Abstract
Adipose tissue dysfunction underpins the association of obesity with type 2 diabetes. Adipogenesis is required for the maintenance of adipose tissue function. It involves the commitment and subsequent differentiation of preadipocytes and is coordinated by autocrine, paracrine, and endocrine factors. We previously reported that fibroblast growth factor-1 (FGF-1) primes primary human preadipocytes and Simpson Golabi Behmel syndrome (SGBS) preadipocytes and increases adipogenesis through a cascade involving extracellular signal–related kinase 1/2 (ERK1/2). Here, we aimed to use the FGF-1 system to identify novel adipogenic regulators. Expression profiling revealed bone morphogenetic protein (BMP) and activin membrane-bound inhibitor (BAMBI) as a putative FGF-1 effector. BAMBI is a transmembrane protein and modulator of paracrine factors that regulate adipogenesis, including transforming growth factor (TGF) superfamily members (TGF-β and BMP) and Wnt. Functional investigations established BAMBI as a negative regulator of adipogenesis and modulator of the anti- and proadipogenic effects of Wnt3a, TGF-β1, and BMP-4. Further studies showed that BAMBI expression levels are decreased in a mouse model of diet-induced obesity. Collectively, these findings establish BAMBI as a novel, negative regulator of adipogenesis that can act as a nexus to integrate multiple paracrine signals to coordinate adipogenesis. Alterations in BAMBI may play a role in the (patho)physiology of obesity, and manipulation of BAMBI may present a novel therapeutic approach to improve adipose tissue function.
Obesity represents a major global health problem. Adipose tissue dysfunction underpins the association of obesity with the development of chronic metabolic diseases, including type 2 diabetes. Thus, research into the molecular and cellular mechanisms governing maintenance of adipose tissue mass and function is critical for the development of effective therapeutic strategies aimed at reducing obesity-related diseases.
Obesity occurs in the setting of chronic positive energy balance, which gives rise to an increase in adipose tissue mass via hypertrophy of existing adipocytes and/or acquisition of new adipocytes via hyperplasia and adipogenesis of mesenchymal stem cells (preadipocytes) within adipose tissue (1,2). Excessive adipocyte hypertrophy in the absence of new, metabolically healthy adipocytes can give rise to adipose tissue dysfunction (3–5). Moreover, impaired adipogenesis may contribute to the etiology of type 2 diabetes (4,6,7). Hence, appropriate rates of hyperplasia and adipogenesis appear to be essential for metabolic homeostasis. Although there is considerable insight into the latter stages of adipogenesis, relatively little is known about mechanisms involved in preadipocyte commitment.
We previously reported that fibroblast growth factor-1 (FGF-1) acts as a paracrine factor, secreted from adipose-derived microvascular endothelial cells, to promote adipogenesis of human preadipocytes via an FGF-1/FGF receptor 1/fibroblast growth factor receptor substrate 2 (FRS2)/mitogen-activated protein kinase (MAPK) pathway (8,9). A key element in the adipogenic actions of FGF-1 is its ability to promote “priming” of adipogenesis by inducing expression of the master adipogenic regulator, peroxisome proliferator–activated receptor γ (PPARγ), before induction of differentiation (8,10,11). As such, the FGF-1 model provides a useful platform for investigations aimed at obtaining a more comprehensive understanding of early adipogenic events at the molecular level. Such understanding may reveal novel therapeutic avenues to improve adipose tissue function and ameliorate obesity-related complications, including type 2 diabetes.
To pursue these aims, we performed microarray analysis to discover proximal FGF-1 effectors and identified BMP and activin membrane-bound inhibitor (BAMBI). BAMBI is a transmembrane protein that has an extracellular domain similar to that of type 1 transforming growth factor-β (TGF-β) and BMP receptors but lacks an intracellular kinase domain. It acts as a decoy receptor for, and antagonizes signaling of, TGF superfamily members, including TGF-β and BMP (12,13). BAMBI also functions as a positive regulator of the canonical Wnt/β-catenin pathway (14). Each of these pathways regulates growth and differentiation of a number of cell types, including preadipocytes. For instance, the canonical Wnt/β-catenin pathway maintains preadipocytes in an undifferentiated proliferative state by inhibiting induction of PPARγ expression (15). TGF-β signaling, particularly TGF-β1, inhibits adipogenesis via phosphorylation of Smad2/3 (16). In contrast, other TGF-β superfamily members, such as BMP-4, promote commitment and differentiation of preadipocytes through pathways involving phosphorylation of Smad1/5/8 (17). Each of these pathways may provide therapeutic antiobesity strategies (18,19).
In light of the above, we have characterized the effects of BAMBI knockdown on adipogenesis of human preadipocytes. We have also determined how deletion of BAMBI modulates the effects of the autocrine/paracrine adipogenic regulators Wnt3a, TGF-β1, and BMP-4 and examined BAMBI expression in a mouse model of diet-induced obesity.
RESEARCH DESIGN AND METHODS
General reagents were obtained from Sigma-Aldrich (Victoria, Australia). Tissue culture reagents were obtained from Invitrogen (Victoria, Australia).
Primary human preadipocytes.
Primary human preadipocytes (phPAs) were isolated from subcutaneous adipose tissue and cultured as described previously (8,9,11). The protocol was approved by the Research Ethics Committees of the University of Queensland, the Princess Alexandra Hospital, and the Mater Adults Hospital. All patients had given their written informed consent.
Simpson Golabi Behmel syndrome PAs.
Simpson Golabi Behmel syndrome (SGBS) PAs, a gift from Martin Wabitsch (University of Ulm, Ulm, Germany) (20), were cultured for ≤30 generations and differentiated as described previously (10,11).
FGF-1 treatment.
Recombinant human FGF-1 (R&D Systems, Minneapolis, MN) was used at 1 ng/mL in the presence of 90 μg/mL heparin to treat phPAs and SGBS PAs.
Inhibitor studies.
