 kinase activation during heat shock.
Bck2 overexpression partially restores Rlm1 activation in cbk1 mutants
Our data suggest that Bck2-mediated dosage suppression of cbk1 mutants occurs by restoring Mpk1 kinase activity and CWI-dependent gene expression. To test this hypothesis, we introduced BCK2 suppressor plasmids into conditional cbk1-8 mutants and quantified Rlm1 reporter activity after heat shock induction and caffeine treatment. We observed that high-copy BCK2 plasmids restored Rlm1 reporter activity in heat-shocked and caffeine-treated cells to ∼30% (heat shock) and ∼20% (caffeine) of the levels of cells expressing wild-type Cbk1 (Figure 2C). It is intriguing that Rph1 overexpression also partially restored the diminished Rlm1 reporter activity in heat-shocked and caffeine-treated cbk1-8 cells, suggesting that Rph1 overexpression elevates Rlm1-dependent transcription (Supplemental Figure S2B). The high-copy BCK2-RPH1 plasmid restores Rlm1 reporter activity in cbk1 mutants to the same degree as the BCK2 and RPH1 plasmids (Supplemental Figure S2B). These data support the hypothesis that Bck2 and Rph1 overexpression suppresses the conditional lethality of cbk1 mutants by elevating Rlm1-dependent gene expression. Our data are consistent with the model that Cbk1 functions via Bck2 to mediate Mpk1 activation during stress signaling. Because Rph1 is a histone H3 demethylase and functions as a transcriptional repressor (Kim et al., 2002), we hypothesize that Rph1 overexpression induces epigenetic changes that indirectly enhance Rlm1-mediated gene expression.
Cbk1, Bck2, and Knr4 are not required for CWI pathway activation
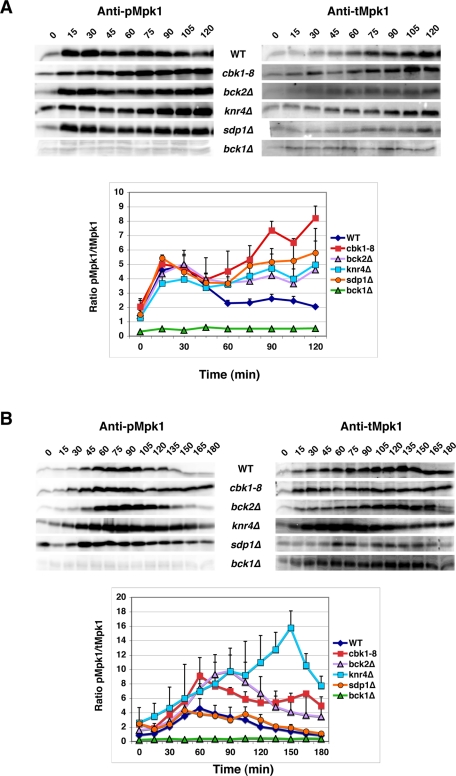
It is well established that heat shock, cell wall stress, and caffeine treatment lead to CWI pathway and Mpk1 activation (Kamada et al., 1995; Harrison et al., 2004; Imazu and Sakurai, 2005; Levin, 2005). MAPKK-dependent Mpk1 Thr-190/Tyr-192 phosphorylation is a common marker for CWI pathway activation and is readily detected via immunoblots probed with a phosphorylation-specific p44/42 MAPK antibody (Martin et al., 2000). To determine whether Cbk1 and Bck2 are required for Mpk1 Thr-190/Tyr-192 phosphorylation, we monitored Mpk1 phosphorylation in heat-shocked and caffeine-treated cbk1-8 and bck2Δ cells. Representative immunoblots are presented (Figure 3). Because heat shock leads to increased Mpk1 expression (Jung and Levin, 1999; Mattison et al., 1999; Hahn and Thiele, 2002), we plotted the ratios of Thr-190/Tyr-192–phosphorylated Mpk1 to total Mpk1 (pMpk1/tMpk1), as determined from two independent experiments (Figure 3 and Supplement Table S1). Within 15 min of heat shock and 45–60 min of caffeine treatment, the levels of phosphorylated Mpk1 increased significantly (approximately fourfold) in cbk1-8, bck2Δ, and wild-type cells (Figure 3, A and B). The same is true for knr4Δ cells (Figure 3, A and B; Martin-Yken et al., 2002). These data indicate that Cbk1, Bck2, and Knr4 are not essential for CWI activation and Mpk1 phosphorylation. Thus Cbk1, Bck2 and Knr4 must function after Mkk1/Mkk2–dependent phosphorylation with respect to Mpk1 kinase activation, similar to the chaperone Hsp90 and the cochaperone Cdc37 (Hawle et al., 2007; Truman et al., 2007).
FIGURE 3:
Cbk1 and Bck2 are not essential for Mpk1 Thr-190/Tyr-192 phosphorylation. Mpk1 Thr-190/Tyr-192 phosphorylation levels in wild-type, cbk1-8, bck2Δ, knr4Δ, sdp1Δ, and bck1Δ cells were monitored by quantitative immunoblots at various times during (A) heat shock and (B) caffeine treatment. bck1Δ cells lack the CWI pathway–specific MAPKKK and thus serve as a negative control for Mpk1 phosphorylation. Representative immunoblots are shown. Immunoblots were probed with anti–phospho Mpk1 Thr-190/Tyr-192 (Mpk1P), stripped and reprobed with anti-Mpk1 antibody. The ratios of phospho-Mpk1 to total Mpk1 protein (pMpk1/tMpk1) were quantified from two independent experiments and plotted. The yeast strains used for these experiments were FLY1300, FLY2884, FLY3270, FLY3276, FLY3277, and FLY3570. The corresponding pMpk1/tMpk1 data for the graphs in A and B are presented in Supplemental Table S1.
Bck2 localizes to the nucleus, cytosol, and bud neck during logarithmic growth
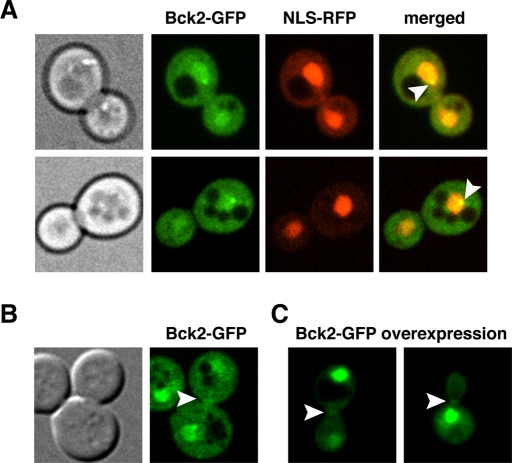
Our data suggest that Cbk1 and Bck2 are important for Mpk1 activation, perhaps by promoting Mpk1 protein interactions. If this model is correct, Bck2 should at least transiently localize to the nucleus or to sites of polarized growth (bud neck and bud cortex) where Mpk1 and Cbk1 localize (Kamada et al., 1995; Hahn and Thiele, 2002; Jung et al., 2002; van Drogen and Peter, 2002; Weiss et al., 2002). To investigate Bck2 localization, we generated strains expressing Bck2–green fluorescent protein (GFP) under the control of its own promoter. The cells expressing Bck2-GFP displayed no obvious phenotypes, suggesting that Bck2-GFP is fully functional. We discovered that during logarithmic growth, Bck2-GFP localizes to the cytosol and is enriched in the nucleoplasm throughout the cell cycle (Figure 4A). We confirmed the nuclear localization by coexpressing a red fluorescent protein (RFP)–tagged nuclear marker. In addition, Bck2-GFP localized to a prominent spot at the nuclear periphery in all cells (n = 100; Figure 4A, arrowhead). We also occasionally observed modest enrichment of Bck2 at the bud neck in a small fraction of cells (<1% of cells; Figure 4B). It is likely that the number of cells with Bck2 at the bud neck is an underestimate because the Bck2-GFP fluorescence signal is difficult to detect over the diffuse cytosolic Bck2-GFP signal. Bck2 overexpression via the constitutive GPD promoter enhanced detection of Bck2 at the bud neck in small- and large-budded cells (Figure 4C; 38%; n = 60). These data support the proposed functional interactions among Bck2, Mpk1, and Rlm1 in the nucleus and cytosol.
FIGURE 4:
Bck2 localization in unstressed cells. (A) The localization of physiologically expressed Bck2-GFP and the nuclear marker NLS-RFP were monitored by spinning disk confocal microscopy. Bck2-GFP constitutively localizes to the cytoplasm, nucleoplasm, and single nuclear spot (arrowhead) in all cells. (B) Physiologically expressed Bck2-GFP faintly localizes to bud necks (arrowhead) in ∼1% of medium- and large-budded cells. (C) Moderate Bck2 overexpression enhances detection of Bck2 at bud necks (arrowhead). For Bck2 overexpression, a plasmid with Bck2-GFP under the control of the constitutive GPD promoter (pGPD-BCK2-GFP) was introduced into bck2Δ cells (FLY3276). All images represent single optical sections.
