Abstract
Diabetic nephropathy (DN) is the major life-threatening complication of diabetes. Abnormal permselectivity of glomerular basement membrane (GBM) plays an important role in DN pathogenesis. Heparanase is the predominant enzyme that degrades heparan sulfate (HS), the main polysaccharide of the GBM. Loss of GBM HS in diabetic kidney was associated with increased glomerular expression of heparanase; however, the causal involvement of heparanase in the pathogenesis of DN has not been demonstrated. We report for the first time the essential involvement of heparanase in DN. With the use of Hpse-KO mice, we found that deletion of the heparanase gene protects diabetic mice from DN. Furthermore, by investigating the molecular mechanism underlying induction of the enzyme in DN, we found that transcription factor early growth response 1 (Egr1) is responsible for activation of heparanase promoter under diabetic conditions. The specific heparanase inhibitor SST0001 markedly decreased the extent of albuminuria and renal damage in mouse models of DN. Our results collectively underscore the crucial role of heparanase in the pathogenesis of DN and its potential as a highly relevant target for therapeutic interventions in patients with DN.
The kidneys represent primary targets of diabetes, and diabetic nephropathy (DN) is the leading cause of end-stage renal disease in the western world (1–3). DN is characterized by glomerular hyperfiltration, increased renal albumin permeability, and cellular and extracellular changes in the glomerular and tubulointerstitial compartments, collectively resulting in progression of proteinuria and renal failure. The puzzle of mechanisms underlying DN pathogenesis involves complex interplay between hemodynamic and metabolic factors (i.e., systemic and intraglomerular pressure, activation of vasoactive hormone pathways, and induction of inflammatory and prosclerotic cytokines) and is still far from being fully understood. Several studies suggested involvement of heparanase in DN (4,5). Heparanase is the only known mammalian endoglycosidase that cleaves heparan sulfate (HS) (6–8), the principle polysaccharide associated with the cell surface and extracellular matrix (ECM) of a wide range of tissues (9). HS binds to and assembles structural basement membrane (BM) proteins, thus contributing to BM integrity and barrier function. In addition, HS moieties in the ECM sequester heparin-binding growth factors, cytokines, and chemokines, thereby controlling their accessibility, function, and mode of action (6,9). Enzymatic degradation of HS by heparanase leads to disassembly of ECM barriers and release of HS-bound bioactive molecules (10,11), and is therefore involved in fundamental biological phenomena associated with tissue remodeling, including morphogenesis, inflammation, angiogenesis, and cancer (6,7,12). The findings linking heparanase to DN and other proteinuric disorders include elevated levels of heparanase in the kidneys and urine of patients with DN (13,14), induction of glomerular heparanase expression in murine models of streptozotocin (STZ)-induced diabetes (14), and passive Heymann nephritis (15), as well as in vitro studies demonstrating that hyperglycemic conditions enhance heparanase expression in rat and human glomerular epithelial cells (16). Induction of glomerular heparanase in the course of diabetes may interfere with kidney function primarily through degradation of HS in the glomerular basement membrane (GBM). Indeed, GBM, along with fenestrated glomerular endothelium and podocyte foot processes/slit diaphragms, serves as the key functional component of the kidney filtration barrier, whereas HS represents a chief polysaccharide constituent of the GBM (17,18), playing a key space-filling and molecular-sieving role in the GBM. Degradation and loss of HS in the GBM were tightly linked to the pathophysiology of DN (19), yet several recent reports challenged the importance of HS degradation in DN development (20,21), and causative involvement of heparanase in DN has not been demonstrated.
Our research was undertaken to elucidate the biological significance and regulation of heparanase in DN pathogenesis. With the use of a heparanase-null (Hpse-KO) mouse model (22), we demonstrated that unlike their wt littermates, Hpse-KO mice fail to develop albuminuria and renal damage in response to STZ-induced diabetes. By investigating the precise molecular mechanism underlying heparanase induction under hyperglycemic conditions, we revealed that early growth response 1 (Egr1) transcription factor critically regulates heparanase overexpression. To block excessive heparanase, brought about in hyperglycemic mice by Egr1, we used a specific heparanase inhibitor SST0001 (non-anticoagulant N-acetylated, glycol split heparin = 100NA,RO-H) (23,24). Administration of SST0001 resulted in a marked decrease in the extent of albuminuria and renal damage in diabetic mice. Taken together, our results validate the role of heparanase in the pathogenesis of DN, reveal the molecular mechanism underlying induction of the enzyme in diabetic kidney, and attest the enzyme as a promising therapeutic target in patients with DN.
RESEARCH DESIGN AND METHODS
Animals.
BALB/c, DBA-2, C57Bl/6 J (Harlan Laboratories; Jerusalem, Israel), and heparanase-null Hpse-KO mice (22) were kept under pathogen-free conditions; all experiments were performed in accordance with the Hebrew University Institutional Animal Care and Use Committee.
STZ-induced diabetes.
Mice were injected intraperitoneally with 40 mg/kg body weight STZ in 100 mmol/L citrate buffer (pH 4.6) for 5 consecutive days (after an overnight fast). Mice received 0.4 IU of insulin every other day when their blood glucose levels increased >350 mg/dL. Blood glucose levels were measured using the Ascensia ELITE Blood Glucose Meter (Bayer, Leverkusen, Germany). Subcutaneous injections of compound SST0001 (300 μg in 100 μL saline, twice per day) were given to mice in the experimental group. Mice in the control group were injected with saline alone. For urine collection, mice were placed in metabolic cages for 24 h. Urinary albumin was measured using an ELISA kit (Bethyl Laboratories Inc., Montgomery, TX). Creatinine was measured using VITROS system 5.1, FS Chemistry System (Johnson & Johnson, New Brunswick, NJ). Animals were killed on indicated time points, half of each kidney was snap-frozen for RNA preparation/protein extraction, and another half was processed for histology and immunostaining.
Renal histopathology.
Kidney tissue was immersion-fixed in 4% paraformaldehyde in PBS and routinely processed, and 2- to 4-μm sections were stained with Masson’s trichrome and periodic acid-Schiff. A semiquantitative score was used to evaluate the extent of glomerular mesangial expansion essentially as described by Qi et al. (25). Briefly, mesangial matrix expansion for each glomerulus was graded from 1 to 4 as follows: 1 represents no lesion, 2 represents sclerosis of >25% of the glomerulus, 3 represents sclerosis of >50% of the glomerulus, and 4 represents sclerosis of >75% of the glomerulus. A whole kidney sclerosis index was obtained by averaging scores from all glomeruli on one section. Four mice per experimental condition were analyzed, and >50 glomeruli were assessed per mouse. Immunohistochemistry of the paraffin-embedded and cryostat kidney sections was performed as previously described (26,27).
