Abstract
B cells participate in the priming of the allo- and autoimmune responses, and their depletion can thus be advantageous for islet transplantation. Herein, we provide an extensive study of the effect of B-cell depletion in murine models of islet transplantation. Islet transplantation was performed in hyperglycemic B-cell–deficient(μMT) mice, in a purely alloimmune setting (BALB/c into hyperglycemic C57BL/6), in a purely autoimmune setting (NOD.SCID into hyperglycemic NOD), and in a mixed allo-/autoimmune setting (BALB/c into hyperglycemic NOD). Inotuzumab ozogamicin murine analog (anti-CD22 monoclonal antibody conjugated with calicheamicin [anti-CD22/cal]) efficiently depleted B cells in all three models of islet transplantation examined. Islet graft survival was significantly prolonged in B-cell–depleted mice compared with control groups in transplants of islets from BALB/c into C57BL/6 (mean survival time [MST]: 16.5 vs. 12.0 days; P = 0.004), from NOD.SCID into NOD (MST: 23.5 vs. 14.0 days; P = 0.03), and from BALB/c into NOD (MST: 12.0 vs. 5.5 days; P = 0.003). In the BALB/c into B-cell–deficient mice model, islet survival was prolonged as well (MST: μMT = 32.5 vs. WT = 14 days; P = 0.002). Pathology revealed reduced CD3+ cell islet infiltration and confirmed the absence of B cells in treated mice. Mechanistically, effector T cells were reduced in number, concomitant with a peripheral Th2 profile skewing and ex vivo recipient hyporesponsiveness toward donor-derived antigen as well as islet autoantigens. Finally, an anti-CD22/cal and CTLA4-Ig–based combination therapy displayed remarkable prolongation of graft survival in the stringent model of islet transplantation (BALB/c into NOD). Anti-CD22/cal–mediated B-cell depletion promotes the reduction of the anti-islet immune response in various models of islet transplantation.
B cells traditionally have been investigated for their impact on hyperacute and chronic rejection (1–3) by virtue of their capacity to produce alloantibodies (4). In the past decade, investigators have focused on the antigen-presenting function of B cells in the priming of autoimmunity (5,6) and activation of allo- and autoreactive T cells (7–12). This notion is emphasized by two recent works from Yale University (11) and from our group (9), which demonstrate that B-cell–depletion strategies are capable of reversing established diabetes in NOD mice. B cells have been shown to be crucial players in the indirect allorecognition pathway as well by displaying a remarkable ability to capture alloantigens and activate alloreactive T cells (5,13). Indeed, Noorchashm et al. (5) demonstrated prolongation of heart allograft survival, disruption of antibody production, and a decrease in CD4+ T-cell activation in a chimeric model of B-cell–restricted MHC class II–mediated antigen presentation deficiency. In the wake of these findings, authors have directed their attention to B-cell–depleting strategies to prolong graft survival (14–17). Among all transplant models, islet transplantation is of particular interest in applying a B-cell–based depleting strategy because islets endure attack from the allo- and autoimmune responses (18–20), but it is surprising that a systemic study of the role of B cells in murine models of islet transplantation is lacking. Indeed, treatment with anti-CD20 monoclonal antibody (mAb)—specifically, rituximab—in association with antithymocyte globulin induction, followed by a sirolimus-based immunosuppressive regimen, was found to improve long-term islet allograft survival in the nonautoimmune model of nonhuman primates (10), and a B-cell–depletion strategy is currently under investigation in a phase II clinical trial (NCT00468442). We have recently proposed the use of a B-cell–targeted cytotoxic immunoconjugate in autoimmune diabetes (anti-CD22 conjugated to calicheamicin, anti-CD22/cal or inotuzumab ozogamicin murine analog) capable of achieving complete depletion of B cells in peripheral blood, spleen, bone marrow, and lymph nodes (21). Given that CD22 also is expressed on CD138+ plasma cells, this compound also has a potential effect on autoantibody production (9). Thus, we aimed to examine the effect of an inotuzumab ozogamicin murine analog–based B-cell–depletion strategy (anti-CD22/cal) in three different models of islet transplantation (BALB/c into C57BL/6, NOD.SCID into NOD, and BALB/c into NOD) in which allo- and autoimmune responses, separately or jointly, are responsible for the destruction of islet grafts (22,23). This approach will ultimately allow us to distinguish the beneficial effects of B-cell depletion on the different paths of the anti-islet immune response. Finally, we propose a novel and highly translational immunosuppressive strategy in islet transplantation with the goal of completely inhibiting indirect alloantigen presentation, based on the disruption of both primary aspects of antigen presentation (i.e., B-cell–mediated and dendritic cell [DC]-mediated T-cell activation).
RESEARCH DESIGN AND METHODS
Mice.
Female C57BL/6, BALB/c, B6.C.H-2bm12 (bm12), NOD, and NOD.SCID mice were obtained from The Jackson Laboratory (Bar Harbor, ME). B-cell–deficient (μMT) mice on a C57BL/6 genetic background were purchased from The Jackson Laboratory as well. CD4+ T-cell receptor transgenic mice specific for I-Abm12 expressed on bm12 mice (ABM) were available in our own laboratory (24).
Study design and treatment protocols.
The inotuzumab ozogamicin murine analog compound (anti-CD22/cal mAb, provided by Pfizer) is a conjugate of an anti-mouse CD22 mAb and N-acetyl-calicheamicin dimethyl acid (9). Three murine models of islet transplantation were tested: 1) a purely alloimmune setting (BALB/c islets transplanted into C57BL/6 mice) labeled as allo, 2) a purely autoimmune setting (NOD.SCID islets transplanted into NOD mice) labeled as auto, and 3) a mixed allo- and autoimmune setting (BALB/c islets transplanted into NOD mice) labeled as allo/auto. Anti-CD22/cal was administered in two injections (160 μg i.p. at days 0 and 5), as was cal treatment, which was given at the same dosage and timing. A group of mice was left untreated. CTLA4-Ig was administered to NOD mice using the following protocol: 500 μg on day 0 and 250 μg on days 2, 4, 6, 8, and 10. NOD mice were transplanted after a minimum of 7 to a maximum of 14 days from the onset of hyperglycemia to avoid a possible confounding effect, since we demonstrated that diabetes may be reverted by B-cell depletion within 3 days from the onset of hyperglycemia (9). When required, streptozotocin-induced diabetic or spontaneously hyperglycemic NOD mice were kept alive by insulin pellet.
ELISpot and Luminex.
ELISpot (BD Biosciences, San Jose, CA) and Luminex assays (Millipore, Billerica, MA) were performed, as described, to measure cytokines (25).
Statistical analysis.
Data are expressed as means ± SEM. Kaplan-Meier analysis was used for survival analysis. To compare groups, we used a two-sided unpaired Student t test (for parametric data) or Mann-Whitney U test (for nonparametric data) according to distribution. P < 0.05 (by two-tailed testing) was considered an indicator of statistical significance. Data were analyzed with an SPSS statistical package for Windows (SPSS, Chicago, IL).
RESULTS
Inotuzumab ozogamicin murine analog (anti-CD22/cal) depletes B cells in islet-transplanted mice.