Bck2 localizes to cytoplasmic puncta upon heat shock
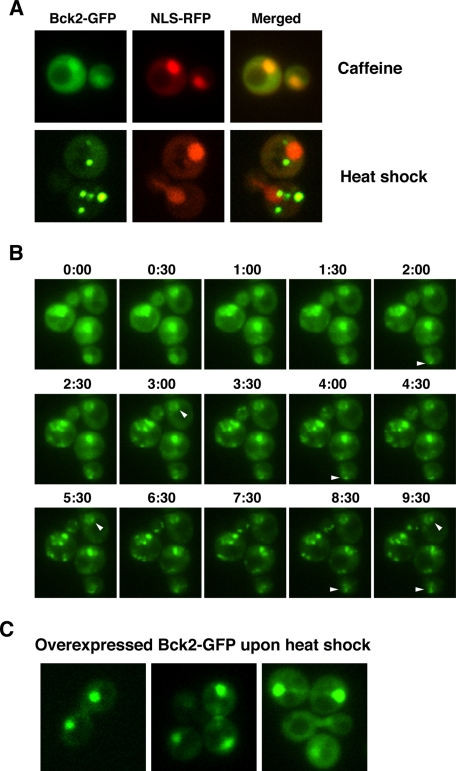
To determine how cellular stress influences Bck2 localization, we monitored Bck2-GFP in caffeine-, CW-, salt-, and heat-stressed cells. Caffeine, CW treatment, and hypertonic stress did not trigger any obvious changes in Bck2 nuclear or cytosolic localizations (Figure 5A and data not shown). In contrast, within 15 min of heat shock, Bck2 radically redistributed from the nucleoplasm and cytosol to 5–11 prominent cytoplasmic puncta (Figure 5, A and B). Corresponding time-lapse microscopy indicates that the puncta derive from redistributed cytoplasmic and nuclear Bck2 (Figure 5B and Supplemental Movie S1). Heat shock did not appear to eliminate or disrupt the single Bck2 nuclear spot (Figure 5B, arrowheads, and Supplemental Movie S1). Significantly, after ∼60 min of continual exposure to high temperature (39°C), most cytoplasmic puncta disappear and Bck2 reaccumulates in the nucleus, reflecting the return of Bck2 to a prestressed localization pattern during heat shock adaptation (Figure 6 and Figure 9 later in this paper).
FIGURE 5:
Bck2 localization significantly changes during heat shock. (A) Representative wide-field fluorescence images of Bck2-GFP cells (FLY3503) 90 min after 15 mM caffeine treatment at 22°C and 30 min after cells were transferred to 39°C (heat shock). Caffeine treatment does not induce changes in Bck2 localization, but heat shock causes Bck2 to relocate from the nucleoplasm to bright cytoplasmic puncta. (B) Time-lapse analysis of heat-shocked Bck2-GFP cells. Bck2-GFP cells were heat shocked at T = 0 and images were captured at 30-s intervals. Arrowheads point to representative nuclear foci that remain visible throughout the experiment. These data are also presented in Supplemental Movie S1. (C) Moderately overexpressed Bck2-GFP (from pGPD-BCK2-GFP) does not localize to mitochondria or other cytoplasmic puncta during heat shock (images were captured 15 min after heat stress induction). All images were captured via wide-field fluorescence microscopy. All images in A and C represent single optical sections, and the images in B are merged from 3 × 0.2 μm optical sections.
FIGURE 6:
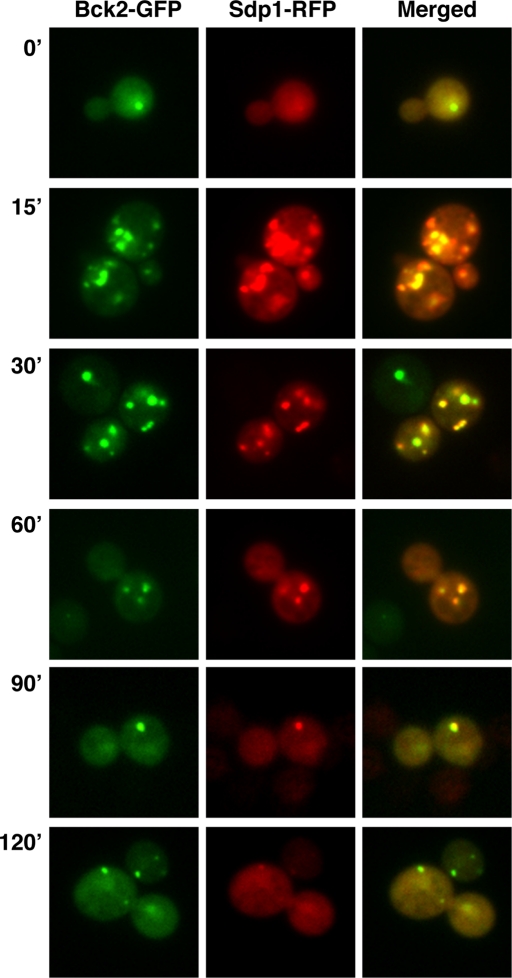
Bck2 colocalizes with the MAP kinase phosphatase Sdp1. Cells expressing Bck2-GFP and Sdp1-RFP were monitored by wide-field fluorescence microscopy prior to (T = 0) and during heat shock. Bck2 and Sdp1 colocalize prior to and throughout heat shock. Greater than 98% of the Sdp1 puncta colocalize with Bck2 (n > 800 puncta in ∼550 cells). The strain and plasmid used in this experiment are FLY3503 and FLE1283, respectively.
FIGURE 9:
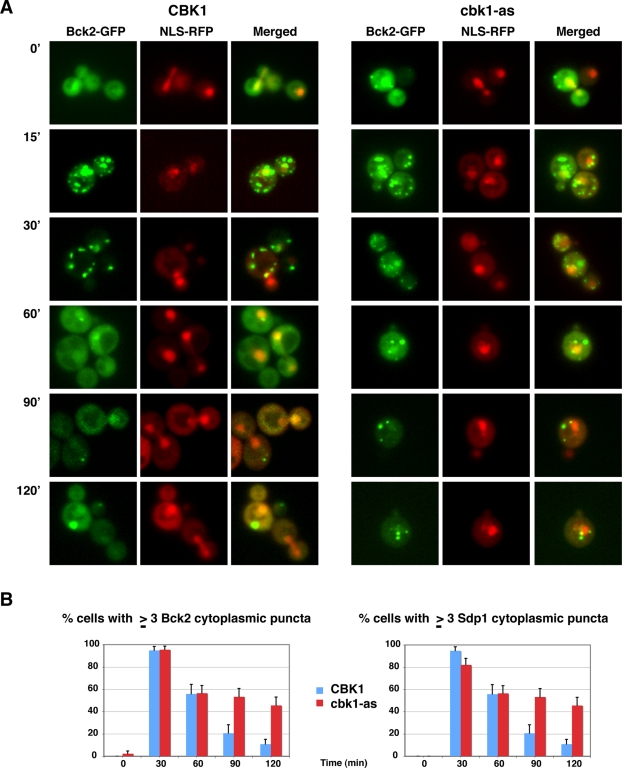
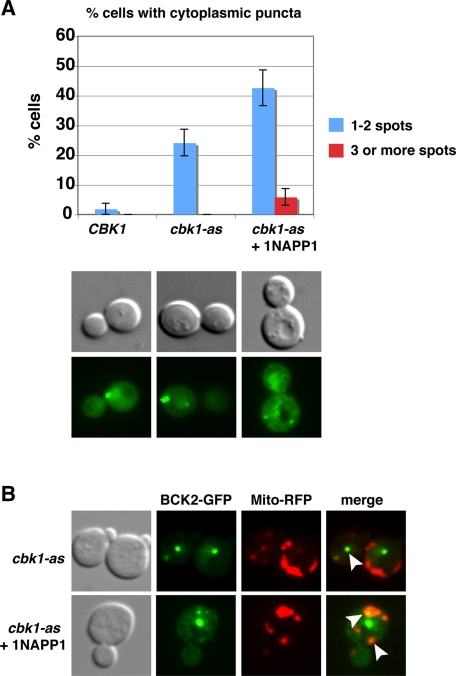
Cbk1 inhibition prolongs Bck2 and Sdp1 puncta localization during heat shock. (A) Bck2-GFP localization was monitored at various intervals after heat shock in wild-type cells (left) and in cbk1-as cells upon Cbk1 inhibition in cbk1-as cells (right). For cbk1-as cells, Cbk1 inhibition (1NA-PP1 addition) and heat shock were done simultaneously. NLS-RFP shows the nuclear localization. Bck2 localized to cytoplasmic puncta in all cells (n = 35) within 15 min of heat shock. All images were captured via wide-field fluorescence microscopy and represent two merged optical sections. See Supplemental Figure S3 for a temporal analysis of Bck2 localization at mitochondria. (B) Parallel experiments were done in cells coexpressing Sdp1-RFP, and the number of cells with three or more Bck2 and Sdp1 cytoplasmic puncta was plotted over time. The data were tabulated from two independent experiments (n = 29–55 cells per time point). Cbk1 kinase inhibition significantly delayed the disappearance of the heat shock–induced Bck2 and Sdp1 puncta. See Supplemental Figure S4 for additional controls. The strains used for these experiments are FLY3503 and FLY3559.
Bck2 overexpression disrupts its recruitment to cytoplasmic puncta
Because Bck2 is spatially regulated and is a robust cbk1 dosage suppressor, we wondered whether Bck2 translocation to cytoplasmic puncta correlates with dosage suppression. We therefore investigated Bck2 localization when expressed from a low-copy plasmid under the control of the constitutive GPD promoter. pGPD-Bck2-GFP suppressed the conditional lethality of cbk1-8 mutants (Supplemental Figure S1C); however, curiously, and in contrast to physiologically expressed Bck2, moderately overexpressed Bck2 did not localize to cytoplasmic puncta during heat shock (Figure 5C). Instead, most Bck2 remained in the nucleus during heat shock. These data indicate that Bck2 overexpression disrupts its recruitment to cytoplasmic puncta and suggest that Bck2 does not need to translocate to cytoplasmic puncta in order to suppress the conditional lethality of cbk1 mutants.
Bck2 colocalizes with the Mpk1-specific phosphatase Sdp1
The localization of Bck2 to cytoplasmic puncta in heat-shocked cells was particularly intriguing because Mpk1, Cbk1, and Rlm1 do not localize similarly during heat shock or cell wall stress (Kamada et al., 1995; Hahn and Thiele, 2002; Jung et al., 2002; and data not shown). The punctate pattern of Bck2 localization in heat-shocked cells was reminiscent of the localization of the MAPK phosphatase Sdp1, which was also shown to localize to the cytoplasm and nucleus in unstressed cells and to cytoplasmic puncta in heat-shocked cells (Hahn and Thiele, 2002). We introduced RFP-tagged Sdp1 into strains expressing Bck2-GFP to investigate whether Bck2 and Sdp1 colocalize. It is striking that Sdp1 mirrors Bck2 localization both prior to and during heat shock (Figure 6). During heat shock, nearly all (∼98%) Bck2 and Sdp1 puncta colocalize. Notably, Mpk1 does not localize similarly during heat shock (Kamada et al., 1995; Hahn and Thiele, 2002), suggesting that Bck2 and Sdp1 are transiently sequestered away from Mpk1 during heat shock.
