Abstract
Amino acids profoundly affect insulin action and glucose metabolism in mammals. Here, we investigated the role of the mediobasal hypothalamus (MBH), a key center involved in nutrient-dependent metabolic regulation. Specifically, we tested the novel hypothesis that the metabolism of leucine within the MBH couples the central sensing of leucine with the control of glucose production by the liver. We performed either central (MBH) or systemic infusions of leucine in Sprague-Dawley male rats during basal pancreatic insulin clamps in combination with various pharmacological and molecular interventions designed to modulate leucine metabolism in the MBH. We also examined the role of hypothalamic ATP-sensitive K+ channels (KATP channels) in the effects of leucine. Enhancing the metabolism of leucine acutely in the MBH lowered blood glucose through a biochemical network that was insensitive to rapamycin but strictly dependent on the hypothalamic metabolism of leucine to α-ketoisocaproic acid and, further, insensitive to acetyl- and malonyl-CoA. Functional KATP channels were also required. Importantly, molecular attenuation of this central sensing mechanism in rats conferred susceptibility to developing hyperglycemia. We postulate that the metabolic sensing of leucine in the MBH is a previously unrecognized mechanism for the regulation of hepatic glucose production required to maintain glucose homeostasis.
Ample evidence has established that protein and amino acids exert profound effects on insulin action, glucose metabolism, and food intake (1–8); however, the mechanisms behind these effects are not yet fully understood. Early work established that postprandial elevations of plasma amino acids stimulate endogenous secretion of both insulin and glucagon (9,10) and thus might modulate hepatic glucose metabolism by changing the portal insulin-to-glucagon ratio (11). Furthermore, amino acids act as substrates for gluconeogenesis (12), a pathway that contributes to glucose production. These direct and indirect effects of amino acids on hepatic glucose metabolism have been studied in various models, mainly using amino acid mixtures. Although these studies have provided a great deal of information, the use of mixtures did not allow for a direct examination of the unique actions of specific amino acids on glucose metabolism. Moreover, the majority of these studies emphasized the actions of amino acids in liver and skeletal muscle. The potential role of the mediobasal hypothalamus (MBH) in the mechanism(s) by which amino acids influence glucose metabolism has not been investigated. The arcuate nucleus of the MBH has emerged as a major center where signals involved in the determination of nutrient availability and energy balance converge. Nutrients engage biochemical sensors in the MBH that in turn exert a negative feedback on food intake and liver glucose production (13–16). Amino acids gain access to the central nervous system through a facilitative transport system (17), and their concentration in the hypothalamus reflects their circulating levels (18). This work focuses on leucine, a branched-chain ketogenic amino acid that plays a critical role in cellular signaling (19–21) via activation of the mammalian target of rapamycin (mTOR) pathway. In fact, leucine has been shown to modulate food intake by activating mTOR in the hypothalamus (6). Leucine metabolism generates acetyl-CoA, which can be oxidized to carbon dioxide or used in de novo lipid synthesis (Fig. 1A). The metabolism of leucine is initiated by its transamination to α-ketoisocaproic acid (KIC), catalyzed by the branched-chain amino acid aminotransferase (BCAT) (22). BCAT is highly expressed in the muscle and brain of humans and rodents (22,23). KIC undergoes oxidative decarboxylation catalyzed by the branched-chain ketoacid dehydrogenase (BCKDH) (22,23), yielding isovaleryl-CoA, which is further oxidized to acetyl-CoA (Fig. 1A), which is subsequently carboxylated to malonyl-CoA by acetyl-CoA carboxylase (ACC). Here, we tested the hypothesis that the metabolism of leucine to acetyl- and malonyl-CoA within the MBH couples the central sensing of leucine with the control of glucose production by the liver and that this regulatory mechanism contributes to the maintenance of euglycemia.
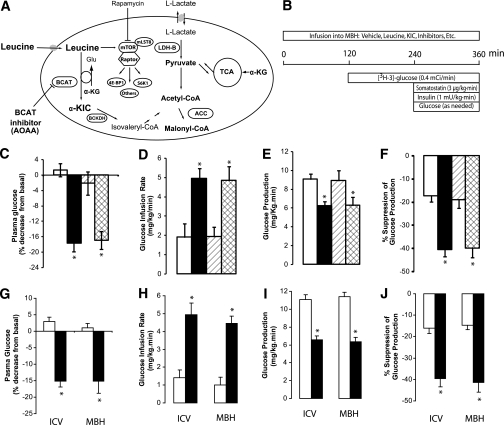
FIG. 1.
Central administration of leucine lowers plasma glucose through KIC-dependent inhibition of liver glucose production. A: Metabolism of leucine and activation of the mTOR pathway. B: Protocol for pancreatic clamp and central infusions (MBH) in rats. C–F: Effect of the central administration (MBH) of vehicle (n = 6) (□), leucine (n = 6) (■), leucine plus BCAT inhibitor (amino-oxyacetic acid [AOAA]; n = 5) (▨), and leucine plus mTOR inhibitor (rapamycin; n = 4) (▩) on basal plasma glucose (C) and whole-body glucose metabolism during pancreatic insulin clamps (D–F). G: Effect of the administration of vehicle (n = 6) (□) or KIC (n = 6) (■) into the third cerebral ventricle (intracerebroventricular) or the MBH of rats on plasma glucose. H–J: Effect of central infusion of vehicle (n = 6) (□) or KIC (n = 5) (■) into the third cerebral ventricle (intracerebroventricular) or the MBH on whole-body glucose metabolism during pancreatic insulin clamps (H–J). All values are means ± SEM. *P < 0.05 vs. vehicle.
RESEARCH DESIGN AND METHODS
Animal preparation
Rats.
We performed the studies in 10- to 12-week-old male Sprague-Dawley rats (24), which first underwent stereotaxic surgery to have inserted either a single cannula into the third cerebral ventricle (intracerebroventricular) or bilateral cannulae into the MBH. The correct placement of the cannulae was verified as previously described (24). A week later, we placed indwelling vascular catheters for pancreatic clamp studies. Satisfactory postoperative recovery was monitored by daily measurements of food intake and body weight gain for 7 days.