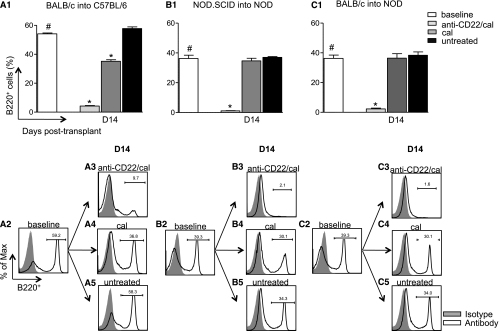
We first evaluated the effect of islet transplantation on peripheral B-cell numbers. In chemically (streptozotocin) induced hyperglycemic C57BL/6 mice receiving BALB/c islets, the percentage of splenic B220+ cells remained unchanged at days 7, 14, and 28 after islet transplantation (data not shown). To assess whether treatment with anti-CD22/cal was able to efficiently deplete B cells in murine models, we performed islet transplantation in three different immune settings: 1) a purely alloimmune setting (BALB/c islets into chemically induced hyperglycemic C57BL/6 mice) or allo, 2) a purely autoimmune setting (NOD.SCID islets into spontaneously hyperglycemic NOD mice) or auto, and 3) a mixed allo- and autoimmune setting (BALB/c islets into spontaneously hyperglycemic NOD mice) or allo/auto. The percentage of B cells in the spleens of mice treated with anti-CD22/cal (160 μg i.p., at days 0 and 5) or cal (160 μg i.p., at days 0 and 5) or untreated was evaluated at different time points by fluorescence-activated cell sorter analysis (Fig. 1). In all three models of islet transplantation, anti-CD22/cal treatment depleted B cells up to 14 days after transplantation (Fig. 1). In cal-treated mice (in C57BL/6 but not NOD recipient mice), a small B-cell depletion was evident. Of note, a strain-dependent effect with regard to the efficacy of B-cell depletion was observed. Indeed, a less profound depletion was evident in anti-CD22/cal–treated C57BL/6 mice receiving BALB/c islets (Fig. 1A, 3), compared with NOD mice receiving NOD.SCID or BALB/c (Fig. 1B, 3 and C, 3). The full recovery of B cells after anti-CD22/cal treatment was observed at ∼8 weeks after transplantation in both C57BL/6 and NOD mice (data not shown).
FIG. 1.
Anti-CD22/cal–mediated B-cell depletion in three immune settings of islet transplantation (allo, auto, and allo/auto). The percentage of B220+ cells was analyzed in the spleen of C57BL/6 and NOD mice at baseline and at 14 days after islet transplantation (n = 4 mice/condition). 1) In allo, BALB/c islets were transplanted into chemically induced C57BL/6 mice and treated with anti-CD22/cal, cal alone, or were left untreated (A1–5). Robust depletion of B220+ cells was evident 14 days after transplantation in mice treated with anti-CD22/cal compared with naïve untransplanted mice (baseline) (#P < 0.0001) and control antibody (cal)-treated mice (*P < 0.0001) (A1–5). 2) In auto, NOD.SCID islets were transplanted under the kidney capsule of spontaneously hyperglycemic NOD mice (B1–5). Complete B-cell depletion was observed 14 days after transplantation in mice treated with anti-CD22/cal compared with naive untransplanted (#P < 0.0001) and control-treated mice (*P < 0.0001) (B1–5), whereas no depleting effect was found when mice were treated with cal or were untreated (NS). 3) In allo/auto, BALB/c islets were transplanted into spontaneously hyperglycemic NOD mice (C1–5). A complete B-cell depletion occurred 14 days after transplantation in mice treated with anti-CD22/cal compared with untransplanted (#P < 0.0001), cal-treated (*P < 0.0001), and untreated mice (*P < 0.0001) (C1–5), whereas no differences in B-cell peripheral percentage were observed in mice treated with cal or untreated (NS) (C4–5).
B-cell depletion and B-cell deficiency prolong islet graft survival without inducing tolerance.
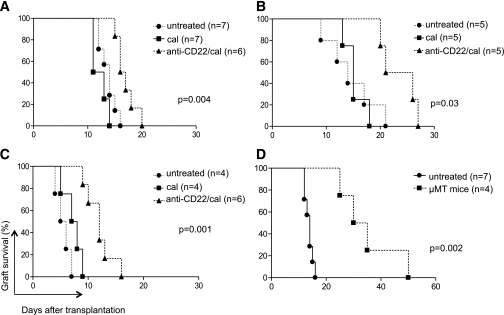
We evaluated the effect of anti-CD22/cal–mediated B-cell depletion on islet allograft survival in the three aforementioned settings of the immune response (Fig. 2A–D). In allo, untreated and control antibody (cal)-treated mice invariably rejected allografts (mean survival time [MST]: untreated = 14.0 days; cal = 12.0 days; NS) (Fig. 2A), whereas mice that were B-cell depleted with anti-CD22/cal treatment displayed a slight prolongation in allograft survival compared with controls (MST: anti-CD22/cal = 16.5 days; anti-CD22/cal vs. all, P < 0.05) (Fig. 2A). In auto, mice treated with anti-CD22/cal showed a significant delay in syngeneic islet graft rejection compared with both cal-treated and untreated mice (MST: anti-CD22/cal = 23.5 days; untreated = 14.0 days; cal = 15.0 days; anti-CD22/cal vs. all, P < 0.05) (Fig. 2B). In allo/auto, in which both auto- and alloimmunity exert their effects, untreated and cal-treated mice rapidly rejected islet grafts within a few days after transplantation, whereas B-cell–depleted mice displayed significant prolongation of graft survival (MST: anti-CD22/cal = 12.0 days; untreated = 5.5; cal = 7.5 days; anti-CD22/cal vs. all, P < 0.05) (Fig. 2C). To study the effect of the absence of B cells on islet allograft survival, we transplanted BALB/c islets into genetically B-cell–deficient μMT mice (on a C57BL/6 background); in this setting, islet rejection was significantly delayed compared with wild-type (WT) control mice (MST: WT = 14.0 vs. μMT = 32.5 days; P = 0.002), but none of the islet-transplanted mice achieved graft tolerance (Fig. 2D).
FIG. 2.
Islet graft survival in B-cell–deficient (µMT) and B-cell–depleted (anti-CD22/cal) mice in the three immune settings of islet transplantation (allo, auto, and allo/auto). 1) In allo, a delay in graft rejection was evident in B-cell–depleted mice compared with control-treated and untreated mice (P = 0.004) (A). 2) In auto, a prolongation of syngeneic graft survival was observed in B-cell–depleted mice compared with cal-treated (P = 0.006) and untreated mice (P = 0.03) (B). 3) In allo/auto, delayed graft rejection was achieved in B-cell–depleted mice compared with cal-treated (P = 0.003) and untreated mice (P = 0.001) (C). 4) When BALB/c islets were transplanted in chemically induced C57BL/6 B-cell–deficient mice, islet graft survival was significantly prolonged compared with WT C57BL/6 mice (P = 0.002) (D).
B-cell depletion preserves islet morphology.
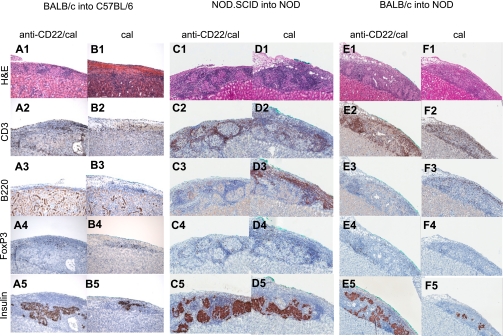
Islet grafts were analyzed at different time points in the three aforementioned immune settings of islet transplantation to evaluate the effect of B-cell depletion on the quality of graft structure and islet infiltrate. Grafts from BALB/c into C57BL/6 and NOD.SCID into NOD transplants were collected 14 days after transplantation, whereas BALB/c into NOD transplant grafts were collected at day 7 (the average time of rejection of control mice was chosen) (Fig. 3). In allo, mice treated with anti-CD22/cal (Fig. 3A, 1–5) displayed preserved islet structure with a mild CD3+ cell infiltrate and no B220+ cells in the graft compared with control treated mice (Fig. 3B, 1–5). In auto, anti-CD22/cal–treated mice showed a clear preservation of islet structure with some CD3+ cells surrounding the islets, few B220+ and FoxP3+ cells, and a clear insulin-positive cell staining (Fig. 3C, 1–5). Cal-treated mice (Fig. 3D, 1–5) maintained preservation of islet structure with some insulin staining and abundant CD3+ and B220+ cells infiltrating the graft. In allo/auto at day 7, islet morphology was maintained in anti-CD22/cal–treated mice (Fig. 3E, 1–5), with clear insulin staining but several CD3+ cells (and some B220+ cells) infiltrating the graft. Conversely, cal-treated mice (Fig. 3F, 1–5) displayed an equal distribution of CD3+ and B220+ cells throughout the islet graft, resulting in a complete disruption of islet architecture and few remaining insulin-positive cells in the remnants of β-cells.