Bck2 and Sdp1 localize to mitochondria upon heat shock
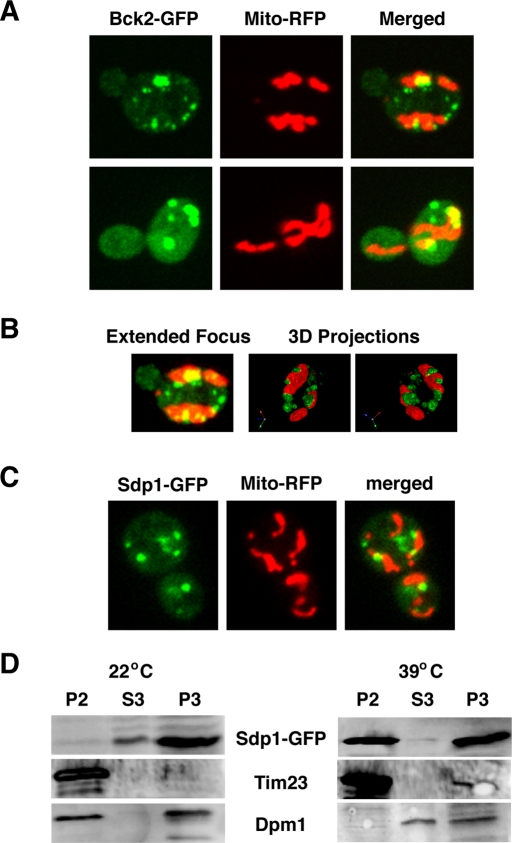
Because mitochondria are known to play an important role in mediating heat shock response (Lanneau et al., 2008), we introduced mitochondrial-binding dyes and mitochondria-targeted RFP into GFP-tagged cells to test whether Bck2 and Sdp1 colocalize with mitochondria during heat shock. It is striking that ∼53% of the Bck2 cytoplasmic puncta (n = 50 cells) colocalized with mitochondria within 15–30 min of heat shock (Figure 7A). Three-dimensional models of the microscopy data support the conclusion that many of the Bck2 puncta colocalize with mitochondria (Figure 7B and Supplemental Movie S2). We obtained similar results with Sdp1-GFP (Figure 7C)
FIGURE 7:
Bck2 and Sdp1 associate with mitochondria during heat shock. (A) Some heat shock–induced Bck2 puncta colocalize with mitochondria. A plasmid expressing an RFP-tagged mitochondrial marker (Mito-RFP; pHCRED) was introduced into Bck2-GFP cells. All images represent single optical sections captured by spinning disk confocal microscopy 15 min after shifting cells to 39°C (heat shock). (B) The cell is presented as a three-dimensional (3D) model via Volocity software (PerkinElmer). Left, a merge/projection of 21 × 0.2 μm Z-sections. Middle and right, different angles of a 3D model of the same cell. See Supplemental Movie S2 for this model in rotation. (C) Sdp1 localizes to mitochondria in heat-shocked cells. Cells expressing Sdp1-GFP and Mito-RFP (FLY3570, plasmids pRS425-SDP1-GFP, pHCRED) were monitored after 15 min of heat stress, as described in A. (D) Immunoblots of organelle fractions of unstressed (22°C) and heat-shocked (39°C) Sdp1-GFP cells (see Materials and Methods). Western blots are probed with antibodies to GFP, Tim23 (a mitochondrial marker), and Dpm1 (ER/microsome marker). Mitochondria are enriched in P2, microsomes and ER are enriched in P3, and S3 is enriched for cytosolic proteins. Note that Sdp1 is enriched in fraction P2 (mitochondria) of heat-shocked cells and not in P2 of unstressed cells. Some Sdp1 is also present in fraction P3 (ER/microsome), regardless of heat shock.
To corroborate the mitochondrial localization of Bck2 and Sdp1, we fractionated unstressed and heat-shocked cells and probed mitochondria fractions for Bck2 and Sdp1 by immunoblot. As a control for specificity, we also probed immunoblots with antibodies to the mitochondrial protein Tim23 and the endoplasmic reticulum (ER) protein Dpm1. We were unable to obtain conclusive fractionation data for Bck2 due to unresolved protein stability issues (data not shown); however, Sdp1 was greatly enriched in the mitochondrial-enriched fraction P2 from heat-shocked cells (Figure 7D). Notably, Sdp1 was not enriched in fraction P2 from unstressed cells. Because Bck2 and Sdp1 colocalize, these experiments suggest that a significant fraction of both proteins associate with mitochondria during heat shock. It is intriguing that Sdp1 was also present in fraction P3 in both unstressed and heat-shocked cells, which is enriched for microsomes and ER. It is not clear whether some of the heat shock–induced Bck2 or Sdp1 puncta associate with microsomes.
Cbk1 promotes the return of Bck2 and Sdp1 to their unstressed localization patterns during heat shock adaptation
The genetic relationship between Cbk1 and Bck2 with respect to Mpk1 activation suggests that Cbk1 regulates Bck2 function. To determine whether Cbk1 influences Bck2 or Sdp1 localization, we monitored Bck2 and Sdp1 in cells carrying the analogue-sensitive cbk1-as allele, which encodes mutant Cbk1-as, which is specifically inhibited by the drug 1NA-PP1 (Weiss et al., 2002), thereby allowing Cbk1 inhibition in the absence of heat shock.
We first explored the effect of Cbk1 inhibition on Bck2 localization in unstressed cbk1-as cells. At 22°C in the absence of 1NA-PP1, the overall pattern of Bck2 nuclear localization was similar to that of wild-type cells. Curiously, Bck2 also localized to approximately one or two cytoplasmic Bck2 puncta in 25% of the cbk1-as cells (Figure 8A). On Cbk1 inhibition (1NA-PP1 addition) at 22°C, the percentage of cells with one or two cytoplasmic puncta increased to ∼42% (n = 90), and the number of cells with three or more cytoplasmic puncta per cell increased from 0 to 7% (Figure 8A). Parallel experiments with cells expressing an RFP-tagged mitochondria marker indicate that ∼49% (n = 63) of the Bck2 cytoplasmic puncta colocalize with mitochondria (Figure 8B, arrowheads). These data suggest that Cbk1 activity is modestly diminished in cbk1-as cells (in the absence of 1NA-PP1) and that robust Cbk1 kinase inhibition enhances the formation of Bck2 cytoplasmic puncta in unstressed cells.
FIGURE 8:
Cbk1 inhibition enhances the appearance of Bck2 puncta. (A) cbk1-as cells expressing Bck2-GFP were monitored before and after Cbk1 inhibition. The percentages of cells with one or two cytoplasmic and three or more cytoplasmic Bck2 puncta are plotted (n = 90). Bck2 localizes to one or two cytoplasmic puncta in ∼24% of cbk1-as cells at 22°C. By 15 min of Cbk1 inhibition (1NA-PP1 addition), the number of cells with one or two Bck2 puncta nearly doubles. Cbk1 inhibition also increases the percentage of cells with three or more Bck2 puncta per cell. Representative cells are shown below the graph. (B) Many (∼49%, n = 63) of the Bck2 puncta colocalize with Mito-RFP. All images were captured via wide-field fluorescence microscopy and represent single optical sections.
We also tested whether Cbk1 inhibition influences Bck2 and Sdp1 localization during heat shock and heat shock adaptation (Figure 9). We inhibited Cbk1 at the same time as heat shock and observed that Bck2 and Sdp1 rapidly (within 15 min) relocated from the nucleus to cytoplasmic puncta in nearly all cells, similar to heat-shocked wild-type cells (Figure 9, A and B). In contrast, Cbk1 inhibition significantly delayed the release of Bck2 and Sdp1 from cytoplasmic puncta during heat shock adaptation. By 90 min of continual heat stress, when ∼20% of wild-type cells contained three or more Bck2 and Sdp1 cytoplasmic puncta, ∼58% of 1NA-PP1-treated cbk1-as cells retained three or more Bck2 and Sdp1 cytoplasmic puncta (Figure 9B). Moreover, the percentage of cbk1-as cells with three or more Bck2 and Sdp1 cytoplasmic puncta remained high for at least 2 h during heat stress. Parallel experiments with cells expressing the RFP-tagged mitochondrial marker established that ∼59% of the Bck2 puncta colocalize with mitochondria in heat-shocked cbk1-as cells throughout the experiment (Supplemental Figure S3). These data indicate that Cbk1 is important for mediating the recovery of Bck2-Sdp1 from a heat shock–induced localization pattern to the unstressed localization pattern and are consistent with the model that Cbk1 regulates heat shock recovery by controlling Bck2 and Sdp1 release from cytoplasmic puncta.
Cbk1, Bck2, and Knr4 are required for Mpk1 dephosphorylation during heat shock
Sdp1 was shown to play a major role in Mpk1 Thr-190/Tyr-192 dephosphorylation during heat shock adaptation (Collister et al., 2002; Hahn and Thiele, 2002). In light of the Bck2-Sdp1 colocalization data and the role of Cbk1 in regulating Bck2-Sdp1 release from cytoplasmic puncta, we postulated that Cbk1, Bck2, and Sdp1 cooperatively function to inactivate Mpk1 during heat shock adaptation. To test this hypothesis, we compared the dynamics of Mpk1 Thr-190/Tyr-192 phosphorylation in heat-stressed wild-type and mutant cells over time (Figure 3A). In heat-shocked wild-type cells, the pMpk1/tMpk1 ratio peaked at ∼30 min and then rapidly decreased to near-basal levels by 60 min. In contrast, Mpk1 phosphorylation levels peaked slightly earlier in heat-shocked sdp1Δ cells (∼15 min), decreased by ∼33% by 60 min, and then steadily increased for the duration of the experiment (>2 h). The transient decrease in Mpk1 phosphorylation in sdp1Δ cells is likely due to the activity of other MAPK phosphatases (Mattison et al., 1999; Flandez et al., 2004; Chen and Thorner, 2007). In heat-shocked cbk1-8 cells, the pMpk1/tMpk1 ratio peaked within 15–30 min, dropped slightly between 30 and 45 min, and then steadily increased, resulting in significantly elevated Mpk1 phosphorylation levels in comparison to similarly treated wild-type cells (Figure 3A). Likewise, the pMpk1/tMpk1 ratios of heat-shocked bck2Δ and knr4Δ cells peaked within 15–30 min, dropped slightly between 30 and 45 min, and remained elevated for >120 min. These data indicate that, like Sdp1, also Cbk1, Bck2, and Knr4 are required for efficient Mpk1 dephosphorylation during heat shock adaptation. Moreover, these data support the model that Bck2 and Sdp1 recruitment to cytoplasmic puncta during heat shock prolongs Mpk1 activity by effectively reducing nuclear and cytosolic MAPK phosphatase activity. Consistent with this model, most Bck2 and Sdp1 disappear from the nucleus shortly after heat shock induction (Hahn and Thiele, 2002) and reappear in the nucleus after prolonged heat stress, concurrent with Mpk1 dephosphorylation (Figure 6).
