Abstract
The Banting Medal for Scientific Achievement Award is the American Diabetes Association's highest scientific award and honors an individual who has made significant, long-term contributions to the understanding of diabetes, its treatment, and/or prevention. The award is named after Nobel Prize winner Sir Frederick Banting, who codiscovered insulin treatment for diabetes. Dr. Barbara E. Corkey received the American Diabetes Association's Banting Medal for Scientific Achievement at the Association's 71st Scientific Sessions, 24–28 June 2011, San Diego, California. She presented the Banting Lecture, “Hyperinsulinemia: Cause or Consequence?” on Sunday, 26 June 2011.
Many environmental changes have accompanied the rising onset of obesity and diabetes. Much has changed in our world to explain this epidemic incidence of obesity and diabetes, and many of those changes have not been carefully studied. Our foods have changed; living conditions, activity levels, the air we breathe have all changed: so where can we start looking for culprits?
Striking correlations between the toxin polybrominated diphenyl ethers, air conditioning, antidepressant prescriptions, and average home temperature and the prevalence of obesity have been shown by Allison and colleagues (1). The worldwide expansion of metabolic diseases across all age-groups decreases the likelihood that our air or unique living conditions are the main culprits. The differences in activity levels among boys and girls, old and young, a farmer and an office worker make it unlikely that decreased activity, though detrimental, can be the only main explanation. However, food is now universally shared across the globe, particularly processed food. Food is different today than it was in the past; over 4,000 new agents have entered our food supply intentionally or inadvertently: almost none of those have been evaluated as potential causes of obesity or diabetes. The body weight and composition of food animals have changed (2): the average weight of cattle has increased as it has in humans; however, the percent body fat has actually declined. There have been dramatic changes in poultry such that the average age at market has decreased from 112 days to 42 days (3). The average weight has more than doubled, and feed efficiency has increased almost threefold with a decrease in mortality. Science has likely helped to increase efficiency and require less food. The mineral content of fruits and vegetables has changed over the past 40 years (4–7), probably because of optimized and standardized growing conditions. The packaging and preparation of our food have also changed leading to an increase in nonedible packing materials in the food (5–8). Many foods contain preservatives, emulsifiers, flavor enhancers, food coloring, and other fillers that have not been previously consumed in significant quantities. Virtually none of these nonfood compounds have been carefully assessed for a potential impact on obesity or diabetes.
There have been extensive studies of pancreatic islets, liver, fat cells, as well as brain, gut, vasculature, and muscle. Evidence now exists to support an important role for each in metabolic homeostasis and for a causative role for several organs in both diabetes and obesity (9–11). Many treatments for, and much of the research in, obesity have focused on the role of diet and physical activity. Most pharmacological research focused on the control of food intake, increasing energy expenditure or improving insulin action. These focused efforts were based on excellent models, but despite evidence to support their utility, they have not yet slowed the growth in rates of obesity or diabetes.
We need an alternative model. My model proposes that environmentally induced elevated background levels of insulin, superimposed on a susceptible genetic background, or basal hyperinsulinemia is the root cause of insulin resistance, obesity, and diabetes.
There is a strong relationship between basal insulin levels, obesity, and diabetes in humans (12). Increasing fasting insulin levels compared with those in lean control subjects have been documented as subjects progress from obesity to impaired glucose tolerance and severe diabetes (13,14). This correlation provides no information on causation, and the same relationship with insulin resistance could be shown. However, there is evidence that hypersecretion of insulin can precede and cause insulin resistance. For example, rodents infused with insulin via an implanted minipump become hyperinsulinemic and insulin resistant with impaired glucose tolerance (14). Furthermore, in human studies, inhibition of hyperinsulinemia with diazoxide actually causes weight loss and decreases insulin levels without impairing glucose tolerance in obese humans (15–17). These studies suggest that hyperinsulinemia can cause insulin resistance and that lowering insulin secretion in hyperinsulinemic individuals may be beneficial.
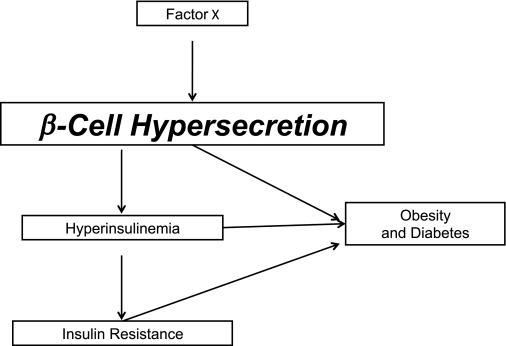
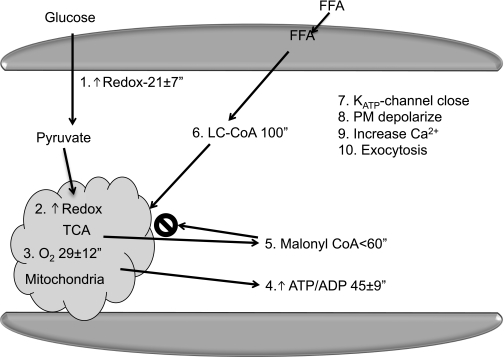
The proposed new model (Fig. 1) is based on the hypothesis that excessive β-cell secretory responses, possibly to environmental agents (Factor X in the scheme), may be a contributing or major cause of obesity and type 2 diabetes. The communication system envisioned involves metabolic signals, specifically redox indicators, which circulate in the blood (Fig. 2). They cause different functional changes in different tissues (Fig. 3). So the same change in redox indicators could change secretion in β-cells, lipolysis in adipocytes, and glucose production in the liver and orchestrate a systemic response to metabolic stress.