Antibodies.
The following antibodies were used: anti-F4/80 (Serotec, Inc., Raleigh, NC), anti-transforming growth factor (TGF)-β (sc 146), anti-Egr1 (sc 588, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-HS HS4C3 (27), and anti-heparanase 733 (28) and 01385–126 (provided by Dr. P. Kussie, ImClone Systems Inc., New York, NY) (29).
Cell culture.
Human embryonic kidney cells 293 (HEK-293) were cultured at 37°C in a 5% CO2 humidified incubator in complete Dulbecco’s modified Eagle's medium with 10% FCS. HK-160 primary human epithelial kidney cells (provided by Dr. A. Katz, Bikur Holim Hospital, Jerusalem, Israel) were cultured at 37°C in an 8% CO2 humidified incubator in complete Roswell Park Memorial Institute medium with 20% FCS. In some experiments, cells at 60–80% confluence were maintained for 24 h in serum-free medium and then incubated in the absence or presence of increasing concentrations of glucose, as indicated.
Reverse transcription and real-time PCR.
RNA isolation, reverse transcription, and real-time quantitative PCR were performed as previously described (26). Mouse RNA polymerase IIA and L-19, and human Gus-β primers were used as internal standards. The following primers were used:
mEgr1: Sense 5′-CCTTTTCTGACATCGCTCTGAA-3′,
Antisense:5′-CGAGTCGTTTGGCTGGGATA-3′.
mHpse: Sense 5′-GGAGCAAACTCCGAGTGTATC-3′,
Antisense 5′-CAGAATTTGACCGTTCAGTTGG-3′; and
Sense 5′-CAAGAAGGAATCAACCTTTGAAG-3′,
Antisense 5′-GTAGTCCAGGAGCAACTGAG-3′.
mPolRIIA: Sense 5′-GTCCTCTACTCATGCTGTCTTGG-3′,
Antisense 5′AAATGCCTGTATCCCAATCAAG-3′.
mL-19: Sense 5′-GAATGGCTCAACAGGTAAACA-3′,
Antisense: 5′-GGGTTCCAGAGTCAAGTTCAG-3′.
hEgr: Sense 5′-GAGCAGCCCTACGAGC-3′,
Antisense: 5′-AGCGGCCAGTATAGGT-3′.
hHpse: Sense 5′-GTTCTAATGCTCAGTTGCTCCT-3′,
Antisense: 5′-ACTGCGACCCATTGATGAAA-3′.
hGUS-β: Sense 5′-CACAAGAGTGGTGCTGAGGA-3′,
Antisense: 5′-GTATTGGATGGTCCCTGGTG-3′.
Immunoblotting.
Equal protein aliquots (20 μg) were subjected to SDS-PAGE (10% acrylamide) under reducing conditions. Proteins were transferred to a polyvinylidene difluoride membrane (Millipore Corporation, Billerica, MA) and probed with the anti-Egr1 rabbit polyclonal antibody sc-588 (Santa Cruz Biotechnology, Inc.) (1:200), followed by horseradish peroxidase-conjugated secondary antibody (KPL, Inc., Gaithersburg, MD) and a chemiluminescent substrate (iNtron Biotechnology, Kyunggi-do, Korea), as described (26). Membrane was stripped and incubated with anti−β-actin (1:1,000) or antilamin (1:1,000) antibodies to ensure equal protein load.
Heparanase activity assay.
For measurements of heparanase enzymatic activity, tissue lysates were incubated (16–36 h, 37°C, pH 6.2) on dishes coated with sulfate-labeled ECM and analyzed as described (8). Nearly intact HS proteoglycans are eluted just after the void volume (peak I, Kav < 0.2, fractions 1–10), and HS degradation fragments are eluted later with 0.5 < Kav < 0.8 (peak II, fractions 15–35) (8). These fragments were shown to be degradation products of HS because they were five- to sixfold smaller than intact HS side chains, resistant to further digestion with papain and chondroitinase ABC, and susceptible to deamination by nitrous acid.
Chromatin immunoprecipitation.
Cross-linking of proteins to DNA, chromatin isolation, and sonication was performed as described (30). Chromatin immunoprecipitation (ChIP) was performed with 5 μg of anti-Egr1 (sc H-250) or anti-LAMP1 antibodies (Santa Cruz Biotechnology, Inc.) preincubated with magnetic bead-conjugated rabbit IgG (Dynal Biotech ASA, Oslo, Norway) at 4°C overnight with rotation. Elution of immune complexes, reverse cross-linking, and DNA extraction were carried out as previously described (30). PCR analysis was performed using 5 μL of immunoprecipitated chromatin or input chromatin, using Titanium Taq PCR kit (Clontech Laboratories, Inc., Mountain View, CA). Amplifications (38 cycles) were performed using a specific primer set that covers functional Egr1 binding site in the heparanase promoter (31). The following primers were used:
Hpse promoter: Sense 5′-TTCGTAAGTGAACGTCACCG-3′,
Antisense: 5′-CTTCTGCATCCCTCCCACT-3′;
L-19: Sense 5′-ATGCCAACTCTCGTCAACAG-3′,
Antisense: 5′-GCGCTTTCGTGCTTCCTT-3′.
Statistical analysis.
Values are expressed as means ± SEM. Statistical analysis was performed using t test or Mann–Whitney test. A P value of <0.05 was considered significant.
RESULTS
Heparanase KO mice fail to develop DN in response to STZ-induced diabetes.
