Abstract
Cellular metabolic memory occurs in diabetic microvascular and macrovascular complications, but the underlying mechanisms remain unclear. Here, we investigate the role of sirtuin 1 (SIRT1) and metformin in this phenomenon. In bovine retinal capillary endothelial cells (BRECs) and retinas of diabetic rats, the inflammatory gene, nuclear factor-κB (NF-κB), and the proapoptotic gene, Bax, induced by hyperglycemia, remained elevated after returning to normoglycemia. BRECs with small interfering RNA–mediated SIRT1 knockdown had increased sensitivity to hyperglycemia stress, whereas SIRT1 overexpression or activation by metformin inhibited the increase of mitochondrial reactive oxygen species–mediated glyceraldehyde-3-phosphate dehydrogenase by poly (ADP-ribose) polymerase (PARP) activity through the upregulation of liver kinase B1/AMP-activated protein kinase (LKB1/AMPK), ultimately suppressing NF-κB and Bax expression. Furthermore, we showed that hyperglycemia led to PARP activation, which in turn may have downregulated SIRT1. Of importance, this study also demonstrated that metformin suppressed the “memory” of hyperglycemia stress in the diabetic retinas, which may be involved in the SIRT1/LKB1/AMPK pathway. Our data suggest that SIRT1 is a potential therapeutic target for the treatment of the cellular metabolic memory, and the use of metformin specifically for such therapy may be a new avenue of investigation in the diabetes field.
The Diabetes Control and Complications Trial and the follow-up Epidemiology of Diabetes Interventions and Complications Study showed that the benefits of instituting tight glycemic control in diabetic patients may not be immediately reflected in the progression of diabetes complications and that these benefits may be seen beyond the period of good glycemic control (1,2). Furthermore, data from the Epidemiology of Diabetes Interventions and Complications Study also suggest that the influence of early glycemic control on the progression to macrovascular events may become more evident with longer follow-up (3,4). The authors of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study reports have referred to this phenomenon as “metabolic memory” (4), suggesting that memory of the early glycemic environment is retained in vascular endothelial cells. However, this metabolic memory phenomenon is poorly understood and poses a major challenge in treating diabetes.
After being first reported by Engerman and Kern (5), the metabolic memory phenomenon was explored in isolated human umbilical vein endothelial cells, where it was shown that glucose-induced overexpression of fibronectin in cells preexposed to high glucose was not readily reversible after exposure to normal glucose (6). Furthermore, it was found that the potential mechanisms for propagating this memory involved an excess of reactive oxygen species (ROS) from mitochondria (7). These observations were in agreement with our own previous studies, as well as in those by others (8–11), and a unifying hypothesis has been proposed whereby production of mitochondrial ROS in response to hyperglycemia may be an initiating cause in the pathogenesis of diabetes complications. That is, hyperglycemia in endothelial cells can lead to overproduction of mitochondrial ROS, which then results in inactivation of glyceraldehyde-3-phosphate dehydrogenase by poly (ADP-ribose) polymerase (PARP) activation and subsequent ADP ribosylation. However, the mechanisms by which hyperglycemia induces the overproduction of mitochondrial ROS remain elusive.
Class III histone deacetylase sirtuin 1 (SIRT1) is a multifunctional protein critically involved in stress responses, cellular metabolism, and aging through deacetylating a variety of substrates, including histones and transcription factors and coregulators, to regulate target gene expression both positively and negatively (12). Recently, it was found that SIRT1 regulates energy homeostasis, cell cycle, apoptosis, inflammatory responses, and levels of ROS (13,14). Therefore, we hypothesized that SIRT1 may play a major role in the pathogenesis of the metabolic memory phenomenon.
AMP-activated protein kinase (AMPK) is a serine/threonine protein kinase that has emerged as a master sensor of cellular energy balance in mammalian cells. AMPK is activated by metabolic stress to promote energy conservation and glucose uptake, allowing cells to survive periods of low availability of energy. In addition to an elevated intracellular AMP-to-ATP ratio, AMPK also is activated by phosphorylation by at least two upstream kinases, the tumor suppressor kinase liver kinase B1 (LKB1) and Ca2+/calmodulin-dependent protein kinase kinase (15,16). Recently, it was shown that the AMPK pathway reduces intracellular ROS levels (17,18). Thus, as a central metabolic switch governing glucose and lipid metabolism in response to alterations in nutrients and intracellular energy levels, AMPK may play an important role in the metabolic memory phenomenon.
In this study, we demonstrate that the dysfunction of the LKB1/AMPK/ROS pathway results in a cellular metabolic memory of high glucose. This can arise from the downregulation of SIRT1, which leads to sustained responses of inflammatory and apoptosis proteins, such as nuclear factor-κB (NF-κB), Bax, and PARP, which have been implicated in diabetic vascular complications including diabetic retinopathy. We also examined the therapeutic response to metformin, a drug widely used to lower blood glucose concentrations in diabetic patients.
RESEARCH DESIGN AND METHODS
All experiments in this study comply with the requirements of the Association for Research in Vision and Ophthalmology as outlined in the Statement for the Use of Animals in Ophthalmic and Vision Research. All chemicals, purchased from Sigma Chemicals (St. Louis, MO), were of reagent-grade quality, unless stated otherwise.
Cell culture and infection.
Primary bovine retinal capillary endothelial cell (BREC) cultures were obtained as described in our previous study (8). The cells were incubated with normal glucose (5 mmol/L glucose) for 3 weeks, high glucose (30 mmol/L glucose) for 3 weeks, or high glucose for 1 week followed by normal glucose for 2 weeks.
A recombinant adenovirus overexpressing SIRT1 cDNA (Ad SIRT1) was constructed as described (19), and small interfering RNA (siRNA) for SIRT1 (20 nmol/L) and LKB1 (100 nmol/L) were from Genesil Biotechnology (Wuhan, China). At 24 h after passage, at a 1:5 ratio, cells were transfected using Lipofectamine 2000 (0.15%, vol/vol) (Invitrogen, Carlsbad, CA) following the protocol provided by the manufacturer. The Lipofectamine 2000 was removed by changing to fresh medium containing 10% FBS 5 h posttransfection, and the cells were analyzed 48 h after transfection.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay.
Apoptotic cells in the BREC cultures were detected using the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) system (Promega, Madison, WI) according to the manufacturer’s instructions.
Animals.