FIG. 1.
Model of β-cell secretion of insulin leading to hyperinsulinemia and causing obesity, diabetes, and insulin resistance.
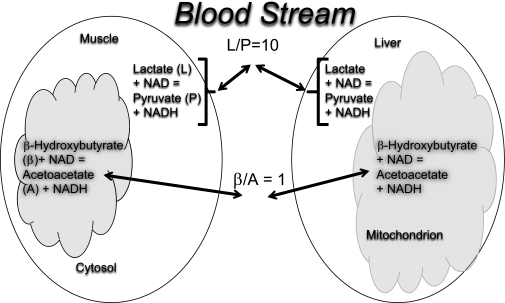
FIG. 2.
Illustration of communication of intracellular redox state to the blood stream: equilibration of cytosolic and mitochondrial redox as reflected in the muscle cytosolic lactate-to-pyruvate ratio (L/P) and liver mitochondrial β/A ratio.
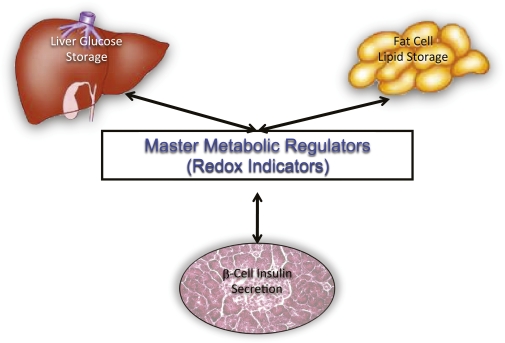
FIG. 3.
Model of redox as master regulator of metabolism affecting insulin secretion, hepatic glucose handling, and adipocyte lipid storage.
Intracellular redox is defined as the ratio of reduced NADH to its oxidized partner NAD. These compounds do not normally pass in and out of cells but are in equilibrium with metabolites that do move across membranes. Thus, the ratio in the cell can be known by the ratio of indicator metabolites. Accordingly, the ratio of lactate to pyruvate in the blood reflects the cytosolic NADH-to-NAD ratio. This is mainly controlled by muscle and is usually about 10 in both muscle and blood (18,19) (Fig. 2). β-Hydroxybutyrate–to–acetoacetate (β/A) ratio reflects the mitochondrial redox state and is mainly controlled by liver and usually around 1 (20,21) (Fig. 2). These circulating metabolites are referred to as redox indicators. A change in redox will influence different organs in different ways. This is conceptually a highly refined system that assures that after ingestion of a meal, all the metabolically important organs in the body respond appropriately: β-cells secrete insulin, the liver stores glucose, adipose tissue increases fat storage, and the brain signals satiety.
Focusing on the β-cell, consider what happens in this model when insulin secretion is increased due to genetic or environmental influences such as a false stimulus (a fictitious example, Factor X) (Fig. 1). How will this impact both our understanding and the model itself?
Our fictitious Factor X may influence insulin secretion by acting directly on the β-cell or indirectly by changing the circulating redox indicators produced through an effect on another organ. If an increase in insulin secretion is sustained, an increase in insulin-generated signals throughout the body occurs. This can cause hepatic insulin resistance and increased fat mass—both key pathophysiological components of obesity and type 2 diabetes.
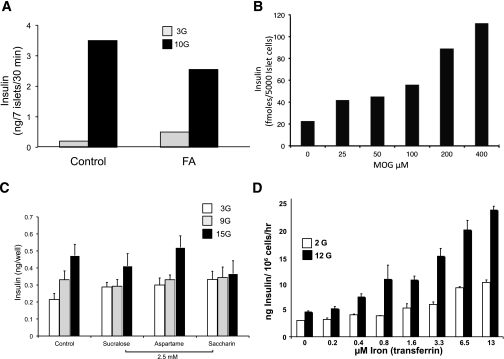
To test a model of hyperinsulinemia as cause of obesity-associated type 2 diabetes, it is necessary to find a way to induce insulin secretion at nonstimulatory glucose levels. It is well established that exposure to free fatty acid (FFA) affects basal insulin secretion, but this takes time. We confirmed that elevated basal and suppressed glucose-stimulated secretion occurs after an 18-h exposure to FFA in isolated islets (Fig. 4A). Infusion studies in humans by Boden and colleagues (22–24) also show a marked ability of FFA to increase circulating insulin levels in normal, obese, and type 2 diabetic subjects.
FIG. 4.
Insulin secretion. A: Effect of 18-h exposure to 100 μmol/L fatty acid (FA) on insulin secretion from isolated rat islets (73). B: Concentration dependence of MOG-stimulated insulin secretion from dissociated rat islets at basal 3 mmol/L glucose (73). C: Effect of artificial sweeteners on insulin secretion in dissociated rat islets (74). Effect of iron exposure in INS-1 (832/13) cells (Deeney et al., unpublished data). Data shown are means ± SEM for at least three experiments.
In order to study basal hyperinsulinemia, we needed a model system and sought a well-controlled cellular system to determine what could rapidly increase basal secretion in the absence of stimulatory glucose. Using cultured INS-1 cells, we screened substances that have entered our food supply in recent years and identified common lipid food additives that increased insulin secretion at basal glucose levels including monoacylglycerides. They are formed and degraded in the gut, and by lipoprotein lipase in peripheral tissues, and are commonly added in small quantities as emulsifiers and preservatives. The ability of mono-oleoylglycerol (MOG) to stimulate insulin secretion at basal glucose was concentration dependent and significant at a concentration as low as 25 μmol/L (Fig. 4B). The physiological relevance of monoglycerides is not established because there appear to be few measurements (25,26) and no standard for the level of circulating or tissue monoglycerides.