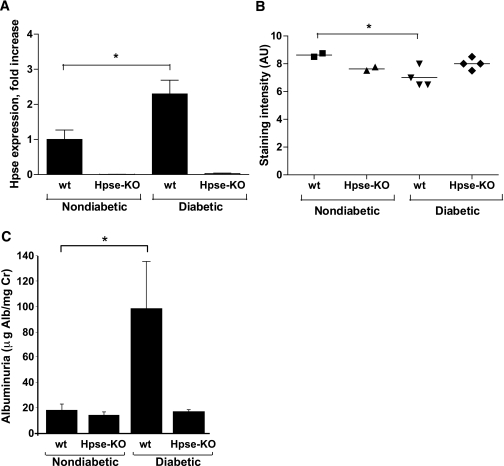
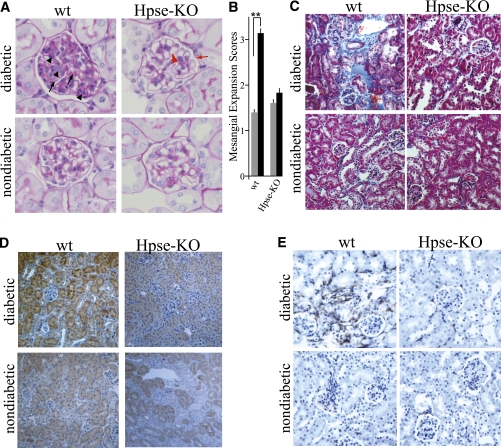
Although several findings link heparanase to DN (5), direct mechanistic demonstration of the role of the enzyme in the pathogenesis of this disease was not available. To provide decisive evidence that heparanase is causally involved in the pathogenesis of DN, we used the model of STZ-induced DN (32) in heparanase-null (Hpse-KO) mice and their wt littermates. The Hpse-KO mice are fertile and viable, and show normal gross appearance (22), including kidney size and renal histology. Blood glucose, serum creatinine, urea nitrogen (not shown), and urinary albumin excretion (Fig. 1C) measured in healthy Hpse-KO mice were not different from those in wt littermates. Hpse-KO and wt mice were made hyperglycemic by applying multiple low-dose STZ administrations (40 mg/kg/day for 5 days) to mitigate the nonspecific renal toxicity of STZ (32). By week 2 of the experiment, 100% of the wt and KO mice developed diabetes as revealed by increased blood glucose levels (>300 mg/dL). Blood glucose levels were maintained at < 350 mg/dL by treatment with insulin (0.4 units/mouse) administered every other day. Mice were kept diabetic for 16 weeks, and their urine samples were collected for analysis of albumin excretion. It should be noted that the body weight and blood glucose concentrations measured throughout the experiment (Table 1) and the amount of insulin required to maintain glycemic control did not differ between Hpse-KO and wt mice. After 16 weeks of the experiment, mice were killed, their kidneys were excised, and heparanase expression in the renal cortex was assessed by quantitative real-time PCR (qRT-PCR). A marked increase in heparanase mRNA was readily detected in the renal cortex of diabetic wt mice compared with nondiabetic wt mice (P = 0.0493, Fig. 1A). As expected, no heparanase mRNA was detected in the kidneys of diabetic or nondiabetic Hpse-KO mice (Fig. 1A). In agreement with the increased expression of heparanase, immunofluorescence staining with anti-HS antibody HS4C3 (27) revealed a statistically significant decrease in HS content along the GBM in the kidneys of diabetic versus nondiabetic wt mice, whereas no change in HS content was detected in the kidneys of diabetic Hpse-KO mice compared with their nondiabetic littermates (Fig. 1B and Supplementary Fig. 1). Examination of albumin excretion in urine samples revealed a fivefold increase in urine albumin excretion (quantified as micrograms of albumin/milligram of creatinine) in diabetic wt mice versus their nondiabetic controls, whereas no increase in urine albumin excretion was noted in diabetic Hpse-KO mice compared with nondiabetic Hpse-KO mice (Fig. 1C). The occurrence of albuminuria was paralleled by glomerular morphologic changes characteristic of DN (i.e., mesangial matrix expansion) in diabetic wt kidney tissue, exemplified by more than a twofold increase in glomerular expansion score, compared with normal glomerular architecture preserved in kidney tissue derived from diabetic Hpse-KO mice (Fig. 2A and B). Along with mesangial matrix expansion in glomeruli, tubulointerstitial injury is considered as a major feature of DN and an important predictor of renal dysfunction (33). During progression of DN, the renal tubule is exposed to glomerular effluent, which includes, in addition to glycation end products and glucose, large quantities of protein. Excessive protein load to the proximal tubule ultimately leads to peritubular inflammation and fibrosis (33). In agreement with this notion, massive interstitial fibrosis (as indicated by increased collagen deposition) was detected in the kidney of diabetic wt but not in diabetic Hpse-KO mice by Masson’s trichrome staining (Fig. 2C). TGF-β is implicated in cellular processes characteristic of DN, including glomerular hypertrophy with mesangial matrix expansion, renal tubular injury, and interstitial fibrosis (34–36). Immunohistochemical evaluation of TGF-β in kidney tissue specimens revealed a marked increase in TGF-β in diabetic wt mice versus their nondiabetic controls (Fig. 2D, left). No increase in TGF-β levels was noted in diabetic versus nondiabetic Hpse-KO mice (Fig. 2D, right). In agreement, twofold increase in TGF-β mRNA expression was detected by qRT-PCR in the kidney of diabetic versus nondiabetic wt mice, whereas no induction of TGF-β mRNA was noted in kidneys of diabetic Hpse-KO mice compared with their nondiabetic littermates (not shown). Macrophage accumulation is another characteristic feature of diabetic kidney disease associated with both experimental and human DN (37–39). Moreover, the intensity of the interstitial macrophage infiltrate is proportional to the rate of subsequent decline in renal function (39). This notion led us to examine the degree of macrophage infiltration in wt and Hpse-KO diabetic kidney. By applying immunostaining with antibody directed against F4/80 (mouse macrophage specific marker) (40), we detected increased macrophage infiltration in the diabetic kidney of wt versus Hpse-KO mice (Fig. 2E). Quantification of F4/80-positive macrophages per microscopic field, based on six sections from three independent mice of each group, revealed a sixfold increase in macrophage accumulation in the cortex of diabetic versus nondiabetic kidney in wt mice (95.3 ± 15.8 vs. 15.9 ± 10.5 macrophages/field, P = 0.001), whereas no statistically significant difference in macrophage infiltration was noted in the kidneys of diabetic versus nondiabetic Hpse-KO mice (63.9 ± 27.7 vs. 40.9 ± 10.3 macrophages/field, P > 0.2).
FIG. 1.