Mice.
Twelve-week-old wild-type (n = 8) and SUR1-null (Sur1−/−) (n = 13) male mice (27–32 g) underwent stereotaxic surgery under anesthesia for implantation of a single cannula in the third cerebral ventricle. A week later, the animals were catheterized for pancreatic clamp studies. Each animal was monitored daily for food intake and body weight gain to ensure complete postoperative recovery. The animal studies were approved by the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine.
Central or systemic infusions and pancreatic clamp procedure in rats.
The studies were carried out in rats fasted for 6 h before the experiments and consisted of central (hypothalamic) or systemic infusions combined with pancreatic clamps (Figs. 1B, 5A, and 5F). The various central infusions were as follows: 5 µl/h i.c.v. and 0.33 µL/h into the MBH. The concentration of inhibitors was chosen based on their half-maximal inhibitory concentration (IC50). Infusions were as follows (intracerebroventricular or MBH vehicle [artificial cerebrospinal fluid]): 90 nmol KIC i.c.v., MBH leucine, and 600 pmol KIC; 400 pmol MBH aminooxyacetic acid and 1 pmol MBH rapamycin; 1 nmol MBH α-chloroisocaproate (α-CIC) (pH ∼7.4); and 1 µg MBH SHU9119. The central infusions lasted 360 min (Fig. 1B). At t = 0 min, a primed continuous infusion, either intracerebroventricular or into the MBH, of each of the various solutions described was initiated and maintained for the remainder of the study. At t = 120 min, a primed continuous infusion of [3-3H] glucose (40 µCi bolus, 0.4 µCi/min; New England Nuclear) was initiated and maintained for the last 4 h of the study. A basal insulin clamp was initiated at t = 240 min and maintained for 2 h (24). Throughout the study, the plasma levels of metabolites were monitored. At the end of the experiments, the rats were killed and tissue samples immediately freeze-clamped in situ and stored at –80°C for subsequent analysis.
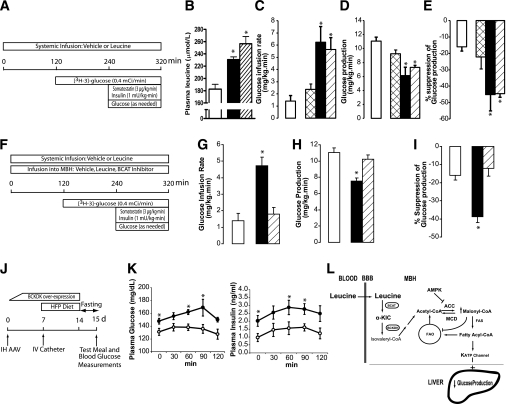
FIG. 5.
Central metabolism of leucine is required for the regulation of endogenous glucose production during physiological hyperleucinemia. A: Protocol for studies of systemic infusions of leucine. B: Plasma leucine: basal (□) or after systemic infusions of leucine at 120 (■) and 240 (▨) µmol/kg/h. C–E: Effect of systemic leucine infusion on whole-body glucose metabolism during pancreatic insulin clamps. Rats received vehicle (n = 6) (□) or 60 µmol/kg/h (n = 5) (▩), 120 µmol/kg/h (n = 6) (■), or 240 µmol/kg/h (n = 6) (▨) leucine. F: Protocol for studies of systemic infusions of leucine during central inhibition of leucine metabolism. G–I: Effect of the systemic vehicle (n = 6) (□) and leucine (120 µmol/kg/h) in combination with central (MBH) infusion of vehicle (n = 6) (■) or BCAT inhibitor (n = 6) (▨) on whole-body glucose metabolism during pancreatic insulin clamps. J: Protocol for meal challenge test. IH, intrahypothalamic; HFP, high-fat and high-protein diet; IV, intravascular. K: Time course of plasma glucose and insulin in rats injected (MBH) with either control AVV (n = 7) (○) or BCKDK AAV (n = 6) (●). L: Proposed mechanism for the regulation of liver glucose production by central leucine sensing. BBB, blood brain barrier; FAO, fatty acid oxidation. All values are means ± SEM. *P < 0.05 vs. vehicle except for K, where P < 0.05 vs. control (GFP) animals.
Central infusions and pancreatic clamp procedure in mice.
The studies lasted a total of 90 min and were carried out in animals fasted for 6 h. The experiments consisted of intracerebroventricular infusions combined with pancreatic insulin clamps (Fig. 4E). Groups of animals received central infusions (1 µL/h) as follows: vehicle (artificial cerebrospinal fluid) or 30 pmol KIC. During the infusions, a basal insulin pancreatic clamp was performed as previously described (25,26).
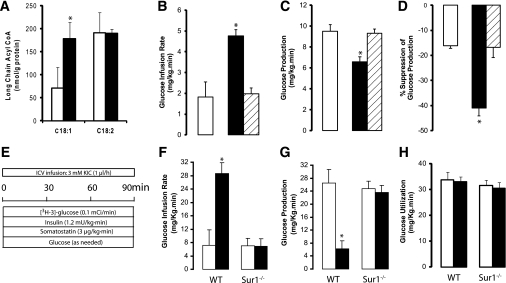
FIG. 4.
Central administration of KIC increases hypothalamic oleyl-CoA levels and lowers plasma glucose through a KATP pathway–dependent inhibition of liver glucose fluxes. A: Effect of central administration (MBH) of vehicle (n = 6) (□) or KIC (n = 6) (■) on hypothalamic long-chain acyl-CoA levels. B–D: Effect of central (MBH) vehicle (□), KIC (■), and KIC plus glibenclamide (▨) on whole-body glucose metabolism during pancreatic insulin clamps in rats. E: Experimental protocol of central infusions of either KIC or vehicle during pancreatic insulin clamps in mice. F–H: Effect of central vehicle (□) or KIC (■) on whole-body glucose metabolism during pancreatic insulin clamps in wild-type (WT) (n = 8) and Sur1-null (n = 13) mice. All values are means ± SEM. *P < 0.05 vs. vehicle.
Biochemical analyses and determination of amino acids in plasma.