Several additional nonlipid stimuli were also identified in our screening, including artificial sweeteners and iron. Artificial sweeteners that are also frequently present in modern foods were found to impact insulin secretion. Shown here is insulin secretion at basal and two stimulatory concentrations of glucose in response to saccharin, aspartame, and sucralose (Fig. 4C). All stimulated basal secretion acutely, but saccharin was most potent and also inhibited glucose-stimulated secretion. Interestingly, only saccharin stimulated basal secretion at concentrations that might be achieved by high levels of consumption, for example, in diet beverages.
Iron consumption has increased as the lean content of food animals has increased, although it is not clear that this has affected tissue iron content. Here we show that iron increased both basal and stimulated insulin secretion (Fig. 4D). Thus, iron, saccharin, and MOG can be used as tools to study the mechanism of basal insulin secretion.
It is well established in the β-cell that metabolism of glucose generates sequential signals (Fig. 5) that increase cytosolic and mitochondrial redox half-maximally at 21 s (27). Respiration or oxygen consumption follows at 29 s resulting in ATP production that is half-maximal at 45 s (27). There is also efflux of intermediates from the citric acid cycle that form malonyl CoA at <1 min (28). Malonyl CoA blocks fat oxidation and causes an increase in cytosolic long-chain acyl-CoA (LC-CoA) at ∼100 s and at 325 s; a final series of steps result in a rise in cytosolic Ca2+ just prior to enhanced insulin exocytosis (27). These changes reflect glucose metabolism leading to signals that depolarize the β-cell and open Ca2+ channels and stimulate the movement of insulin-containing secretory vesicles to the membrane where they release their contents. Several of these signals were examined in response to the nonfood compounds we had identified.
FIG. 5.
Time course of glucose-induced metabolic changes after glucose addition (27). PM, plasma membrane. TCA, tricarboxylic acid cycle.
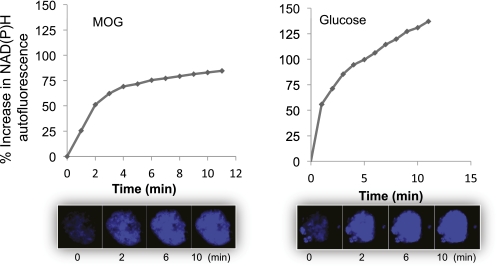
MOG neither changed Ca2+ nor altered the normal responses to glucose (data not shown). Likewise there was no affect on respiration in the absence or presence of stimulatory glucose (data not shown). The expected response to glucose was not altered by MOG between 25 and 100 μmol/L. In contrast, redox increased rapidly above control in response to MOG at basal glucose (Fig. 6), with an area under the curve that was more than double basal values.
FIG. 6.
Effect of MOG (left panel) and glucose (right panel) on rat islet redox state (73). (A high-quality digital representation of this figure is available in the online issue.)
Reactive oxygen species (ROS) are chemically reactive molecules containing oxygen. In high amounts ROS cause damage known as oxidative stress. However, ROS form as a natural byproduct of metabolism, and modest ROS production has important roles in cell signaling (29,30). Conditions that increase redox, as we found with stimulatory glucose and MOG, can lead to production of modest amounts of ROS in mitochondria. Thus, redox and ROS are candidate signals for basal insulin secretion, and we asked whether the putative signal was essential or sufficient.
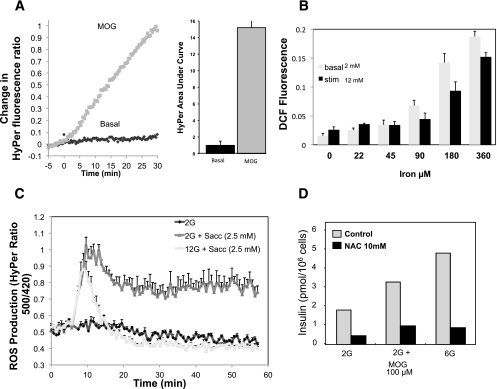
Here we show that MOG induced a robust increase in ROS measured using the fluorescent indicator HyPer (Fig. 7A). It is well known that Fe can induce ROS (31), and we illustrate this (Fig. 7B) at both basal and stimulatory glucose. Finally, we found that saccharin, but not the other artificial sweeteners, increased ROS generation at basal glucose (Fig. 7C). These data indicate that the compounds that stimulated basal insulin secretion most effectively also generated ROS.
FIG. 7.
Effect on ROS of agents that stimulate basal insulin secretion in INS-1 (832/13) cells. A: ROS generation by MOG measured in islet cells virally infected with the ROS indicator HyPercyto (73). B: Iron increases ROS as documented by the ROS indicator dichlorofluorescein (DCF) (Deeney et al., unpublished data). C: Effect of saccharin (Sacc) on ROS in cells virally infected with the ROS indicator HyPercyto (74). D: Effect of ROS scavengers on insulin secretion from INS-1 cells (73). Data shown are means ± SEM for at least three experiments.
To test the notion that ROS generation was essential, we used ROS scavengers to deplete intracellular ROS. This not only prevented MOG-induced basal insulin secretion but also markedly decreased secretion from basal and 6 mmol/L glucose (Fig. 7D). The ability of the ROS scavengers to prevent MOG-induced basal secretion implicated an obligatory role for ROS in hyperinsulinemia and possibly even in normal basal secretion. It should be noted that ROS scavenging is likely to have effects that can be either beneficial or detrimental depending on the ROS level (32).