Changes in heparanase expression, HS content, and urinary albumin excretion in wt and Hpse-KO mice in response to STZ-induced diabetes. Kidney tissue samples were harvested from diabetic wt and Hpse-KO mice at 16 weeks after induction of diabetes and from their age-matched nondiabetic littermates. A: Heparanase expression in nondiabetic and diabetic kidney lysates was assessed by qRT-PCR (n = 5 per experimental condition). B: Semiquantitative analysis of the glomerular HS recognized by antibody HS4C3. Staining intensity of HS was scored by three independent observers on a scale between 0 and 10 in arbitrary units expressed as means ± SEM (two nondiabetic and four diabetic mice per genotype). C: Urinary albumin excretion; 24-h urine samples were collected from diabetic wt and Hpse-KO mice 16 weeks after induction of diabetes and from age-matched nondiabetic littermates. The experiment was repeated three times (n ≥ 5 per experimental condition). Albuminuria is expressed as microgram albumin/milligram creatinine. Urinary albumin and creatinine were determined as described in research design and methods. *P < 0.05. Alb, albumin; AU, arbitrary units; Cr, creatinine.
TABLE 1.
Average blood glucose levels and body weight of Hpse-KO and wt mice on week 16 of the experiment
FIG. 2.
Histopathologic changes in the kidneys of wt and Hpse-KO mice in response to STZ-induced diabetes. Kidney sections obtained from diabetic wt and Hpse-KO mice at 16 weeks after induction of diabetes and from age-matched nondiabetic littermates were processed for staining with periodic acid-Schiff (A) and Masson-Trichrome (C), and for immunohistochemistry with antibodies directed against TGF-β-1 (D) and F4/80 (E). Representative microphotographs are shown (n ≥ 4 mice per experimental condition). A and B: Glomerular mesangial expansion. A: Representative glomerular histopathology. Note mesangial matrix expansion (black arrow), focal hypercellularity (black arrowhead), and absence of visible clear vascular space in diabetic wt kidney (top, left) compared with diabetic Hpse-KO kidney (top, right) displaying normal-looking glomerular structure, clear Bowman’s capsule (red arrow), clear vascular space (red arrowhead), and normal tuft architecture, resembling that of nondiabetic kidney (bottom). Original magnification ×400. B: Glomerular mesangial expansion scores in diabetic (black bars) wt and Hpse-KO mice and age-matched nondiabetic littermates (gray bars). The scores were determined using light microscopy at ×400, as described in research design and methods. **P < 0.001. C: Representative Masson’s trichrome–stained kidney sections. Note higher degree of tubulointerstitial fibrosis (blue staining) in diabetic kidneys derived from wt versus Hpa-KO mice. D and E: Immunohistochemistry with antibodies directed against TGF-β-1 (D) and F4/80 (E) reveals induction of TGF-β-1 (D) and increased accumulation of macrophages (E) in diabetic wt kidney (top, left) compared with both diabetic Hpse-KO (top, right) and wt nondiabetic (bottom, left) kidney. (A high-quality digital representation of this figure is available in the online issue.)
Induction of heparanase in diabetic kidney is mediated by Egr-1 transcription factor.
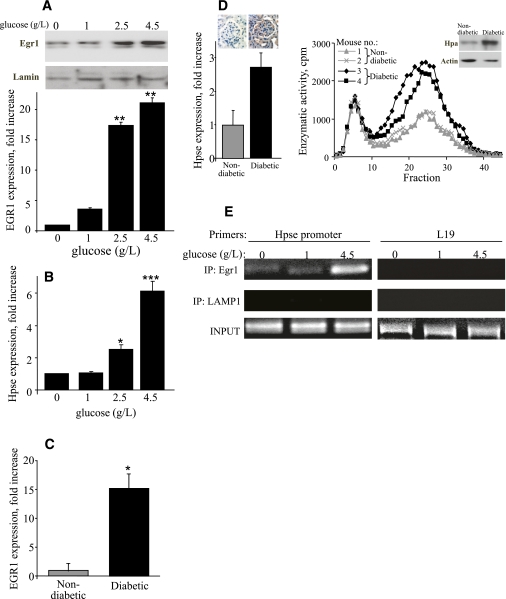
Causal involvement of heparanase in DN, along with the increased levels of heparanase observed in diabetic kidney tissue specimens (14) and in the urine and plasma of diabetic patients (13), prompted us to investigate how this enzyme is regulated under diabetic conditions. Highly relevant to the DN setting, heparanase expression is induced in renal epithelial cells by high glucose (16). However, the precise mechanism responsible for induction of heparanase by high glucose has not been fully elucidated. Among several factors controlling heparanase expression, the Egr1 transcription factor is implicated in inducible transcription of the heparanase gene in immune cells and many (but not all) cancer cell types (31,41,42). In light of the previously reported inducibility of Egr1 by glucose (43), it is logical to assume that when glomerular cells are exposed to high glucose levels (i.e., in the kidneys of diabetic patients), glucose-induced Egr1 may affect heparanase gene promoter activity, leading to overexpression of heparanase. To test this hypothesis, we first investigated the effect of glucose on Egr1 levels in kidney-derived cells. HEK-293 cells were exposed to increasing concentrations of glucose. Western blot analysis and qRT-PCR revealed a dose-dependent increase in Egr1 expression after exposure to glucose (Fig. 3A). Similar results were obtained with HK-160 human kidney epithelial cells (not shown). In agreement, a significant increase in Egr1 mRNA and protein levels was revealed in mouse kidneys of diabetic C57BL/6 mice 10 weeks after induction of diabetes by STZ, compared with healthy mice (Fig. 3C and not shown), corroborating the relevance of the in vitro findings. This increase in Egr1 levels was invariably associated with induction of heparanase expression both in vitro (Fig. 3B) and in the kidney tissue of diabetic mice, as demonstrated by real-time PCR, immunohistochemistry, immunoblotting, and enzymatic activity assay (Fig. 3D).
FIG. 3.