FIG. 3.
Islet pathology in the three immune settings of islet transplantation (allo, auto, and allo/auto). Islets from BALB/c or NOD.SCID mice were transplanted under the kidney capsule of chemically induced hyperglycemic C57BL/6 or spontaneously hyperglycemic NOD mice treated with anti-CD22/cal or cal and were retrieved for histological analysis at different time points (n = 3 mice/group). Grafts from both BALB/c into C57BL/6– and NOD.SCID into NOD–transplanted mice were collected 14 days after transplantation, whereas BALB/c into NOD grafts were collected at day 7 at the time of graft rejection of control mice. 1) In allo at day 14, anti-CD22/cal–treated mice displayed preserved islet architecture (A1) with mild CD3+ cell infiltrate (A2), no B220+ cells (A3), and few FoxP3+ cells (A4). Insulin staining was clearly preserved (A5). Cal-treated and untreated mice showed severe cellular infiltration with a complete disruption of islet architecture (B1) and several CD3+ (B2) and B220+ cells (B3); some CD3+ cells also coexpressed FoxP3+ (B4). Very few cells were positive for insulin (B5). 2) In auto at day 14, anti-CD22/cal–treated mice displayed clear preservation of islet structure (C1) with several CD3+ cells in the infiltrate (C2) surrounding the islets, along with few B220+ (C3) and FoxP3+ cells (C4) as well as abundant insulin-positive cells (C5). Cal-treated mice maintained preservation of islet structure (D1) with insulin-positive cells (D5) but with a massive CD3+ (D2) and B220+ (D3) cell infiltrate and with some FoxP3+ (D4) cells evident in the islet mass. 3) In allo/auto at day 7, islet architecture appeared preserved in anti-CD22/cal–treated mice (E1) with clear insulin staining (E5) but with severe CD3+ cell (E2) infiltrate and few FoxP3+ cells (E4) surrounding the islets, whereas B cells were absent (E3). Conversely, cal-treated mice displayed equally distributed CD3+ (F2) and B220+ (F3) cell infiltration, resulting in complete disruption of islet architecture (F1) and scant insulin staining (F5). Almost no infiltrating FoxP3+ cells were evident (F4). H&E, hematoxylin-eosin. (A high-quality digital representation of this figure is available in the online issue.)
B-cell depletion halts the expansion of effector T cells.
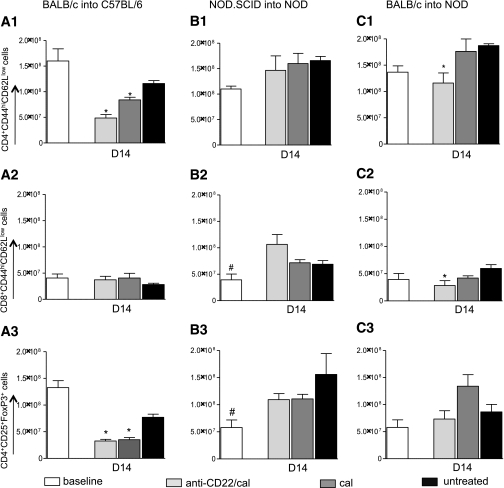
We studied the effect of B-cell depletion on the immune system and particularly on effector T cells (Teffs) and regulatory T cells (Tregs) 14 days after islet transplantation in allo and auto and at day 7 in allo/auto. Teffs and Tregs were quantified in the spleens of anti-CD22/cal–treated, cal-treated, or untreated mice. In allo, anti-CD22/cal–treated mice displayed a significant reduction in CD4+CD44hiCD62Llow (CD4+ effector) cell number compared with control mice (anti-CD22/cal = 4.68 × 107 ± 6.8 × 106; untreated = 11.6 × 107 ± 5.6 × 106; cal = 8.4 × 107 ± 5.2 × 106, absolute number of cells; anti-CD22/cal vs. all, P < 0.05) (Fig. 4A, 1), whereas cal-treated mice showed a partial reduction in CD4+ effector cell number (cal vs. untreated, P = 0.01) (Fig. 4A, 1). No differences in the number of peripheral CD8+CD44hiCD62Llow (CD8+ effector) cells were evident among the three groups (Fig. 4A, 2). In this model, B-cell depletion is associated with an overall reduction of peripheral CD4+CD25+FoxP3+ (Tregs) both in anti-CD22/cal– and cal alone–treated mice compared with untreated mice (Fig. 4A, 3). In auto, no significant differences in the absolute numbers of Teffs and Tregs were evident among the three groups of mice studied (Fig. 4B, 1–3). Indeed, anti-CD22/cal treatment did not affect CD4+ and CD8+ Teff number, nor did it affect Treg number. In allo/auto, a reduction in the absolute number of Teffs was found in anti-CD22/cal–treated mice compared with cal-treated and untreated mice. Both CD4+ and CD8+ Teffs (Fig. 4C, 1–2) were reduced in anti-CD22/cal–treated mice, whereas no differences were evident in the absolute number of Tregs (Fig. 4C, 3).
FIG. 4.
Effect of B-cell depletion on the immune system in the three immune settings of islet transplantation (allo, auto, and allo/auto). Splenocytes were analyzed in the three groups of islet-transplanted mice at day 14 in the BALB/c into C57BL/6 and NOD.SCID into NOD groups and at day 7 in the BALB/c into NOD group (n = 3 mice/experiment). 1) In allo, anti-CD22/cal treatment induced a decrease in the absolute number of CD4+CD44hiCD62Llow cells compared with untreated (*P = 0.0002) and cal-treated (*P = 0.01) mice (A1). Cal-treated mice, which experience a partial B-cell depletion, displayed a significant reduction in CD4+CD44hiCD62Llow cell (Teff) numbers as well (*P = 0.01) (A1). No differences in the absolute numbers of CD8+CD44hiCD62Llow cells (Teffs) were observed in this model (A2). In the same immune setting, a reduction in CD4+CD25+FoxP3+ cell numbers was evident in the anti-CD22/cal–treated (*P < 0.0001) and in cal-treated (*P = 0.0006) groups compared with the untreated group (A3). 2) In auto, no differences were evident with regard to either CD4+CD44hiCD62Llow or CD8+CD44hiCD62Llow cell numbers as well as in the peripheral numbers of CD4+CD25+FoxP3+ cells in the B-cell–depleted group compared with both untreated and cal-treated mice (B1–3). Naïve untransplanted mice displayed reduced numbers of CD8+CD44hiCD62Llow T cells compared with anti-CD22/cal–treated mice (#P = 0.03) (B2), as well as reduced numbers of CD4+CD25+FoxP3+ cells compared with both anti-CD22/cal–treated (#P = 0.04) and cal-treated mice (#P = 0.03) (B3). 3) In allo/auto, anti-CD22/cal–treated mice displayed a significant reduction in CD4+CD44hiCD62Llow (*P = 0.02) and CD8+CD44hiCD62Llow (*P = 0.04) cell numbers (C1–2), with no significant differences in CD4+CD25+FoxP3+ cell numbers (C3).