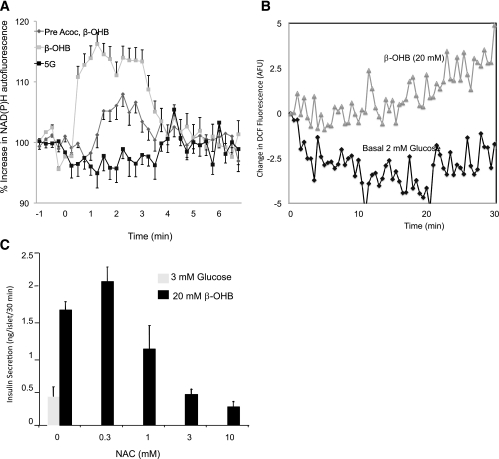
The focus on ROS was based on the relationship between ROS and mitochondrial redox. Clearly MOG increased redox and generated ROS; but if they were causally related, a change in redox alone should have the same effect. To test this idea, we used β-hydroxybutyrate (β-OHB) that increases redox specifically in the mitochondria (33) (Fig. 2). We asked whether an increase in redox induced by β-OHB could cause an increase in ROS and secretion. As shown in Fig. 8A, β-OHB greatly increased redox in the isolated islet cells, an effect that was attenuated by the oxidized member of the couple, acetoacetate. As can be seen in Fig. 8B, increasing mitochondrial redox in this way indeed generated ROS. Data shown here demonstrated that β-OHB also stimulated insulin secretion at 3 mmol/L glucose (Fig. 8C).
FIG. 8.
Effects of 20 mmol/L β-OHB. A: NAD(P)H autofluorescence in islet cells (73). B: ROS generation in INS-1 (832/13) cells virally infected with the ROS indicator HyPercyto (73). C: Effect of β-OHB and ROS scavenging by NAC on insulin secretion from islet cells (73). Data shown are means ± SEM for at least three experiments.
We found that, consistent with a direct and essential role of ROS, scavenging with N-acetylcysteine (NAC) prevented insulin secretion (Fig. 8C). Previous studies showed that ROS are sufficient signals for insulin secretion. Studies performed by Pi et al. (29) show that ROS, added as peroxide or generated internally through addition of diethyl maleate, stimulated insulin secretion in a dose-dependent manner.
Taken together, these data suggest that agents that increase redox or generate ROS, result in stimulation of basal insulin secretion. These data further indicate that hypersecretion of insulin can be caused directly by ROS and that ROS are essential and sufficient signals.
However, ROS are not the only essential and sufficient signals. There is abundant evidence in the literature that an increase in cytosolic Ca2+ directly stimulates and its removal prevents secretion (34,35). However, Ca2+ does not change with MOG at basal glucose. Another important signal is LC-CoA, the active form of FFA, that is derived from both internal and external sources. Prentki and I have published many studies documenting an important role for LC-CoA in glucose-stimulated insulin secretion (28,36–39).
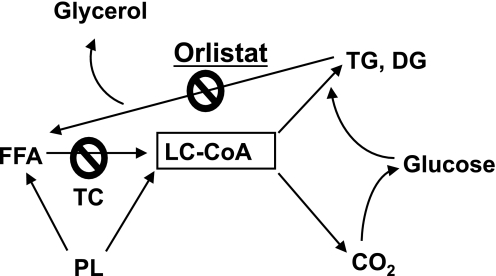
Exocytosis of insulin is enhanced in permeabilized β-cells in response to increasing Ca2+ with a further increase in secretion induced by the addition of LC-CoA at each Ca2+ concentration (40). Stein et al. (41) were the first to show that insulin secretion requires FFA. There is little or no glucose-stimulated insulin secretion in perfused pancreas from fasted rats without the addition of FFA (41). Presumably this happens because fasted rats have depleted islet fat stores—so robust secretion in vitro required added fat. Additional evidence for a role for the active form of FFA was obtained by blocking LC-CoA formation to prevent insulin secretion. Figure 9 shows the sites where we can either inhibit FFA production with the lipase inhibitor orlistat (42) or prevent FFA activation with triacsin C (43,44).
FIG. 9.
Inhibition of LC-CoA formation by orlistat (lipase inhibitor) and triacsin C (TC) (acyl-CoA synthetase inhibitor). DG, diacylglycerol; PL, phospholipids; TG, triglyceride.
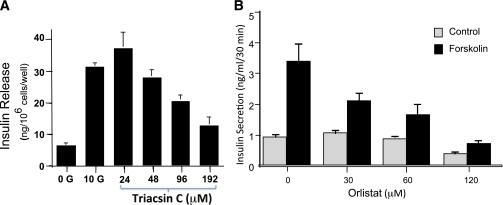
We documented a concentration-dependent decrease in glucose-stimulated secretion, using triacsin C (Fig. 10A). We also found that inhibiting lipolysis with orlistat blocked insulin secretion from glucose alone or glucose plus forskolin (Fig. 10B). These data are consistent with an essential role for LC-CoA in insulin secretion. Interestingly, although LC-CoA levels also increased with MOG (data not shown), we do not yet know whether inhibition of LC-CoA formation from MOG blocks secretion.
FIG. 10.
Insulin secretion in response to lipid modulators in clonal β-cells. A: Inhibiting LC-CoA formation with triacsin C inhibits secretion (Deeney et al., unpublished data). B: Inhibiting lipolysis with orlistat inhibits secretion (75). Data shown are means ± SEM for at least three experiments.
These and other data lead to the conclusion that Ca2+, LC-CoA, and ROS may all be essential signals for insulin secretion under some circumstances, but ROS is so far the only documented signal essential for basal hypersecretion in the absence of fuel stimuli. Interestingly, FFAs contribute to both LC-CoA and ROS generation (45), thus providing two essential signals.
