Abstract
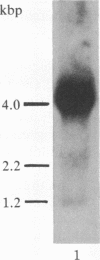
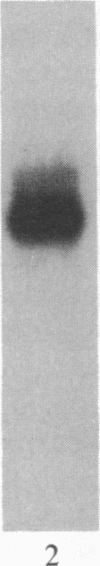
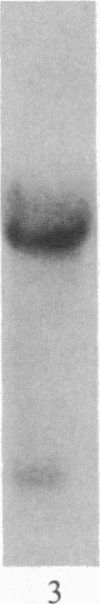
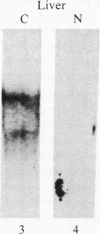
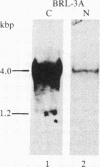
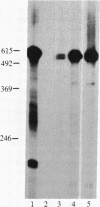
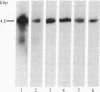
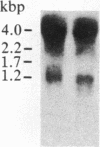
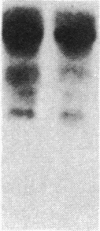
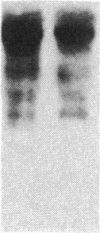
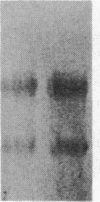
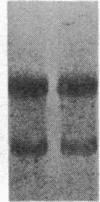
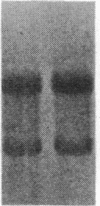
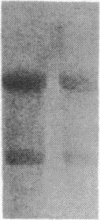
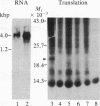
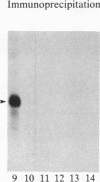
Insulin-like growth factor II (IGF-II) is a mitogenic polypeptide that is thought to play a role in fetal growth and development. To study the hormonal and developmental regulation of IGF-II gene expression, we have isolated a cDNA clone for rat IGF-II (rIGF-II) from a 12S [1.2-kilobase-pair (kbp)] fraction of mRNA from a rat liver cell line (BRL-3A) that directs the cell-free synthesis of pre-pro-rIGF-II. In the present study, the rIGF-II probe was used to determine the size of IGF-II RNA. Surprisingly, in BRL-3A cells and in neonatal liver, the probe hybridized under stringent conditions 10-20 times more strongly to a larger (4 kbp) RNA than to 1.2-kbp RNA. The 4-kbp RNA is almost exclusively cytoplasmic and is colinear with a 551-base fragment of the rIGF-II cDNA insert containing coding and 3' noncoding regions. The 4-kbp and 1.2-kbp RNA species are regulated coordinately with developmental age, being high in liver from neonatal rats but not detectable in liver from older animals, suggesting that both IGF-II mRNA species arise from a single primary transcript by alternative RNA processing. Although oligodeoxynucleotide hybridization and S1 nuclease protection experiments suggest that the 4-kbp RNA contains an intact protein-coding region, fractions enriched in 4-kbp RNA do not direct the translation of pre-pro-rIGF-II in vitro. This may indicate that the 4-kbp RNA specifies an altered protein product that has not yet been recognized, or alternatively that it contains a normal protein-coding region but requires further RNA processing to be activated for translation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acquaviva A. M., Bruni C. B., Nissley S. P., Rechler M. M. Cell-free synthesis of rat insulin-like growth factor II. Diabetes. 1982 Jul;31(7):656–658. doi: 10.2337/diab.31.7.656. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Gerhard D. S., Fong N. M., Sanchez-Pescador R., Rall L. B. Isolation of the human insulin-like growth factor genes: insulin-like growth factor II and insulin genes are contiguous. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6450–6454. doi: 10.1073/pnas.82.19.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Chrapkiewicz N. B., Beale E. G., Granner D. K. Induction of the messenger ribonucleic acid coding for phosphoenolpyruvate carboxykinase in H4-II-E cells. Evidence for a nuclear effect of cyclic AMP. J Biol Chem. 1982 Dec 10;257(23):14428–14432. [PubMed] [Google Scholar]
- Cooper T. A., Ordahl C. P. A single cardiac troponin T gene generates embryonic and adult isoforms via developmentally regulated alternate splicing. J Biol Chem. 1985 Sep 15;260(20):11140–11148. [PubMed] [Google Scholar]
- Dull T. J., Gray A., Hayflick J. S., Ullrich A. Insulin-like growth factor II precursor gene organization in relation to insulin gene family. 1984 Aug 30-Sep 5Nature. 310(5980):777–781. doi: 10.1038/310777a0. [DOI] [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Gluckman P. D., Butler J. H. Parturition-related changes in insulin-like growth factors-I and -II in the perinatal lamb. J Endocrinol. 1983 Nov;99(2):223–232. doi: 10.1677/joe.0.0990223. [DOI] [PubMed] [Google Scholar]