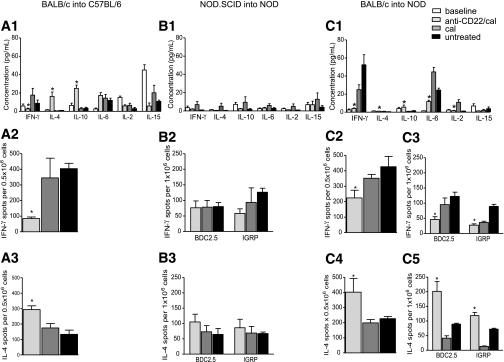
B-cell depletion reshapes the peripheral cytokine profile by skewing Th1 to Th2.
We then evaluated the effect of B-cell depletion on peripheral cytokine profile after islet transplantation using the Luminex assay. In allo 14 days after islet transplantation, peripheral levels of the Th1 cytokine γ-interferon (IFN-γ) were reduced in the anti-CD22/cal–treated group, whereas an increase in peripheral levels of the Th2 cytokines interleukin (IL)-4 and IL-10 was evident (Fig. 5A, 1). No differences in the proinflammatory cytokines IL-6, IL-2, and IL-15 peripheral levels were noted (Fig. 5A, 1). In auto, no polarization of the immune response was observed by serum analysis after transplantation (Fig. 5B, 1). In this model, peripheral levels of Th1, Th2 (IL-4 and IL-10), and proinflammatory cytokines (IL-6) were unaffected by B-cell depletion. In allo/auto 7 days after islet transplantation, an overall suppression of the Th1 response was evident, with B-cell–depleted mice showing lower peripheral levels of IFN-γ (anti-CD22/cal = 4.2 ± 2.1 pg/mL; untreated = 52.5 ± 11.1 pg/mL; cal = 24.9 ± 5.5 pg/mL; anti-CD22/cal vs. all, P < 0.01) (Fig. 5C, 1). Moreover, B-cell–depleted mice showed an increase in peripheral levels of the Th2 cytokines IL-4 (anti-CD22/cal = 1.1 ± 0.1 pg/mL; untreated = 0.5 ± 0.1 pg/mL; cal = 1.0 ± 0.1 pg/mL; anti-CD22/cal vs. untreated, P < 0.0001) and IL-10 (anti-CD22/cal = 5.5 ± 2.3 pg/mL; untreated = 1.5 ± 0.3 pg/mL; cal = 1.0 ± 0.0 pg/mL; anti-CD22/cal vs. untreated, P = 0.03), thus confirming the skewing of Th1 to Th2 immune response (Fig. 5C, 1). Finally, proinflammatory cytokines IL-2 (anti-CD22/cal = 1.6 ± 0.3 pg/mL; untreated = 1.2 ± 0.1 pg/mL; cal = 11.0 ± 2.9 pg/mL; anti-CD22/cal vs. cal, P = 0.009) and IL-6 (anti-CD22/cal = 11.7 ± 1.4 pg/mL; untreated = 24.4 ± 2.1 pg/mL; cal = 44.5 ± 5.0 pg/mL; anti-CD22/cal vs. all, P < 0.01) were reduced compared with controls (Fig. 5C, 1).
FIG. 5.
Th1 to Th2 skewing of the immune response in the three immune settings of islet transplantation (allo, auto, and allo/auto) after B-cell depletion. Serum samples (n = 3 samples/experiment) and splenocytes (n = 3 mice/experiment) were analyzed in the three groups of islet-transplanted mice at day 14 in the BALB/c into C57BL/6 and NOD.SCID into NOD groups and at day 7 in the BALB/c into NOD group. Peripheral cytokine profiles and polarization of the auto- and alloimmune response were examined. 1) In allo, a decrease in the Th1 cytokine IFN-γ (*P < 0.05) and an increase in the Th2-relevant cytokines IL-4 (*P < 0.01) and IL-10 (*P < 0.001) were evident in B-cell–depleted mice compared with cal-treated and untreated mice (A1). These data were confirmed by the ELISpot assay in which donor-derived antigens (irradiated BALB/c splenocytes) were plated with splenocytes harvested from transplant recipients undergoing the different treatments. We found that anti-CD22/cal–treated mice exhibit a reduced number of IFN-γ–secreting cells compared with cal-treated and untreated mice (*P < 0.05) (A2), whereas IL-4–secreting cell numbers were significantly increased (*P < 0.05) (A3). 2) In auto, no change in peripheral cytokine levels was evident in the three groups of mice studied (B1), and similar numbers of IFN-γ– and IL-4–producing cells (B2 and B3, respectively) were observed after ex vivo restimulation with β-cell peptides (BDC2.5 and IGRP). 3) In allo/auto, examination of the cytokine profile (C1) revealed a systemic suppression of the inflammatory response (IL-6, *P < 0.01) with increased levels of the Th2-relevant cytokines IL-4 (*P < 0.01) and IL-10 (*P < 0.05) and a complete suppression in the Th1 immune response (IFN-γ, *P < 0.01; IL-2, *P < 0.01). In this animal model, both the allo- and autoimmune responses were examined (C2–5). Donor-derived antigen (BALB/c splenocytes) and islet peptides (BDC2.5 and IGRP) were plated with splenocytes harvested from the three groups of mice studied at day 7 after transplantation. B-cell–depleted mice displayed reduced numbers of IFN-γ–producing cells (*P < 0.05) and increased numbers of IL-4–producing cells (*P < 0.05) among both donor-specific T cells (stimulated using BALB/c splenocytes) (C2 and 4) and CD4+ (BDC2.5-stimulated, *P < 0.05) and CD8+ (IGRP-stimulated, *P < 0.05) (C3 and 5) autoreactive T cells.
B-cell depletion promotes antidonor hyporesponsiveness.
We also examined the effect of B-cell depletion on in vitro rechallenge with donor-derived alloantigens or islet-derived autoantigens using the ELISpot assay. In allo, splenocytes from transplanted mice were harvested 14 days after islet transplantation and rechallenged ex vivo with donor-derived antigen (i.e., BALB/c irradiated splenocytes) to stimulate an antidonor-specific alloimmune response. Anti-CD22/cal–treated mice displayed a reduction in global responsiveness toward alloantigens with a skewing of the Th1 response toward a Th2 profile. IFN-γ–producing cells were substantially reduced in B-cell–depleted mice (anti-CD22/cal = 84.6 ± 10.3; untreated = 404.3 ± 34.2; cal = 345.3 ± 125.7, counted as number of IFN-γ–producing cells per 5 × 105 total splenocytes; anti-CD22/cal vs. all, P < 0.05), with an increase in IL-4–producing cells (anti-CD22/cal = 293.6 ± 24.5; untreated = 133.3 ± 27.2; cal = 174.8 ± 28.1, counted as number of IL-4–producing cells per 5 × 105 total splenocytes; anti-CD22/cal vs. all, P < 0.05) (Fig. 5A, 2–3). In auto, the rechallenge of splenocytes 14 days after transplantation with islet autoantigens (i.e., BDC2.5 and islet-specific glucose-6-phosphatase catalytic subunit-related protein [IGRP]) did not show any differences in responsiveness toward autoantigens in anti-CD22/cal–treated, untreated, or cal-treated mice (Fig. 5B, 2). In allo/auto, splenocytes from transplanted mice 7 days after transplantation were rechallenged ex vivo with donor-derived antigen (i.e., BALB/c irradiated splenocytes) and islet-derived peptides. The ex vivo challenge with alloantigens confirmed that B-cell–depleted mice were donor hyporesponsive, with fewer IFN-γ–producing cells (anti-CD22/cal = 224.5 ± 49.7; untreated = 426.5 ± 66.9; cal = 352.0 ± 24.5, counted as number of IFN-γ–producing cells per 5 × 105 total splenocytes; anti-CD22/cal vs. all, P < 0.05) and more IL-4–producing cells (anti-CD22/cal = 402.2 ± 91.2; untreated = 227.0 ± 13.9; cal = 199.1 ± 22.0, counted as number of IL-4–producing cells per 5 × 105 total splenocytes; anti-CD22/cal vs. all, P < 0.05) (Fig. 5C, 2 and 4). These results were confirmed by ex vivo challenge of splenocytes harvested from islet-transplanted mice with CD4+ and CD8+ T-cell–restricted islet peptides (BDC2.5 and IGRP, respectively), demonstrating hyporesponsiveness toward autoantigens as well as a clear shift from a Th1 to a Th2 immune response in B-cell–depleted mice compared with both untreated and cal-treated controls (Fig. 5C, 3 and 5).