The potency of redox to directly impact ROS and insulin secretion suggests that changes in redox could be induced in other cells or organs and transmitted to the β-cell via the blood stream. Redox ratios vary with nutritional state and in response to obesity, diabetes, and high fatty acids (19,46–48). There is additional evidence in the literature that redox metabolites affect cell function. As an example, Shaw and Wolfe (49,50) have shown that β-OHB infusion decreases glucose production and FA release in dogs.
Redox has more than one meaning. I have focused on the NADH-to-NAD ratio. It should be noted that this ratio interacts with the thiol redox state because NADH and NADPH can be interconverted and change the oxidation state of glutathione as follows:
Mitochondria
NADPH + NAD = NADP + NADH
Cytosol
GSSG + NADPH = 2GSH + NADP
H2O2 + 2GSH = GSSG + H2O
Citrate = Isocitrate
Isocitrate + NADP = αKG + NADPH
Isocitrate + NAD = αKG + NADH
Elegant work by Jones et al. (51–54) has shown regulation by the redox state established by reduced to oxidized thiols involving glutathione and cysteine. Changes in thiol redox correlate with aging, diabetes, heart disease, and some cancers. They regulate intracellular signal transduction and mitochondrial ROS production. Thus, it is important to consider redox as an integrated system that involves the pyridine nucleotides, glutathione, thioredoxins, and multiple redox-sensitive proteins.
Diabetes and obesity are associated with increased circulating levels of several metabolites that are known to alter redox. These include the redox indicator lactate and the essential branched-chain amino acids (BCAAs). Recent metabolomic studies by Wang et al. (55) and Laferrère et al. (56) measuring hundreds of blood metabolites have emphasized a strong and predictive association with BCAA. Interestingly, elevated FFAs have often been associated with obesity and diabetes; however, a recent review of the literature suggests that there is no consistent relationship between FFA and BMI in the absence of diabetes (57), consistent with the effectiveness of hyperinsulinemia to suppress lipolysis. Thus, there can be metabolic adaptation to hyperinsulinemia that permits maintenance of normal circulating metabolites. This can also be observed in patients with insulinoma who develop adaptive mechanisms such as insulin resistance and short periods of fasting and only infrequently suffer from symptoms of hypoglycemia (58).
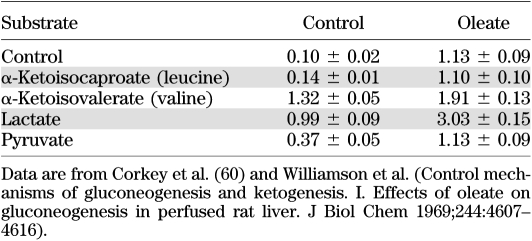
We previously documented an increase in mitochondrial redox indicated by the β/A ratio in the liver that occurred in response to branched-chain ketoacids, as well as lactate, and was exaggerated in the presence of elevated FFA (Table 1) (59–63). Since elevated BCAA, FFA, lactate, and combinations of these metabolites are associated with diabetes and increase the liver redox state, they are expected to increase the blood redox state reflected in the β/A ratio (Fig. 2). Such an increase in redox could contribute to metabolic alteration in other organs and possibly sustained hyperinsulinemia in the β-cell.
TABLE 1.
Effect of branched-chain ketoacids and oleate on hepatic mitochondrial redox state
Much evidence indicates that redox changes with nutritional state and may serve to communicate the metabolic status to all tissues. These redox changes may influence various tissue-specific functions probably through ROS generation. Previous studies have explored the role of intracellular redox in regulating metabolism (30,64–68). The capacity of extracellular redox to communicate to the inside of the cell is potentially an important form of interorgan communication that may prove exciting for further investigation and possible intervention.
If the concept that redox-driven ROS generation is validated, particularly in humans, it may be possible to use this knowledge to prevent a cascade from β-cell hypersecretion leading to diabetes. The most striking example of rapid diabetes reversal is gastric bypass surgery (12,69).
An apparent cure of diabetes following Roux-en-Y gastric bypass surgery has been reported in the majority of patients with type 2 diabetes or impaired glucose tolerance (13). There is no evidence for a sustained β-cell defect. This even occurs in individuals who were insulin-requiring patients with diabetes before surgery. It will be important to determine whether changes in redox accompany the transition from diabetes to normoglycemia and especially to ascertain whether the relationship between redox and insulinemia can explain these findings.
In summary, there is evidence that lowering basal insulin can be achieved through gastric surgery (12,69), fat loss (70–72), or drug inhibition of secretion (15–17). Validation of β-cell–mediated insulin resistance via hypersecretion would lead to radically different and novel strategies for the treatment of insulin resistance and type 2 diabetes. Such validation would suggest possible early interventions for prevention of basal hypersecretion rather than early interventions that stimulate even more insulin secretion. It may even be possible to use natural nontoxic extracellular metabolites or diet to modulate intracellular signal transduction and fluxes based on this concept.
The approach I have discussed and the model I have presented (Fig. 3) introduce the novel concept of redox as a master regulator of metabolism. Metabolism generates signals to alter metabolic function in β-cells and other tissues thus regulating anabolic and catabolic function appropriately. This is perhaps analogous to the generally accepted concept of transcriptional master switches that regulate families of anabolic and catabolic genes. I have also suggested that it is important to assess environmental factors that have arisen in recent decades as modifiers of redox or ROS.