- Graham D. E., Medina D., Smith G. H. Increased concentration of an indigenous proviral mouse mammary tumor virus long terminal repeat-containing transcript is associated with neoplastic transformation of mammary epithelium in C3H/Sm mice. J Virol. 1984 Mar;49(3):819–827. doi: 10.1128/jvi.49.3.819-827.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselbacher G. K., Schwab M. E., Pasi A., Humbel R. E. Insulin-like growth factor II (IGF II) in human brain: regional distribution of IGF II and of higher molecular mass forms. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2153–2157. doi: 10.1073/pnas.82.7.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselbacher G., Humbel R. Evidence for two species of insulin-like growth factor II (IGF II and "big" IGF II) in human spinal fluid. Endocrinology. 1982 May;110(5):1822–1824. doi: 10.1210/endo-110-5-1822. [DOI] [PubMed] [Google Scholar]
- Hoopes B. C., McClure W. R. Studies on the selectivity of DNA precipitation by spermine. Nucleic Acids Res. 1981 Oct 24;9(20):5493–5504. doi: 10.1093/nar/9.20.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen R., Jat P., Treisman R., Favaloro J., Folk W. R. 5' termini of polyoma virus early region transcripts synthesized in vivo by wild-type virus and viable deletion mutants. J Mol Biol. 1982 Aug 5;159(2):189–224. doi: 10.1016/0022-2836(82)90493-4. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Takagaki Y., Furuto S., Tanaka T., Nawa H., Nakanishi S. A single gene for bovine high molecular weight and low molecular weight kininogens. Nature. 1983 Oct 6;305(5934):545–549. doi: 10.1038/305545a0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Maki R., Roeder W., Traunecker A., Sidman C., Wabl M., Raschke W., Tonegawa S. The role of DNA rearrangement and alternative RNA processing in the expression of immunoglobulin delta genes. Cell. 1981 May;24(2):353–365. doi: 10.1016/0092-8674(81)90325-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McCarty K. S., Stafford D., Brown O. Resolution and fractionation of macromolecules by isokinetic sucrose density gradient sedimentation. Anal Biochem. 1968 Aug;24(2):314–329. doi: 10.1016/0003-2697(68)90185-1. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Curran T., Verma I. M. c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell. 1984 Jan;36(1):51–60. doi: 10.1016/0092-8674(84)90073-4. [DOI] [PubMed] [Google Scholar]
- Moses A. C., Nissley S. P., Short P. A., Rechler M. M., White R. M., Knight A. B., Higa O. Z. Increased levels of multiplication-stimulating activity, an insulin-like growth factor, in fetal rat serum. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3649–3653. doi: 10.1073/pnas.77.6.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnich A., Daegelen D., Besmond C., Marie J., Dreyfus J. C., Kahn A. Cell-free translation of messenger RNAs from human muscle biopsies: a miniaturized tool for investigation of neuromuscular diseases. Pediatr Res. 1982 May;16(5):335–339. doi: 10.1203/00006450-198205000-00001. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Lumelsky N., Lerman L. S., Maniatis T. Detection of single base substitutions in total genomic DNA. Nature. 1985 Feb 7;313(6002):495–498. doi: 10.1038/313495a0. [DOI] [PubMed] [Google Scholar]
- Rechler M. M., Nissley S. P. The nature and regulation of the receptors for insulin-like growth factors. Annu Rev Physiol. 1985;47:425–442. doi: 10.1146/annurev.ph.47.030185.002233. [DOI] [PubMed] [Google Scholar]