B-cell absence induces Th1 to Th2 skewing in vitro.
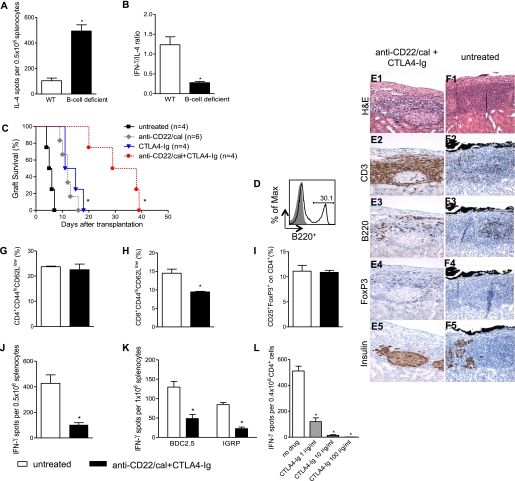
To provide clear evidence of the link between the absence of B cells and the skewing of the immune system from a Th1 toward a Th2 response, we performed an in vitro mixed-lymphocytes reaction where 0.5 × 106 irradiated splenocytes of BALB/c mice were challenged with 0.5 × 106 splenocytes harvested from B-cell–deficient (µMT) or C57BL/6 WT mice in an ELISpot assay. The number of IFN-γ–producing cells was similar between the two groups (WT = 115 ± 28; B-cell deficient = 139 ± 28 number of IFN-γ–producing cells; NS), whereas the number of IL-4–producing cells increased in the absence of B cells (WT = 104 ± 20; B-cell deficient = 493 ± 48 number of IL-4–producing cells; P = 0.001) (Fig. 6A), with a lower IFN-γ–to–IL-4 ratio (WT = 1.2 ± 0.2; B-cell deficient = 0.2 ± 0.03 IFN-γ–to–IL-4 ratio; P = 0.009) (Fig. 6B).
FIG. 6.
In vitro Th1 to Th2 skewing in B-cell–deficient mice and combinational immunosuppressive strategy in NOD mice. BALB/c splenocytes were challenged with B-cell–deficient or C57BL/6 splenocytes. IL-4–producing cells were increased in B-cell–deficient mice (n = 3) (*P = 0.001) (A) with a reduction of IFN-γ–to–IL-4 ratio (*P = 0.009) in the absence of B cells (B). Spontaneously hyperglycemic NOD mice were transplanted with BALB/c islets and treated with anti-CD22/cal alone or in combination with CTLA4-Ig; control treatments were anti-CD22/cal and CTLA4-Ig alone (C). Mice treated with anti-CD22/cal+CTLA4-Ig displayed prolonged islet graft survival compared with CTLA4-Ig (*P = 0.006) or anti-CD22/cal (*P = 0.006) treatment alone (C). At almost 40 days after transplantation, B220+ cells reemerged in the spleens of anti-CD22/cal+CTLA4-Ig NOD mice (D). At the time of rejection, islet structure appeared well preserved in anti-CD22/cal+CTLA4-Ig–treated mice (E1). As expected, severe infiltration of CD3+ cells (E2) with some FoxP3+ (E4) expression was evident in the graft area in anti-CD22/cal+CTLA4-Ig–treated mice. Few B220+ cells infiltrated the graft (E3), and insulin staining remained clearly preserved despite the complete loss of graft function (E5). At the day of rejection, untreated mice displayed a complete disruption of islet architecture (F1), an equally distributed CD3+ (F2) and B220+ (F3) cell infiltrate, few FoxP3+ cells (F4), and scant insulin staining (F5). Splenocytes harvested from islet-transplanted NOD mice treated with anti-CD22/cal+CTLA4-Ig or from untreated mice were analyzed at the time of rejection (n = 3 mice/experiment). The percentages of CD4+CD44hiCD62Llow (G) and CD4+CD25+FoxP3+ cells (I) were similar in the two groups of mice, whereas a reduction in CD8+CD44hiCD62Llow cells (H) was evident in anti-CD22/cal+CTLA4-Ig–treated NOD mice (*P < 0.05). Splenocytes harvested from anti-CD22/cal+CTLA4-Ig–treated NOD mice were stimulated with donor-derived antigen (BALB/c) or islet-specific peptides (BDC2.5 and IGRP) and compared with untreated mice at the time of rejection (n = 3 mice/experiment) (J and K). Reduced numbers of IFN-γ–producing cells were evident in mice treated with the combinational strategy upon stimulation of the alloantigen immune response (*P = 0.003) (J). In a similar manner, CD4+ and CD8+ autoreactivity to BDC2.5 (*P = 0.02) and IGRP (*P = 0.04), respectively (K), was significantly reduced in NOD mice treated with anti-CD22/cal+CTLA4-Ig compared with untreated NOD mice. bm12-derived B220+ cells were challenged with ABM-derived CD4+ cells with serial concentrations of CTLA4-Ig (1, 10, and 100 ng/mL). IFN-γ–producing cells, 96 h after stimulation, showed a dose-dependent reduction in the presence of CTLA4-Ig (n = 3) (no drug vs. all, *P < 0.001) (L). H&E, hematoxylin-eosin. (A high-quality digital representation of this figure is available in the online issue.)
Combinational targeting of indirect antigen presentation with B-cell depletion and CTLA4-Ig prolongs islet allograft survival in NOD mice.