In this conceptual model, insulin resistance is caused by hyperinsulinemia and is an appropriate adaptation to the increased need to store fat in adipose tissue without causing hypoglycemia. Thus, insulin resistance is an adaptive response that successfully maintains normal circulating levels of fat and glucose as long as the β-cell is able to maintain sufficiently elevated insulin levels (57). Perhaps the time has come to expand our research focus to carefully investigate the environmental changes that have accompanied the epidemic of obesity and diabetes.
ACKNOWLEDGMENTS
It has been a unique privilege to have had some of the finest mentors in the world: Otto Loewi, Robert Steele, Britton Chance, and John Williamson; many outstanding long-term collaborators: Jude Deeney, Marc Prentki, Christopher Rhodes, Orian Shirihai, Sheila Collins, and P.-O. Berggren; and my current mentor and Chair David Coleman. Support for the experimental work that forms the basis for this article was provided by the High Throughput Core, Cellular Imaging Core, and Analytical Instrumentation Core of the Department of Medicine, Boston University, and the National Institutes of Health grants DK35914, DK56690, and DK46200.
REFERENCES
- 1.Keith SW, Redden DT, Katzmarzyk PT, et al. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int J Obes (Lond) 2006;30:1585–1594 [DOI] [PubMed] [Google Scholar]
- 2.Elam TE, Preston RL. Fifty years of pharmaceutical technology and its impact on the beef we provide to consumers [article online], 2004. Available from http://www.feedstuffsfoodlink.com/Media/MediaManager/whitePaper-summary.pdf. Accessed August 2004
- 3.U.S. Environmental Protection Agency Poultry production [Internet]. Available from http://www.epa.gov/oecaagct/ag101/printpoultry.html. Accessed 25 September 2009
- 4.Mayer AM. Historical changes in the mineral content of fruits and vegetables. Br Food J 1997;99:207–211 [Google Scholar]
- 5.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol 2007;24:139–177 [DOI] [PubMed] [Google Scholar]
- 6.Leranth C, Hajszan T, Szigeti-Buck K, Bober J, MacLusky NJ. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc Natl Acad Sci U S A 2008;105:14187–14191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacLusky NJ, Hajszan T, Leranth C. The environmental estrogen bisphenol A inhibits estradiol-induced hippocampal synaptogenesis. Environ Health Perspect 2005;113:675–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith DL, Jr, Elam CF, Jr, Mattison JA, et al. Metformin supplementation and life span in Fischer-344 rats. J Gerontol A Biol Sci Med Sci 2010;65:468–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masuzaki H, Flier JS. Tissue-specific glucocorticoid reactivating enzyme, 11 beta-hydroxysteroid dehydrogenase type 1 (11 beta-HSD1)—a promising drug target for the treatment of metabolic syndrome. Curr Drug Targets Immune Endocr Metabol Disord 2003;3:255–262 [DOI] [PubMed] [Google Scholar]
- 10.Shinozaki S, Choi CS, Shimizu N, et al. Liver-specific inducible nitric-oxide synthase expression is sufficient to cause hepatic insulin resistance and mild hyperglycemia in mice. J Biol Chem 2011;286:34959–34975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonner C, Farrelly AM, Concannon CG, et al. Bone morphogenetic protein 3 controls insulin gene expression and is down-regulated in INS-1 cells inducibly expressing a hepatocyte nuclear factor 1A-maturity-onset diabetes of the young mutation. J Biol Chem 2011;286:25719–25728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reed MA, Pories WJ, Chapman W, et al. Roux-en-Y gastric bypass corrects hyperinsulinemia implications for the remission of type 2 diabetes. J Clin Endocrinol Metab 2011;96:2525–2531 [DOI] [PubMed] [Google Scholar]
- 13.Pories WJ, MacDonald KG, Jr, Morgan EJ, et al. Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am J Clin Nutr 1992;55(Suppl):582S–585S [DOI] [PubMed] [Google Scholar]
- 14.Destefano MB, Stern JS, Castonguay TW. Effect of chronic insulin administration on food intake and body weight in rats. Physiol Behav 1991;50:801–806 [DOI] [PubMed] [Google Scholar]
- 15.Alemzadeh R, Jacobs W, Pitukcheewanont P. Antiobesity effect of diazoxide in obese Zucker rats. Metabolism 1996;45:334–341 [DOI] [PubMed] [Google Scholar]
- 16.Alemzadeh R, Langley G, Upchurch L, Smith P, Slonim AE. Beneficial effect of diazoxide in obese hyperinsulinemic adults. J Clin Endocrinol Metab 1998;83:1911–1915 [DOI] [PubMed] [Google Scholar]
- 17.Greenwood RH, Mahler RF, Hales CN. Improvement in insulin secretion in diabetes after diazoxide. Lancet 1976;1:444–447 [DOI] [PubMed] [Google Scholar]
- 18.Astiz M, Rackow EC, Weil MH, Schumer W. Early impairment of oxidative metabolism and energy production in severe sepsis. Circ Shock 1988;26:311–320 [PubMed] [Google Scholar]
- 19.Wasserman K, Beaver WL, Davis JA, Pu JZ, Heber D, Whipp BJ. Lactate, pyruvate, and lactate-to-pyruvate ratio during exercise and recovery. J Appl Physiol 1985;59:935–940 [DOI] [PubMed] [Google Scholar]
- 20.Foster KJ, Alberti KG, Hinks L, et al. Blood intermediary metabolite and insulin concentrations after an overnight fast: reference ranges for adults, and interrelations. Clin Chem 1978;24:1568–1572 [PubMed] [Google Scholar]