For inhibition of ERK, p38 MAPK, and phosphatidylinositol 3-kinase (PI3K), SGBS PAs were pretreated for 30 min with 10 μmol/L UO126, 10 μmol/L SB202190, and 50 μmol/L LY294002, respectively (Merck, Rahway, NJ), before treatment with FGF-1 for 24 h (in continued presence of inhibitor). Total RNA was extracted for real-time RT-PCR. No cellular toxicity was observed after individual treatments, although combined treatment with UO126 and LY294002 resulted in significant (≥50%) cell loss.
Real-time RT-PCR.
Real-time RT-PCR was performed as previously described (11). In brief, expression of cyclophilin, glyceraldehyde-3-phosphate dehydrogenase, and transferrin receptor was assessed as potential housekeeping genes. Transferrin receptor expression was low and was not assessed further. Expression of glyceraldehyde-3-phosphate dehydrogenase exhibited significant changes upon differentiation of preadipocytes to adipocytes, whereas cyclophilin did not (change in Ct values upon differentiation were 1.7 ± 0.29 [P = 0.001] and 0.10 ± 0.20, respectively). Relative expression of the gene of interest (GOI) was therefore determined using cyclophilin as the housekeeping gene using the calculation = 2(Ct cyclophilin gene – Ct GOI). Primers were from Sigma-Aldrich. Sequences are available on request.
Western blot analysis.
Western blot analysis was carried out as previously described (21). Primary antibodies included BAMBI (R&D Systems); β-catenin and PPARγ (Santa Cruz Biotechnology Inc., Santa Cruz, CA); adiponectin (Abcam, Cambridge, MA); perilipin, phospho-Smad1/5/8, phospho-Smad2, and phospho-Smad3 (Cell Signaling, Danvers, MA); and β-tubulin (Sigma-Aldrich).
BAMBI knockdown/overexpression.
BAMBI small interfering RNA (siRNA) and scrambled (SCR) control (AllStars negative control siRNA) were from QIAGEN (Valencia, CA). In preliminary experiments, three independent BAMBI siRNAs gave similar results (data not shown; sequences available on request). SGBS PAs or phPAs were seeded on six-well plates at 50–80% confluence in serum-containing medium without antibiotics. Transfections were carried out 24 h later using NanoJuice transfection reagents according to the manufacturer’s protocol (Novagen). Knockdown was assessed 72 h posttransfection by real-time PCR (RT-PCR) and Western blot. Transfected cells were then cultured in serum-containing medium for an additional 3 days before induction of differentiation under standard differentiation conditions (10). At differentiation days 0, 3, 7, and 14, cells were harvested for subsequent analysis. BAMBI overexpression was performed using pCMV5-hBAMBI, provided by Ye-Guang Chen (Tsinghua University, Beijing, China). Control plasmid, pcDNA5-green fluorescent protein (GFP), was generated in the host laboratory. Transfections were carried out using Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen).
Measurement of lipid accumulation.
Lipid accumulation was assessed by phase-contrast microscopy (200× magnification) (Nikon Eclipase TE300) and Oil Red O staining (22).
Adiponectin secretion.
Conditioned medium from SGBS PAs was collected at the indicated time points, and adiponectin concentration was determined by ELISA (Invitrogen). Adiponectin secretion was expressed in nanograms per milliliter of medium per 24 h.
2-Deoxyglucose uptake assay.
Glucose uptake was measured in SGBS PAs after 14 days differentiation (10).
Treatment of SGBS PAs with adipogenic factors.
After treatment with SCR or BAMBI siRNA, SGBS PAs were grown to confluence and differentiated in the absence or presence of Wnt3a (10–25 ng/mL), TGF-β1 (0.1–1 ng/mL), or BMP-4 (10–50 ng/mL) (concentrations based on preliminary dose-response studies; all factors were from R&D Systems). Cells were harvested at the indicated times for assessment of differentiation.
Analysis of BAMBI expression in high-fat diet–induced and ob/ob obese mice.
Animal studies were approved under the St. Vincent’s Hospital Animal Experimentation Ethics Committee. Seven-week-old C57BL/6 mice were maintained on chow (n = 4) or high-fat diet (HFD) (n = 4) for 16 weeks as previously described (23). BAMBI expression in epididymal adipose tissue was determined by RT-PCR.
Statistical analyses.
Statistical analyses were performed in GraphPad Prism 5.0 using the following tests: Student t test (used unless otherwise stated), one-way ANOVA (repeated measures) for differences across experimental groups in conjunction with Tukey post hoc test to compare differences between treatment groups, and two-way ANOVA for differences from different groups in the same treatment. Data are expressed as means ± SEM. P values ≤0.05 were considered statistically significant.
RESULTS
BAMBI expression is reduced in response to FGF-1 and during differentiation.