We sought to find a clinically relevant B-cell depletion–based immunosuppressive strategy to fully target indirect allorecognition in NOD mice. We combined anti-CD22/cal treatment (to inhibit B-cell–mediated T-cell activation) and CTLA4-Ig, a fusion protein that prevents the interaction between B7.1 and CD28 (to inhibit DC-mediated T-cell activation) (25). CTLA4-Ig treatment alone significantly prolonged islet graft survival with similar kinetic as anti-CD22/cal compared with untreated mice (MST: CTLA4-Ig = 13.0 days; anti-CD22/cal = 12.0 days; untreated = 5.5 days; untreated vs. all, P < 0.01) (Fig. 6C) and anti-CD22/cal+CTLA4-Ig treatment was able to further prolong islet allograft survival compared with monotherapeutic anti-CD22/cal or CTLA4-Ig (MST: anti-CD22/cal+CTLA4-Ig = 33.5 days; anti-CD22/cal+CTLA4-Ig vs. all, P < 0.01) (Fig. 6C). The relevance of this strategy was confirmed in the chemically induced hyperglycemic B-cell–deficient (μMT) mice transplanted with BALB/c islet, which treated with CTLA4-Ig showed indefinite islet allograft survival (data not shown). It is interesting that anti-CD22/cal+CTLA4-Ig–treated mice displayed a conspicuous number of peripheral reemerging B cells at the time of rejection (Fig. 6D). Histological analysis of the grafts of these anti-CD22/cal+CTLA4-Ig–treated mice revealed preserved islet structure and insulin staining with severe CD3+- and B220+-cell infiltration (Fig. 6E, 1–5) compared with untreated mice, which displayed no insulin staining with lesser CD3+ and B220+ cell infiltration (Fig. 6F, 1–5). We then analyzed the frequency of Teffs and Tregs in the spleens of transplanted mice at the time of rejection, and a reduction in the number of CD8+ effector cells was observed in anti-CD22/cal+CTLA4-Ig–treated mice compared with untreated control mice (Fig. 6H), whereas no difference was seen in the percentages of CD4+ effector cells (Fig. 6G) or Tregs (Fig. 6I) at the time point examined. Moreover, splenocytes from transplanted mice were rechallenged in vitro with donor-derived antigen (BALB/c irradiated splenocytes), and the donor-specific immune response was evaluated using the ELISpot assay. We observed a significant reduction in IFN-γ–producing cells in anti-CD22/cal+CTLA4-Ig–treated mice compared with untreated mice at the time of rejection (Fig. 6J). Moreover, the status of the autoimmune response in treated mice was evaluated by restimulating splenocytes from transplanted mice ex vivo with BDC2.5 (to activate the CD4+ autoreactive T-cell response) and IGRP (to activate the CD8+ autoreactive T-cell response) peptides (Fig. 6K). We confirmed that the immune response toward autoantigen was suppressed in anti-CD22/cal+CTLA4-Ig–treated mice compared with untreated mice at the time of rejection (BDC2.5-stimulated IFN-γ–producing cells: anti-CD22/cal+CTLA4-Ig = 84.4 ± 5.9; untreated = 129.8 ± 14.5, counted as number of IFN-γ–producing cells per 1 × 106 total splenocytes; P = 0.02; IGRP-stimulated IFN-γ–producing cells: anti-CD22/cal+CTLA4-Ig = 22.5 ± 4.0; untreated = 48.5 ± 10.5, counted as number of IFN-γ–producing cells per 1 × 106 total splenocytes; P = 0.04) (Fig. 6K). We evaluated whether CTLA4-Ig is endowed with an immunologic effect on B/T-cell interaction. Thus, we took advantage of T-cell receptor transgenic mice and we plated 0.1 × 106 CD11c+ or 0.2 × 106 B220+ cells harvested from bm12 mice with 0.4 × 106 CD4+ cells harvested from ABM mice in an ELISpot assay adding serial concentrations of CTLA4-Ig (1, 10, and 100 ng/mL). We found that CTLA4-Ig inhibited, in a dose-dependent manner, the number of IFN-γ–producing cells induced by DCs (no drug = 332 ± 23; CTLA4-Ig 1 μg/mL = 127 ± 29; CTLA4-Ig 10 μg/mL = 31 ± 5; CTLA4-Ig 100 μg/mL = 15 ± 3, counted as number of IFN-γ–producing cells per 1 × 106 total splenocytes; no drug vs. all, P < 0.01) (data not shown) and B cells (no drug = 510 ± 37; CTLA4-Ig 1 μg/mL = 119 ± 29; CTLA4-Ig 10 μg/mL = 14 ± 4; CTLA4-Ig 100 µg/mL = 2 ± 0, counted as number of IFN-γ–producing cells per 1 × 106 total splenocytes; no drug vs. all, P < 0.001) (Fig. 6L).
DISCUSSION
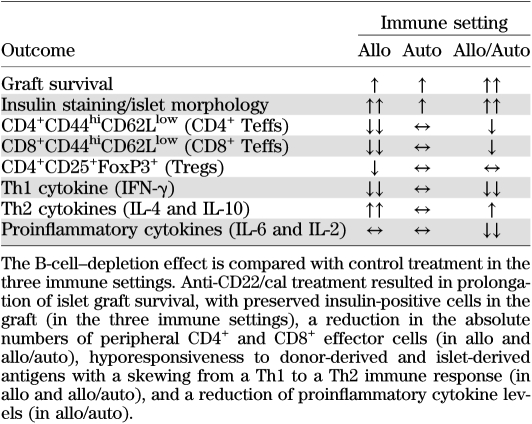
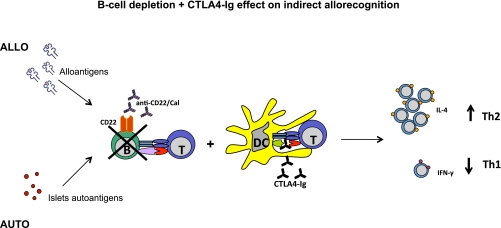
Islet transplantation represents a transplant model in which the alloimmune response and recurrence of autoimmunity coexist, jeopardizing long-term islet function and ultimately leading to graft rejection (9,18,26). B cells have been shown to have an essential role as antigen-presenting cells (APCs) in the priming of T cells during the allo- and autoimmune responses (6,8,27), thus constituting a suitable target to modulate the immune response in islet transplantation (28). It is interesting that although clinical trials based on B-cell depletion in islet transplantation are ongoing (28), the literature is lacking in murine studies examining the effect of B-cell depletion in islet transplantation. Herein, we delineate the effect of B-cell depletion in three different models of murine islet transplantation: 1) in a purely alloimmune setting (BALB/c into chemically induced hyperglycemic C57BL/6 mice), 2) in a purely autoimmune setting (NOD.SCID into spontaneously hyperglycemic NOD mice), and 3) in a mixed allo- and autoimmune setting (BALB/c into spontaneously hyperglycemic NOD mice). We used the novel B-cell–depleting agent inotuzumab ozogamicin murine analog (anti-CD22/cal), which targets the CD22 receptor and, thus, depletes mature B cells from lymphoid organs (9,21), and cal alone, as control antibody. We observed a nearly complete depletion of B cells in the spleen 14 days after transplantation (depletion was more robust in NOD mice compared with the C57BL/6 strain), with a full recovery 8 weeks after treatment. When anti-CD22/cal was used as B-cell–depleting agent, a significant delay in islet graft rejection was found in all three models tested; however, B-cell depletion per se cannot induce tolerance, which was confirmed in B-cell–deficient μMT knockout mice. Graft pathology confirmed the complete absence of B cells in the graft infiltrate of the three models tested, with a prominent CD3+ cell infiltrate. Mechanistic studies revealed that mice treated with anti-CD22/cal experienced prolongation of graft survival, with preserved insulin staining in the graft, an overall reduction in peripheral Teff numbers in allo and allo/auto, and hyporesponsiveness to donor alloantigens (in allo) and to islet-derived autoantigens (in allo/auto), with a reshaping of the peripheral cytokine profile and an increase in IL-4 and IL-10 peripheral levels (Table 1). Of interest, we found that the absence of B cells leads to a polarization of allo- and autoimmune responses toward a Th2 response, with an inversion of IFN-γ/IL-4 production in the periphery, in the ex vivo challenge of T cells with donor-derived antigen and islet autoantigens and the in vitro mixed-lymphocytes reaction. Unfortunately, the control antibody (cal), being a DNA-damaging cytotoxic agent, showed some depleting activity on B and T cells, but since there was no effect on islet