Cbk1, Bck2, and Knr4 influence Mpk1 phosphorylation in caffeine-treated cells in an Sdp1-independent manner
Because Sdp1 localization is not affected by caffeine treatment (data not shown), we hypothesized that Sdp1 would not affect the timing of Mpk1 dephosphorylation during prolonged caffeine exposure. To test this hypothesis, we compared the timing of Mpk1 Thr-190/Tyr-192 dephosphorylation in caffeine-treated sdp1Δ cells to that of wild-type cells. In caffeine-treated wild-type and sdp1Δ cells, we observed that pMpk1/tMpk1 ratios peaked at ∼60 min and then steadily dropped until reaching basal levels at ∼120–180 min (Figure 3B). Thus sdp1Δ had no major effect on the timing or extent of Mpk1 dephosphorylation in caffeine-treated cells, consistent with the model that Sdp1 is specific for heat shock adaptation (Figure 3B).
We conducted parallel experiments to determine whether Cbk1, Bck2, or Knr4 influenced adaptation to caffeine stress. Of note, the pMpk1/tMpk1 ratios consistently peaked higher (greater than twofold) in caffeine-treated cbk1-8, bck2Δ, and knr4Δ cells than in corresponding wild-type cells. Moreover, Mpk1 phosphorylation remained higher at every subsequent time point for the duration of the experiment (180 min). In cbk1-8 cells, Mpk1 Thr-190/Tyr-192 phosphorylation levels peaked at ∼60 min, similar to wild-type cells, before steadily decreasing to ∼50% of peak levels by 120 min (Figure 3B). In bck2Δ and knr4Δ cells, the pMpk1/tMpk1 ratios peaked 30 and 90 min later than in similarly treated wild-type cells. Collectively, these data indicate that Cbk1, Bck2, and Knr4 influence the proper timing and extent of Mpk1 dephosphorylation (adaptation) during prolonged caffeine exposure; however, they likely regulate Mpk1 dephosphorylation during caffeine treatment independent of the MAPK phosphatase Sdp1.
DISCUSSION
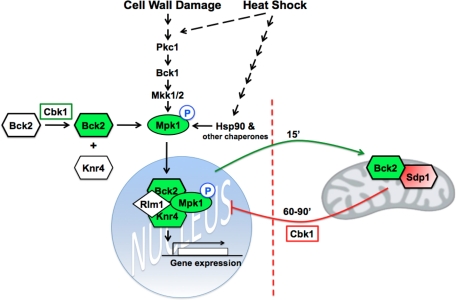
Our data support the working model that Cbk1 controls two Bck2 functions with regard to stress signaling (Figure 10). The first Cbk1-dependent Bck2 function is to promote Mpk1 activation and Mpk1-dependent gene expression during heat shock and cell wall stress, in collaboration with Knr4 and Hsp90. It is significant that Cbk1, Bck2, and Knr4 are not essential for Mpk1 Thr-190/Tyr-192 phosphorylation during heat shock, caffeine treatment, and cell wall stress; however each protein is essential for Mpk1 kinase activity and Rlm1 activation. These data indicate that Cbk1 and Bck2 function after MAPKK-dependent Mpk1 phosphorylation with regard to CWI pathway signaling, similar to the chaperone Hsp90 and cochaperone Cdc37 (Hawle et al., 2007; Truman et al., 2007). Given the similar phenotypes of cbk1-8, bck2Δ, krn4Δ, and hsp90 cells with respect to Mpk1 activation, we propose that Bck2 and Knr4 cooperate with or function in parallel to Hsp90 for Mpk1-dependent Rlm1 activation during heat shock and cell wall stress. Furthermore, because Bck2 overexpression suppresses the stress sensitivity of mpk1Δ mutants (Lee et al., 1993), it is likely that in the absence of Mpk1, Bck2 stimulates other MAP kinases to activate Rlm1 and other Mpk1-dependent transcription factors during heat shock and cell wall stress. It is intriguing that Bck2 contains consensus sequences for Cbk1 phosphorylation, which is consistent with the model that Cbk1 promotes Mpk1 activation via Bck2 phosphorylation. Alternatively, Cbk1 and Bck2 may promote Mpk1 activation by inhibiting an Mpk1 inhibitor. Nevertheless, because Mpk1 Thr-190/Tyr-192 phosphorylation occurs on schedule in heat-shocked and caffeine-treated cbk1-8, bck2Δ, and knr4Δ cells, it is unlikely that Cbk1 and Bck2 promote Mpk1 activation by inactivating MAPK phosphatases, such as Sdp1.
FIGURE 10:
Model for Cbk1 and Bck2 function with regard to CWI pathway signaling. There are two distinct roles for Cbk1 and Bck2 in CWI-pathway signaling: 1) Cbk1 promotes Mpk1 activation via Bck2 during stress-dependent CWI activation. Cbk1 and Bck2 function after MAPKK-dependent Mpk1 phosphorylation, similar to Knr4 and the chaperone Hsp90. 2) Cbk1, Bck2, and the MAPK phosphatase Sdp1 cooperatively function to inactivate Mpk1 during heat shock adaptation. During heat shock, but not other CWI-activating stresses, Bck2 and Sdp1 localize to mitochondria and other cytoplasmic puncta, where they are sequestered from Mpk1. During heat shock adaptation, Cbk1 mediates the release of Bck2 and Sdp1 phosphatase from mitochondria/cytoplasmic puncta, leading to Mpk1 dephosphorylation and inactivation.
A second function for Cbk1 and Bck2 is to mediate Mpk1 dephosphorylation during heat stress adaptation (Figure 10). In support, Cbk1 and Bck2 are essential for efficient Mpk1 dephosphorylation during heat stress adaptation, and Cbk1 influences Bck2 and Sdp1 release from heat stress–induced cytoplasmic/mitochondrial puncta. During heat shock (but not during caffeine treatment), both Bck2 and Sdp1 are rapidly targeted to cytoplasmic puncta, many of which colocalize with mitochondria, where they are sequestered from Mpk1. On heat shock adaptation and CWI signaling attenuation (as detected by Mpk1 dephosphorylation), Bck2 and Sdp1 disappear from the mitochondria and other cytoplasmic puncta and concurrently reappear in the cytosol and nucleus. We propose that the Cbk1-dependent release of Bck2 and Sdp1 from puncta promotes Mpk1 dephosphorylation/inactivation during heat shock adaptation. We speculate that Cbk1 regulates Bck2-Sdp1 release from puncta via indirect mechanisms because it does not localize to cytoplasmic puncta during heat shock.
Prior to heat shock, a significant fraction of Bck2 and Sdp1 localizes to the nucleus. This is particularly noteworthy with respect to Mpk1 regulation because Mpk1-mediated Rlm1 phosphorylation is thought to take place in the nucleus since Rlm1 is only detectable in the nucleus (Jung et al., 2002; data not shown). Thus we propose that Bck2 and Sdp1 sequestration in cytoplasmic puncta prevents premature Mpk1 dephosphorylation/inactivation during heat stress.
The role of Bck2 in heat shock signaling
Our data clearly establish that Bck2 is subject to heat shock–specific regulation, as previously shown for Sdp1 (Hahn and Thiele, 2002). Most notably, heat shock causes a rapid change in Bck2 and Sdp1 phosphatase localization that is not brought about by other CWI pathway–activating stresses (caffeine or cell wall–disrupting drugs). Sdp1-mediated Mpk1 dephosphorylation also appears to be specific for heat shock adaptation (Hahn and Thiele, 2002; Figure 3). Likewise, Bck2 has a more pronounced effect on Mpk1 dephosphorylation during heat stress adaptation than during caffeine stress adaptation. We cannot rule out the possibility that Sdp1 contributes to Mpk1 dephosphorylation during caffeine treatment or other stresses; however, our data indicate that other MAPK phosphatases must play a more significant role in Mpk1 Thr-190/Tyr-192 dephosphorylation during cell wall or caffeine stress adaptation, as previously suggested (Mattison et al., 1999; Hahn and Thiele, 2002; Flandez et al., 2004; Chen and Thorner, 2007). Because Cbk1, Bck2, and Knr4, but not Sdp1, also influence Mpk1 dephosphorylation after prolonged caffeine treatment, we suggest that Cbk1, Bck2, and Knr4 at least indirectly regulate other MAPK phosphatases. Collectively, these data provide further evidence that the type of stress dictates the mechanisms for modulating Mpk1 activity and indicate that Sdp1 and Bck2 have a greater role in heat shock adaptation than in adaptation to other CWI-activating stresses.
There is precedent for stress-dependent differences in CWI signaling. Heat shock and cell wall stress stimulate the same plasma membrane receptors that activate the CWI signaling, whereas caffeine activates the CWI pathway independent of cell wall sensors by disrupting Tor1 signaling (Kuranda et al., 2006). Caffeine also leads to Mpk1 phosphorylation on Ser-423 and Ser-428 in addition to Thr-190 and Tyr-192, reflecting the presence of important stress-specific Mpk1 modifications (Truman et al., 2009). There is also evidence that heat shock stimulates Mkk1/2 or Mpk1 independent of upstream CWI pathway components (Inagaki et al., 1999; Ketela et al., 1999; Martin et al., 2000; Harrison et al., 2004). Heat shock also stimulates parallel physiological and transcriptional responses via Hsf1 transcription factor, which induces the expression and activity of a variety of proteins and chaperones (Imazu and Sakurai, 2005; Truman et al., 2007), including Hsp90, which was shown to mediate Mpk1 activation and the mitochondrial targeting of a variety of proteins (Young et al., 2003).