Egr1 mediates glucose-induced overexpression of heparanase in the kidney. A and B: Effects of glucose on Egr1 and heparanase expression in vitro. HEK-293 cells were incubated (1.5 h) in triplicates in the absence or presence of increasing concentrations of glucose (1, 2.5, and 4.5 g/L). A: Egr1 expression was assessed by immunoblotting of nuclear extracts (top) and qRT-PCR determination of mRNA levels (bottom). B: Heparanase mRNA expression was assessed by qRT-PCR. *P < 0.05, **P < 0.01, ***P < 0.001. C and D: Egr1 induction in the kidneys of diabetic mice correlates with overexpression of heparanase. C: Induction of Egr1 in the kidneys of diabetic mice. Kidney tissue was harvested from nondiabetic and diabetic mice 10 weeks after induction of diabetes by STZ, lysed, and analyzed for Egr1 expression by qRT-PCR determination of mRNA levels. Error bars represent ± SE (n = 4 mice for each group). *P < 0.05. D: Heparanase expression in nondiabetic and diabetic kidney lysates was assessed by qRT-PCR (left), immunostaining (left, inset), enzymatic activity assay (right), and immunoblotting (right, inset). E: Increased recruitment of Egr1 to the heparanase promoter in the presence of high glucose. After cross-linking of proteins to DNA, chromatin was isolated from HEK-293 cells incubated (1.5 h) in the presence of increasing concentrations of glucose (0, 1, and 4.5 g/L), sonicated into fragments of average length ≤ 500 base pairs and immunoprecipitated with antibodies against Egr1 (top), or an unrelated protein LAMP1 (middle), as described in research design and methods. The final DNA extractions were amplified using primer set that covers functional Egr1 binding site in the heparanase promoter (left), or primer set specific to unrelated L-19 gene sequence, used as control (right). Samples were equilibrated for DNA loading amounts using primers specific to the heparanase promoter and DNA that was PCR amplified from chromatin preparations before immunoprecipitation (bottom). The results are representative of three independent experiments. (A high-quality color representation of this figure is available in the online issue.)
These observations are in further support of our assumption that similar to immune and some cancer cells, in kidney tissue Egr1 acts as an activator of heparanase transcription. To further validate this hypothesis, we applied the ChIP approach to study the effect of elevated levels of glucose on occupancy of the heparanase promoter by Egr1 in HEK-293 cells. For this purpose, HEK-293 cells were incubated in the presence of increasing concentrations of glucose (0, 1, and 4.5 g/L). After chemical cross-linking, chromatin was isolated, sonicated to ∼500-base pair fragments, and immunoprecipitated with anti-Egr1 antibody. DNA obtained from the immunoprecipitated chromatin was amplified using heparanase promoter–specific primers, as well as a set of primers specific to the unrelated L-19 gene sequence, to normalize for equal chromatin amounts. As shown in Figure 3E (top), markedly increased occupancy of the heparanase gene promoter by Egr1 was detected in cells that were incubated with high glucose. No enrichment was observed when antibody directed against an irrelevant protein (LAMP1) was used for the ChIP analysis (Fig. 3E, middle).
Finally, to validate the ability of Egr1 to induce heparanase expression in our system, HEK-293 cells were cotransfected with plasmids encoding for luciferase driven by heparanase promoter (44) together with Egr1 expressing vector (pEgr1) or empty pcDNA3 vector and incubated in the absence or presence of 4.5 g/L glucose. Luciferase activity was measured in cell lysates 24 h post-transfection and normalized with β-galactosidase. A threefold increase in heparanase promoter activity was detected in cells (incubated in medium without glucose) that were cotransfected with the pEgr1, compared with cells cotransfected with empty vector (P < 0.05; Supplementary Fig. 2). Of note, both cotransfection with pEgr1 or incubation with high glucose levels activated heparanase promoter to a similar extent (Supplementary Fig. 2), in further support of the role of Egr1 in glucose-induced heparanase expression. These results collectively demonstrate that in the presence of elevated glucose levels, Egr1 directly binds to the heparanase promoter and stimulates its transcriptional activity.
Specific heparanase inhibitor decreases albuminuria in mouse models of DN.
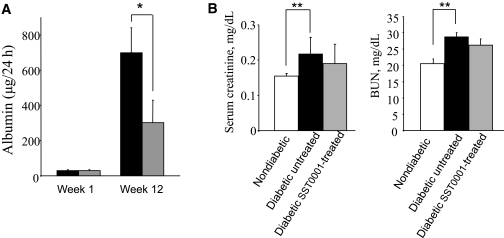
The above results led us to hypothesize that inhibition of excessive heparanase (induced in diabetic kidney via an Egr1-dependent mechanism) may prevent the progression of DN in a manner similar to that observed in Hpse-KO mice (Figs. 1 and 2). To validate this hypothesis, we investigated the effect of the specific heparanase inhibitor SST0001 on the development of proteinuria in diabetic mice. Compound SST0001 is 100% N-acetylated, 25% glycol-split heparin that effectively inhibits heparanase enzymatic activity in vitro and, unlike unmodified heparin, is devoid of anticoagulant and proangiogenic activities (23,24,45). The effectiveness of SST0001 in suppressing the biological activity of heparanase in vivo was demonstrated in models of heparanase-driven processes other than DN, such as inflammation, tumor growth, and metastatic spread (24,26,46). To test the effect of heparanase inhibition on DN, we used BALB/c mouse strain, known to be particularly prone to DN (25). Diabetes was induced by multiple low-dose STZ administrations (40 mg/kg/day for 5 days). Five days after the last STZ administration, diabetic mice (blood glucose levels > 300 mg/dL) were divided into two groups (n = 7) and treated with SST0001 (administered intraperitoneally twice per day, 300 μg per injection) or vehicle (saline) alone. Blood glucose levels in experimental mice were maintained at ∼300 mg/dL by treatment with 0.4 units insulin administered every other day. As expected, 12 weeks after induction of diabetes, a marked increase in 24-h albumin excretion was detected in the urine of vehicle-treated diabetic BALB/c mice (Fig. 4, black bars). Of note, SST0001 treatment resulted in a statistically significant (P < 0.0351) twofold decrease in 24-h albumin excretion (Fig. 4, gray bars). The difference between 24-h albumin excretion in SST0001-treated versus vehicle-treated mice was maintained on week 16 of the experiment as well, although it did not reach statistical significance (not shown). In addition, assessment of renal function in vehicle-treated diabetic mice on experimental week 16 revealed a significant increase in serum creatinine (P = 0.016) and blood urea nitrogen (P = 0.0171) (Fig. 4B, black bars) compared with healthy nondiabetic mice, whereas in SST0001-treated diabetic mice the corresponding values did not increase significantly compared with those observed in nondiabetic mice (Fig. 4B, gray bars). In a similar manner, treatment with SST0001 resulted in a twofold or greater decrease in 24-h urinary albumin excretion in two additional in vivo experimental systems: STZ-induced type 1 DN in DBA-2 mice (Supplementary Fig. 3), which, along with BALB/c strain, is considered as one of the most useful platforms for DN modeling (25), and in type 2 DN in obese db/db mice (P = 0.010) (Supplementary Fig. 4), regarded as one of the best genetic models of diabetic renal disease (47), further validating heparanase inhibition as a possible therapeutic approach in DN.