Rats were categorized as diabetic when blood glucose exceeded 16.7 mmol/L at 48 h after intraperitoneal administration with 60 mg/kg streptozotocin. The rats were then randomized into five groups for the 6-week experiment and given treatment or not as follows: (1) normal (nondiabetic) rats, (2) diabetic rats not treated with insulin, (3) diabetic rats treated with insulin during the last 4 weeks, (4) diabetic rats treated with insulin plus metformin by oral route (100 mg/kg body wt/day) during the last 4 weeks, and (5) diabetic rats treated with insulin plus Ad SIRT1 vector by intravitreal injection (1.2 × 1010 pfu/mL) during the 4 weeks. The rats in which good control was intended received insulin twice daily (6–8 units total) to maintain their blood glucose levels <8 mmol/L. Blood glucose levels were in the following ranges: 4.7–6.3 mmol/L in nondiabetic rats, 17.0–31.8 mmol/L in diabetic rats, and 4.3–7.9 mmol/L in insulin-treated animals.
Retinal digest procedures and measurement of ROS.
The eyes enucleated from the animals were immediately placed in 4% buffered paraformaldehyde for 24 h. Retinal trypsin digestion and measurement of ROS were performed according to the methods described in our previous study (8).
Measurement of retinal blood vessel leakage using Evans blue dye.
Retinal blood vessel leakage was quantitated using Evans blue dye, which noncovalently binds to plasma albumin in the blood stream (8).
Real-time RT-PCR.
The primer sequences (sense/antisense) used were as follows: SIRT1, 5′-CCTGTGAAAGTGATGAGGAGGATAG-3′/5′-TTGGATTCCCGCAACCTG-3′; and β-actin, 5′-GCACCGCAAATGCTTCTA-3′/5′-GGTCTTTACGGATGTCAACG-3′. Relative quantification of the signals was performed by normalizing the signals of different genes to that of the β-actin signal.
Western blotting.
Fifty micrograms of protein obtained from each sample (BRECs or retinas) were subjected to SDS-PAGE in a Bio-Rad miniature slab gel apparatus and electrophoretically transferred onto a nitrocellulose membrane. The membrane was blocked in a 5% nonfat dried-milk solution and incubated overnight with partially purified mouse anti-SIRT1 monoclonal antibody (mAb; Chemicon, Temecula, CA), mouse anti-LKB1 mAb (Cell Signaling Technology, Beverly, MA), rabbit anti-AMPK mAb (Cell Signaling Technology), mouse anti–acetylated lysine antibody (Upstate Biotechnology, Inc., Lake Placid, NY), rabbit anti–phospho-AMPK mAb (Cell Signaling Technology), mouse anti–poly (ADP-ribose) (PAR) mAb (R&D Systems, Minneapolis, MN), human anti–NF-κB antibody (Enzo Life Sciences, Inc., Farmingdale, NY), or mouse anti-Bax mAb (R&D Systems).
SIRT1, LKB1, and AMPK activity.
SIRT1 deacetylase activity was quantified following the protocols of the SIRT1 fluorometric assay kit (Sigma-Aldrich, St. Louis, MO). For the LKB1 activity assay, LKB1 was immunoprecipitated with the N-19 LKB1 antibody and protein G beads, and AMPK activity was measured as previously described (20).
Statistical analysis.
Group means were compared by one-way ANOVA using the GraphPad Prism 4.0 software system (GraphPad, San Diego, CA) and the statistical software program SPSS version 17.0 for Windows (SPSS, Chicago, IL). Pearson correlation tests also were performed. P values <0.05 were considered significant in all cases.
RESULTS
Persistence of increased NF-κB, Bax, and PAR protein and inhibition of SIRT1 induced by high glucose after glucose normalization in BRECs.
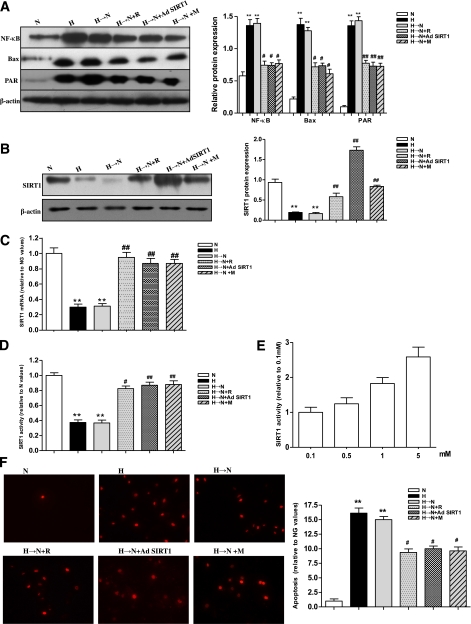
The transcription factor NF-κB is a central regulator of inflammatory responses (21), and Bax, a mitochondrial proapoptotic protein BCL-2 family member, is a marker of mitochondrial stress associated with vascular diabetes complications (22). Moreover, PAR, a product of activated PARP, was an indicator of PARP activity, which was shown to be a critical factor in the development of vascular diabetes complications (23). We examined whether there was a persistence, or cellular memory, of hyperglycemic stress, such as inflammation and apoptosis, after normalization of glucose levels. As shown in Fig. 1A, chronic high glucose resulted in significantly increased levels of NF-κB, Bax, and PAR protein. Compared with exposure to continuous normal glucose, their levels remained increased in cells treated with high glucose for 1 week followed by normal glucose for 2 weeks. In contrast, chronic high glucose led to a significant decrease of SIRT1 at the mRNA and protein levels as well as in its activity. Moreover, the level and activity of SIRT1 remained decreased in cells treated with high glucose for 1 week followed by normal glucose for 2 weeks compared with exposure to continuous normal glucose (Fig. 1B–D). We also observed the level of cellular apoptosis using the TUNEL assay. The chronic exposure to high glucose caused a significant increase in cellular apoptosis, which remained increased in the cells exposed to high glucose for 1 week followed by normal glucose for 2 weeks (Fig. 1F). These results suggested that there was a cellular memory of hyperglycemia stress. Pearson correlation tests showed a negative correlation between the level of SIRT1 and those of NF-κB, Bax, PARP activity, or cellular apoptosis (data not shown).
FIG. 1.
Increased NF-κB, Bax, PAR protein, and apoptosis and decreased SIRT1 in BRECs after culture in high glucose or in high glucose followed by normal glucose. A: Western blotting (left) and quantification (right) of NF-κB, Bax, and PAR protein expression profiles in cell treatment groups: normal glucose (N), high glucose (H), high glucose followed by normal glucose (H→N), H→N plus resveratrol (H→N+R), H→N plus adenovirus overexpressing SIRT1 (H→N+Ad SIRT1), and H→N plus metformin (H→N+M). B–D: Western blotting (left) and quantification (right) of SIRT1 protein (B) or real-time RT-PCR analysis of mRNA (C) expression and activity (D) profiles in the 6 groups. E: Effects of metformin (0.1, 0.5, 1.0, and 5.0 mmol/L) on SIRT1 activity in H→N. F: Analysis of cellular apoptosis levels in the 6 groups by TUNEL. Bars indicate SDs. A representative experiment of the three is shown. **P < 0.01 vs. N; #P < 0.05 vs. H→N; ##P < 0.01 vs. H→N. (A high-quality digital representation of this figure is available in the online issue.)