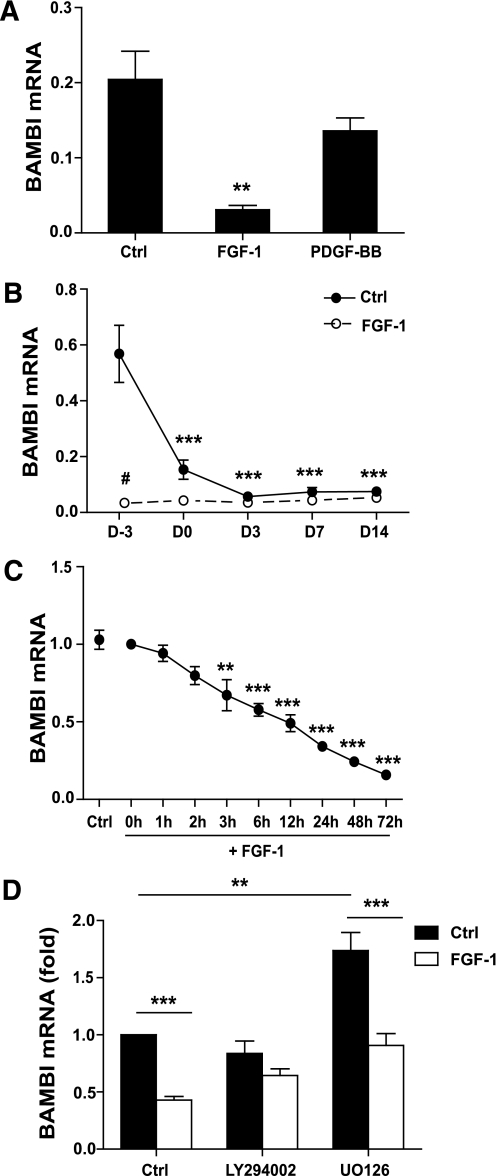
Microarray expression profiling of phPAs cultured in the absence or presence of FGF-1 for 1 week revealed that BAMBI was downregulated 13-fold by FGF-1 (P = 0.01; B-value = 50.8) (data not shown). These results were confirmed by RT-PCR of phPAs from five independent subjects, which also demonstrated the specificity of FGF-1’s effects on BAMBI relative to platelet-derived growth factor-BB (PDGF-BB) (Fig. 1A), a factor that we have previously shown to be unable to promote adipogenesis (11). Expression of BAMBI was also reduced in the widely used human SGBS PA cell strain after treatment with FGF-1 or induction of adipogenesis (Fig. 1B) (24). Time-course studies revealed a progressive decline in BAMBI mRNA upon FGF-1 treatment (Fig. 1C). FGF-1 is known to stimulate a number of downstream molecules implicated in adipogenesis, including ERK, PI3K, and p38 MAPK (25–29). To identify signaling pathways responsible for the decrease in BAMBI expression, we used a pharmacological approach. Inhibition of PI3K with LY294002 inhibited the effects of FGF-1 on BAMBI expression (Fig. 1D). Surprisingly, inhibition of ERK using UO126 increased BAMBI expression in control cells but did not prevent the reduction of BAMBI in response to FGF-1 (Fig. 1D). Inhibition of p38 MAPK with SB202190 was without effect in control or FGF-1–treated cells (data not shown). Simultaneous inhibition of ERK and PI3K resulted in major cell loss, preventing detailed investigations of the combined effects of inhibition of these two pathways. Overall, these findings demonstrate that BAMBI expression is under complex regulation involving both PI3K and ERK signaling pathways and indicate that activation of PI3K plays a role in FGF-1’s inhibitory effects on BAMBI expression. In addition, the results support the hypothesis that a reduction in BAMBI represents a key step in adipogenesis that is enhanced by FGF-1.
FIG. 1.
BAMBI expression is reduced in response to FGF-1 and during differentiation. A: Subcutaneous phPAs were grown to confluence in serum-containing medium (SCM) with either 1 ng/mL FGF-1 or 2 ng/mL platelet-derived growth factor-BB (PDGF-BB). BAMBI mRNA expression was determined by RT-PCR of total RNA. Data represent the ratio of BAMBI:cyclophilin and are the mean ± SEM of samples derived from five individuals (ANOVA, **P < 0.05 relative to control [Ctrl] SCM cells). B: SGBS PAs were grown and differentiated in the presence or absence of 1 ng/mL FGF-1. Cells were harvested at the indicated time points and BAMBI mRNA expression was measured by RT-PCR of total RNA. Data represent the ratio of BAMBI:cyclophilin and are the mean ± SEM of three to four independent experiments (ANOVA, ***P < 0.001 relative to untreated cells at differentiation day −3; Tukey #P < 0.05 for FGF-1 compared with Ctrl at day −3). C: SGBS PAs were treated with FGF-1 for the indicated times, and BAMBI mRNA expression was assessed by RT-PCR of total RNA. Data are normalized to expression at 0 h, and Ctrl represents cells harvested at 72 h without FGF-1 (mean ± SEM; n = 4 independent experiments; ANOVA, **P < 0.01; ANOVA, ***P < 0.001). D: SGBS PAs were pretreated with LY294002 or UO126 for 30 min prior to 24 h treatment with FGF-1 (in continued presence of inhibitor). BAMBI mRNA expression was determined using RT-PCR. Results are expressed as fold over vehicle-treated “Ctrl” cells (mean ± SEM; n = 3 independent experiments; **P < 0.01; ***P < 0.001).
BAMBI knockdown increases priming of SGBS PAs.
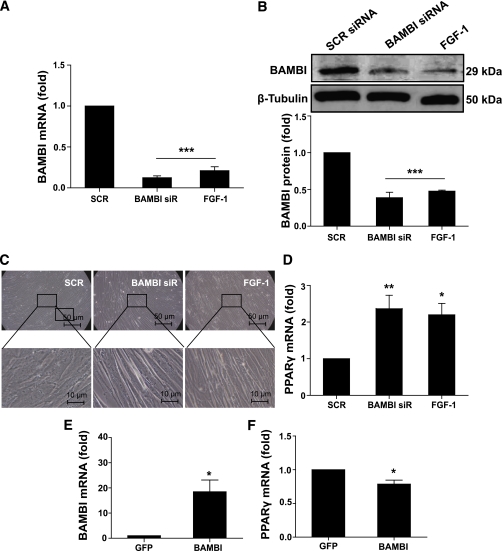
To investigate a putative role for BAMBI in regulation of adipogenesis, we used siRNA. BAMBI mRNA and protein levels were determined 72 h after transfection of SGBS PAs with SCR control siRNA or BAMBI siRNA. We observed significant decreases of BAMBI mRNA and protein, and these were similar to those induced by FGF-1 (Fig. 2A and B). We also observed differences in morphology between cells treated with BAMBI siRNA or FGF-1 compared with those treated with SCR siRNA. Treatment with BAMBI siRNA or FGF-1 promoted the generation of elongated cells arranged in a relatively orderly pattern in comparison with the SCR siRNA–treated cells, which were larger, more spread out, and less uniform (Fig. 2C) (the elongated morphology is maintained for 2–3 days, after which the cells begin to attain their characteristic rounded shape). It is noteworthy that there was a concomitant increase in PPARγ mRNA expression after BAMBI knockdown (prior to induction of differentiation) and this also mirrored the effects of FGF-1 (Fig. 2D).