allograft survival, we believe that this drug may be suitable as negative control antibody for anti-CD22/cal. Our data, for the first time, elucidate the effect of B-cell depletion on the immune system in three settings of islet-transplanted mice and demonstrate that the absence of B cells (using depletion strategies or via genetic deletion) is not sufficient to induce islet graft tolerance. We speculate that B cells may be replaced by DCs as APCs or that the maintenance of some regulatory B cells (28–30) may be required to achieve graft tolerance (31,32). Indeed, our group previously demonstrated that B cells are required to obtain anti-CD45RB–mediated allograft tolerance and to inhibit alloreactive T-cell activation (33). The lack of efficacy of B-cell depletion in prolonging islet allograft survival may be related to the fact that B cells play a more relevant role in initial expansion of pathogenic T cells in NOD mice, but as soon as autoreactive T cells have become memory cells, B-cell depletion resulted in a limited effect. This can also explain why depleting B cells is slightly more effective in an alloimmune setting, in which T cells are naïve at the time of transplantation. Our data revealed that B-cell depletion has a mild effect in prolonging islet graft survival, in reducing Teffs and skewing the immune response to Th2 profile in the auto setting, whereas autoimmune response was severely reduced in the mixed allo/auto setting. This may suggest that the reduced anti-islet response in the latter recipient group could be secondary to the suppressed alloimmune response. In summary, we demonstrate that B-cell depletion is effective in prolonging islet graft survival but is unable to induce allograft tolerance. Inotuzumab ozogamicin murine analog (anti-CD22/cal) is available for clinical use (34,35), thus representing a translational therapeutic approach (36,37). It is likely that depleting strategies (e.g., anti-T/B cells) are not fully effective when used as a monotherapy because of the redundancy of the immune response (38,39). Therefore, to completely halt T-cell activation and block residual APC function, we used an immunosuppressive regimen based on the combination of B-cell depletion and CTLA4-Ig treatment in the benchmark model of islet transplantation: the NOD mouse (Fig. 7). CTLA4-Ig is a clinically approved compound that prevents the interaction between CD80/CD86 (B7.1/B7.2) and CD28, leading to a reduction in clonal expansion, to T-cell anergy, and to inhibition of T-cell activation (40–42). In this study, we confirm that CTLA4-Ig inhibits DC/T-cell interaction in vitro, but for the first time, we show that B-cell–mediated T-cell activation is strongly inhibited by CTLA4-Ig as well. Our combination strategy prolonged allograft survival in a stringent model of islet transplantation, in which both allo- and autoimmune responses take part in the rejection process. A repeated, extended administration of a B-cell–depleting agent may be tested in clinical trials to inhibit reemerging B-cell–mediated chronic activation of the immune system, but because we show that islet allograft rejection occurs before B-cell recovery, we believe that B cells are partially responsible for triggering allograft rejection.
TABLE 1.
The effect of B-cell depletion using inotuzumab ozogamicin murine analog (anti-CD22/cal) treatment on the anti-islet immune response in three murine models of islet transplantation
FIG. 7.
Mechanism of action of the novel immunomodulatory regimen tested in islet transplantation in NOD mice. T-cell activation is mediated by APCs made up of B cells and DCs, as depicted. Our strategy is based on the complete blockade of T-cell activation in response to allo- and autoantigens through combining a B-cell–depleting agent (anti-CD22/cal) and CTLA4-Ig, which by blocking the CD28-B7.1 interaction, inhibits DC-mediated T-cell activation. This approach prolongs islet graft survival, induces hyporesponsiveness toward allo- and autoantigens, and tips the balance toward a Th2 anti-inflammatory phenotype of the immune response. (A high-quality color representation of this figure is available in the online issue.)
In conclusion, B-cell depletion significantly affects allo- and autoimmune responses, albeit to a lower extent in the latter process. B-cell depletion in monotherapy cannot be considered to be a tolerogenic strategy per se and must be accompanied by complementary or synergistic immunological strategies (e.g., costimulatory blockade).
ACKNOWLEDGMENTS
P.F. is the recipient of a Juvenile Diabetes Research Foundation Career Development Award, an American Society of Nephrology Career Development Award, an American Diabetes Association mentor-based fellowship, and a grant from the Italian Ministero dell'Istruzione, dell'Università e della Ricerca (Staminali RF-FSR-2008-1213704). K.D.J. is a paid employee of Pfizer. Pfizer provided anti-CD22/cal and control antibody compounds. No other potential conflicts of interest relevant to this article were reported.
M.Ca. and A.P. performed research, analyzed data, and wrote the manuscript. A.V., K.M.L., S.T., and M.Ch. performed research. E.O., C.S., A.S., M.H.S., and J.F.M. designed research. K.D.-J. contributed vital new reagents. P.F. designed research, edited the manuscript, and is the guarantor for the article.
REFERENCES
- 1.Rifle G, Mousson C, Martin L, Guignier F, Hajji K. Donor-specific antibodies in allograft rejection: clinical and experimental data. Transplantation 2005;79(Suppl. 3):S14–S18 10.1097/01.TP.0000153292.49621.60 [DOI] [PubMed] [Google Scholar]
- 2.Kwun J, Knechtle SJ. Overcoming chronic rejection—can it B? Transplantation 2009;88:955–961 10.1097/TP.0b013e3181b96646 [DOI] [PubMed] [Google Scholar]
- 3.Zarkhin V, Li L, Sarwal M. “To B or not to B?” B-cells and graft rejection. Transplantation 2008;85:1705–1714 10.1097/TP.0b013e318177793e [DOI] [PubMed] [Google Scholar]
- 4.Tinckam KJ, Chandraker A. Mechanisms and role of HLA and non-HLA alloantibodies. Clin J Am Soc Nephrol 2006;1:404–414 10.2215/CJN.00270106 [DOI] [PubMed] [Google Scholar]
- 5.Noorchashm H, Reed AJ, Rostami SY, et al. B cell-mediated antigen presentation is required for the pathogenesis of acute cardiac allograft rejection. J Immunol 2006;177:7715–7722 [DOI] [PubMed] [Google Scholar]
- 6.Wong FS, Wen L, Tang M, et al. Investigation of the role of B-cells in type 1 diabetes in the NOD mouse. Diabetes 2004;53:2581–2587 10.2337/diabetes.53.10.2581 [DOI] [PubMed] [Google Scholar]
- 7.Serreze DV, Chapman HD, Varnum DS, et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD.Ig mu null mice. J Exp Med 1996;184:2049–2053 10.1084/jem.184.5.2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol 1998;161:3912–3918 [PubMed] [Google Scholar]
- 9.Fiorina P, Vergani A, Dada S, et al. Targeting CD22 reprograms B-cells and reverses autoimmune diabetes. Diabetes 2008;57:3013–3024 10.2337/db08-0420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu C, Noorchashm H, Sutter JA, et al. B lymphocyte-directed immunotherapy promotes long-term islet allograft survival in nonhuman primates. Nat Med 2007;13:1295–1298 10.1038/nm1673 [DOI] [PubMed] [Google Scholar]