Bck2 and Sdp1 mitochondrial functions
Our data support the model that Bck2 and Sdp1 are specifically sequestered at mitochondria and other cytoplasmic puncta during heat shock to prevent premature Mpk1 dephosphorylation/inactivation. Bck2 and Sdp1 mitochondrial localization may also reflect specific mitochondrial functions during heat shock. Indeed, many proteins that transiently associate with mitochondria are known to mediate heat shock, oxidative stress response, and apoptosis (Fairn et al., 2007; Lanneau et al., 2008). Both Hsf1 and the chaperone Hsp90 were shown to associate with mitochondrial proteins (Young et al., 2003; Reinders et al., 2006). Thus it is tempting to speculate that Hsp90 or other chaperones contribute to Bck2 and Sdp1 mitochondrial localization and Mpk1 signaling. Alternatively, Bck2 or Sdp1 may be subject to functionally important posttranslational modifications or protein interactions at mitochondria that in turn are important for Mpk1 inhibition during heat shock adaptation.
Regardless of the specific function of mitochondrial Bck2 and Sdp1, it is unlikely that Bck2-Sdp1 mitochondrial recruitment is essential for cbk1 dosage suppression because moderate Bck2 overexpression disrupts mitochondrial targeting. Rather, we expect that Bck2 overexpression suppresses the lethality of cbk1 mutants by enhancing Bck2 protein interactions in the nucleus or cytosol that promote Mpk1 signaling. Although it is not known how Bck2 overexpression disrupts mitochondrial recruitment, we speculate that Bck2 overexpression interferes with the function or expression of important Bck2 regulators or mitochondrial-targeting factors.
Other Bck2 functions
Our data regarding Bck2-dependent Mpk1 activation also have important implications regarding cell cycle and growth control. We expect that Bck2 is required for Mpk1 activation and Mpk1-dependent gene expression during logarithmic growth. In agreement with this, Mpk1 and Bck2 influence G1 gene expression via the Swi4–Swi6 transcription complex (Epstein and Cross, 1994; Di Como et al., 1995; Wijnen and Futcher, 1999; Ferrezuelo et al., 2009). Bck2 overexpression elevates cell cycle gene expression, especially genes controlled by the Swi4–Swi6 complex (Di Como et al., 1995; Ferrezuelo et al., 2009). Similarly, Bck2 overexpression or deletion also causes aberrant cell size regulation, consistent with Swi4/Swi6 misregulation (Di Como et al., 1995; data not shown). In addition, Cbk1, Mpk1, and other CWI proteins, such as Pkc1, were implicated in actin cytoskeleton regulation during polarized growth (Helliwell et al., 1998; Weiss et al., 2002; Levin, 2005). Thus the transient bud neck localization of Bck2, Mpk1, and Cbk1 may suggest a cooperative role in polarized growth regulation.
Multiple Cbk1-dependent cell integrity mechanisms
This study reveals a new mechanism for Cbk1 in regulating yeast cell integrity and provides the first evidence that Cbk1 and Mpk1 signaling networks are functionally linked. Previous studies indicated that a major function for Cbk1 in the maintenance of cell integrity is to regulate the mRNA-binding protein Ssd1 (Jansen et al., 2009; Kurischko et al., 2011a). In the absence of Cbk1 phosphorylation or in response to cellular stress, Ssd1 localizes to P-bodies and stress granules, where its associated mRNAs are translationally repressed (Kurischko et al., 2011a). The findings presented here represent a separate function for Cbk1 in cell integrity, as Ssd1 and the CWI pathway modulate cell integrity and gene expression via largely distinct sets of genes. Given the conservation of the Cbk1 and MAPK signaling pathways among eukaryotes, we expect that future studies will establish that a key function for Cbk1-related LATS/NDR kinases in other organisms is to regulate MAPK signaling via similar regulatory circuits.
MATERIALS AND METHODS
Yeast growth conditions and strain construction
Standard yeast genetics and culture methods were used as described (Guthrie and Fink, 1991; Kurischko et al., 2005). For heat shock, mid-log-phase cells were transferred from 25 to 39°C. For caffeine-mediated CWI pathway activation, cells were transferred to media containing 15 mM caffeine and maintained at 25°C. The strains used in this study are listed in Table 1. The strain expressing C-terminally GFP-tagged Bck2 was constructed by integration of a PCR-based GFP cassette using the oligos FLO655 and FLO656 (Table 2), as described (Longtine et al., 1998).
TABLE 1:
Yeast strains.
| Name | Genotype | Source |
|---|---|---|
| FLY1299 | MATa his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 | Deletion consortium |
| FLY1300 | MATα his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 | Deletion consortium |
| FLY2084 | MATa cbk1-as-HIS3::cbk1Δ::KANMX leu2Δ0 ura3Δ0 his3Δ | Kurischko et al. (2008) |
| FLY3270 | MATα bck1Δ::KANMX his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 | Deletion consortium |
| FLY3271 | MATα mpk1Δ::KANMX his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 | Deletion consortium |
| FLY3276 | MATα bck2Δ::KANMX his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 | Deletion consortium |
| FLY3277 | MATα knr4Δ::KANMX his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 | Deletion consortium |
| FLY3278 | MATα rph1Δ::KANMX his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 | Deletion consortium |
| FLY2884 | MATa/α cbk1-8-HIS3::cbk1Δ::KANMX/cbk1-8-HIS3::cbk1Δ::KANMX his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 met15Δ0/MET15 lys2Δ0/LYS2 | Kurischko et al. (2008) |
| FLY3503 | MATα BCK2-GFP::KANMX his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 | This study |
| FLY3570 | MATα sdp1Δ::KANMX his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 | Deletion consortium |
| FLY3559 | MATa BCK2-GFP::KANMX cbk1-as-HIS3::cbk1Δ::KANMX leu2Δ0 ura3Δ0 his3Δ | This study |
TABLE 2:
Oligonucleotides.
| Oligo | Sequence | Gene |
|---|---|---|
| FLO655 | 5′-ATAACGACATTGATAATAATTTACAGTCTTTTTATTTTGATAATAGCA ACGGTGGTCCCGGTGGTCGGATCCCCGGGTTAATTAA-3′ | BCK2 |
| FLO656 | 5′-AAGATATCTGTTACTATTTTTGAACTTTTTTTTTTTTTTTTCATTCCTT TTGAATTCGAGCTCGTTTAAAC-3′ | BCK2 |
| FLO686 | 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTCGAAGGAGATAGAAC CATGCCGAAGAATAGTCACCACCATCGTT-3′ | BCK2 |
| FLO687 | 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTGGTTGCTATTATCAAA ATAAAAAGACTG-3′ | BCK2 |
| FLO696 | 5′-CTAGGAGCTCGATCGCTATTATTCAAGGAC-3′ | BCK2 |
| FLO697 | 5′-CTAGCTCGAGCGAAAATGAAATAATATCTC-3′ | BCK2 |
| FLO704 | 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTCGAAGGAGATAGAAC CATGAACATATACACATCACCCAC-3′ | SDP1 |
| FLO705 | 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTGCGGTACTTTTCTATAA CTGTTGG-3′ | SDP1 |
cbk1-8 dosage suppressor screen
We performed a genome-wide screen for dosage suppressors of the cbk1-8 mutant strain (FLY2884), using an ordered array of 1588 high-copy plasmids of overlapping yeast genomic DNA that was obtained from Open Biosystems/Thermo Fisher (Waltham, MA) and described in Jones et al. (2008). Plasmid YGPM13c23, which contained both BCK2 and RPH1, suppressed the conditional lethality of cbk1-8 cells at 34 and 37°C.
Plasmid construction
The oligonucleotides and plasmids used in this study are listed in Tables 2 and 3. pGP564-BCK2 (FLE1255) was generated by inserting PCR-amplified BCK2 into the SacI and XhoI sites of pGP564. BCK2 (from base pair −550 to +220) was amplified from YGPM13c23 with oligos FLO696 and FLO697. pGP564-RPH1 (FLE1254) was constructed by digesting YGPM13c23 (containing both BCK2 and RPH1) with XhoI and subcloning the 12.0-kb fragment containing RPH1 into pGP564. pENTRY-BCK2 and pENTRY-SDP1 were generated by PCR amplifying BCK2 and SDP1 open reading frames with oligos FLO686, FLO687, FLO704, and FLO705 (Table 2) and subcloning the PCR products into the Gateway vector pDONR221 (Invitrogen, Carlsbad, CA). BCK2 and SDP1 were transferred from their pENTRY vectors into pAG416-GDP-ccdB-GFP to yield pGPD-BCK2-GFP and pGPD-SDP1-GFP (C-terminally tagged), as described (Alberti et al., 2007). pAG416-GDP-ccdB-GFP was provided by Aaron Gitler (University of Pennsylvania, Philadelphia, PA). All constructs were confirmed by sequencing.