- 21.Zammit VA. Regulation of hepatic fatty acid metabolism. The activities of mitochondrial and microsomal acyl-CoA:sn-glycerol 3-phosphate O-acyltransferase and the concentrations of malonyl-CoA, non-esterified and esterified carnitine, glycerol 3-phosphate, ketone bodies and long-chain acyl-CoA esters in livers of fed or starved pregnant, lactating and weaned rats. Biochem J 1981;198:75–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boden G, Chen X. Effects of fatty acids and ketone bodies on basal insulin secretion in type 2 diabetes. Diabetes 1999;48:577–583 [DOI] [PubMed] [Google Scholar]
- 23.Boden G. Fatty acids and insulin resistance. Diabetes Care 1996;19:394–395 [DOI] [PubMed] [Google Scholar]
- 24.Boden G, Chen X, Rosner J, Barton M. Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes 1995;44:1239–1242 [DOI] [PubMed] [Google Scholar]
- 25.Fielding BA, Humphreys SM, Frayn KN. Mono- and di-acylglycerol concentrations in human plasma in relation to lipoprotein lipase activity. Biochem Soc Trans 1993;21:235S. [DOI] [PubMed] [Google Scholar]
- 26.Fielding BA, Humphreys SM, Allman RF, Frayn KN. Mono-, di- and triacylglycerol concentrations in human plasma: effects of heparin injection and of a high-fat meal. Clin Chim Acta 1993;216:167–173 [DOI] [PubMed] [Google Scholar]
- 27.Civelek VN, Deeney JT, Kubik K, Schultz V, Tornheim K, Corkey BE. Temporal sequence of metabolic and ionic events in glucose-stimulated clonal pancreatic beta-cells (HIT). Biochem J 1996;315:1015–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prentki M, Vischer S, Glennon MC, Regazzi R, Deeney JT, Corkey BE. Malonyl-CoA and long chain acyl-CoA esters as metabolic coupling factors in nutrient-induced insulin secretion. J Biol Chem 1992;267:5802–5810 [PubMed] [Google Scholar]
- 29.Pi J, Bai Y, Zhang Q, et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 2007;56:1783–1791 [DOI] [PubMed] [Google Scholar]
- 30.Pi J, Zhang Q, Fu J, et al. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol 2010;244:77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011;283:65–87 [DOI] [PubMed] [Google Scholar]
- 32.Tanaka Y, Gleason CE, Tran PO, Harmon JS, Robertson RP. Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc Natl Acad Sci U S A 1999;96:10857–10862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Civelek VN, Deeney JT, Shalosky NJ, et al. Regulation of pancreatic beta-cell mitochondrial metabolism: influence of Ca2+, substrate and ADP. Biochem J 1996;318:615–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henquin JC. Tolbutamide stimulation and inhibition of insulin release: studies of the underlying ionic mechanisms in isolated rat islets. Diabetologia 1980;18:151–160 [DOI] [PubMed] [Google Scholar]
- 35.Jonas JC, Gilon P, Henquin JC. Temporal and quantitative correlations between insulin secretion and stably elevated or oscillatory cytoplasmic Ca2+ in mouse pancreatic beta-cells. Diabetes 1998;47:1266–1273 [DOI] [PubMed] [Google Scholar]
- 36.Corkey BE, Deeney JT. Acyl CoA regulation of metabolism and signal transduction. Prog Clin Biol Res 1990;321:217–232 [PubMed] [Google Scholar]
- 37.Corkey BE, Glennon MC, Chen KS, Deeney JT, Matschinsky FM, Prentki M. A role for malonyl-CoA in glucose-stimulated insulin secretion from clonal pancreatic beta-cells. J Biol Chem 1989;264:21608–21612 [PubMed] [Google Scholar]
- 38.Deeney JT, Prentki M, Corkey BE. Metabolic control of beta-cell function. Semin Cell Dev Biol 2000;11:267–275 [DOI] [PubMed] [Google Scholar]
- 39.Prentki M, Corkey BE. Are the beta-cell signaling molecules malonyl-CoA and cystolic long-chain acyl-CoA implicated in multiple tissue defects of obesity and NIDDM? Diabetes 1996;45:273–283 [DOI] [PubMed] [Google Scholar]
- 40.Deeney JT, Gromada J, Høy M, et al. Acute stimulation with long chain acyl-CoA enhances exocytosis in insulin-secreting cells (HIT T-15 and NMRI beta-cells). J Biol Chem 2000;275:9363–9368 [DOI] [PubMed] [Google Scholar]
- 41.Stein DT, Esser V, Stevenson BE, et al. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J Clin Invest 1996;97:2728–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heck AM, Yanovski JA, Calis KA. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy 2000;20:270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corkey BE, Deeney JT, Yaney GC, Tornheim K, Prentki M. The role of long-chain fatty acyl-CoA esters in beta-cell signal transduction. J Nutr 2000;130(Suppl):299S–304S [DOI] [PubMed] [Google Scholar]
- 44.Tomoda H, Igarashi K, Omura S. Inhibition of acyl-CoA synthetase by triacsins. Biochim Biophys Acta 1987;921:595–598 [PubMed] [Google Scholar]
- 45.Vial G, Dubouchaud H, Couturier K, et al. Effects of a high-fat diet on energy metabolism and ROS production in rat liver. J Hepatol 2011;54:348–356 [DOI] [PubMed] [Google Scholar]
- 46.Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr 2006;26:1–22 [DOI] [PubMed] [Google Scholar]
- 47.Thiess S, Becskei C, Tomsa K, Lutz TA, Wanner M. Effects of high carbohydrate and high fat diet on plasma metabolite levels and on i.v. glucose tolerance test in intact and neutered male cats. J Feline Med Surg 2004;6:207–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williamson DH, Lund P, Krebs HA. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J 1967;103:514–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaw JH, Wolfe RR. Energy and substrate kinetics and oxidation during ketone infusion in septic dogs: role of changes in insulin and glucagon. Circ Shock 1984;14:63–79 [PubMed] [Google Scholar]