FIG. 2.
BAMBI manipulation affects priming of SGBS PAs. The effect of BAMBI knockdown/overexpression on priming of SGBS PAs was assessed using RT-PCR, Western blot, and microscopy of cells 72 h posttransfection with SCR or BAMBI siRNA or 72 h with FGF-1 (A–D) or transfection with GFP or BAMBI cDNA (E and F). A: BAMBI mRNA expression was assessed by RT-PCR. Results are expressed as fold compared with SCR (control) cells (mean ± SEM; n = 7; ANOVA, ***P < 0.001). B: BAMBI protein expression was assessed by Western blot (β-tubulin was used as a loading control). A representative blot (top) and quantitation of data from three to six independent experiments (bottom) are shown. Results are expressed as fold compared with SCR (control) cells (mean ± SEM; ANOVA, ***P < 0.001). C: Photomicrographs of SGBS PA cells showing cell morphology (images are representative of seven independent experiments). D: PPARγ mRNA expression was assessed relative to SCR (control) cells, by RT-PCR (ANOVA, *P < 0.05; **P < 0.01). E and F: BAMBI and PPARγ expression assessed by RT-PCR. Results are expressed as fold compared with SCR (control) cells (mean ± SEM; n = 4–7; ANOVA, *P < 0.05). (A high-quality color representation of this figure is available in the online issue.)
We recently described a putative role for reduced FGF-2 in the early adipogenic actions of FGF-1 (30). Similar to FGF-1, knockdown of BAMBI significantly reduced the expression of FGF-2 (Supplementary Fig. 1). Collectively, these observations suggest that BAMBI knockdown is sufficient to recapitulate the effects of FGF-1 by promoting commitment of the cells to the adipocyte lineage.
BAMBI overexpression decreases priming of SGBS PAs.
Overexpression studies were performed to investigate the effects of increasing cellular BAMBI levels. Using a standard lipid-based approach to transfect the cells, 72 h after transfection, BAMBI expression was increased, and this resulted in a significant decrease in PPARγ expression compared with GFP–transfected cells (Fig. 2E and F). We did not observe any morphological differences between GFP- and BAMBI-transfected cells. This probably reflects the limited transfection efficiency, as we routinely achieved ∼15–20% transfection efficiency in the SGBS PAs, as determined by GFP expression (data not shown).
BAMBI knockdown increases differentiation of SGBS PAs.
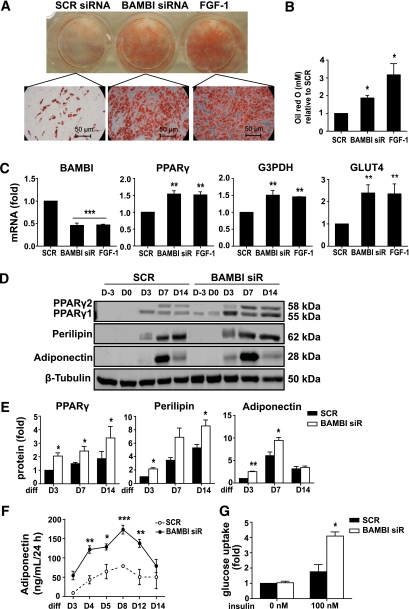
We next examined the effects of BAMBI knockdown on the later stages of differentiation. BAMBI knockdown resulted in increased lipid accumulation throughout differentiation, with results similar to those in FGF-1–treated cells (Fig. 3A and B and data not shown). Additional features of differentiating SGBS PAs were assessed by RT-PCR and Western blot analysis of adipogenic markers (Fig. 3C–E). There were significant increases in the mRNA levels of adipogenic markers, including PPARγ, glycerol-3-phosphate dehydrogenase (G3PDH), and glucose transporter type 4 (GLUT4), and the effects of BAMBI knockdown were comparable to those of FGF-1 (Fig. 3C). Analysis of protein expression confirmed the adipogenic effects of BAMBI knockdown. PPARγ levels were significantly increased throughout differentiation after BAMBI knockdown (Fig. 3D and E). PPARγ1 was detected before induction of differentiation (days −3 and 0) and PPARγ2 was detected early in the differentiation period (day 3) in BAMBI knockdown cells (Fig. 3D and E). Expression of perilipin and adiponectin was also increased during differentiation of BAMBI knockdown cells (Fig. 3D and E).
FIG. 3.
BAMBI knockdown induces differentiation of SGBS PAs. The effect of BAMBI knockdown on differentiation of SGBS PAs on day 14 of differentiation (unless indicated otherwise) was assessed using Oil Red O staining, RT-PCR, Western blot, and functional assays in cells treated with SCR siRNA, BAMBI siRNA, or FGF-1. A: Micrographs showing Oil Red O staining (magnification 200×). B: Quantification of Oil Red O staining from three independent experiments. Data are expressed as fold compared with SCR (control) cells (mean ± SEM; ANOVA, *P < 0.05). C: mRNA expression of BAMBI, PPARγ, G3PDH, and glucose transporter type 4 (GLUT4) as determined by RT-PCR. Results are expressed as fold compared with SCR (control) cells (mean ± SEM; n = 4–6; ANOVA, **P < 0.01; ***P < 0.001). D: Representative Western blots of whole cell lysates showing induction of adipocyte proteins PPARγ, perilipin, and adiponectin throughout differentiation (β-tubulin is a loading control). E: Quantification of Western blot analysis from three to six independent experiments. Results are expressed as fold compared with SCR (control) cells (mean ± SEM; ANOVA, *P < 0.05; **P < 0.01). F: Adiponectin secretion was measured by ELISA. Results show mean ± SEM from three to four independent experiments (ANOVA, *P < 0.05; ** P < 0.01; *** P < 0.001 comparing SCR [control] with BAMBI at each time point). G: Basal and insulin-stimulated 2-deoxyglucose uptake was measured as outlined in research design and methods. Results are expressed as fold compared with SCR (control) cells (mean ± SEM; n = 3–4 independent experiments; ANOVA, *P < 0.05). (A high-quality digital representation of this figure is available in the online issue.)