Plasma metabolites and hormones were measured as previously described (25,26). Leucine, isoleucine, and valine were measured in samples of plasma (rats) as previously described (27,28) (Scientific Research Consortium, St. Paul, MN).
BCKDH kinase overexpression and assay.
A cDNA for full-length rat liver BCKDH kinase (BCKDK) was cloned into the pAAV-CMV-MCS plasmid (Stratagene, La Jolla, CA), and the construct, phospho–adeno-associated virus (pAAV)-cytomegalovirus (CMV)-BCKDK, designed to overexpress BCKDK under control of the CMV promoter, was used for the generation of the adeno-associated virus (AAV, serotype 2) vector AAV-BCKDK. The pAAV-BCKDK plasmid was transfected into human embryonic kidney (HEK) 293 cells, which were lysed 48 h later for BCKDK Western blot analysis and activity assays. Aliquots from either an AAV-BCKDK or a control protein (green fluorescent protein [GFP]) vector stock (8 × 1011 genome copies/mL; Applied Viromics, Freemont, CA) were used to transduce HEK 293 cells, which after 5–7 days were analyzed by Western blot. BCKDK activity assays were performed using a commercial kit (Globozymes, Carlsbad, CA). Briefly, aliquots of cell lysates were incubated in the presence of purified BCKDH (substrate) and [32P]-γ-ATP at 37°C for 10 min. Next, the extracts were precipitated with trichloroacetic acid and washed onto filter paper. The filters were dried, placed in scintillation fluid, and counted in a scintillation counter.
Malonyl-CoA decarboxylase overexpression and injection of AAV vector in the MBH of rats.
The AAV vector used for the overexpression of malonyl-CoA decarboxylase (MCD) has previously been described (29). Male Sprague-Dawley rats equipped with chronic bilateral cannulae in the MBH were injected with 2 µl per side of the indicated viral preparations. Seven days later, the animals received intravascular catheters, and upon recovery central infusions combined with euglycemic pancreatic insulin clamps were performed.
Meal challenge test.
Groups of rats overexpressing either a control protein (GFP) or BCKDK in the MBH were equipped with jugular catheters. The animals were fed a high-fat, high-protein diet for 7 days. After an 18-h fast, the animals were offered a small meal (8 g) of the same diet (Fig. 5J). Samples of blood were collected right before the meal and postprandially at regular intervals for glucose and insulin determinations.
Western blot analysis and quantification and S6 kinase and S6 ribosomal protein phosphorylation analysis.
Western blot analysis and quantifications were carried out using the Odyssey Infrared Imaging System (Li-Cor Biotechnology, Lincoln, Nebraska). Hypothalamic wedges were obtained from animals that received central infusions (MBH) of each of the following: 1) vehicle, 2) 1 µmol/L rapamycin (2 µL/side), 3) insulin (4 µU), 4) insulin plus rapamycin, 5) leucine (3 mmol/L), and 6) leucine plus rapamycin. The wedges were processed for Western blot analysis of phosphorylated S6 kinase (S6 K) and S6 ribosomal protein. Additional samples of liver and skeletal muscle from rats that received systemic infusions of either vehicle or leucine during pancreatic clamps were similarly analyzed for phosphorylated S6 K and S6 ribosomal protein.
α-KIC infusions into the paraventricular nucleus of the hypothalamus.
Chronic bilateral cannulae were implanted into the paraventricular nucleus of rats followed by vascular catheterization as previously described. Either vehicle or a 3 mmol/L KIC solution was infused into the paraventricular nucleus over a period of 6 h, and pancreatic clamps were performed.
Third cerebral ventricle infusions of KIC and KIC plus SHU9119 and statistical analyses.
A single cannula was implanted into the third cerebral ventricle of rats, and upon recovery the animals received vascular catheters. The animals were randomized into three groups to receive 6-h central infusions of the following: vehicle, 3 mmol/L KIC, or 3 mmol/L KIC plus 3 µg SHU9119. Pancreatic clamp studies were performed as described above. Statistical comparisons were assessed by unpaired Student t test or ANOVA.
RESULTS
Brain leucine and glucose homeostasis.
To selectively increase the central availability of leucine, we infused this amino acid into the MBH of conscious rats via bilateral cannulae (Fig. 1B). During the first 4 h of the study, we monitored the effects of the intrahypothalamic infusions on plasma glucose and insulin. The infusion of leucine into the MBH lowered plasma glucose and insulin levels (Fig. 1C and Supplementary Table 1, available in Supplementary Data). To investigate the mechanism by which intrahypothalamic leucine lowered glucose levels despite lower insulin levels, we performed a pancreatic basal insulin clamp study (Fig. 1B). During the clamps, we had to infuse more glucose to prevent hypoglycemia when leucine was infused into the MBH compared with vehicle (Fig. 1D). This central effect of leucine was due to a robust suppression of endogenous glucose production (Fig. 1E and F) accompanied by marked decreases in the in vivo rate of glycogenolysis and gluconeogenesis (Supplementary Fig. 1A and B). Conversely, the levels of glucoregulatory hormones and the rate of glucose utilization were unchanged (Supplementary Table 1 and Supplementary Fig. 1C). No significant change in the levels of plasma leucine was observed throughout the experiments (Supplementary Fig. 1D).
In eukaryotic cells, leucine activates a nutrient-sensing mechanism that controls cellular growth and differentiation (20,21,30) through the mTOR pathway (mTOR complex 1), which is inhibited by rapamycin (Fig. 1A). To test whether the central glucoregulatory effect of leucine requires activation of mTOR, we repeated the central infusion of leucine in rats preinfused and then coinfused with rapamycin into the MBH at a dose sufficient to inhibit the stimulatory effect of leucine or insulin on the S6 kinase pathway (Supplementary Fig. 2). Coinfusion of rapamycin failed to alter the effects of central leucine on plasma glucose (Fig. 1C), glucose infusion rate, glucose production (Fig. 1 D–F), and liver glycogenolysis and gluconeogenesis (Supplementary Fig. 1A and B), indicating that the glucoregulatory action of leucine was mTOR independent.
Leucine metabolism to KIC and glucose homeostasis.