- Reynolds G. A., Goldstein J. L., Brown M. S. Multiple mRNAs for 3-hydroxy-3-methylglutaryl coenzyme A reductase determined by multiple transcription initiation sites and intron splicing sites in the 5'-untranslated region. J Biol Chem. 1985 Aug 25;260(18):10369–10377. [PubMed] [Google Scholar]
- Rosenfeld M. G., Amara S. G., Evans R. M. Alternative RNA processing: determining neuronal phenotype. Science. 1984 Sep 21;225(4668):1315–1320. doi: 10.1126/science.6089345. [DOI] [PubMed] [Google Scholar]
- Saito H., Richardson C. C. Processing of mRNA by ribonuclease III regulates expression of gene 1.2 of bacteriophage T7. Cell. 1981 Dec;27(3 Pt 2):533–542. doi: 10.1016/0092-8674(81)90395-0. [DOI] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenle E., Zapf J., Humbel R. E., Froesch E. R. Insulin-like growth factor I stimulates growth in hypophysectomized rats. Nature. 1982 Mar 18;296(5854):252–253. doi: 10.1038/296252a0. [DOI] [PubMed] [Google Scholar]
- Scott J., Cowell J., Robertson M. E., Priestley L. M., Wadey R., Hopkins B., Pritchard J., Bell G. I., Rall L. B., Graham C. F. Insulin-like growth factor-II gene expression in Wilms' tumour and embryonic tissues. Nature. 1985 Sep 19;317(6034):260–262. doi: 10.1038/317260a0. [DOI] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Nunberg J. H., Schimke R. T. Size heterogeneity in the 3' end of dihydrofolate reductase messenger RNAs in mouse cells. Cell. 1980 Nov;22(2 Pt 2):361–370. doi: 10.1016/0092-8674(80)90346-3. [DOI] [PubMed] [Google Scholar]
- Soares M. B., Ishii D. N., Efstratiadis A. Developmental and tissue-specific expression of a family of transcripts related to rat insulin-like growth factor II mRNA. Nucleic Acids Res. 1985 Feb 25;13(4):1119–1134. doi: 10.1093/nar/13.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield H. J., Bruni C. B., Frunzio R., Terrell J. E., Nissley S. P., Rechler M. M. Isolation of a cDNA clone encoding rat insulin-like growth factor-II precursor. Nature. 1984 Nov 15;312(5991):277–280. doi: 10.1038/312277a0. [DOI] [PubMed] [Google Scholar]
- Yang Y. W., Rechler M. M., Nissley S. P., Coligan J. E. Biosynthesis of rat insulin-like growth factor II. II. Localization of mature rat insulin-like growth factor II (7484 daltons) to the amino terminus of the approximately 20-kilodalton biosynthetic precursor by radiosequence analysis. J Biol Chem. 1985 Feb 25;260(4):2578–2582. [PubMed] [Google Scholar]
- Yang Y. W., Romanus J. A., Liu T. Y., Nissley S. P., Rechler M. M. Biosynthesis of rat insulin-like growth factor II. I. Immunochemical demonstration of a approximately 20-kilodalton biosynthetic precursor of rat insulin-like growth factor II in metabolically labeled BRL-3A rat liver cells. J Biol Chem. 1985 Feb 25;260(4):2570–2577. [PubMed] [Google Scholar]
- Young R. A., Hagenbüchle O., Schibler U. A single mouse alpha-amylase gene specifies two different tissue-specific mRNAs. Cell. 1981 Feb;23(2):451–458. doi: 10.1016/0092-8674(81)90140-9. [DOI] [PubMed] [Google Scholar]
- Zapf J., Froesch E. R., Humbel R. E. The insulin-like growth factors (IGF) of human serum: chemical and biological characterization and aspects of their possible physiological role. Curr Top Cell Regul. 1981;19:257–309. doi: 10.1016/b978-0-12-152819-5.50024-5. [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. Mutationally altered 3' ends of yeast CYC1 mRNA affect transcript stability and translational efficiency. J Mol Biol. 1984 Jul 25;177(1):107–135. doi: 10.1016/0022-2836(84)90060-3. [DOI] [PubMed] [Google Scholar]