We further investigated the effects of BAMBI knockdown on adipocyte function by characterizing adiponectin secretion and glucose uptake. Compared with control cells, BAMBI knockdown cells secreted higher levels of adiponectin at all stages of differentiation, with adiponectin secretion peaking at around day 8 (Fig. 3F). Basal glucose uptake levels were unaffected but insulin-stimulated glucose uptake was significantly increased in BAMBI knockdown cells (Fig. 3G). In summary, BAMBI knockdown is sufficient to enhance adipogenesis of the SGBS PAs, and this mirrors the effects of FGF-1 treatment.
BAMBI knockdown increases priming and differentiation of phPAs.
To extend our observations, we performed similar experiments in phPAs (which have a low adipogenic potential, in the absence of FGF-1, and show intrinsic variability between individuals). BAMBI mRNA was reduced by ∼90%, 72 h posttransfection with BAMBI siRNA, concomitant with a significant increase in PPARγ mRNA (Supplementary Fig. 2A). Knockdown was maintained throughout differentiation, and this correlated with increased expression of adipocyte mRNAs and proteins and increased lipid accumulation (Supplementary Fig. 2B–D).
These results demonstrate that BAMBI plays an inhibitory role in adipogenesis of phPAs and SGBS PAs. In particular, the changes in cell morphology and increase in PPARγ expression upon BAMBI knockdown suggest a role for BAMBI in cell fate determination and commitment. Although there is relatively little understanding of the mechanisms involved in preadipocyte commitment to the adipocyte lineage, it is known that there is a complex interplay between autocrine and paracrine factors, including those modulated by BAMBI, namely Wnts, TGF-β, and BMPs. Hence, we next examined whether BAMBI knockdown would alter the effects of these adipogenic regulators.
BAMBI knockdown attenuates the antiadipogenic effects of Wnt3a.
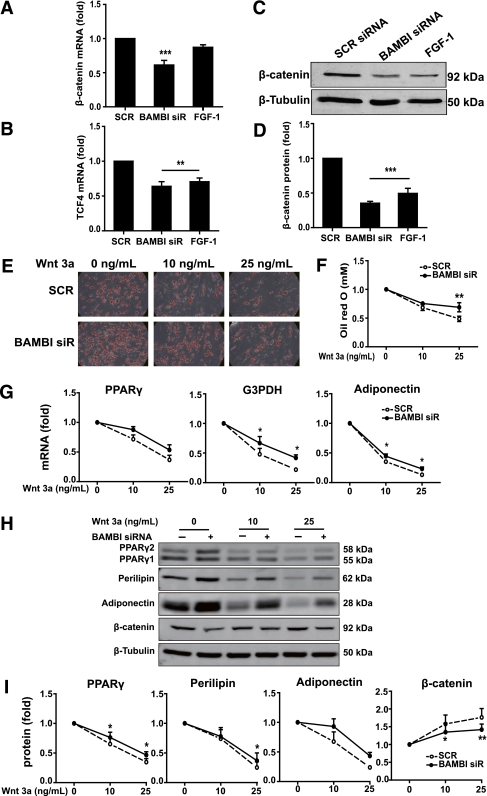
First, we determined the effects of BAMBI knockdown on the expression of genes involved in the canonical Wnt pathway. BAMBI knockdown promoted a decrease in the mRNA levels of both β-catenin and T cell transcription factor-4 (TCF-4) (Fig. 4A and B). Although only TCF-4 mRNA was significantly reduced by FGF-1 treatment (Fig. 4A and B), both BAMBI knockdown and FGF-1 treatment decreased β-catenin protein levels to a similar extent (Fig. 4C and D). These results suggest that the adipogenic effects of BAMBI knockdown and FGF-1 may be at least partly mediated by the modulation of canonical Wnt/β-catenin signaling.
FIG. 4.
BAMBI knockdown attenuates the antiadipogenic effect of Wnt3a. A and B: β-Catenin (A) and TCF-4 (B) mRNA expression was assessed by RT-PCR at 72 h posttransfection of SGBS PAs with SCR or BAMBI siRNA or after 72 h with FGF-1. Results are expressed as fold compared with SCR (control) cells (mean ± SEM; n = 4–7; ANOVA, **P < 0.01; ***P < 0.001). C: β-Catenin protein expression was assessed by Western blot at 72 h posttransfection (β-tubulin was used as a loading control). D: Quantification of Western blot analysis from three independent experiments. Results are expressed as fold compared with SCR (control) cells (mean ± SEM; ANOVA, ***P < 0.001). E–I: SGBS PAs were transfected with SCR or BAMBI siRNA prior to incubation with Wnt3a (as indicated) during maintenance and differentiation. For all graphs, values obtained for SCR and BAMBI siRNA cells without Wnt3a treatment were set at 1 and other values were normalized to these to indicate relative sensitivity to Wnt3a (n = 3 throughout). E: Photomicrographs show Oil Red O staining of cells on day 14 of differentiation (magnification 200×). F: Quantification of Oil Red O staining (mean ± SEM; two-way ANOVA, **P < 0.01). In the absence of Wnt3a treatment, lipid accumulation was 1.9-fold greater in BAMBI knockdown compared with SCR control (**P < 0.01). G: mRNA expression of adipogenic markers PPARγ, G3PDH, and adiponectin was assessed by RT-PCR (mean ± SEM; two-way ANOVA, *P < 0.05). In the absence of Wnt3a treatment, PPARγ, G3PDH, and adiponectin were all 1.3-fold in BAMBI knockdown compared with SCR control (all *P < 0.05). H: Western blot analysis of whole cell lysates was used to determine the expression of PPARγ, perilipin, adiponectin, and β-catenin (β-tubulin is a loading control). I: Quantification of Western blot analysis (mean ± SEM; two-way ANOVA, *P < 0.05; **P < 0.01). In the absence of Wnt3a treatment, PPARγ, perilipin, adiponectin, and β-catenin were 1.5, 1.7, 2.0, and 0.8-fold in BAMBI knockdown compared with SCR control (all *P < 0.05). (A high-quality color representation of this figure is available in the online issue.)