TABLE 3:
Plasmids.
| Name/alias | Relevant markers | Source |
|---|---|---|
| YGPM13c23 pGP564-BCK2-RPH1 | LEU2, 2 micron | Jones et al. (2008) |
| YGPM11e20 pGP564-CBK1 | LEU2, 2 micron | Jones et al. (2008) |
| pGP564 | LEU2, 2 micron | Jones et al. (2008) |
| pGP564-BCK2 FLE1255 | LEU2, 2 micron | This study |
| pGP564-RPH1 FLE1254 | LEU2, 2 micron | This study |
| pENTRY-BCK2 FLE1246 | This study | |
| pGPD-BCK2-GFP FLE1256 | URA3, CEN | This study |
| pENTRY-SDP1 FLE1280 | This study | |
| pGPD-SDP1-RFP FLE1283 | URA3, CEN | This study |
| pHPS100L RLM1 reporter plasmid | LEU2, CEN | Kirchrath et al. (2000) |
| pHPS100U RLM1 reporter plasmid | URA3, CEN | Kirchrath et al. (2000) |
| pRS425-Mpk1-GFP | LEU2, 2 micron | Hahn and Thiele (2002) |
| pRS425-SDP1-GFP | LEU2, 2 micron | Hahn and Thiele (2002) |
| pHCRED Mito-RFP | URA3, 2 micron | Fehrenbacher et al. (2004) |
| pKW1219 pRS425-NLS-mRFP | LEU2, 2 micron | Madrid et al. (2006) |
Mpk1 immunoblot analysis
For Mpk1 immunoblots, yeast cells were grown to mid-logarithmic phase (OD600 = 0.6) in synthetic complete media at 25°C. Proteins from 1.8 ml of the yeast culture were precipitated with 10% trichloroacetic acid, as described (Baerends et al., 2000). Protein precipitates were washed twice in ice-cold acetone and dissolved in equal volume of 0.1 N NaOH 1% SDS, and SDS protein sample buffer and processed for immunoblots. To detect phosphorylated Mpk1, the immunoblots were probed with 1/2500 rabbit anti–phospho-p44/42 MAPK (Thr-202/Tyr-204) antibody (4370S; Cell Signaling Technology, Beverly, MA) as described (Martin et al., 2000), followed by secondary alkaline phosphatase (AP)–conjugated anti-rabbit immunoglobulin G (IgG; S373B; Promega, Madison, WI). The immunoblots were processed for enhanced chemifluorescence (ECF), as described by the manufacturer's protocol (GE Healthcare, Piscataway, NJ), and analyzed with a STORM phosphorimager (GE Healthcare).
Total Mpk1 was probed on the same immunoblots after stripping them from antibodies and ECF substrates with 0.2 N NaOH. The blots were reprobed with 1/500 dilution of goat anti-Mpk1 antibody (SC 6802; Santa Cruz Biotechnology, Santa Cruz, CA), followed by secondary AP-conjugated donkey anti-goat IgG (Sc2022; Santa Cruz Biotechnology). Immunoblots were scanned with a STORM phosphorimager and quantified using ImageQuant Tl software (GE Healthcare). The ratios of phosphorylated Mpk1/total Mpk1 from two independent experiments were calculated and plotted.
In vitro Mpk1 kinase assays
For Mpk1-GFP immunoprecipitation, cells containing pRS425-Mpk1-GFP (kindly provided by Dennis J Thiele, Duke University, Durham, NC) were grown to mid-log phase (OD600 = 0.8–1.0) and heat shocked for 30 min at 39°C. The cells were harvested and lysed as described (Kamada et al., 1995; Kurischko et al., 2011a). The cell extracts were normalized for protein concentration, and 100 μg was immunoprecipitated with anti-GFP antibody (11814460001; Roche, Indianapolis, IN). In vitro kinase assays were carried out with immunoprecipitated Mpk1-GFP and exogenous MBP as substrate, as described (Kamada et al., 1995). MBP phosphorylation was detected by a STORM phosphorimager and quantified with ImageQuant software.
Subcellular fractionation and isolation of mitochondria
Mitochondrial fractionation was carried out as described (Sepuri et al., 2007). Briefly, yeast cells were grown in selective synthetic media to mid-log phase (OD600 = 1.2) at 22°C and heat shocked for 30 min at 39°C. Unstressed and heat-shocked cells were harvested and converted to protoplasts by treating them with zymolyase (MP Biomedicals, Solon, OH). Differential centrifugations of protoplasts were carried out as described previously. The mitochondrial-enriched fraction was collected as pellet P2 from 8000 × g centrifugation for 10 min. P2 was resuspended in 0.5 ml SEM buffer (250 mM sucrose, 1 mM EDTA, 10 mM 3-(N-morpholino)propanesulfonic acid, pH, 7.2, 1 mM phenylmethylsulfonyl fluoride). The 8000 × g supernatant (S1) was further subjected to 35,000 × g for 2 h to enrich for ER/microsome (P3) and cytosolic proteins (S3). After fractionation, protein concentrations were measured, and 50 μg of each fraction was loaded onto 10% polyacrylamide gels and immunoblotted. Immunoblots of cytosol, mitochondrial, and microsomal fractions were probed with antibodies for the mitochondrial marker Tim23 (Santa Cruz Biotechnology), the ER protein Dpm1 (A6429; Invitrogen), and anti-GFP antibody (Roche) for Sdp1-GFP protein. Anti-Dpm1 and anti-Tim23 antibodies were kindly provided by Narayan Avadhani.
Rlm1 reporter assays
Rlm1-lacZ reporter plasmid, which bears both a lexA-RLM1 fusion and a lacZ reporter gene with upstream lexA-binding sites, was obtained from Jürgen Heinisch (Universtität Osnabrück, Osnabrück, Germany). The Rlm1-lacZ reporter plasmid was introduced into yeast cells, and β-galactosidase activity was measured at the designated time points following heat shock and caffeine treatment, as previously described (Kirchrath et al., 2000; Martin-Yken et al., 2003). Rlm1-dependent lacZ expression was quantified by measuring the nanomoles per minute per milligram of o-nitrophenyl phosphate produced from o-nitrophenyl β-d-galactoside substrate, as previously described (Kirchrath et al., 2000; Martin-Yken et al., 2003). Rlm1 activity was represented as the mean value of three independent experiments.
Fluorescence microscopy
All microscopy was carried out as described (Kurischko et al., 2011a). Wide-field fluorescence and time-lapse microscopy was carried out with a Leica (Wetzlar, Germany) DMR5 fluorescence microscope equipped with a 100× PL Apo 1.46 numerical aperture (NA) oil objective and an ImagEM 16-bit cooled Hamamatsu EMCCD camera (Hamamatsu, Japan), as previously described (Nelson et al., 2003; Kurischko et al., 2008). For time-lapse microscopy, cells were heat shocked by raising the temperature on an objective heater (Bioptics, Butler, PA) from room temperature to 37°C. Spinning disk confocal microscopy was conducted with a Leica Inverted DMI4000 microscope equipped with a 100× HCX PL Apo 1.46 NA oil objective, a Yokogawa (Sugarland, TX) CSU-10 spinning disk confocal system, and an ImagEM 16-bit cooled Hamamatsu EMCCD camera. Laser excitation was provided by a 488-nm laser (Spectra Physics, Newport Corporation, Irvine, CA) and a 561-nm laser (Cobolt Jive, Solna, Sweden) controlled through LMM5 (Spectral Applied Research, Richmond Hill, Canada). The emissions were collected at 503–552 nm for GFP and 583–650 nm for RFP. Z-stacks were taken for a total thickness of 1.8–3.4 μm at a step size of 0.2 μm. Image capture was controlled by MetaMorph software (MDS Analytical Technologies, Sunnyvale, CA), and image analysis and three-dimensional modeling were conducted with Volocity software (PerkinElmer, Waltham, MA).
Supplementary Material
Acknowledgments
We thank Neeraja Konuthula and Nattha Wannissorn for technical assistance, Lingli Zhang and Bruce Freedman (University of Pennsylvania School of Veterinary Medicine Core Imaging Facility, Philadelphia, PA) and Andrea Stout (University of Pennsylvania School of Medicine Core Imaging Facility, Philadelphia, PA) for assistance with spinning disk confocal microscopy. We also thank Narayan Avadhani and Seema Bhansal for assistance with mitochondrial fractionation and Erfei Bi and Aaron Gitler (University of Pennsylvania) for helpful discussions. We are grateful to Aaron Gitler, Jürgen Heinisch (Universtität Osnabrück), Dennis Thiele (Duke University, Durham, NC), Liza Pon (Columbia University, New York, NY), Karsten Weis (University of California, Berkeley, CA), and David Levin (Boston University, Boston, MA) for providing various plasmids and reagents. This work was supported by grants from the American Cancer Society (RSG0508401), the National Institutes of Health (GM 060575), and the Mari Lowe Center for Comparative Oncology.
Abbreviations used:
- CR
Congo red
- CW
calcofluor white
- CWI
Cell Wall Integrity
- Hyg B
hygromycin B
- MAPK
mitogen-activated protein kinase
- RAM
Regulation of Ace2 and Morphogenesis
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-04-0371) on October 26, 2011.