The first step in the oxidative metabolism of leucine is its transamination to KIC catalyzed by BCAT (Fig. 1A) (22,23). Amino-oxyacetic acid is a potent BCAT inhibitor (31). To test whether the transamination of leucine to KIC is required for modulation of glucose homeostasis, we examined the effect of intrahypothalamic leucine in the presence of BCAT inhibition. Coinfusion of BCAT inhibitor into the MBH markedly blunted the effect of leucine on plasma glucose (Fig. 1C) and on hepatic glucose metabolism (Fig. 1D–F). Compared with findings in vehicle controls, the central administration of BCAT inhibitor alone did not significantly modify glucose infusion rate (2.3 ± 0.88 vs. 2.4 ± 0.80 mg/kg/min, respectively), glucose utilization (11 ± 0.5 vs. 11 ± 1.4 mg/kg/min), or the ability of insulin to inhibit endogenous glucose production (23 ± 8 vs. 21 ± 8%). Thus, the metabolism of leucine to KIC within the MBH is required for its inhibitory effect on glucose production.
We reasoned that since interfering with the conversion of leucine to KIC obliterated its central effects, a primary increase in the central availability of KIC might be sufficient to regulate glucose homeostasis. The infusion of KIC into the third ventricle of rats lowered plasma glucose levels (Fig. 1G), and during pancreatic basal insulin clamps glucose had to be infused to prevent hypoglycemia (Fig. 1H). This effect of KIC was due to suppression of hepatic glucose production (Fig. 1I and J) and liver glycogenolysis and gluconeogenesis (Supplementary Fig. 3A and B) rather than to increased glucose utilization (Supplementary Fig. 3C).
For localization of its central glucoregulatory effect, KIC was directly infused bilaterally within the MBH. KIC lowered plasma glucose levels (Fig. 1G) and increased the rate of glucose infusion required to maintain euglycemia during pancreatic clamps (Fig. 1H). The latter was due to a robust suppression of liver glucose production (Fig. 1I and J), while glucose utilization was not significantly affected (Supplementary Fig. 3C). Of note, a similar infusion of KIC within the paraventricular nuclei failed to inhibit glucose production (Supplementary Fig. 4A). Thus, the central effects of leucine on glucose metabolism can be entirely replicated by a primary increase of KIC in the MBH. To examine the specificity of BCAT inhibition in blocking the action of leucine, we coinfused KIC with the BCAT inhibitor in the MBH. BCAT inhibition failed to alter the effects of KIC on liver glucose metabolism (Supplementary Fig. 4B).
KIC metabolism to acetyl-CoA and glucose homeostasis.
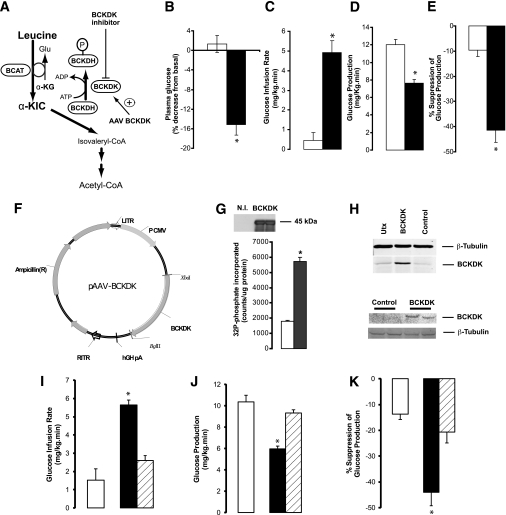
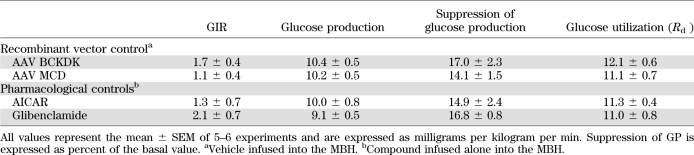
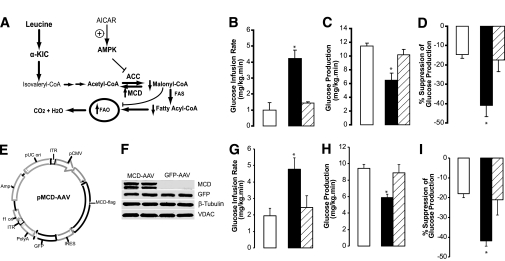
Leucine-derived KIC is further metabolized to acetyl-CoA by the consecutive action of five enzymes (22,23). The rate-limiting step is the oxidative decarboxylation of KIC, catalyzed by BCKDH. This enzyme is inhibited through phosphorylation by BCKDK (Fig. 2A). We reasoned that if the metabolism of KIC to acetyl-CoA is a key signal generated by the central administration of leucine or KIC, then 1) the direct stimulation of KIC flux through BCKDH should recapitulate while 2) the inhibition of KIC flux through BCKDH should blunt the effects of leucine. BCKDH is activated by α-CIC, a potent and specific inhibitor of BCKDK (32). As expected, the infusion of α-CIC into the MBH lowered circulating glucose levels (Fig. 2B) and, during pancreatic clamps, increased the rate of glucose infusion required to maintain euglycemia (Fig. 2C). This effect was due to suppression of liver glucose production (Fig. 2D and E) and liver glycogenolysis and gluconeogenesis (Supplementary Fig. 5A and B) rather than to increased glucose utilization (Supplementary Fig. 5C). Next, to selectively inhibit KIC flux through BCKDH, we overexpressed BCKDK in the MBH. We constructed the pAAV-BCKDK plasmid, which drives the expression of BCKDK under the control of the CMV promoter (Fig. 2F). Transfection of this plasmid into HEK 293 cells resulted in efficient expression of functional BCKDK (Fig. 2G and H). An AAV BCKDK vector prepared from this plasmid was first tested in HEK 293 cells. Five days after transduction, the cells overexpressed BCKDK (Fig. 2H). AAV BCKDK vectors were injected bilaterally into the MBH of 10- to 12-week-old rats. Ten days later, the expression of BCKDK was demonstrated by Western blot of MBH extracts (Fig. 2H). Importantly, while overexpression of BCKDK in the MBH did not alter glucose metabolism in animals receiving central vehicle (Table 1), it markedly blunted the effect of central leucine on hepatic glucose metabolism (Fig. 2I–K) without modifying glucose utilization (Supplementary Fig. 5D). Taken together, these results clearly indicate that the metabolism of KIC to acetyl-CoA within the MBH is required for the inhibitory effect of leucine on endogenous glucose production.