To further explore the modulatory role of BAMBI in the context of the antiadipogenic effects of Wnt, we examined the effects of BAMBI knockdown on sensitivity to Wnt3a (31). SGBS PAs were treated with SCR or BAMBI siRNA and then cultured and differentiated in the presence of submaximal (10 ng/mL) or maximal (25 ng/mL) inhibitory concentrations of Wnt3a (determined in preliminary dose-response studies) (Supplementary Fig. 3). As expected, lipid accumulation was reduced by Wnt3a in a dose-dependent manner in the presence or absence of BAMBI (Fig. 4E). Quantification of this effect revealed a significant amelioration of the inhibitory effect of Wnt3a in BAMBI knockdown cells (Fig. 4F). Similar results were observed when we measured changes in gene (Fig. 4G) and protein expression (Fig. 4H and I). In all cases, the relative effects of Wnt3a were reduced in cells lacking BAMBI. Moroever, the ability of Wnt3a to increase β-catenin protein was reduced in BAMBI knockdown cells (Fig. 4H and I). These data demonstrate that BAMBI promotes the antiadipogenic effects of Wnt3a by enhancing the canonical Wnt/β-catenin pathway.
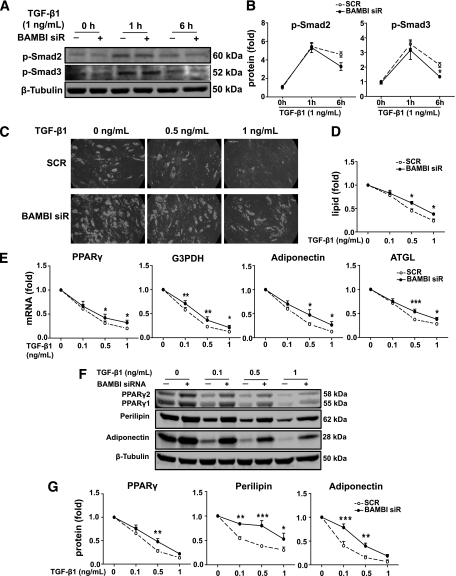
BAMBI knockdown reduces the antiadipogenic effects of TGF-β1.
TGF-β, most notably TGF-β1, has been shown to play a negative role in adipogenesis (16,32), and BAMBI is thought to inhibit TGF-β signaling through its actions as a pseudoreceptor (13). Hence we anticipated that the antiadipogenic effects of TGF-β1 would be increased after BAMBI knockdown. Preliminary dose-response studies confirmed that TGF-β1 stimulated phosphorylation of Smad2/3 and inhibited differentiation of SGBS PAs in a dose-dependent manner (Supplementary Fig. 4). Surprisingly, however, after BAMBI knockdown, maximal TGF-β1–stimulated phosphorylation of Smad2/3 was attenuated relative to control cells, showing reduced phosphorylation after 6 h (Fig. 5A and B). The inhibitory effects of TGF-β1 on lipid accumulation were also reduced in BAMBI knockdown cells (Fig. 5C and D). In addition, the negative impact of TGF-β1 on induction of adipogenic markers was reduced (Fig. 5E–G). These findings indicate that BAMBI is a positive regulator of canonical TGF-β1 signaling and action in human preadipocytes.
FIG. 5.
BAMBI knockdown reduces the antiadipogenic effect of TGF-β1. SGBS PAs were transfected with SCR (−) or BAMBI siRNA. After 72 h, cells were treated with TGF-β1 for 1 or 6 h. Phosphorylation of Smad2/3 was determined by Western blot analysis of whole cell lysates (A) and quantified (B). Results are expressed as fold compared with SCR (control) cells at 0 h (mean ± SEM; n = 3; two-way ANOVA, *P < 0.05). C–G: SGBS PAs were transfected with SCR or BAMBI siRNA prior to incubation with TGF-β1 (as indicated) during maintenance and differentiation. For all graphs, values obtained for SCR and BAMBI siRNA cells without TGF-β1 treatment were set at 1 and other values were normalized to these to indicate relative sensitivity to TGF-β1 (n = 3 throughout). C: Photomicrographs show lipid accumulation on day 14 of differentiation (magnification 200×). D: Quantification of lipid accumulation (mean ± SEM; two-way ANOVA, *P < 0.05). E: mRNA expression of adipogenic markers PPARγ, G3PDH, adiponectin, and adipose triglyceride lipase (ATGL) was assessed by RT-PCR (mean ± SEM; two-way ANOVA, *P < 0.05; **P < 0.01; ***P < 0.001). In the absence of TGF-β1 treatment, PPARγ, G3PDH, adiponectin, and ATGL were 1.5, 1.8, 1.8, and 1.8-fold in BAMBI knockdown compared with SCR control (all *P < 0.05). F: Western blot analysis of whole cell lysates showing PPARγ, perilipin, and adiponectin (β-tubulin is a loading control). G: Quantification of Western blot analysis (mean ± SEM; two-way ANOVA, *P < 0.05; **P < 0.01; ***P < 0.001). In the absence of TGF-β1 treatment, PPARγ, perilipin, and adiponectin were 1.8 (*P < 0.05), 1.2, and 1.8 (*P < 0.05) in BAMBI knockdown compared with SCR control.