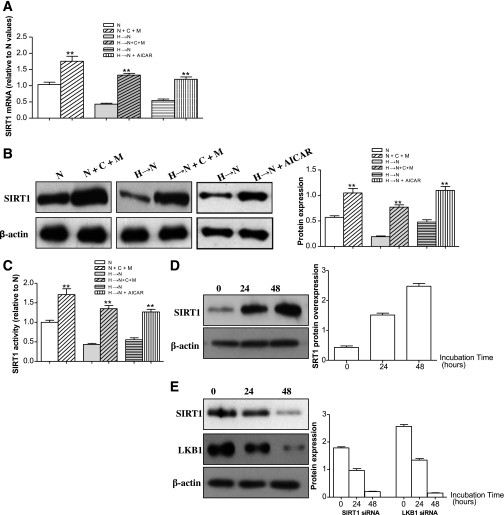
To explore whether SIRT1 activation can interrupt the cellular memory of high-glucose stress, the cells were treated with resveratrol (100 μmol/L, a putative SIRT1 activator), a SIRT1 overexpression vector (Fig. 1A), or metformin (1 mmol/L) during the incubation of cells in high glucose for 1 week followed by normal glucose for 2 weeks. The increase in NF-κB, Bax, and PAR protein expression induced by high glucose was attenuated by resveratrol or SIRT1 overexpression, and metformin was found to have a similar effect through upregulation of SIRT1 expression and activity (Fig. 1A–D). Moreover, the effect of metformin on SIRT1 activity was shown to be dose dependent (Fig. 1E). Consequently, the heightened cellular apoptosis was inhibited by the three treatments (Fig. 1F). In addition, in the presence of compound C, an inhibitor of AMPK, metformin could still upregulate SIRT1 expression and activity (Fig. 2A–C), suggesting that the effect of metformin on SIRT1 was at least partly independent on AMPK, although the AMPK activator 5’-aminoimidazole-4-carboxymide-1-β-d-ribofuranoside (AICAR) directly upregulated SIRT1 expression and activity in cells treated with high glucose for 1 week followed by normal glucose for 2 weeks (Fig. 2A–C). To rule out the influence of osmolarity on the cellular memory of hyperglycemia stress, 25 mmol/L mannitol was added to cells along with normal glucose at 5 mmol/L for 3 weeks to equalize the osmolarity with the high-glucose treatment at 30 mmol/L, and no effects on the memory were observed (data not shown).
FIG. 2.
Analysis of SIRT1 mRNA, protein and activity, and LKB1 protein expression in BRECs. A: Real-time RT-PCR analysis of SIRT1 mRNA in cell treatment groups: normal glucose (N), N plus compound C plus metformin (10 μmol/L) (N+C+M), high glucose followed by normal glucose (H→N), H→N plus compound C plus metformin (10 μmol/L) (H→N+C+M), H→N, H→N+AICAR. B: Western blotting (left) and quantification (right) of SIRT1 protein in the six groups. C: SIRT1 activity profiles in the six groups. D: Effect of adenovirus overexpressing SIRT1 (Ad SIRT1) on SIRT1 expression in BRECs incubated in N. E: Effect of siRNA knockdown on SIRT1 and LKB1 expression in BRECs incubated in N. One representative experiment of the three is shown. **P < 0.01 vs. N.
SIRT1 mediates LKB1/AMPK activity in retinal endothelial cells.
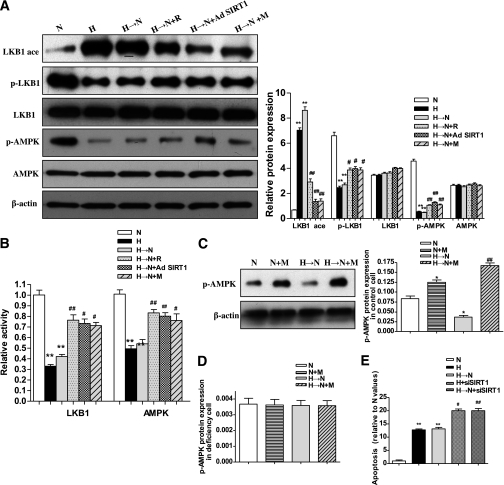
Next, we attempted to clarify the mechanism by which SIRT1 inhibited the cellular memory of high-glucose stress. A previous study demonstrated that the LKB1/AMPK pathway is regulated by SIRT1 in 293T cells (24). In this study, we found that LKB1 and AMPK expression were not changed in retinal endothelial cells incubated with high glucose (Fig. 3A). As described above, chronic high glucose induced a significant decrease in the SIRT1, which remained decreased in cells exposed to high glucose for 1 week followed by normal glucose for 2 weeks, compared with exposure to continuous normal glucose; and similar changes occurred for LKB1 and AMPK activity and phosphorylation (Fig. 3A and B). In addition, the acetylation status of LKB1 was evaluated by an immunoprecipitation assay. We found that chronic high glucose significantly increased LKB1 acetylation, and the level remained elevated in cells exposed to high glucose for 1 week followed by normal glucose for 2 weeks compared with exposure to continuous normal glucose (Fig. 3B). Furthermore, Pearson tests demonstrated positive correlations between the SIRT1 level and LKB1 or AMPK activities (data not shown).
FIG. 3.
Increases of LKB1/AMPK activity by SIRT1 and therapeutic effects of metformin in BRECs. A: Western blotting (left) and quantification (right) of LKB1 and AMPK protein, acetylation of immunoprecipitated-LKB1 (LKB1ace), phosphorylation-LKB1 (p-LKB1), and phosphorylation-AMPK (p-AMPK) expression profiles in cell treatment groups: normal glucose (N), high glucose (H), high glucose followed by normal glucose (H→N), H→N plus resveratrol (H→N + R), H→N plus adenovirus overexpressing SIRT1 (H→N + Ad SIRT1), and H→N plus metformin (H→N + M). B: Relative activity of LKB1 and AMPK in the six groups. C and D: Effect of LKB1 siRNA knockdown on p-AMPK. Western blotting (left) and quantification (right) of p-AMPK protein expression profiles in N, N+M, H→N, and H→N+M in control cells (C) and in LKB1 siRNA knockdown cells (D). E: High glucose–induced cellular apoptosis was increased in SIRT1 siRNA knockdown cells. Apoptosis cells in N, H, and H→N in control cells and H and H→N in siRNA knockdown cells were evaluated by the TUNEL assay. Bars indicate SDs. A representative experiment of the three is shown. *P < 0.05 vs. N; **P < 0.01 vs. N; #P < 0.05 vs. H→N; ##P < 0.01 vs. H→N.