FIG. 4.
Effect of SST0001 on albuminuria and renal damage in diabetic BALB/c mice. Mice were made hyperglycemic applying multiple low-dose STZ administrations as described in research design and methods and remained untreated (black bars) or treated with compound SST0001 (300 μg/mouse injected twice per day intraperitoneally) for 12 weeks (gray bar). A: ELISA-monitored 24-h albumin excretion on weeks 1 and 12 after the onset of diabetes. Seven mice per experimental condition were used. Treatment of diabetic mice with SST0001 resulted in more than twofold decrease in urine albumin (*P = 0.035). B: Vehicle-treated diabetic mice exhibited a significant increase in serum creatinine (left, **P = 0.0157) and blood urea nitrogen (right, **P = 0.0171) compared with healthy mice (n = 5 mice per group). The corresponding values in SST0001-treated diabetic mice did not increase significantly compared with basal levels. BUN, blood urea nitrogen.
DISCUSSION
Diabetes and its complications represent one of the most important health problems worldwide, which is likely to worsen to critical levels in the next decades (3). Therefore, DN (one of the most relevant complications secondary to diabetes) is becoming one of the primary medical concerns (1–3). Thus, more data about the identity of downstream effectors responsible for DN pathogenesis are needed to properly address the disease treatment. Considerable progress has been made in deciphering the role of multiple signaling pathways leading to kidney damage in diabetic patients. Less is known about the exact role of ECM-degrading enzymes in DN pathophysiology. We demonstrate for the first time the essential involvement of heparanase in experimental DN and describe a molecular mechanism underlying heparanase induction under hyperglycemic conditions.
Despite the lack of experimental data directly confirming the causative role of heparanase in the pathogenesis of DN, several reports discussed a possible link between heparanase and DN (4,5). On the other hand, a relationship between heparanase and DN progression was disputed in recent publications, opposing the involvement of the enzyme in diabetic kidney disease (20,21). In light of this controversy, recent generation of heparanase-null (Hpse-KO) mice (22) offered a unique opportunity to assess the relevance of heparanase to the development of DN. Of note, all Hpse-KO mice used in our experiments failed to develop proteinuria in response to STZ-induced diabetes, and their urinary albumin excretion rate remained at the same level as before the onset of diabetes. In contrast, a greater than fivefold increase in urinary excretion rate of albumin was detected in wt mice after STZ-induced diabetes (Fig. 1). In addition, heparanase deficiency resulted in amelioration of mesangial matrix expansion, macrophage infiltration, tubulointerstitial fibrosis, and TGF-β overexpression in the diabetic kidneys of Hpse-KO versus wt mice (Fig. 2). In further support of a causal involvement of heparanase in DN are our findings showing a lower degree of albuminuria in type 1 (Fig. 4 and Supplementary Fig. 3) and type 2 (Supplementary Fig. 4) diabetic mice treated with the heparanase inhibitor SST0001 versus mice treated with vehicle alone. Amelioration of albuminuria by SST0001 represents a proof of concept for heparanase inhibition as a relevant therapeutic approach in DN and warrants further studies aimed at identifying the most effective dose and schedule administration schemes for SST0001 treatment, toward future translation to a clinical setting.
In addition, elucidation of the precise molecular mechanism(s) underlying heparanase induction and pathogenic action in diabetic kidney is critically important toward effective implementation of anti-heparanase treatment modalities in the future. We report that the transcription factor Egr1 mediates overexpression of heparanase in diabetic kidney. In agreement with the previously reported glucose responsiveness of Egr1 in other cell types (48), we have demonstrated dose-dependent induction of Egr1 expression in cultured kidney cells in vitro and in diabetic kidney in vivo (Fig. 3). Of note, Egr1 can both induce and repress the expression of heparanase, depending on the tissue/cell type; it was shown to activate heparanase expression in T lymphocytes and carcinomas of the breast, prostate, and colon, but to inhibit heparanase transcription in melanoma and pancreatic carcinoma cells (31,49). Transactivation studies using Egr1 expression vector, cotransfected with a heparanase promoter-reporter construct, showed that in kidney cells Egr1 acts to stimulate heparanase transcription (Supplementary Fig. 2). At the same experimental setting, ChIP analysis revealed increased occupancy of the heparanase promoter by Egr1 in the presence of elevated glucose levels (Fig. 3E), further confirming the role of Egr1 in glucose-dependent induction of heparanase in the kidney.
A limitation of the current study is that our findings do not provide enough information regarding the exact mode of heparanase action in DN pathogenesis. In the past it was generally accepted that induction of glomerular heparanase in the course of diabetes may contribute to DN progression primarily through degradation of HS in the GBM. Findings supporting the importance of HS integrity in permselectivity of the glomerular filtration (19) include 1) occurrence of massive proteinuria after administration of monoclonal anti-HS antibody to rats, 2) increased GBM permeability as a result of HS removal, and 3) decreased GBM HS content in human/experimental diabetes, which inversely correlated with the degree of proteinuria. Nevertheless, the impact of GBM-residing HS and its enzymatic degradation on DN development was questioned by several recent studies showing no change in glomerular HS content/structure in early human and experimental DN (20,21). These reports challenged the notion that heparanase-mediated loss of HS in the GBM is the primary mechanism implicating the enzyme in DN. We previously suggested that additional mechanisms (i.e., changes in glomerular cell–GBM interactions due to loss of HS, or release of HS-bound growth factors, cytokines, and bioactive HS fragments in the glomeruli) may drive heparanase-mediated renal failure (4,5). In addition, our unpublished results indicate that a previously unappreciated mechanism (i.e., heparanase-driven activation of macrophages leading to unresolved inflammation) (26) may be critically important in DN, as well as in additional kidney pathologies previously linked to heparanase (4,5). The important role of chronic inflammation and macrophages in DN (37–39,50), taken together with the reduced number of macrophages infiltrating Hpse-KO diabetic kidneys (Fig. 2E) and the recently revealed ability of heparanase to facilitate macrophage activation by lipopolysaccharide (26) and by components of the diabetic milieu (i.e., glucose, our unpublished data), strongly suggests that under diabetic conditions, heparanase, induced in the kidney epithelium via an Egr1-dependent mechanism, sustains continuous activation of kidney-damaging macrophages, thus fostering DN development and progression. Studies are under way to accurately evaluate the pathophysiologic significance of each of the above-mentioned heparanase-dependent mechanisms in DN progression and thus better define future combination treatment options, as well as target patient populations in whom future anti-heparanase therapies may be particularly beneficial.