- 11.Hu CY, Rodriguez-Pinto D, Du W, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest 2007;117:3857–3867 10.1172/JCI32405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Neill SK, Shlomchik MJ, Glant TT, Cao Y, Doodes PD, Finnegan A. Antigen-specific B cells are required as APCs and autoantibody-producing cells for induction of severe autoimmune arthritis. J Immunol 2005;174:3781–3788 [DOI] [PubMed] [Google Scholar]
- 13.Sayegh MH, Carpenter CB. Role of indirect allorecognition in allograft rejection. Int Rev Immunol 1996;13:221–229 10.3109/08830189609061749 [DOI] [PubMed] [Google Scholar]
- 14.Schröder C, Azimzadeh AM, Wu G, Price JO, Atkinson JB, Pierson RN. Anti-CD20 treatment depletes B-cells in blood and lymphatic tissue of cynomolgus monkeys. Transpl Immunol 2003;12:19–28 10.1016/S0966-3274(03)00059-5 [DOI] [PubMed] [Google Scholar]
- 15.Genberg H, Hansson A, Wernerson A, Wennberg L, Tydén G. Pharmacodynamics of rituximab in kidney transplantation. Transplantation 2007;84(Suppl. 12):S33–S36 10.1097/01.tp.0000296122.19026.0f [DOI] [PubMed] [Google Scholar]
- 16.Kelishadi SS, Azimzadeh AM, Zhang T, et al. Preemptive CD20+ B cell depletion attenuates cardiac allograft vasculopathy in cyclosporine-treated monkeys. J Clin Invest 2010;120:1275–1284 10.1172/JCI41861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai MK, Chien HF, Tzeng MC, Lee PH. Effects of B cell depletion on T cell allogeneic immune responses: a strategy to reduce allogeneic sensitization. Transpl Immunol 2009;21:215–220 10.1016/j.trim.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 18.Makhlouf L, Kishimoto K, Smith RN, et al. The role of autoimmunity in islet allograft destruction: major histocompatibility complex class II matching is necessary for autoimmune destruction of allogeneic islet transplants after T-cell costimulatory blockade. Diabetes 2002;51:3202–3210 10.2337/diabetes.51.11.3202 [DOI] [PubMed] [Google Scholar]
- 19.Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol 2004;4:259–268 10.1038/nri1332 [DOI] [PubMed] [Google Scholar]
- 20.Bosi E, Braghi S, Maffi P, et al. Autoantibody response to islet transplantation in type 1 diabetes. Diabetes 2001;50:2464–2471 10.2337/diabetes.50.11.2464 [DOI] [PubMed] [Google Scholar]
- 21.Dunussi-Joannopoulos K, Hancock GE, Kunz A, et al. B-cell depletion inhibits arthritis in a collagen-induced arthritis (CIA) model, but does not adversely affect humoral responses in a respiratory syncytial virus (RSV) vaccination model. Blood 2005;106:2235–2243 10.1182/blood-2004-11-4547 [DOI] [PubMed] [Google Scholar]
- 22.Pearson T, Weiser P, Markees TG, et al. Islet allograft survival induced by costimulation blockade in NOD mice is controlled by allelic variants of Idd3. Diabetes 2004;53:1972–1978 10.2337/diabetes.53.8.1972 [DOI] [PubMed] [Google Scholar]
- 23.Rossini AA. Autoimmune diabetes and the circle of tolerance. Diabetes 2004;53:267–275 10.2337/diabetes.53.2.267 [DOI] [PubMed] [Google Scholar]
- 24.Sayegh MH, Wu Z, Hancock WW, et al. Allograft rejection in a new allospecific CD4+ TCR transgenic mouse. Am J Transplant 2003;3:381–389 10.1034/j.1600-6143.2003.00062.x [DOI] [PubMed] [Google Scholar]
- 25.Vergani A, D’Addio F, Jurewicz M, et al. A novel clinically relevant strategy to abrogate autoimmunity and regulate alloimmunity in NOD mice. Diabetes 2010;59:2253–2264 10.2337/db09-1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiorina P, Vergani A, Petrelli A, et al. Metabolic and immunological features of the failing islet-transplanted patient. Diabetes Care 2008;31:436–438 10.2337/dc07-1831 [DOI] [PubMed] [Google Scholar]
- 27.Kirk AD, Turgeon NA, Iwakoshi NN. B cells and transplantation tolerance. Nat Rev Nephrol 2010;6:584–593 10.1038/nrneph.2010.111 [DOI] [PubMed] [Google Scholar]
- 28.Fiorina P, Sayegh MH. B cell-targeted therapies in autoimmunity: rationale and progress [Internet], 28 May 2009. F1000 Biol Rep 2009;1:39 [DOI] [PMC free article] [PubMed]
- 29.Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev 2008;224:201–214 10.1111/j.1600-065X.2008.00661.x [DOI] [PubMed] [Google Scholar]
- 30.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 2008;28:639–650 10.1016/j.immuni.2008.03.017 [DOI] [PubMed] [Google Scholar]
- 31.Watanabe R, Ishiura N, Nakashima H, et al. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol 2010;184:4801–4809 10.4049/jimmunol.0902385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mauri C. Regulation of immunity and autoimmunity by B cells. Curr Opin Immunol 2010;22:761–767 10.1016/j.coi.2010.10.009 [DOI] [PubMed] [Google Scholar]
- 33.Deng S, Moore DJ, Huang X, et al. Cutting edge: transplant tolerance induced by anti-CD45RB requires B lymphocytes. J Immunol 2007;178:6028–6032 [DOI] [PubMed] [Google Scholar]
- 34.Dijoseph JF, Dougher MM, Armellino DC, Evans DY, Damle NK. Therapeutic potential of CD22-specific antibody-targeted chemotherapy using inotuzumab ozogamicin (CMC-544) for the treatment of acute lymphoblastic leukemia. Leukemia 2007;21:2240–2245 10.1038/sj.leu.2404866 [DOI] [PubMed] [Google Scholar]
- 35.Ogura M, Tobinai K, Hatake K, et al. Phase I study of inotuzumab ozogamicin (CMC-544) in Japanese patients with follicular lymphoma pretreated with rituximab-based therapy. Cancer Sci 2010;101:1840–1845 10.1111/j.1349-7006.2010.01601.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DiJoseph JF, Dougher MM, Kalyandrug LB, et al. Antitumor efficacy of a combination of CMC-544 (inotuzumab ozogamicin), a CD22-targeted cytotoxic immunoconjugate of calicheamicin, and rituximab against non-Hodgkin’s B-cell lymphoma. Clin Cancer Res 2006;12:242–249 10.1158/1078-0432.CCR-05-1905 [DOI] [PubMed] [Google Scholar]
- 37.Advani A, Coiffier B, Czuczman MS, et al. Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin’s lymphoma: results of a phase I study. J Clin Oncol 2010;28:2085–2093 10.1200/JCO.2009.25.1900 [DOI] [PubMed] [Google Scholar]
- 38.Haudebourg T, Poirier N, Vanhove B. Depleting T-cell subpopulations in organ transplantation. Transpl Int 2009;22:509–518 10.1111/j.1432-2277.2008.00788.x [DOI] [PubMed] [Google Scholar]
- 39.Thaunat O, Morelon E, Defrance T. Am”B”valent: anti-CD20 antibodies unravel the dual role of B cells in immunopathogenesis. Blood 2010;116:515–521 10.1182/blood-2010-01-266668 [DOI] [PubMed] [Google Scholar]
- 40.Vincenti F, Larsen C, Durrbach A, et al. ; Belatacept Study Group Costimulation blockade with belatacept in renal transplantation. N Engl J Med 2005;353:770–781 10.1056/NEJMoa050085 [DOI] [PubMed] [Google Scholar]
- 41.Sayegh MH, Turka LA. The role of T-cell costimulatory activation pathways in transplant rejection. N Engl J Med 1998;338:1813–1821 10.1056/NEJM199806183382506 [DOI] [PubMed] [Google Scholar]
- 42.Rothstein DM, Sayegh MH. T-cell costimulatory pathways in allograft rejection and tolerance. Immunol Rev 2003;196:85–108 10.1046/j.1600-065X.2003.00088.x [DOI] [PubMed] [Google Scholar]