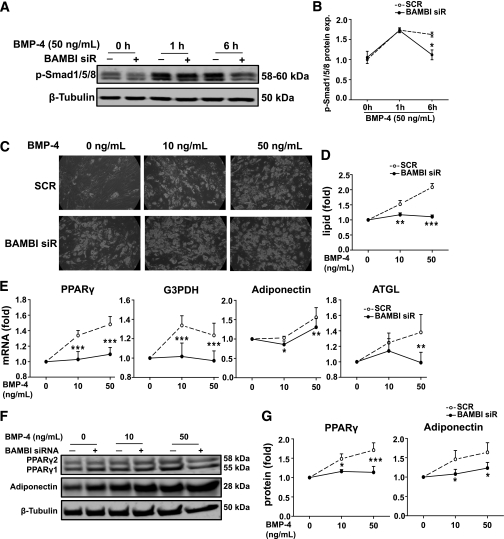
BAMBI knockdown impairs the adipogenic effect of BMP-4.
BMP-4 has been shown to promote preadipocyte commitment and subsequent differentiation (17). Although BAMBI is reported to be a negative regulator of BMP signaling in various cell types, in light of our findings showing a positive role for BAMBI in TGF-β1 signaling in preadipocytes, we hypothesized that BAMBI may facilitate BMP signaling in preadipocytes. Preliminary experiments were performed to identify submaximal and maximal BMP-4 concentrations. Although we observed a dose-dependent increase in BMP-4–stimulated phosphorylation of Smad1/5/8 in SGBS PAs (Supplementary Fig. 5A), treatment of cells with BMP-4 for 7 days prior to induction of differentiation failed to promote differentiation (Supplementary Fig. 5B and C). In contrast, extended treatment with BMP-4, present both prior to and throughout differentiation, enhanced adipogenesis (see below).
In BAMBI knockdown cells, the duration of BMP-4–stimulated Smad1/5/8 phosphorylation was attenuated (Fig. 6A and B). BMP-4 treatment promoted a dose-dependent increase in differentiation, as determined by lipid accumulation and expression of adipogenic markers (Fig. 6C–G). The proadipogenic actions of BMP-4 were significantly reduced in BAMBI knockdown cells (Fig. 6C–G). Collectively these results demonstrate that BAMBI facilitates canonical BMP-4 signaling and action in human preadipocytes.
FIG. 6.
BAMBI knockdown impaired adipogenic effect of BMP-4. SGBS PAs were transfected with SCR (−) or BAMBI siRNA. After 72 h, cells were treated with BMP-4 for 1 or 6 h. Phosphorylation of Smad1/5/8 was determined by Western blot analysis of whole cell lysates (A) and quantified (B). Results are expressed as fold compared with SCR (control) cells at 0 h (mean ± SEM; n = 3; two-way ANOVA, *P < 0.05). C–G: SGBS PAs were transfected with SCR or BAMBI siRNA prior to incubation with BMP-4 (as indicated) during maintenance and differentiation. For all graphs, values obtained for SCR and BAMBI siRNA cells without BMP-4 treatment were set at 1 and other values were normalized to these to indicate relative sensitivity to BMP-4 (n = 3 throughout). C: Photomicrographs show lipid accumulation on day 14 of differentiation (magnification 200×). D: Quantification of lipid accumulation (mean ± SEM; two-way ANOVA, **P < 0.01; ***P < 0.001). E: mRNA expression of adipogenic markers PPARγ, G3PDH, adiponectin, and ATGL was assessed by RT-PCR (mean ± SEM; two-way ANOVA, *P < 0.05; **P < 0.01; ***P < 0.001). In the absence of BMP-4 treatment, PPARγ, G3PDH, adiponectin, and ATGL were 1.5, 1.6, 1.5, and 1.5-fold in BAMBI knockdown compared with SCR control (all *P < 0.05). F: Western blot analysis of whole cell lysates showing PPARγ and adiponectin (β-tubulin is a loading control). G: Quantification of Western blot analysis (mean ± SEM; two-way ANOVA, *P < 0.05; ***P < 0.001). In the absence of BMP-4 treatment, PPARγ and adiponectin were 1.4 and 1.5-fold in BAMBI knockdown compared with SCR control (both *P < 0.05).
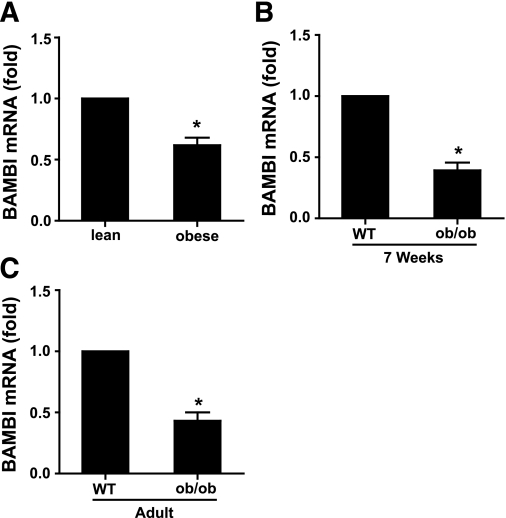
BAMBI expression is downregulated in HFD-induced obese mice.
Having established BAMBI as a negative regulator of adipogenesis whose expression is decreased under conditions that promote adipogenesis (FGF-1 treatment and/or chemical induction) and that BAMBI modulates the effects of known autocrine/paracrine adipogenic factors, we examined BAMBI expression in adipose tissue from lean and obese mice. Obesity was induced in mice by maintenance on an HFD for 16 weeks (23), a time of extensive epididymal adipose tissue remodeling when adipocyte cell number reaches a transient nadir (33). Compared with lean mice, BAMBI mRNA expression was decreased in adipose tissue from HFD-induced obese mice (Fig. 7A). We also examined BAMBI expression in adipose tissue from genetically obese ob/ob mice. BAMBI expression was decreased in 7-week-old and adult ob/ob mice compared with wild-type littermates (Fig. 7B and C).
FIG. 7.
BAMBI expression is reduced in adipose tissue of obese mice. A: C57BL/6 mice were maintained on a standard chow diet or HFD for 16 weeks (n = 4 for each). B and C: ob/ob mice and wild-type littermates were maintained on a standard chow diet. BAMBI mRNA expression in epididymal fat pads was analyzed by RT-PCR. Results are normalized to BAMBI mRNA expression levels in lean/wild-type mice (t test *P < 0.05 obese relative to lean).