To further investigate the effect of SIRT1 in the LKB1/AMPK pathway, we used two approaches that either knocked down (siRNA) or overexpressed SIRT1 (Fig. 2D and E). Transfection with the SIRT1 siRNA vector for 48 h caused decreases in LKB1 and AMPK activity (Fig. 3B). Of note, SIRT1 knockdown cells were sensitive to high-glucose stress and showed increased levels of cellular apoptosis (Fig. 3E) as well as NF-κB and Bax expression (data not shown). For assessing the effects of increased SIRT1 levels, the SIRT1 activator resveratrol, SIRT1 overexpression vector, or metformin were applied to the cells during the incubation with high glucose for 1 week followed by normal glucose for 2 weeks. Treatment with resveratrol caused an increase in LKB1 and AMPK activity and phosphorylation (Fig. 3A and B). Similar findings also were obtained with the SIRT1 overexpression vector and metformin (Fig. 3A and B). By immunoprecipitation, LKB1 acetylation was shown to be decreased by the three treatments through SIRT1 activation (Fig. 3A).
We also examined whether metformin treatment of BRECs increased AMPK activity in an LKB1-dependent manner. AMPK phosphorylation was increased in the control cells treated with metformin with or without high glucose but not in the cells deficient in LKB1 by knocked-down (siRNA) LKB1 (Fig. 2E and Fig. 3C and D), although AMPK protein expression was unaffected (data not shown).
AMPK inhibits ROS pathway activation in high glucose in BRECs.
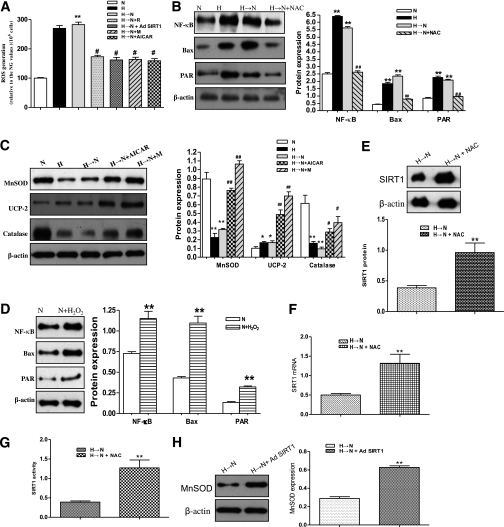
To examine whether mitochondrial ROS remained increased after glucose normalization, the isolated mitochondria from BRECs treated as above were loaded with the cell-permeable ROS-sensitive fluorescent dye CM-H2DCFDA. Continuous exposure to high glucose (30 mmol/L) for 3 weeks resulted in a significant increase in fluorescence, indicating increased ROS levels, compared with cells exposed to normal glucose (5 mmol/L) levels; furthermore, the levels of fluorescence remained elevated in cells exposed to high glucose for 1 week followed by normal glucose for 2 weeks (Fig. 4A). Consistent with the findings in our previous studies and other studies (8,9,11,25), the results indicated that the mitochondria could be a major contributor of ROS, although other sources are possible. Furthermore, an ROS scavenger, N-acetylcysteine (NAC), was used to explore whether decreasing levels of intracellular ROS could interrupt the cellular memory of high-glucose stress. The addition of NAC during the last 2 weeks of normal glucose after 1 week of high-glucose exposure blocked the induction of all markers of high-glucose stress, such as NF-κB, Bax, and PAR protein expression (Fig. 4B), whereas H2O2 (500 μmol/L) induced the increase of NF-κB, Bax, and PAR protein expression, similar to that found with chronic high glucose (Fig. 4D). Furthermore, NAC treatment upregulated SIRT1 expression and activity in the cells incubated with high glucose for 1 week followed by normal glucose for 2 weeks, indicating that ROS may modulate SIRT1 expression (Fig. 4E–G).
FIG. 4.
Inhibition of the ROS pathway activation by AMPK in BRECs. A: ROS production in BRECs was identified by isolating and diluting mitochondria suspensions to 1 mg protein/mL with the fluorescent probe CM-H2DCFDA in cell treatment groups: normal glucose (N), high glucose (H), high glucose followed by normal glucose (H→N), H→N + resveratrol (H→N + R), H→N + adenovirus overexpressing SIRT1 (H→N + Ad SIRT1), H→N + metformin (H→N + M), and H→N+AICAR. B: Effects of an ROS scavenger NAC on the markers of high-glucose stress. Western blotting (left) and quantification (right) of NF-κB, Bax, and PAR protein expression profiles in N, H, H→N, and H→N+NAC. C: AMPK activation upregulates MnSOD, UCP-2, and catalase with AICAR or metformin treatment by Western blotting. D: NF-κB, Bax, and PAR protein expression profiles in N and N+H2O2. E–G: Western blotting (top) and quantification (bottom) of SIRT1 protein (E), mRNA expression (determined by real-time RT-PCR) (F), and activity profiles (G) in H→N and in H→N+NAC. H: MnSOD expression in H→N and in H→N+Ad SIRT1 by Western blotting (left). Bars indicate SDs. A representative experiment of the three is shown. *P < 0.05 vs. N; **P < 0.01 vs. N or H→N; #P < 0.05 vs. H→N; ##P < 0.01 vs. H→N.
Next, we investigated whether AMPK activation can interrupt the cellular memory of high-glucose stress. Treatment with an AMPK activator AICAR (1 mmol/L) or metformin resulted in a decrease in ROS generation (Fig. 4A). Similar findings were obtained with the resveratrol or SIRT1 overexpression vector. We further explored the mechanism by which AMPK inhibited the mitochondrial ROS of high-glucose stress and found that AICAR or metformin could upregulate the expression of manganese superoxide dismutase (MnSOD) uncoupling protein (UCP)-2, and catalase (Fig. 4C). In addition, we found that SIRT1 directly increased the expression of MnSOD (Fig. 4H).
Sustained inhibition of SIRT1 expression is associated with PARP activation.