FIG. 2.
Central metabolism of leucine/KIC is required for the inhibition of glucose production during physiological hyperleucinemia. A: KIC is metabolized to acetyl-CoA by the BCKDH complex, which is inhibited through phosphorylation by BCKDK. BCKDH is indirectly activated by α-CIC. BCKDH activity is inhibited by overexpression of BCKDK. B–E: Effect of central infusion (MBH) of vehicle (n = 6) (□) or α-CIC (n = 5) (■) on basal plasma glucose (B) and whole-body glucose metabolism during pancreatic insulin clamps (C–E). F: Schematic of vector used for the overexpression of BCKDK. LITR, left AAV2 inverted terminal repeat sequence; RITR, right AAV2 inverted terminal repeat sequence; hGH, human growth hormone polyA signal; P CMV, cytomegalovirus promoter. G: Western blot analysis (top panel; N.I., control (no insert) plasmid.) and activity assay (bottom panel; □, control plasmid; ■, BCKDK plasmid.) of overexpression (plasmid) of BCKDK in HEK 293 cells. H: Western blot analysis of AAV-driven overexpression of BCKDK in HEK 293 cells (top panel) or MBH of rats (bottom panel). I–K: Effect of the central infusion (MBH) of leucine in rats overexpressing either a control protein (GFP, n = 4) (■) or BCKDK (n = 4) (▨) in the MBH and vehicle-only (n = 6) (□) animals. All values are means ± SEM. *P < 0.05 vs. vehicle.
TABLE 1.
Glucose kinetics parameters during euglycemic pancreatic clamps in rats: recombinant viruses and pharmacological modulators control groups
KIC metabolism to malonyl-CoA and glucose homeostasis.
Acetyl-CoA moieties derived from the metabolism of KIC (Fig. 3A) can be further metabolized to malonyl-CoA through the action of ACC, which is in turn inhibited by AMP-activated protein kinase (AMPK). To test whether the conversion of KIC to malonyl-CoA was required for the effects of intrahypothalamic KIC, we coinfused the AMPK activator 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) (Fig. 3A) with KIC. The coinfusion of AICAR blunted the effects of KIC on glucose metabolism and hepatic glucose production (Fig. 3B–D and Supplementary Fig. 6A), supporting the idea that formation of malonyl-CoA in the MBH is necessary for the glucoregulatory action of KIC. AICAR alone did not alter glucose fluxes under similar experimental conditions (Table 1). Malonyl-CoA is converted back to acetyl-CoA through the action of MCD (Fig. 3A); thus, an alternative approach to test the role of malonyl-CoA was to enhance the activity of MCD in the MBH (29). We injected AAV MCD (Fig. 3E) into the MBH of rats, and overexpression of MCD was demonstrated by Western blot analysis (Fig. 3F). Importantly, overexpression of MCD in the MBH blunted the effect of central leucine on glucose homeostasis and on hepatic glucose metabolism (Fig. 3G–I and Supplementary Fig. 6B). No significant effect of MCD overexpression per se was observed (29) (Table 1). These results showed that the metabolism of KIC → acetyl-CoA → malonyl-CoA within the MBH is required for the inhibitory effect of leucine on liver glucose production. To further validate the specificity of these effects, we asked whether the activation of the central melanocortin pathway was required for the action of leucine. The pharmacological antagonism of central melanocortin receptors by SHU9119 failed to alter the effects of central leucine/KIC on glucose production (Supplementary Fig. 4C). To directly test the hypothesis that leucine/KIC-derived hypothalamic malonyl-CoA leads to an increase in the levels of oleyl-CoA (Fig. 3A), we measured the levels of long-chain acyl-CoAs in the MBH. Animals that received central infusions of leucine or KIC displayed a significant increase in the levels of oleyl-CoA (C18:1), while other long-chain acyl-CoAs (e.g., linoeyl-CoA, C18:2) did not change compared with vehicle-infused animals (Fig. 4A).
FIG. 3.
Hypothalamic metabolism of leucine or KIC to acetyl-CoA and malonyl-CoA is required for the glucoregulatory action of leucine. A: Metabolism of leucine. B–D: Effect of the central (MBH) infusion of vehicle (□), KIC (■), or KIC plus AICAR (▨) on whole-body glucose metabolism during pancreatic insulin clamps. E: pAAV-MCD vector used for the overexpression of MCD in the hypothalamus. ITR, inverted terminal repeat sequence; pUC ori, pUC vector origin of replication; IRES, internal ribosome entry site; Amp, ampicillin resistance gene; ori, origin of replication. F: Western blot analysis of hypothalamic wedges from rats injected into the MBH with either control (GFP) (AAV GFP) or AAV MCD. G–I: Effect of intrahypothalamic infusion of vehicle (□) or leucine in animals overexpressing a control protein (GFP) (■) in the MBH or of intrahypothalamic leucine in animals overexpressing MCD (▨) in the MBH on whole-body glucose metabolism during pancreatic insulin clamps. All values are means ± SEM. n = 4–6. *P < 0.05 vs. vehicle. VDAC, voltage-dependent anion channel; FAS, fatty acid synthase; FAO, fatty acid oxidation.
Amino acid sensing and ATP-sensitive K+ channels.
The activation of hypothalamic ATP-sensitive K+ channels (KATP channels) is critical for modulation of blood glucose levels and liver glucose metabolism (26) and for the metabolic effects of other nutrient-dependent signals (15,24,25). To examine the role of central KATP channels in leucine sensing, we studied the effects of the central infusion of glibenclamide on the modulation of glucose metabolism by KIC. In the presence of the KATP channel blocker glibenclamide, the infusion of KIC into the MBH failed to modulate the metabolism and production of glucose (Fig. 4B–D and Supplementary Fig. 6C). Glibenclamide alone did not produce significant changes (Table 1). Furthermore, as shown in Fig. 4E–H central administration of KIC also failed to modulate liver glucose metabolism in mice lacking functional KATP channels in the brain (Sur1−/− mice) (33). Taken together, these findings indicate that the hypothalamic sensing of leucine regulates liver glucose fluxes through a mechanism that requires functional KATP channels.