ACKNOWLEDGMENTS
This work was supported by grants from the Juvenile Diabetes Research Foundation (2006-695 and 2009-635), Israel Science Foundation (Grant 593/10), Dutch Kidney Foundation (Grant C09.2296), the EFSD/D-Cure Young Investigator Award, and by the EFSD/Novo Nordisk research grant.
C.P. is employed by Sigma-Tau Industrie Farmaceutiche Riunite S.p.A. and is listed as inventor on a patent for SST0001. No other potential conflicts of interest relevant to this article were reported.
N.G., R.G., T.N., M.G., E.Z., and A.M.R. researched data. T.v.K. generated anti-HS antibody and reviewed and edited the manuscript. A.M. researched data and contributed to discussion. C.P. generated heparanase-inhibiting compound and reviewed and edited the manuscript. J.-P.L. generated/characterized Hpse-KO mice and contributed to discussion. J.v.d.V. researched data and reviewed and edited the manuscript. I.V. supervised part of the work and participated in writing the manuscript. M.E. supervised the entire project, researched data, wrote the manuscript, and is the guarantor.
The authors thank Dr. Angelique Rops (Radboud University Nijmegen Medical Centre, Nijmegen, the Netherlands) for critical support.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1024/-/DC1.
REFERENCES
- 1.Ibrahim HN, Hostetter TH. Diabetic nephropathy. J Am Soc Nephrol 1997;8:487–493 [DOI] [PubMed] [Google Scholar]
- 2.Gilbertson DT, Liu J, Xue JL, et al. Projecting the number of patients with end-stage renal disease in the United States to the year 2015. J Am Soc Nephrol 2005;16:3736–3741 [DOI] [PubMed] [Google Scholar]
- 3.Atkins RC, Zimmet P; World Kidney Day Steering Committee Diabetic kidney disease: act now or pay later. J Am Soc Hypertens 2010;4:3–6 [DOI] [PubMed] [Google Scholar]
- 4.Szymczak M, Kuźniar J, Klinger M. The role of heparanase in diseases of the glomeruli. Arch Immunol Ther Exp (Warsz) 2010;58:45–56 [DOI] [PubMed] [Google Scholar]
- 5.van den Hoven MJ, Rops AL, Vlodavsky I, Levidiotis V, Berden JH, van der Vlag J. Heparanase in glomerular diseases. Kidney Int 2007;72:543–548 [DOI] [PubMed] [Google Scholar]
- 6.Vreys V, David G. Mammalian heparanase: what is the message? J Cell Mol Med 2007;11:427–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parish CR, Freeman C, Hulett MD. Heparanase: a key enzyme involved in cell invasion. Biochim Biophys Acta 2001;1471:M99–M108 [DOI] [PubMed] [Google Scholar]
- 8.Vlodavsky I, Friedmann Y, Elkin M, et al. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat Med 1999;5:793–802 [DOI] [PubMed] [Google Scholar]
- 9.Iozzo RV. Heparan sulfate proteoglycans: intricate molecules with intriguing functions. J Clin Invest 2001;108:165–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkin M, Ilan N, Ishai-Michaeli R, et al. Heparanase as mediator of angiogenesis: mode of action. FASEB J 2001;15:1661–1663 [DOI] [PubMed] [Google Scholar]
- 11.Kato M, Wang H, Kainulainen V, et al. Physiological degradation converts the soluble syndecan-1 ectodomain from an inhibitor to a potent activator of FGF-2. Nat Med 1998;4:691–697 [DOI] [PubMed] [Google Scholar]
- 12.Li JP, Vlodavsky I. Heparin, heparan sulfate and heparanase in inflammatory reactions. Thromb Haemost 2009;102:823–828 [DOI] [PubMed] [Google Scholar]
- 13.Katz A, Van-Dijk DJ, Aingorn H, et al. Involvement of human heparanase in the pathogenesis of diabetic nephropathy. Isr Med Assoc J 2002;4:996–1002 [PubMed] [Google Scholar]
- 14.van den Hoven MJ, Rops AL, Bakker MA, et al. Increased expression of heparanase in overt diabetic nephropathy. Kidney Int 2006;70:2100–2108 [DOI] [PubMed] [Google Scholar]
- 15.Levidiotis V, Freeman C, Punler M, et al. A synthetic heparanase inhibitor reduces proteinuria in passive Heymann nephritis. J Am Soc Nephrol 2004;15:2882–2892 [DOI] [PubMed] [Google Scholar]
- 16.Maxhimer JB, Somenek M, Rao G, et al. Heparanase-1 gene expression and regulation by high glucose in renal epithelial cells: a potential role in the pathogenesis of proteinuria in diabetic patients. Diabetes 2005;54:2172–2178 [DOI] [PubMed] [Google Scholar]
- 17.Conde-Knape K. Heparan sulfate proteoglycans in experimental models of diabetes: a role for perlecan in diabetes complications. Diabetes Metab Res Rev 2001;17:412–421 [DOI] [PubMed] [Google Scholar]
- 18.Miner JH. Renal basement membrane components. Kidney Int 1999;56:2016–2024 [DOI] [PubMed] [Google Scholar]
- 19.Raats CJ, Van Den Born J, Berden JH. Glomerular heparan sulfate alterations: mechanisms and relevance for proteinuria. Kidney Int 2000;57:385–400 [DOI] [PubMed] [Google Scholar]
- 20.van den Born J, Pisa B, Bakker MA, et al. No change in glomerular heparan sulfate structure in early human and experimental diabetic nephropathy. J Biol Chem 2006;281:29606–29613 [DOI] [PubMed] [Google Scholar]
- 21.Harvey SJ, Miner JH. Revisiting the glomerular charge barrier in the molecular era. Curr Opin Nephrol Hypertens 2008;17:393–398 [DOI] [PubMed] [Google Scholar]
- 22.Zcharia E, Jia J, Zhang X, et al. Newly generated heparanase knock-out mice unravel co-regulation of heparanase and matrix metalloproteinases. PLoS ONE 2009;4:e5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naggi A, Casu B, Perez M, et al. Modulation of the heparanase-inhibiting activity of heparin through selective desulfation, graded N-acetylation, and glycol splitting. J Biol Chem 2005;280:12103–12113 [DOI] [PubMed] [Google Scholar]