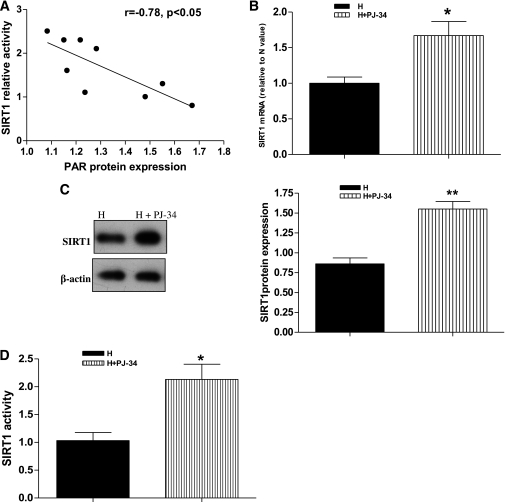
As described above, SIRT1 can regulate the LKB1/AMPK/ROS pathway. Next, we explored the mechanisms for the sustained inhibition of SIRT1. Because both SIRT1 and PARP-1 are nuclear enzymes that use the same nuclear NAD+ cofactor pool, and both respond to similar stimuli in cellular functions (26), we wanted to determine whether high glucose–induced activation of PARP-1 depletes NAD+ and inhibits SIRT1 and whether the sustained inhibition of SIRT1 is associated with PARP activation in retinal endothelial cells. As shown in Fig. 1A–D, chronic high glucose resulted in the significant increase of PARP activity and decrease of SIRT1 levels compared with exposure to continuous normal glucose, and the change remained in cells exposed to high glucose for 1 week followed by normal glucose for 2 weeks. Statistical analysis showed that there was a negative correlation between the SIRT1 levels and PARP activity (Fig. 5A). A PARP-specific inhibitor, PJ-34, was used to further observe the effect of PARP inhibition on SIRT1 expression. As expected, PJ-34 prevented the decrease of SIRT1 expression for mRNA and protein as well as its activity in response to high glucose (Fig. 5B–D). These data suggest that PARP activation may be at least partly involved in the high-glucose–induced sustained inhibition of SIRT1 expression and activity.
FIG. 5.
Association of PARP activation with sustained inhibition of SIRT1 in BRECs. A: Negative correlation between PAR protein expression levels and SIRT1 activity in BRECs. B–D: mRNA level determined by real-time RT-PCR (B), protein level measured by Western blotting (C), and activity assayed by a fluorometric assay kit (D) of SIRT1 in high glucose followed by normal glucose (H→N) and H→N plus a PARP-specific inhibitor PJ-34 (H→N+PJ-34). Bars indicate SDs. A representative experiment of the three is shown. *P < 0.05 vs. H→N; **P < 0.01 vs. H→N.
Memory of hyperglycemia stress in the retina of diabetic animals.
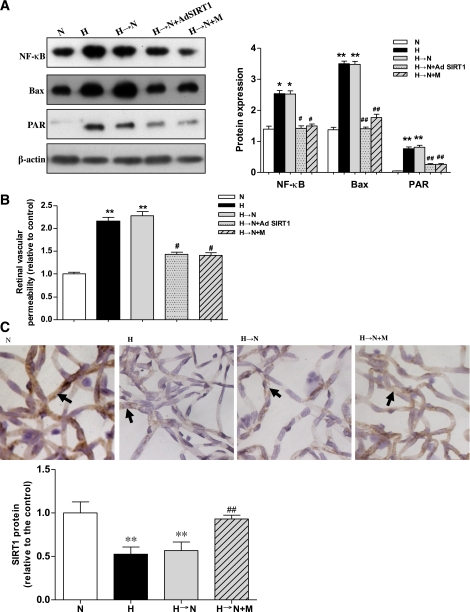
Levels of NF-κB, Bax, and PARP activity also were assessed in the retina of rats that maintained (1) normoglycemia for 6 weeks, (2) hyperglycemia for 6 weeks, or (3) hyperglycemia for 2 weeks followed by treatment to obtain normoglycemia for 4 weeks (memory). As expected, the levels of these hyperglycemia stress markers were increased in the retina of diabetic animals, which remained elevated for 4 weeks after normalization of glucose levels (Fig. 6A). Furthermore, an increase in retinal vascular permeability was detected in diabetic rats, and the hyperglycemia-induced increase even maintained for 4 weeks after glucose normalization (Fig. 6B). In addition, by immunohistochemical staining, we also found that hyperglycemia induced the decrease of SIRT1 protein expression in trypsin-digested retinal blood vessels compared with exposure to continuous normal glucose, and this decreased level was maintained even 4 weeks after glucose normalization (Fig. 6C).
FIG. 6.
Increased markers of high-glucose stress and vascular injury during hyperglycemia or hyperglycemia followed by normoglycemia in rat retinas and their inhibition by Ad SIRT1 or metformin. A: Western blotting (left) and quantification (right) of NF-κB, Bax, and PAR protein expression profiles in normoglycemia (N), hyperglycemia (H), hyperglycemia followed by normoglycemia (H→N), H→N plus adenovirus overexpressing SIRT1 (H→N + Ad SIRT1), and H→N plus metformin (H→N + M). B: Retinal vascular permeability by Evans blue dye in the five groups. C: Immunohistochemical staining for SIRT1 in trypsin-digested retinal blood vessels in N, H, H→N, and H→N + M. Sections were counterstained with hematoxylin. Original magnification was ×400. Bars indicate SDs. A representative experiment of the three is shown. **P < 0.01 vs. N, n = 8; *P < 0.05 vs. N; #P < 0.05 vs. H→N; ##P < 0.01 vs. H→N. (A high-quality digital representation of this figure is available in the online issue.)
Changes in the SIRT1/LKB1/AMPK/ROS pathway in diabetic retinas.