REFERENCES
- Alberti S, Gitler AD, Lindquist S. A suite of Gateway cloning vectors for high-throughput genetic analysis in Saccharomyces cerevisiae. Yeast. 2007;24:913–919. doi: 10.1002/yea.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerends RJ, Faber KN, Kram AM, Kiel JA, van der Klei IJ, Veenhuis M. A stretch of positively charged amino acids at the N terminus of Hansenula polymorpha Pex3p is involved in incorporation of the protein into the peroxisomal membrane. J Biol Chem. 2000;275:9986–9995. doi: 10.1074/jbc.275.14.9986. [DOI] [PubMed] [Google Scholar]
- Baetz K, Moffat J, Haynes J, Chang M, Andrews B. Transcriptional coregulation by the cell integrity mitogen-activated protein kinase Slt2 and the cell cycle regulator Swi4. Mol Cell Biol. 2001;21:6515–6528. doi: 10.1128/MCB.21.19.6515-6528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer MJ, Huttenhower C, Airoldi EM, Rosenstein R, Matese JC, Gresham D, Boer VM, Troyanskaya OG, Botstein D. Coordination of growth rate, cell cycle, stress response, and metabolic activity in yeast. Mol Biol Cell. 2008;19:352–367. doi: 10.1091/mbc.E07-08-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RE, Thorner J. Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 2007;1773:1311–1340. doi: 10.1016/j.bbamcr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clotet J, Posas F. Control of cell cycle in response to osmostress: lessons from yeast. Methods Enzymol. 2007;428:63–76. doi: 10.1016/S0076-6879(07)28004-8. [DOI] [PubMed] [Google Scholar]
- Collister M, Didmon MP, MacIsaac F, Stark MJ, MacDonald NQ, Keyse SM. YIL113w encodes a functional dual-specificity protein phosphatase which specifically interacts with and inactivates the Slt2/Mpk1p MAP kinase in S. cerevisiae. FEBS Lett. 2002;527:186–192. doi: 10.1016/s0014-5793(02)03220-9. [DOI] [PubMed] [Google Scholar]
- Dean N. Yeast glycosylation mutants are sensitive to aminoglycosides. Proc Natl Acad Sci USA. 1995;92:1287–1291. doi: 10.1073/pnas.92.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Como CJ, Chang H, Arndt KT. Activation of CLN1 and CLN2 G1 cyclin gene expression by BCK2. Mol Cell Biol. 1995;15:1835–1846. doi: 10.1128/mcb.15.4.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodou E, Treisman R. The Saccharomyces cerevisiae MADS-box transcription factor Rlm1 is a target for the Mpk1 mitogen-activated protein kinase pathway. Mol Cell Biol. 1997;17:1848–1859. doi: 10.1128/mcb.17.4.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du LL, Novick P. Pag1p, a novel protein associated with protein kinase Cbk1p, is required for cell morphogenesis and proliferation in Saccharomyces cerevisiae. Mol Biol Cell. 2002;13:503–514. doi: 10.1091/mbc.01-07-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrenberger F, Kronstad J. The ukc1 gene encodes a protein kinase involved in morphogenesis, pathogenicity and pigment formation in Ustilago maydis. Mol Gen Genet. 1999;261:281–289. doi: 10.1007/s004380050968. [DOI] [PubMed] [Google Scholar]
- Epstein CB, Cross FR. Genes that can bypass the CLN requirement for Saccharomyces cerevisiae cell cycle START. Mol Cell Biol. 1994;14:2041–2047. doi: 10.1128/mcb.14.3.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairn GD, Macdonald K, McMaster CR. A chemogenomic screen in Saccharomyces cerevisiae uncovers a primary role for the mitochondria in farnesol toxicity and its regulation by the Pkc1 pathway. J Biol Chem. 2007;282:4868–4874. doi: 10.1074/jbc.M610575200. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher KL, Yang HC, Gay AC, Huckaba TM, Pon LA. Live cell imaging of mitochondrial movement along actin cables in budding yeast. Curr Biol. 2004;14:1996–2004. doi: 10.1016/j.cub.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Ferrezuelo F, Aldea M, Futcher B. Bck2 is a phase-independent activator of cell cycle-regulated genes in yeast. Cell Cycle. 2009;8:239–252. doi: 10.4161/cc.8.2.7543. [DOI] [PubMed] [Google Scholar]
- Flandez M, Cosano IC, Nombela C, Martin H, Molina M. Reciprocal regulation between Slt2 MAPK and isoforms of Msg5 dual-specificity protein phosphatase modulates the yeast cell integrity pathway. J Biol Chem. 2004;279:11027–11034. doi: 10.1074/jbc.M306412200. [DOI] [PubMed] [Google Scholar]
- Fuchs BB, Mylonakis E. Our paths might cross: the role of the fungal cell wall integrity pathway in stress response and cross talk with other stress response pathways. Eukaryot Cell. 2009;8:1616–1625. doi: 10.1128/EC.00193-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R, Bermejo C, Grau C, Perez R, Rodriguez-Pena JM, Francois J, Nombela C, Arroyo J. The global transcriptional response to transient cell wall damage in Saccharomyces cerevisiae and its regulation by the cell integrity signaling pathway. J Biol Chem. 2004;279:15183–15195. doi: 10.1074/jbc.M312954200. [DOI] [PubMed] [Google Scholar]
- Guthrie C, Fink GR. Guide to yeast genetics and molecular biology. Methods Enzymol. 1991;194:1–933. [PubMed] [Google Scholar]
- Hahn JS, Thiele DJ. Regulation of the Saccharomyces cerevisiae Slt2 kinase pathway by the stress-inducible Sdp1 dual specificity phosphatase. J Biol Chem. 2002;277:21278–21284. doi: 10.1074/jbc.M202557200. [DOI] [PubMed] [Google Scholar]
- Harrison JC, Bardes ES, Ohya Y, Lew DJ. A role for the Pkc1p/Mpk1p kinase cascade in the morphogenesis checkpoint. Nat Cell Biol. 2001;3:417–420. doi: 10.1038/35070104. [DOI] [PubMed] [Google Scholar]
- Harrison JC, Zyla TR, Bardes ES, Lew DJ. Stress-specific activation mechanisms for the “cell integrity” MAPK pathway. J Biol Chem. 2004;279:2616–2622. doi: 10.1074/jbc.M306110200. [DOI] [PubMed] [Google Scholar]
- Hawle P, Horst D, Bebelman JP, Yang XX, Siderius M, van der Vies SM. Cdc37p is required for stress-induced high-osmolarity glycerol and protein kinase C mitogen-activated protein kinase pathway functionality by interaction with Hog1p and Slt2p (Mpk1p) Eukaryot Cell. 2007;6:521–532. doi: 10.1128/EC.00343-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinisch JJ, Lorberg A, Schmitz HP, Jacoby JJ. The protein kinase C-mediated MAP kinase pathway involved in the maintenance of cellular integrity in Saccharomyces cerevisiae. Mol Microbiol. 1999;32:671–680. doi: 10.1046/j.1365-2958.1999.01375.x. [DOI] [PubMed] [Google Scholar]
- Helliwell SB, Schmidt A, Ohya Y, Hall MN. The Rho1 effector Pkc1, but not Bni1, mediates signalling from Tor2 to the actin cytoskeleton. Curr Biol. 1998;8:1211–1214. doi: 10.1016/s0960-9822(07)00511-8. [DOI] [PubMed] [Google Scholar]
- Hergovich A, Stegert MR, Schmitz D, Hemmings BA. NDR kinases regulate essential cell processes from yeast to humans. Nat Rev Mol Cell Biol. 2006;7:253–264. doi: 10.1038/nrm1891. [DOI] [PubMed] [Google Scholar]
- Hirata D, et al. Fission yeast Mor2/Cps12, a protein similar to Drosophila Furry, is essential for cell morphogenesis and its mutation induces Wee1-dependent G(2) delay. EMBO J. 2002;21:4863–4874. doi: 10.1093/emboj/cdf495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan DJ, Riordan DP, Gerber AP, Herschlag D, Brown PO. Diverse RNA-binding proteins interact with functionally related sets of RNAs, suggesting an extensive regulatory system. PLoS Biol. 2008;6:e255. doi: 10.1371/journal.pbio.0060255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou MC, Wiley DJ, Verde F, McCollum D. Mob2p interacts with the protein kinase Orb6p to promote coordination of cell polarity with cell cycle progression. J Cell Sci. 2003;116:125–135. doi: 10.1242/jcs.00206. [DOI] [PubMed] [Google Scholar]
- Imazu H, Sakurai H. Saccharomyces cerevisiae heat shock transcription factor regulates cell wall remodeling in response to heat shock. Eukaryot Cell. 2005;4:1050–1056. doi: 10.1128/EC.4.6.1050-1056.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki M, Schmelzle T, Yamaguchi K, Irie K, Hall MN, Matsumoto K. PDK1 homologs activate the Pkc1-mitogen-activated protein kinase pathway in yeast. Mol Cell Biol. 1999;19:8344–8352. doi: 10.1128/mcb.19.12.8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YK, Wang L, Sancar GB. RPH1 and GIS1 are damage-responsive repressors of PHR1. Mol Cell Biol. 1999;19:7630–7638. doi: 10.1128/mcb.19.11.7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen JM, Wanless AG, Seidel CW, Weiss EL. Cbk1 regulation of the RNA-binding protein Ssd1 integrates cell fate with translational control. Curr Biol. 2009;19:2114–2120. doi: 10.1016/j.cub.2009.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GM, Stalker J, Humphray S, West A, Cox T, Rogers J, Dunham I, Prelich G. A systematic library for comprehensive overexpression screens in Saccharomyces cerevisiae. Nat Methods. 2008;5:239–241. doi: 10.1038/nmeth.1181. [DOI] [PubMed] [Google Scholar]
- Jorgensen P, Nelson B, Robinson MD, Chen Y, Andrews B, Tyers M, Boone C. High-resolution genetic mapping with ordered arrays of Saccharomyces cerevisiae deletion mutants. Genetics. 2002;162:1091–1099. doi: 10.1093/genetics/162.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung US, Levin DE. Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol Microbiol. 1999;34:1049–1057. doi: 10.1046/j.1365-2958.1999.01667.x. [DOI] [PubMed] [Google Scholar]
- Jung US, Sobering AK, Romeo MJ, Levin DE. Regulation of the yeast Rlm1 transcription factor by the Mpk1 cell wall integrity MAP kinase. Mol Microbiol. 2002;46:781–789. doi: 10.1046/j.1365-2958.2002.03198.x. [DOI] [PubMed] [Google Scholar]
- Kamada Y, Jung US, Piotrowski J, Levin DE. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 1995;9:1559–1571. doi: 10.1101/gad.9.13.1559. [DOI] [PubMed] [Google Scholar]