Circulating leucine and glucose homeostasis.
To examine the physiological relevance of our observations, we performed systemic infusions of leucine to generate increases in its plasma concentration similar to those observed after consumption of diets enriched in protein as well as more moderate increases (34–36) (Fig. 5A). Increasing doses of systemic leucine produced dose-dependent elevations in plasma leucine (Fig. 5B) accompanied by gradual decreases in plasma isoleucine and valine (Supplementary Fig. 7A and B). The latter is a known effect of leucine (36). In the presence of basal insulin and vehicle in the MBH, systemic leucine markedly inhibited glucose production in a dose-independent manner (Fig. 5C–E) without modifying glucose utilization (Supplementary Fig. 7C). More importantly, the infusion of a BCAT inhibitor into the MBH blunted the effect of systemic leucine (Fig. 5F) on glucose production (Fig. 5G–I) without changes in glucose utilization (Supplementary Fig. 7D). Interestingly, the increase of circulating leucine activated the mTOR pathway in skeletal muscle (Supplementary Fig. 7E) and liver (Supplementary Fig. 7F). Taken together, these results show that physiological increases of circulating leucine are sufficient to markedly inhibit hepatic glucose production through a mechanism keenly dependent on the metabolism of leucine within the MBH. We next examined the consequences of attenuating the hypothalamic leucine-sensing mechanism on glycemic control. We fed a lard-enriched (10%) diet containing 45% protein (high protein) during 7 days to both control rats and rats with an acquired defect in the metabolism of leucine in the MBH (Fig. 5J). This defect was induced by AAV-driven overexpression of BCKDK in the MBH, which impairs leucine sensing (Fig. 2F–H). Rats overexpressing BCKDK developed not only higher fasting plasma glucose and insulin but also larger plasma glucose and insulin excursions in response to the meal load compared with control animals (Fig. 5K). These results indicate that hypothalamic leucine sensing is required to maintain proper glycemic control.
DISCUSSION
The arcuate nucleus of the hypothalamus has emerged as a critical site for the integration of multiple nutritional cues designed to inform the brain of the nutritional status of the body (37). Here, we have identified a novel circuit coupling the metabolism of leucine in the MBH with the regulation of glucose homeostasis in the liver (Fig. 5L). We postulate that in the postabsortive state, the hypothalamic sensing of leucine plays a role in the acute regulation of glucose production in response to elevations of circulating levels of leucine. The stimulation of this central pathway is sufficient to lower plasma glucose levels through inhibition of liver glucose output through a mechanism keenly dependent on the metabolism of leucine to acetyl-CoA and malonyl-CoA in the MBH. In contrast with recent studies in rats showing that activation of the mTOR pathway is required for the suppressive effect of centrally administered leucine on food intake (6), the effect of leucine on liver glucose production does not require activation of mTOR. These differential mechanisms of leucine sensing potentially allow for selective and independent regulation of food intake and glucose production. The central metabolism of leucine or KIC led to an increase in oleyl-CoA in the MBH, which has been postulated to activate hypothalamic KATP channels generating the signal(s) that reach the liver via vagal outflow (14,25). In fact, the actions of leucine described here also required functional KATP channels.
Although systemic infusions of leucine, designed to increase circulating leucine to levels similar to those achieved after the consumption of a protein-rich meal (34,35), were sufficient to inhibit liver glucose output, more limited elevations were also effective, supporting the physiological relevance of central leucine sensing. Furthermore, the glucoregulatory effect of systemic leucine is strictly dependent on its metabolism within the MBH, indicating a central mechanism of action. In other studies, leucine-dependent activation of the mTOR pathways in skeletal muscle has been shown to induce insulin resistance as a result of decreased glucose uptake (38). Of interest, in our studies high circulating leucine inhibited liver glucose production despite activation of the mTOR–S6 K pathway in liver and skeletal muscle. Now, it could be argued that the decrease in circulating isoleucine and valine associated with hyperleucinemia (36) may have contributed to the glucoregulatory mechanism. However, this possibility is unlikely because 1) central leucine alone, which does not change the circulating levels of valine or isoleucine, inhibits glucose production to the same extent as systemic leucine and 2) inhibition of leucine metabolism in the MBH markedly blunted the effect of systemic leucine even in the presence of lower circulating valine and isoleucine. Findings of recent studies in mice unable to metabolize leucine owing to a global deletion of BCAT further support the central leucine-sensing mechanism described here. These BCAT-deficient animals display a lean phenotype with high circulating leucine, enhanced insulin sensitivity, and resistance to diet-induced obesity (39). Interestingly, in these mice only the brain was still capable of metabolizing leucine owing to the presence of an alternative isoform of BCAT unaffected by the targeted disruption. Furthermore, studies of human subjects receiving systemic infusions of leucine have also shown a decrease in glucose production (40,41), although the magnitude of the effect was smaller than in our rodent studies. In contrast to our findings in these human studies, a decrease in glucose utilization was also observed. The reasons for these discrepancies are not immediately clear. Moreover, our findings on the central glucoregulatory effects of leucine are in keeping with recent reports showing that dietary leucine supplementation improved glucose metabolism in both nondiabetic and diabetic mice (42,43). Conceivably, hypothalamic leucine sensing is one of the mechanisms by which high-protein low-fat diets improve insulin action in humans (44–46). Of interest, recent reports in humans (47,48) linked high levels of circulating leucine to the development of insulin resistance and diabetes. However, it is unclear whether the elevated leucine levels are a cause or a consequence of insulin resistance. Our observation that attenuating the hypothalamic sensing of leucine in rats led to hyperglycemia emphasizes the pathophysiological consequences of the faltering of this mechanism. Finally, it is conceivable that neurons of the arcuate nucleus, which are known to play a key role in the regulation of energy balance and glucose metabolism, could be involved in the leucine-sensing mechanism described here. In this regard, the inability of SHU9119 to block the central glucoregulatory action of leucine as well as recent work from several groups (16,49) on the central regulation of glucose metabolism by insulin suggests that agouti-related peptide neurons could be the sensors. Future studies will be required to delineate the cellular anatomy of hypothalamic leucine sensing.