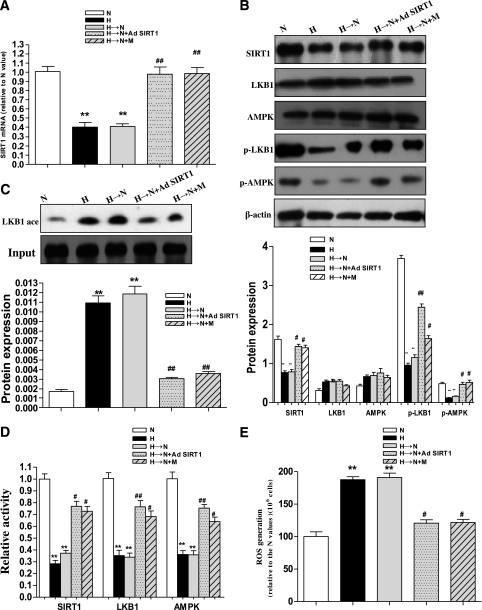
Based on the findings above, it was important to ask whether the SIRT1-mediated effects on the memory of high-glucose stress in diabetic retinas were related to the LKB1/AMPK/ROS pathway. Therefore, we examined the levels of SIRT1, LKB1, AMPK, and ROS in the retinas of diabetic animals. We found that that hyperglycemia caused a significant decrease in the mRNA and protein levels as well as activity of SIRT1. Moreover, the changes remained for 4 weeks after glucose normalization compared with exposure to continuous normal glucose (Fig. 7A–C). Likewise, the expressions of p-LKB1 and p-AMPK and the activities of LKB1 and AMPK also were decreased, although LKB1 and AMPK protein expressions were not changed, whereas ROS generation increased (Fig. 7B–E). These levels remained decreased or elevated for 4 weeks after normalization of glucose levels (Fig. 7A–E). In addition, LKB1 acetylation was found to be decreased via deacetylation through SIRT1 overexpression or metformin treatment (Fig. 7C).
FIG. 7.
Increased ROS generation by inhibition of the SIRT1/LKB1/AMPK pathway in hyperglycemia or hyperglycemia followed by normoglycemia in rat retinas and the effects of Ad SIRT1 and metformin. A: SIRT1 mRNA by real-time RT-PCR in normoglycemia (N), hyperglycemia (H), hyperglycemia followed by normoglycemia (H→N), H→N + adenovirus overexpressing SIRT1 (H→N + Ad SIRT1), and H→N + metformin (H→N + M). B: Western blotting (top) and quantification (bottom) of SIRT1, LKB1, AMPK, p-LKB1, and p-AMPK expression profiles in the five groups. C: Western blotting (top) and quantification (bottom) of LKB1 acetylation level in the five groups. D: SIRT1, LKB1, and AMPK activity profiles in the five groups. E: ROS production was identified by isolating mitochondria and diluting suspensions to 1 mg protein/mL with the fluorescent probe CM-H2DCFDA in the five groups. Bars indicate SDs. A representative experiment of the three is shown. **P < 0.01 vs. N; #P < 0.05 vs. H→N; ##P < 0.01 vs. H→N.
We next wanted to determine whether the persistence of hyperglycemia stress could be interrupted in the rat diabetic model in vivo using the SIRT1 overexpression vector. Administration of Ad SIRT1 during the last month of normalized glucose resulted in significant increases of LKB1 and AMPK activity and decreases in the hyperglycemia stress markers and ROS in the retinas of diabetic animals (Fig. 6A and Fig. 7D and E).
Metformin suppresses memory of hyperglycemia stress in the retinas of diabetic animals.
Metformin, a widely used antidiabetes drug, has been demonstrated to activate SIRT1 (27), consistent with our in vitro results in this present study. Therefore, we investigated the role of metformin in the SIRT1/LKB1/AMPK/ROS-mediated memory of hyperglycemia stress in the retinas of diabetic animals. After hyperglycemia was induced for 2 weeks, we treated the diabetic rats with this drug for 4 weeks, during which normal glycemia was maintained. As expected, metformin significantly inhibited the decreases of SIRT1 expression and LKB1 and AMPK activity and reduced the increase of ROS (Fig. 7A–E). Furthermore, it inhibited the activation of NF-κB, Bax, and PAR protein expression in the retinas of the rats with normalization of glucose for 4 weeks after 2 weeks of hyperglycemia (Fig. 6A).
DISCUSSION
In the current study, we provide evidence that SIRT1 confers resistance to the cellular metabolic memory induced by high glucose. SIRT1-deficient cells had increased sensitivity to high-glucose stress, whereas SIRT1 activation reduced the cellular metabolic memory of high glucose in the retinal endothelial cells. SIRT1 protected the cells from metabolic memory in response to high glucose through at least two mechanisms: suppressing production of the cellular inflammatory gene NF-κB and attenuating the expression of the cellular apoptosis gene Bax. The activation of SIRT1 is likely involved in the direct activation of LKB1/AMPK signaling in retinal endothelial cells, subsequently resulting in the inhibition of the mitochondrial ROS/PARP pathway, which is upstream of the cellular inflammatory and apoptosis pathways. Furthermore, we show here that high glucose led to the activation of PARP, which in turn, downregulated the level of SIRT1. This downregulation then was maintained and propagated in retinal endothelial cells even after 2 weeks in normal glucose following 1 week of exposure to high glucose. Of importance, this study also determined that metformin suppressed the memory of hyperglycemia stress in BRECs and the retina of diabetic animals. This effect may have been mediated by the ability of metformin to reverse the increase in ROS induced by SIRT1/LKB1/AMPK.
To our knowledge, our study is the first to link SIRT1 with the cellular metabolic memory of high glucose. First, we found that the decreased level of SIRT1 correlated with the cellular memory of hyperglycemia stress, as evidenced by markers of inflammation (NF-κB), apoptosis (Bax), and PARP in the cells or in rat retina tissues after glucose levels were normalized. Second, we found that the activation of SIRT1 by resveratrol or SIRT1 overexpression could inhibit the cellular memory of hyperglycemia stress, whereas the inhibition of SIRT1 by SIRT1 siRNA increased sensitivity to high-glucose stress in the cells, suggesting that there may be causal relationships between the reduced level of SIRT1 and the cellar metabolic memory of high glucose. Recent studies demonstrated a link between epigenetic changes by SIRT1 and gene expression (28–31), and dysregulation of epigenetic histone modifications may be an underlying mechanism for the metabolic memory of diabetic cells (32,33). Therefore, the role of SIRT1 in the metabolic memory, as it relates to epigenetic changes, should be investigated in future studies.
The mechanisms by which SIRT1 suppresses inflammatory and apoptosis reactions are poorly understood. This study demonstrated that knockdown of SIRT1 cells reduced the activation of AMPK, whereas activation of SIRT1 increased AMPK activity. Furthermore, activation of AMPK by AICAR inhibited the increase of ROS generation, resulting in the suppression of NF-κB, Bax, and PAR protein expression. We also reported that LKB1 was an upstream activating kinase for AMPK in retinal endothelial cells, and when LKB1 was knocked down in the cells, AMPK activity was decreased and the activation of AMPK by resveratrol or metformin was blocked. These results indicated that the effects of SIRT1 on the suppression of inflammation and apoptosis were mediated by the LKB1/AMPK/ROS pathway in retinal endothelial cells, and these effects continued even after 2 weeks of normal glucose following 1 week of high glucose. In contrast, the previous studies demonstrated that AMPK enhances SIRT1 activity by increasing cellular NAD+ levels (34). However, the effects of AMPK on SIRT1 will need to be further investigated under our experimental conditions.