- 24.Ritchie JP, Ramani VC, Ren Y, et al. SST0001, a chemically modified heparin, inhibits myeloma growth and angiogenesis via disruption of the heparanase/syndecan-1 axis. Clin Cancer Res 2011;17:1382–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi Z, Fujita H, Jin J, et al. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 2005;54:2628–2637 [DOI] [PubMed] [Google Scholar]
- 26.Lerner I, Hermano E, Zcharia E, et al. Heparanase powers a chronic inflammatory circuit that promotes colitis-associated tumorigenesis in mice. J Clin Invest 2011;121:1709–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Kuppevelt TH, Dennissen MA, van Venrooij WJ, Hoet RM, Veerkamp JH. Generation and application of type-specific anti-heparan sulfate antibodies using phage display technology. Further evidence for heparan sulfate heterogeneity in the kidney. J Biol Chem 1998;273:12960–12966 [DOI] [PubMed] [Google Scholar]
- 28.Doviner V, Maly B, Kaplan V, et al. Spatial and temporal heparanase expression in colon mucosa throughout the adenoma-carcinoma sequence. Mod Pathol 2006;19:878–888 [DOI] [PubMed] [Google Scholar]
- 29.Kelly T, Miao HQ, Yang Y, et al. High heparanase activity in multiple myeloma is associated with elevated microvessel density. Cancer Res 2003;63:8749–8756 [PubMed] [Google Scholar]
- 30.Cohen I, Maly B, Simon I, et al. Tamoxifen induces heparanase expression in estrogen receptor-positive breast cancer. Clin Cancer Res 2007;13:4069–4077 [DOI] [PubMed] [Google Scholar]
- 31.de Mestre AM, Rao S, Hornby JR, Soe-Htwe T, Khachigian LM, Hulett MD. Early growth response gene 1 (EGR1) regulates heparanase gene transcription in tumor cells. J Biol Chem 2005;280:35136–35147 [DOI] [PubMed] [Google Scholar]
- 32.Breyer MD, Böttinger E, Brosius FC, 3rd, et al. ; AMDCC Mouse models of diabetic nephropathy. J Am Soc Nephrol 2005;16:27–45 [DOI] [PubMed] [Google Scholar]
- 33.Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int 1999;56:1627–1637 [DOI] [PubMed] [Google Scholar]
- 34.Ziyadeh FN. Mediators of diabetic renal disease: the case for tgf-Beta as the major mediator. J Am Soc Nephrol 2004;15(Suppl. 1):S55–S57 [DOI] [PubMed] [Google Scholar]
- 35.Lu A, Miao M, Schoeb TR, Agarwal A, Murphy-Ullrich JE. Blockade of TSP1-dependent TGF-β activity reduces renal injury and proteinuria in a murine model of diabetic nephropathy. Am J Pathol 2011;178:2573–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci U S A 1993;90:1814–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tesch GH. Macrophages and diabetic nephropathy. Semin Nephrol 2010;30:290–301 [DOI] [PubMed] [Google Scholar]
- 38.Tuttle KR. Linking metabolism and immunology: diabetic nephropathy is an inflammatory disease. J Am Soc Nephrol 2005;16:1537–1538 [DOI] [PubMed] [Google Scholar]
- 39.Nguyen D, Ping F, Mu W, Hill P, Atkins RC, Chadban SJ. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology (Carlton) 2006;11:226–231 [DOI] [PubMed] [Google Scholar]
- 40.Starkey PM, Turley L, Gordon S. The mouse macrophage-specific glycoprotein defined by monoclonal antibody F4/80: characterization, biosynthesis and demonstration of a rat analogue. Immunology 1987;60:117–122 [PMC free article] [PubMed] [Google Scholar]
- 41.Ogishima T, Shiina H, Breault JE, et al. Increased heparanase expression is caused by promoter hypomethylation and up-regulation of transcriptional factor early growth response-1 in human prostate cancer. Clin Cancer Res 2005;11:1028–1036 [PubMed] [Google Scholar]
- 42.de Mestre AM, Staykova MA, Hornby JR, Willenborg DO, Hulett MD. Expression of the heparan sulfate-degrading enzyme heparanase is induced in infiltrating CD4+ T cells in experimental autoimmune encephalomyelitis and regulated at the level of transcription by early growth response gene 1. J Leukoc Biol 2007;82:1289–1300 [DOI] [PubMed] [Google Scholar]
- 43.Hasan RN, Phukan S, Harada S. Differential regulation of early growth response gene-1 expression by insulin and glucose in vascular endothelial cells. Arterioscler Thromb Vasc Biol 2003;23:988–993 [DOI] [PubMed] [Google Scholar]
- 44.Elkin M, Cohen I, Zcharia E, et al. Regulation of heparanase gene expression by estrogen in breast cancer. Cancer Res 2003;63:8821–8826 [PubMed] [Google Scholar]
- 45.Casu B, Vlodavsky I, Sanderson RD. Non-anticoagulant heparins and inhibition of cancer. Pathophysiol Haemost Thromb 2008;36:195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y, MacLeod V, Dai Y, et al. The syndecan-1 heparan sulfate proteoglycan is a viable target for myeloma therapy. Blood 2007;110:2041–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allen TJ, Cooper ME, Lan HY. Use of genetic mouse models in the study of diabetic nephropathy. Curr Diab Rep 2004;4:435–440 [DOI] [PubMed] [Google Scholar]
- 48.Josefsen K, Sørensen LR, Buschard K, Birkenbach M. Glucose induces early growth response gene (Egr-1) expression in pancreatic beta cells. Diabetologia 1999;42:195–203 [DOI] [PubMed] [Google Scholar]
- 49.Meirovitz A, Hermano E, Lerner I, et al. Role of heparanase in radiation-enhanced invasiveness of pancreatic carcinoma. Cancer Res 2011;71:2772–2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 2008;19:433–442 [DOI] [PubMed] [Google Scholar]