The maintenance of glucose homeostasis is a vital requirement for mammalian survival and a critical priority for the central nervous system. The gathering of accurate biological information is the basic underpinning of any closed-loop homeostatic system. Consistent with a homeostatic loop, the central effects of leucine described herein appear to oppose its peripheral actions on insulin signaling and on gluconeogenesis (2,38,50). In summary, the novel biochemical mechanism for central leucine sensing reported here may be helpful in the development of nutritional and pharmacological interventions for the treatment of diabetes.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health to R.G.-J. (DK45024) and to the Diabetes Research and Training Center (DK20541).
No potential conflicts of interest relevant to this article were reported.
Y.S. researched data and reviewed the manuscript. T.K.T.L. researched data and reviewed the manuscript. W.H. researched data and reviewed the manuscript. A.P. researched data and reviewed the manuscript. J.B. researched data and reviewed the manuscript. L.A.-B. researched data and reviewed the manuscript. R.G.-J. designed experiments, researched data, wrote and edited the manuscript, and is the guarantor of the article.
The authors thank the following individuals at the Albert Einstein College of Medicine for their expert technical assistance in these studies: Bing Liu (animal surgery), Stanislaw Gaweda (biochemical analyses), Clive Baveghems (biochemical analyses), Hong Zhang (assistance with animal studies), Cyndra Liu (animal surgery), and Isabel Arrieta-Cruz (advice and assistance with stereotaxic surgery and injections).
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0857/-/DC1.
T.K.T.L. is currently affiliated with the Departments of Physiology and Medicine, University Health Network and University of Toronto, Toronto, Canada. W.H. is currently affiliated with the New York Blood Center, Lindsley F. Kimball Research Institute, New York, New York. A.P. is currently affiliated with Merck Research Laboratories, Rahway, New Jersey.
REFERENCES
- 1.Rossetti L, Rothman DL, DeFronzo RA, Shulman GI. Effect of dietary protein on in vivo insulin action and liver glycogen repletion. Am J Physiol 1989;257:E212–E219 [DOI] [PubMed] [Google Scholar]
- 2.Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR. Bidirectional modulation of insulin action by amino acids. J Clin Invest 1998;101:1519–1529 10.1172/JCI1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nuttall FQ, Gannon MC, Saeed A, Jordan K, Hoover H. The metabolic response of subjects with type 2 diabetes to a high-protein, weight-maintenance diet. J Clin Endocrinol Metab 2003;88:3577–3583 10.1210/jc.2003-030419 [DOI] [PubMed] [Google Scholar]
- 4.Gannon MC, Nuttall FQ, Saeed A, Jordan K, Hoover H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am J Clin Nutr 2003;78:734–741 [DOI] [PubMed] [Google Scholar]
- 5.Scharrer E, Baile CA, Mayer J. Effect of amino acids and protein on foot intake of hyperphagic and recovered aphagic rats. Am J Physiol 1970;218:400–404 [DOI] [PubMed] [Google Scholar]
- 6.Cota D, Proulx K, Smith KA, et al. Hypothalamic mTOR signaling regulates food intake. Science 2006;312:927–930 10.1126/science.1124147 [DOI] [PubMed] [Google Scholar]
- 7.Blouet C, Jo YH, Li X, Schwartz GJ. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci 2009;29:8302–8311 10.1523/JNEUROSCI.1668-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tremblay F, Lavigne C, Jacques H, Marette A. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu Rev Nutr 2007;27:293–310 10.1146/annurev.nutr.25.050304.092545 [DOI] [PubMed] [Google Scholar]
- 9.Floyd JC, Jr, Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest 1966;45:1487–1502 10.1172/JCI105456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohneda A, Parada E, Eisentraut AM, Unger RH. Characterization of response of circulating glucagon to intraduodenal and intravenous administration of amino acids. J Clin Invest 1968;47:2305–2322 10.1172/JCI105916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roden M, Perseghin G, Petersen KF, et al. The roles of insulin and glucagon in the regulation of hepatic glycogen synthesis and turnover in humans. J Clin Invest 1996;97:642–648 10.1172/JCI118460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felig P. Amino acid metabolism in man. Annu Rev Biochem 1975;44:933–955 10.1146/annurev.bi.44.070175.004441 [DOI] [PubMed] [Google Scholar]
- 13.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes 2002;51:271–275 10.2337/diabetes.51.2.271 [DOI] [PubMed] [Google Scholar]
- 14.Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab 2005;1:53–61 10.1016/j.cmet.2004.11.001 [DOI] [PubMed] [Google Scholar]
- 15.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 2002;8:1376–1382 10.1038/nm1202-798 [DOI] [PubMed] [Google Scholar]
- 16.Könner AC, Janoschek R, Plum L, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 2007;5:438–449 10.1016/j.cmet.2007.05.004 [DOI] [PubMed] [Google Scholar]
- 17.Smith QR. Transport of glutamate and other amino acids at the blood-brain barrier. J Nutr 2000;130(Suppl):1016S–1022S [DOI] [PubMed] [Google Scholar]
- 18.Choi YH, Fletcher PJ, Anderson GH. Extracellular amino acid profiles in the paraventricular nucleus of the rat hypothalamus are influenced by diet composition. Brain Res 2001;892:320–328 10.1016/S0006-8993(00)03267-4 [DOI] [PubMed] [Google Scholar]
- 19.Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Léopold P. A nutrient sensor mechanism controls Drosophila growth. Cell 2003;114:739–749 10.1016/S0092-8674(03)00713-X [DOI] [PubMed] [Google Scholar]