This study also elucidated the mechanisms underlying the role of AMPK in the inhibition of mitochondrial ROS. Our results showed that AMPK activation by AICAR increased MnSOD, UCP-2, and catalase expression in the cells. MnSOD is a major antioxidant enzyme of mitochondria (35), and UCP-2 was found to mediate mitochondrial ROS production in a chronic hyperglycemia setting in our previous study (8,36). Of note, the induction of anti-ROS genes is not limited to those of the mitochondria: catalase is present substantially or totally in the nonmitochondrial cytoplasm and peroxisomes (37).
A previous study demonstrated that ROS may affect SIRT1 levels/activity (38), although the underlying mechanism is not clear. In this study, we reported that sustained inhibition of SIRT1 expression was involved in the activation of PARP. PARP is a member of a family of eukaryotic nuclear enzymes that play important roles in regulating DNA repair, gene transcription, cell cycle progression, chromatin function, genomic stability, and cell death. Previous studies by our group and others demonstrated that high glucose leads to overproduction of mitochondrial ROS, which inactivates glyceraldehyde-3-phosphate dehydrogenase by PARP activation and subsequent ADP ribosylation in the endothelial cells, further promoting inflammatory and apoptosis cascades (9,11,23,39). This study showed that the inhibition of PARP activity by PJ-34 upregulated SIRT1 expression and activity, suggesting that ROS-induced PARP activation was at least partly involved in the decrease of SIRT1 expression in the cells. This may have created an amplifying auto-feedback loop regulating SIRT1 expression and a vicious cycle that further propagated vascular inflammation and apoptosis in retinal endothelial cells, even after removal of the cells from high-glucose exposure and returning to normal glucose conditions for 2 weeks.
Of importance, we also found that metformin suppressed memory of hyperglycemia stress in vitro and in vivo in this study. Metformin is an oral biguanide that is one of the most widely prescribed drugs not only for type 2 diabetic patients but also for patients with obesity, metabolic syndrome, or prediabetes (40–44). Decreased hepatic gluconeogenesis and, to a lesser extent, increased glucose uptake into skeletal muscle cells have been proposed as mechanisms of metformin action (45). Recent studies suggest that metformin decreases intracellular ROS and inhibits diabetes-induced renal hypertrophy through AMPK (46,47). Until now, our study was the first to demonstrate that activation of SIRT1 by metformin inhibited cellular inflammation and apoptosis through the LKB1/AMPK/ROS/PARP pathway, and we further confirmed these results in vivo. Gundewar et al. (48) previously reported that metformin upregulated AMPK, whereas AMPK may have modulated SIRT1 (34). Under high-glucose conditions, we found that metformin increases SIRT1 level/activity directly.
In conclusion, our results provide molecular insight into the regulation of the cellular metabolic memory induced by high glucose in cells and retinas. Using in vitro and in vivo models, we demonstrated the crucial role of SIRT1 in the control of inflammation and apoptosis through LKB1/AMPK-dependent pathways, and SIRT1 expression and activity were inversely correlated with PARP, an upstream gene of the inflammatory and apoptotic pathways. We also provided evidence that metformin inhibited the cellular metabolic memory resulting from the suppression of ROS/PARP signaling, which was linked to the upregulation of the SIRT1/LKB1/AMPK pathway. Our data suggest that SIRT1 can be a potential therapeutic target for treatments aimed at reducing cellular metabolic memory of high glucose. The use of metformin in such treatments to provide some additional benefits for microvascular and macrovascular complications beyond its antihyperglycemia activity may be a new avenue of investigation in the diabetes field.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (nos. 30872828, 30871204, and 81070739) and the National Key Basic Research Program (2010CB535006).
No potential conflicts of interest relevant to this article were reported.
Z.Z. is the guarantor of this article. Z.Z. and H.C. researched data, contributed to the discussion, and wrote, reviewed, and edited the manuscript. J.L., T.L., B.Z., Y.Z., H.J., Y.H., and X.X. researched data and contributed to the discussion. Q.G. researched data.
REFERENCES
- 1.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002;287:2563–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan DM, Lachin J, Cleary P, et al. ; Diabetes Control and Complications Trial; Epidemiology of Diabetes Interventions and Complications Research Group Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 2003;348:2294–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan DM, Cleary PA, Backlund JY, et al. ; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engerman RL, Kern TS. Progression of incipient diabetic retinopathy during good glycemic control. Diabetes 1987;36:808–812 [DOI] [PubMed] [Google Scholar]
- 6.Roy S, Sala R, Cagliero E, Lorenzi M. Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc Natl Acad Sci USA 1990;87:404–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ihnat MA, Thorpe JE, Kamat CD, et al. Reactive oxygen species mediate a cellular ‘memory’ of high glucose stress signaling. Diabetologia 2007;50:1523–1531 [DOI] [PubMed] [Google Scholar]
- 8.Zheng Z, Chen H, Ke G, et al. Protective effect of perindopril on diabetic retinopathy is associated with decreased vascular endothelial growth factor-to-pigment epithelium-derived factor ratio: involvement of a mitochondria–reactive oxygen species pathway. Diabetes 2009;58:954–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng Z, Chen H, Wang H, et al. Improvement of retinal vascular injury in diabetic rats by statins is associated with the inhibition of mitochondrial reactive oxygen species pathway mediated by peroxisome proliferator-activated receptor γ coactivator 1α. Diabetes 2010;59:2315–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000;404:787–790 [DOI] [PubMed] [Google Scholar]
- 11.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 12.Yu J, Auwerx J. Protein deacetylation by SIRT1: an emerging key post-translational modification in metabolic regulation. Pharmacol Res 2010;62:35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kao CL, Chen LK, Chang YL, et al. Resveratrol protects human endothelium from H(2)O(2)-induced oxidative stress and senescence via SirT1 activation. J Atheroscler Thromb 2010;17:970–979 [DOI] [PubMed] [Google Scholar]