- 50.Shaw JH, Wolfe RR. Influence of beta-hydroxybutyrate infusion on glucose and free fatty acid metabolism in dogs. Am J Physiol 1984;247:E756–E764 [DOI] [PubMed] [Google Scholar]
- 51.Adimora NJ, Jones DP, Kemp ML. A model of redox kinetics implicates the thiol proteome in cellular hydrogen peroxide responses. Antioxid Redox Signal 2010;13:731–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Go YM, Park H, Koval M, et al. A key role for mitochondria in endothelial signaling by plasma cysteine/cystine redox potential. Free Radic Biol Med 2010;48:275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol 2002;348:93–112 [DOI] [PubMed] [Google Scholar]
- 54.Moriarty-Craige SE, Jones DP. Extracellular thiols and thiol/disulfide redox in metabolism. Annu Rev Nutr 2004;24:481–509 [DOI] [PubMed] [Google Scholar]
- 55.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laferrère B, Reilly D, Arias S, et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med 2011;3:80re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 2011;60:2441–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Black KO, Birnstingl MA. Insulinoma with symptoms for thirty years. Proc R Soc Med 1964;57:675–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corkey BE, Martin-Requero A, Brandt M, Williamson JR. Regulation of α-ketoisocaproate and α-ketoisovalerate metabolism and their interactions with the citric acid cycle in isolated hepatocytes. In Metabolism and Clinical Implications of Branched Chain Amino and Ketoacids. Walser M, Williamson JR, Eds. New York, Elsevier, 1981, p. 119–127 [Google Scholar]
- 60.Corkey BE, Martin-Requero A, Walajtys-Rode E, Williams RJ, Williamson JR. Regulation of the branched chain alpha-ketoacid pathway in liver. J Biol Chem 1982;257:9668–9676 [PubMed] [Google Scholar]
- 61.Martin-Requero A, Corkey BE, Cerdan S, Walajtys-Rode E, Parrilla RL, Williamson JR. Interactions between alpha-ketoisovalerate metabolism and the pathways of gluconeogenesis and urea synthesis in isolated hepatocytes. J Biol Chem 1983;258:3673–3681 [PubMed] [Google Scholar]
- 62.Williamson JR, Corkey BE, Martin-Requero A, Walajtys-Rode E, Coll KE. Metabolic repercussions of branched chain ketoacid metabolism in liver. In Problems and Potential of Branched Chain Amino Acids in Physiology and Medicine. Odessey R, Ed. Amsterdam, Elsevier, 1986, p. 135–172 [Google Scholar]
- 63.Williamson JR, Martin-Requero A, Corkey BE, Brandt M, Rothman R. Interactions between a-ketoisovalerate, propoinate, and fatty acids on gluconeogenesis and ureogenesis in isolated hepatocytes. In Metabolism and Clinical Implications of Branched Chain Amino and Ketoacids. Walser M, Williamson JR, Eds. New York, Elsevier, 1981, p. 105–117 [Google Scholar]
- 64.Ahn BH, Kim HS, Song S, et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A 2008;105:14447–14452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zee RS, Yoo CB, Pimentel DR, et al. Redox regulation of sirtuin-1 is mediated by S-glutathiolation. Antioxid Redox Signal 2010;13:1023–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Powers SK, Talbert EE, Adhihetty PJ. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol 2011;589:2129–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delmastro MM, Piganelli JD. Oxidative stress and redox modulation potential in type 1 diabetes. Clin Dev Immunol 2011;2011:593863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rath E, Haller D. Inflammation and cellular stress: a mechanistic link between immune-mediated and metabolically driven pathologies. Eur J Nutr 2011;50:219–233 [DOI] [PubMed] [Google Scholar]
- 69.Gavin TP, Sloan RC, 3rd, Lukosius EZ, et al. Duodenal-jejunal bypass surgery does not increase skeletal muscle insulin signal transduction or glucose disposal in Goto-Kakizaki type 2 diabetic rats. Obes Surg 2011;21:231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aasen G, Fagertun H, Halse J. Effect of loss of regional fat assessed by DXA on insulin resistance and dyslipidaemia in obese men. Scand J Clin Lab Invest 2010;70:547–553 [DOI] [PubMed] [Google Scholar]
- 71.Aasen G, Fagertun H, Halse J. Effect of regional fat loss assessed by DXA on insulin resistance and dyslipidaemia in obese women. Scand J Clin Lab Invest 2010;70:229–236 [DOI] [PubMed] [Google Scholar]
- 72.Seidell JC, Björntorp P, Sjöström L, Kvist H, Sannerstedt R. Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism 1990;39:897–901 [DOI] [PubMed] [Google Scholar]
- 73.Saadeh M, Ferrante T, Kane A, Shirihai O, Corkey BE, Deeney J.Reactive oxygen species stimulate insulin secretion in rat pancreatic islets: studies using mono-oleoyl-glycerol. PLoS One. In press [DOI] [PMC free article] [PubMed]
- 74.Al-Saleh A. Effect of Artificial Sweeteners on Insulin Secretion, ROS and Oxygen Consumption in Pancreatic β-Cells. Boston, MA, Boston University School of Medicine, 2010
- 75.Yaney GC, Civelek VN, Richard AM, et al. Glucagon-like peptide 1 stimulates lipolysis in clonal pancreatic beta-cells (HIT). Diabetes 2001;50:56–62 [DOI] [PubMed] [Google Scholar]