- 20.Cutler NS, Pan X, Heitman J, Cardenas ME. The TOR signal transduction cascade controls cellular differentiation in response to nutrients. Mol Biol Cell 2001;12:4103–4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nobukuni T, Joaquin M, Roccio M, et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci USA 2005;102:14238–14243 10.1073/pnas.0506925102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch CJ, Halle B, Fujii H, et al. Potential role of leucine metabolism in the leucine-signaling pathway involving mTOR. Am J Physiol Endocrinol Metab 2003;285:E854–E863 [DOI] [PubMed] [Google Scholar]
- 23.Suryawan A, Hawes JW, Harris RA, Shimomura Y, Jenkins AE, Hutson SM. A molecular model of human branched-chain amino acid metabolism. Am J Clin Nutr 1998;68:72–81 [DOI] [PubMed] [Google Scholar]
- 24.Lam TK, Gutierrez-Juarez R, Pocai A, Rossetti L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science 2005;309:943–947 10.1126/science.1112085 [DOI] [PubMed] [Google Scholar]
- 25.Lam TK, Pocai A, Gutierrez-Juarez R, et al. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med 2005;11:320–327 10.1038/nm1201 [DOI] [PubMed] [Google Scholar]
- 26.Pocai A, Lam TK, Gutierrez-Juarez R, et al. Hypothalamic K(ATP) channels control hepatic glucose production. Nature 2005;434:1026–1031 10.1038/nature03439 [DOI] [PubMed] [Google Scholar]
- 27.Moore S, Stein WH. Procedures for the chromatographic determination of amino acids on four per cent cross-linked sulfonated polystyrene resins. J Biol Chem 1954;211:893–906 [PubMed] [Google Scholar]
- 28.Stein WH. A chromatographic investigation of the amino acid constituents of normal urine. J Biol Chem 1953;201:45–58 [PubMed] [Google Scholar]
- 29.He W, Lam TK, Obici S, Rossetti L. Molecular disruption of hypothalamic nutrient sensing induces obesity. Nat Neurosci 2006;9:227–233 10.1038/nn1626 [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Fang Y. A novel pathway regulating the mammalian target of rapamycin (mTOR) signaling. Biochem Pharmacol 2002;64:1071–1077 10.1016/S0006-2952(02)01263-7 [DOI] [PubMed] [Google Scholar]
- 31.Gao Z, Young RA, Li G, et al. Distinguishing features of leucine and alpha-ketoisocaproate sensing in pancreatic beta-cells. Endocrinology 2003;144:1949–1957 10.1210/en.2002-0072 [DOI] [PubMed] [Google Scholar]
- 32.Harris RA, Paxton R, DePaoli-Roach AA. Inhibition of branched chain alpha-ketoacid dehydrogenase kinase activity by alpha-chloroisocaproate. J Biol Chem 1982;257:13915–13918 [PubMed] [Google Scholar]
- 33.Seghers V, Nakazaki M, DeMayo F, Aguilar-Bryan L, Bryan J. Sur1 knockout mice. A model for K(ATP) channel-independent regulation of insulin secretion. J Biol Chem 2000;275:9270–9277 10.1074/jbc.275.13.9270 [DOI] [PubMed] [Google Scholar]
- 34.Colombo JP, Cervantes H, Kokorovic M, Pfister U, Perritaz R. Effect of different protein diets on the distribution of amino acids in plasma, liver and brain in the rat. Ann Nutr Metab 1992;36:23–33 10.1159/000177695 [DOI] [PubMed] [Google Scholar]
- 35.Peters JC, Harper AE. Adaptation of rats to diets containing different levels of protein: effects on food intake, plasma and brain amino acid concentrations and brain neurotransmitter metabolism. J Nutr 1985;115:382–398 [DOI] [PubMed] [Google Scholar]
- 36.Block KP, Harper AE. High levels of dietary amino and branched-chain alpha-keto acids alter plasma and brain amino acid concentrations in rats. J Nutr 1991;121:663–671 [DOI] [PubMed] [Google Scholar]
- 37.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 2000;404:661–671 [DOI] [PubMed] [Google Scholar]
- 38.Tremblay F, Krebs M, Dombrowski L, et al. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes 2005;54:2674–2684 10.2337/diabetes.54.9.2674 [DOI] [PubMed] [Google Scholar]
- 39.She P, Reid TM, Bronson SK, et al. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab 2007;6:181–194 10.1016/j.cmet.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherwin RS. Effect of starvation on the turnover and metabolic response to leucine. J Clin Invest 1978;61:1471–1481 10.1172/JCI109067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nair KS, Matthews DE, Welle SL, Braiman T. Effect of leucine on amino acid and glucose metabolism in humans. Metabolism 1992;41:643–648 10.1016/0026-0495(92)90057-H [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 2007;56:1647–1654 10.2337/db07-0123 [DOI] [PubMed] [Google Scholar]
- 43.Guo K, Yu YH, Hou J, Zhang Y. Chronic leucine supplementation improves glycemic control in etiologically distinct mouse models of obesity and diabetes mellitus. Nutr Metab (Lond) 2010;7:57. 10.1186/1743-7075-7-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McAuley KA, Hopkins CM, Smith KJ, et al. Comparison of high-fat and high-protein diets with a high-carbohydrate diet in insulin-resistant obese women. Diabetologia 2005;48:8–16 10.1007/s00125-004-1603-4 [DOI] [PubMed] [Google Scholar]
- 45.Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med 2005;142:403–411 [DOI] [PubMed] [Google Scholar]
- 46.McAuley KA, Smith KJ, Taylor RW, McLay RT, Williams SM, Mann JI. Long-term effects of popular dietary approaches on weight loss and features of insulin resistance. Int J Obes (Lond) 2006;30:342–349 [DOI] [PubMed] [Google Scholar]
- 47.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–326 10.1016/j.cmet.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O'Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–453 [DOI] [PMC free article] [PubMed]
- 49.Lin HV, Plum L, Ono H, et al. Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in agouti-related protein and POMC neurons. Diabetes 2010;59:337–346 10.2337/db09-1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Um SH, Frigerio F, Watanabe M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 2004;431:200–205 10.1038/nature02866 [DOI] [PubMed] [Google Scholar]