- 14.Pardo PS, Mohamed JS, Lopez MA, Boriek AM. Induction of Sirt1 by mechanical stretch of skeletal muscle through the early response factor EGR1 triggers an antioxidative response. J Biol Chem 2011;286:2559–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawley SA, Boudeau J, Reid JL, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol 2003;2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woods A, Dickerson K, Heath R, et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2005;2:21–33 [DOI] [PubMed] [Google Scholar]
- 17.Li XN, Song J, Zhang L, et al. Activation of the AMPK-FOXO3 pathway reduces fatty acid–induced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes 2009;58:2246–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colombo SL, Moncada S. AMPKalpha1 regulates the antioxidant status of vascular endothelial cells. Biochem J 2009;421:163–169 [DOI] [PubMed] [Google Scholar]
- 19.Breitenstein A, Stein S, Holy EW, et al. Sirt1 inhibition promotes in vivo arterial thrombosis and tissue factor expression in stimulated cells. Cardiovasc Res 2011;89:464–472 [DOI] [PubMed] [Google Scholar]
- 20.Murase T, Misawa K, Haramizu S, Hase T. Catechin-induced activation of the LKB1/AMP-activated protein kinase pathway. Biochem Pharmacol 2009;78:78–84 [DOI] [PubMed] [Google Scholar]
- 21.Ashida H, Kim M, Schmidt-Supprian M, Ma A, Ogawa M, Sasakawa C. A bacterial E3 ubiquitin ligase IpaH9.8 targets NEMO/IKKgamma to dampen the host NF-kappaB-mediated inflammatory response. Nat Cell Biol 2010;12:66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Podestà F, Romeo G, Liu WH, et al. Bax is increased in the retina of diabetic subjects and is associated with pericyte apoptosis in vivo and in vitro. Am J Pathol 2000;156:1025–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du X, Matsumura T, Edelstein D, et al. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest 2003;112:1049–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1: possible role in AMP-activated protein kinase activation. J Biol Chem 2008;283:27628–27635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui Y, Xu X, Bi H, et al. Expression modification of uncoupling proteins and MnSOD in retinal endothelial cells and pericytes induced by high glucose: the role of reactive oxygen species in diabetic retinopathy. Exp Eye Res 2006;83:807–816 [DOI] [PubMed] [Google Scholar]
- 26.Kolthur-Seetharam U, Dantzer F, McBurney MW, de Murcia G, Sassone-Corsi P. Control of AIF-mediated cell death by the functional interplay of SIRT1 and PARP-1 in response to DNA damage. Cell Cycle 2006;5:873–877 [DOI] [PubMed] [Google Scholar]
- 27.Lee JS, Park KY, Min HG, et al. Negative regulation of stress-induced matrix metalloproteinase-9 by Sirt1 in skin tissue. Exp Dermatol 2010;19:1060–1066 [DOI] [PubMed] [Google Scholar]
- 28.Holloway KR, Calhoun TN, Saxena M, et al. SIRT1 regulates Dishevelled proteins and promotes transient and constitutive Wnt signaling. Proc Natl Acad Sci USA 2010;107:9216–9221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellet MM, Sassone-Corsi P. Mammalian circadian clock and metabolism: the epigenetic link. J Cell Sci 2010;123:3837–3848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajendrasozhan S, Yao H, Rahman I. Current perspectives on role of chromatin modifications and deacetylases in lung inflammation in COPD. COPD 2009;6:291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holness MJ, Caton PW, Sugden MC. Acute and long-term nutrient-led modifications of gene expression: potential role of SIRT1 as a central co-ordinator of short and longer-term programming of tissue function. Nutrition 2010;26:491–501 [DOI] [PubMed] [Google Scholar]
- 32.Villeneuve LM, Reddy MA, Lanting LL, Wang M, Meng L, Natarajan R. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci USA 2008;105:9047–9052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brasacchio D, Okabe J, Tikellis C, et al. Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes 2009;58:1229–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantó C, Gerhart-Hines Z, Feige JN, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009;458:1056–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kukidome D, Nishikawa T, Sonoda K, et al. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes 2006;55:120–127 [PubMed] [Google Scholar]
- 36.Zheng Z, Chen H, Zhao H, et al. Inhibition of JAK2/STAT3-mediated VEGF upregulation under high glucose conditions by PEDF through a mitochondrial ROS pathway in vitro. Invest Ophthalmol Vis Sci 2010;51:64–71 [DOI] [PubMed] [Google Scholar]
- 37.St-Pierre J, Drori S, Uldry M, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006;127:397–408 [DOI] [PubMed] [Google Scholar]
- 38.Lee YA, Cho EJ, Yokozawa T. Protective effect of persimmon (Diospyros kaki) peel proanthocyanidin against oxidative damage under H2O2-induced cellular senescence. Biol Pharm Bull 2008;31:1265–1269 [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Jia W, Xu X, et al. Upregulation of PEDF expression by PARP inhibition contributes to the decrease in hyperglycemia-induced apoptosis in HUVECs. Biochem Biophys Res Commun 2008;369:718–724 [DOI] [PubMed] [Google Scholar]
- 40.Srinivasan S, Ambler GR, Baur LA, et al. Randomized, controlled trial of metformin for obesity and insulin resistance in children and adolescents: improvement in body composition and fasting insulin. J Clin Endocrinol Metab 2006;91:2074–2080 [DOI] [PubMed] [Google Scholar]
- 41.Park MH, Kinra S, Ward KJ, White B, Viner RM. Metformin for obesity in children and adolescents: a systematic review. Diabetes Care 2009;32:1743–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meaney E, Vela A, Samaniego V, et al. Metformin, arterial function, intima-media thickness and nitroxidation in metabolic syndrome: the Mefisto Study. Clin Exp Pharmacol Physiol 2008;35:895–903 [DOI] [PubMed] [Google Scholar]
- 43.Bulcão C, Ribeiro-Filho FF, Sañudo A, Roberta Ferreira SG. Effects of simvastatin and metformin on inflammation and insulin resistance in individuals with mild metabolic syndrome. Am J Cardiovasc Drugs 2007;7:219–224 [DOI] [PubMed] [Google Scholar]
- 44.Rhee MK, Herrick K, Ziemer DC, et al. Many Americans have pre-diabetes and should be considered for metformin therapy. Diabetes Care 2010;33:49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bosi E. Metformin: the gold standard in type 2 diabetes: what does the evidence tell us? Diabetes Obes Metab 2009;11(Suppl. 2):3–8 [DOI] [PubMed] [Google Scholar]
- 46.Piwkowska A, Rogacka D, Jankowski M, Dominiczak MH, Stepiński JK, Angielski S. Metformin induces suppression of NAD(P)H oxidase activity in podocytes. Biochem Biophys Res Commun 2010;393:268–273 [DOI] [PubMed] [Google Scholar]
- 47.Lee MJ, Feliers D, Mariappan MM, et al. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol 2007;292:F617–F627 [DOI] [PubMed] [Google Scholar]
- 48.Gundewar S, Calvert JW, Jha S, et al. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res 2009;104:403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]