- Kanai M, Kume K, Miyahara K, Sakai K, Nakamura K, Leonhard K, Wiley DJ, Verde F, Toda T, Hirata D. Fission yeast MO25 protein is localized at SPB and septum and is essential for cell morphogenesis. EMBO J. 2005;24:3012–3025. doi: 10.1038/sj.emboj.7600782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketela T, Green R, Bussey H. Saccharomyces cerevisiae mid2p is a potential cell wall stress sensor and upstream activator of the PKC1-MPK1 cell integrity pathway. J Bacteriol. 1999;181:3330–3340. doi: 10.1128/jb.181.11.3330-3340.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EM, Jang YK, Park SD. Phosphorylation of Rph1, a damage-responsive repressor of PHR1 in Saccharomyces cerevisiae, is dependent upon Rad53 kinase. Nucleic Acids Res. 2002;30:643–648. doi: 10.1093/nar/30.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchrath L, Lorberg A, Schmitz HP, Gengenbacher U, Heinisch JJ. Comparative genetic and physiological studies of the MAP kinase Mpk1p from Kluyveromyces lactis and Saccharomyces cerevisiae. J Mol Biol. 2000;300:743–58. doi: 10.1006/jmbi.2000.3916. [DOI] [PubMed] [Google Scholar]
- Kuranda K, Leberre V, Sokol S, Palamarczyk G, Francois J. Investigating the caffeine effects in the yeast Saccharomyces cerevisiae brings new insights into the connection between TOR, PKC and Ras/cAMP signalling pathways. Mol Microbiol. 2006;61:1147–1166. doi: 10.1111/j.1365-2958.2006.05300.x. [DOI] [PubMed] [Google Scholar]
- Kurischko C, Kim HK, Kuravi VK, Pratzka J, Luca FC. The yeast Cbk1 kinase regulates mRNA localization via the mRNA-binding protein Ssd1. J Cell Biol. 2011a;192:583–598. doi: 10.1083/jcb.201011061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurischko C, Kuravi VK, Herbert CJ, Luca FC. Nucleocytoplasmic shuttling of Ssd1 defines the destiny of its bound mRNAs. Mol Microbiol. 2011b;81:831–849. doi: 10.1111/j.1365-2958.2011.07731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurischko C, Kuravi VK, Wannissorn N, Nazarov PA, Husain M, Zhang C, Shokat KM, McCaffery JM, Luca FC. The yeast LATS/Ndr kinase Cbk1 regulates growth via Golgi-dependent glycosylation and secretion. Mol Biol Cell. 2008;19:5559–5578. doi: 10.1091/mbc.E08-05-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurischko C, Weiss G, Ottey M, Luca FC. A role for the Saccharomyces cerevisiae regulation of Ace2 and polarized morphogenesis signaling network in cell integrity. Genetics. 2005;171:443–455. doi: 10.1534/genetics.105.042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C. Heat shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med. 2008;12:743–761. doi: 10.1111/j.1582-4934.2008.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Hines LK, Levin DE. A pair of functionally redundant yeast genes (PPZ1 and PPZ2) encoding type 1-related protein phosphatases function within the PKC1-mediated pathway. Mol Cell Biol. 1993;13:5843–5853. doi: 10.1128/mcb.13.9.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DE. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2005;69:262–291. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang CY, Hsu PH, Chou DF, Pan CY, Wang LC, Huang WC, Tsai MD, Lo WS. The histone H3K36 demethylase Rph1/KDM4 regulates the expression of the photoreactivation gene PHR1. Nucleic Acids Res. 2011;39:4151–4165. doi: 10.1093/nar/gkr040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A 3rd, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Madden K, Sheu YJ, Baetz K, Andrews B, Snyder M. SBF cell cycle regulator as a target of the yeast PKC-MAP kinase pathway. Science. 1997;275:1781–1784. doi: 10.1126/science.275.5307.1781. [DOI] [PubMed] [Google Scholar]
- Madrid AS, Mancuso J, Cande WZ, Weis K. The role of the integral membrane nucleoporins Ndc1p and Pom152p in nuclear pore complex assembly and function. J Cell Biol. 2006;173:361–371. doi: 10.1083/jcb.200506199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager WH, De Kruijff AJ. Stress-induced transcriptional activation. Microbiol Rev. 1995;59:506–531. doi: 10.1128/mr.59.3.506-531.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H, Flandez M, Nombela C, Molina M. Protein phosphatases in MAPK signalling: we keep learning from yeast. Mol Microbiol. 2005;58:6–16. doi: 10.1111/j.1365-2958.2005.04822.x. [DOI] [PubMed] [Google Scholar]
- Martin H, Rodriguez-Pachon JM, Ruiz C, Nombela C, Molina M. Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J Biol Chem. 2000;275:1511–1519. doi: 10.1074/jbc.275.2.1511. [DOI] [PubMed] [Google Scholar]
- Martin-Yken H, Dagkessamanskaia A, Basmaji F, Lagorce A, Francois J. The interaction of Slt2 MAP kinase with Knr4 is necessary for signalling through the cell wall integrity pathway in Saccharomyces cerevisiae. Mol Microbiol. 2003;49:23–35. doi: 10.1046/j.1365-2958.2003.03541.x. [DOI] [PubMed] [Google Scholar]
- Martin-Yken H, Dagkessamanskaia A, Talibi D, Francois J. KNR4 is a member of the PKC1 signalling pathway and genetically interacts with BCK2, a gene involved in cell cycle progression in Saccharomyces cerevisiae. Curr Genet. 2002;41:323–332. doi: 10.1007/s00294-002-0299-6. [DOI] [PubMed] [Google Scholar]
- Mattison CP, Spencer SS, Kresge KA, Lee J, Ota IM. Differential regulation of the cell wall integrity mitogen-activated protein kinase pathway in budding yeast by the protein tyrosine phosphatases Ptp2 and Ptp3. Mol Cell Biol. 1999;19:7651–7660. doi: 10.1128/mcb.19.11.7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni C, Zarov P, Rambourg A, Mann C. The SLT2 (MPK1) MAP kinase homolog is involved in polarized cell growth in Saccharomyces cerevisiae. J Cell Biol. 1993;123:1821–1833. doi: 10.1083/jcb.123.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza M, Redemann S, Brunner D. The fission yeast MO25 protein functions in polar growth and cell separation. Eur J Cell Biol. 2005;84:915–926. doi: 10.1016/j.ejcb.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Nelson B, et al. RAM: a conserved signaling network that regulates ace2p transcriptional activity and polarized morphogenesis. Mol Biol Cell. 2003;14:3782–3803. doi: 10.1091/mbc.E03-01-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama Y, Kasahara K, Kokubo T. Saccharomyces cerevisiae Ssd1p promotes CLN2 expression by binding to the 5′-untranslated region of CLN2 mRNA. Genes Cells. 2010;15:1169–1188. doi: 10.1111/j.1365-2443.2010.01452.x. [DOI] [PubMed] [Google Scholar]
- Pearce AK, Humphrey TC. Integrating stress-response and cell-cycle checkpoint pathways. Trends Cell Biol. 2001;11:426–433. doi: 10.1016/s0962-8924(01)02119-5. [DOI] [PubMed] [Google Scholar]
- Reinders J, Zahedi RP, Pfanner N, Meisinger C, Sickmann A. Toward the complete yeast mitochondrial proteome: multidimensional separation techniques for mitochondrial proteomics. J Proteome Res. 2006;5:1543–1554. doi: 10.1021/pr050477f. [DOI] [PubMed] [Google Scholar]
- Sepuri NB, Yadav S, Anandatheerthavarada HK, Avadhani NG. Mitochondrial targeting of intact CYP2B1 and CYP2E1 and N-terminal truncated CYP1A1 proteins in Saccharomyces cerevisiae—role of protein kinase A in the mitochondrial targeting of CYP2E1. FEBS J. 2007;274:4615–4630. doi: 10.1111/j.1742-4658.2007.05990.x. [DOI] [PubMed] [Google Scholar]
- Song Y, Cheon SA, Lee KE, Lee SY, Lee BK, Oh DB, Kang HA, Kim JY. Role of the RAM network in cell polarity and hyphal morphogenesis in Candida albicans. Mol Biol Cell. 2008;19:5456–5477. doi: 10.1091/mbc.E08-03-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres J, Di Como CJ, Herrero E, De La Torre-Ruiz MA. Regulation of the cell integrity pathway by rapamycin-sensitive TOR function in budding yeast. J Biol Chem. 2002;277:43495–43504. doi: 10.1074/jbc.M205408200. [DOI] [PubMed] [Google Scholar]
- Truman AW, Kim KY, Levin DE. Mechanism of Mpk1 mitogen-activated protein kinase binding to the Swi4 transcription factor and its regulation by a novel caffeine-induced phosphorylation. Mol Cell Biol. 2009;29:6449–6461. doi: 10.1128/MCB.00794-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman AW, Millson SH, Nuttall JM, Mollapour M, Prodromou C, Piper PW. In the yeast heat shock response, Hsf1-directed induction of Hsp90 facilitates the activation of the Slt2 (Mpk1) mitogen-activated protein kinase required for cell integrity. Eukaryot Cell. 2007;6:744–752. doi: 10.1128/EC.00009-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drogen F, Peter M. Spa2p functions as a scaffold-like protein to recruit the Mpk1p MAP kinase module to sites of polarized growth. Curr Biol. 2002;12:1698–1703. doi: 10.1016/s0960-9822(02)01186-7. [DOI] [PubMed] [Google Scholar]
- Verde F, Wiley DJ, Nurse P. Fission yeast orb6, a ser/thr protein kinase related to mammalian rho kinase and myotonic dystrophy kinase, is required for maintenance of cell polarity and coordinates cell morphogenesis with the cell cycle. Proc Natl Acad Sci USA. 1998;95:7526–7531. doi: 10.1073/pnas.95.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton FJ, Heitman J, Idnurm A. Conserved elements of the RAM signaling pathway establish cell polarity in the basidiomycete Cryptococcus neoformans in a divergent fashion from other fungi. Mol Biol Cell. 2006;17:3768–3780. doi: 10.1091/mbc.E06-02-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Irie K, Matsumoto K. Yeast RLM1 encodes a serum response factor-like protein that may function downstream of the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol Cell Biol. 1995;15:5740–5749. doi: 10.1128/mcb.15.10.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EL, Kurischko C, Zhang C, Shokat K, Drubin DG, Luca FC. The Saccharomyces cerevisiae Mob2p-Cbk1p kinase complex promotes polarized growth and acts with the mitotic exit network to facilitate daughter cell-specific localization of Ace2p transcription factor. J Cell Biol. 2002;158:885–900. doi: 10.1083/jcb.200203094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnen H, Futcher B. Genetic analysis of the shared role of CLN3 and BCK2 at the G(1)-S transition in Saccharomyces cerevisiae. Genetics. 1999;153:1131–1143. doi: 10.1093/genetics/153.3.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JC, Hoogenraad NJ, Hartl FU. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell. 2003;112:41–50. doi: 10.1016/s0092-8674(02)01250-3. [DOI] [PubMed] [Google Scholar]
- Zarzov P, Mazzoni C, Mann C. The SLT2(MPK1) MAP kinase is activated during periods of polarized cell growth in yeast. EMBO J. 1996;15:83–91. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.