DISCUSSION
Increased understanding of the molecular mechanisms that govern the physiological and pathophysiological development and function of adipose tissue are required to facilitate effective therapeutic strategies aimed at reducing obesity-related diseases such as type 2 diabetes. We previously reported a novel role for FGF-1 as a potent regulator of human adipogenesis (8,10,11). FGF-1 acts in a paracrine manner to enhance the adipogenic program by increasing preadipocyte proliferation, commitment, and subsequent differentiation. Our previous studies established the involvement of an FGF-1/FGF receptor 1/FRS2/MAPK pathway in FGF-1’s adipogenic effects. In the current investigation we have extended these observations by identifying and characterizing a novel adipogenic regulator, BAMBI.
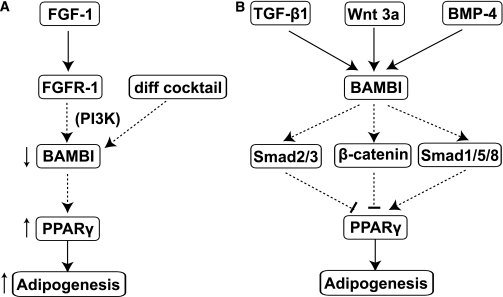
Although BAMBI has been shown to play an important role in several types of cancer (34–36), the expression and function of BAMBI in adipocyte development has not been investigated. In the current report, we first observed that BAMBI expression was decreased upon FGF-1 treatment, or induction of differentiation, in phPAs and SGBS cells, and this was dependent on activation of the PI3K pathway. These findings lead us to hypothesize that BAMBI may be an inhibitor of adipogenesis and that the observed decrease in BAMBI expression may represent an integral part of the adipogenic process that is enhanced by FGF-1. To test this, we characterized the effects of BAMBI knockdown on differentiation at various stages of adipogenesis. BAMBI knockdown resulted in induction of PPARγ, changes in cell morphology, and reduced FGF-2 expression prior to induction of differentiation, consistent with increased priming and commitment to the adipocyte lineage (11,30). Further biochemical and functional analysis revealed enhanced adipogenesis of mature adipocytes, with cells exhibiting increased adiponectin secretion and insulin-stimulated glucose uptake. These findings demonstrate that BAMBI knockdown is sufficient to recapitulate the adipogenic effects of FGF-1 and lead us to conclude that BAMBI represents a critical, and most likely proximal, adipogenic effector of FGF-1 (Fig. 8A).
FIG. 8.
Summarizing the role of BAMBI in adipogenesis. A: Schematic showing BAMBI’s position in the adipogenic program. BAMBI is downregulated in response to FGF-1, in a PI3K-dependent manner, or induction of adipogenesis. Decreased BAMBI promotes increased PPARγ, leading to increased adipogenesis. B: Schematic summarizing BAMBI’s modulatory effects on autocrine/paracrine adipogenic factors. BAMBI facilitates (arrows) the antiadipogenic actions of TGF-β1 signaling and Wnt3a signaling (blocks) and the proadipogenic actions of BMP-4 signaling on PPARγ, leading to decreased or increased adipogenesis, respectively.
Since its discovery as an inhibitor of TGF-β signaling (12), BAMBI has been shown to play negative and positive roles in the BMP and Wnt signaling pathways, respectively (14,37). The molecular mechanisms underpinning these diverse effects have, to some extent, been previously described (14,38), and recent work has added new insights. BAMBI serves as a positive regulator of the canonical Wnt/β-catenin pathway by facilitating the interaction between Wnt, its membrane receptor frizzled (Fzd), and the cytosolic protein dishevelled (Dvl), a process essential for Wnt pathway activation (14). More recently, BAMBI has been shown to expand the dynamic range of BMP-4 signaling (39) and has also been reported to undergo nuclear translocation upon treatment of ovarian cancer cells with TGF-β (34), although the potential relevance of this remains to be determined.
BAMBI has the potential to regulate adipogenesis via modulation of the effects of known autocrine/paracrine adipogenic factors described above. Our finding that BAMBI is a potent negative regulator of adipogenesis leads us to speculate that BAMBI may inhibit adipogenesis, at least in part, by facilitating Wnt signaling and/or by inhibition of BMP signaling. Hence, we characterized the effects of BAMBI knockdown on sensitivity to the anti- or proadipogenic effects of these factors. BAMBI knockdown decreased signaling through the canonical Wnt/β-catenin pathway, as evidenced by decreased β-catenin protein, which is afforded protection from proteasomal degradation by Wnt-mediated inhibition of glycogen synthase kinase-3 (GSK3) (40), and reduced TCF-4 mRNA. Treatment with FGF-1 produced similar effects. Sensitivity to the antiadipogenic effect of Wnt3a was significantly reduced in BAMBI knockdown cells as was Wnt3a-mediated stabilization of β-catenin protein, which provides further evidence of attenuated signaling by the canonical Wnt/β-catenin pathway.
Although BAMBI has been described as a downstream target of the Wnt/β-catenin, TGF-β, and BMP signaling pathways in a variety of cell types (41–43), we did not observe any change in BAMBI expression after treatment of SGBS preadipocytes with these factors (data not shown). Such discrepancies may reflect cell type–specific differences.
Similar to the Wnts, TGF-β ligands inhibit adipogenesis (19,32). However, in contrast to its positive role in Wnt signaling (14,37), BAMBI is thought to play a negative role in TGF-β signaling (12,13). In contrast to our expectations, BAMBI knockdown decreased sensitivity to the antiadipogenic effects of TGF-β1, and this correlated with reduced Smad2/3 phosphorylation (at 6 h), indicative of rapid signal attenuation. These surprising findings suggest that BAMBI may play a positive role in TGF-β signaling, serving to maintain activation of canonical TGF-β signaling in human preadipocytes.
The BMPs represent a subclass of the TGF-β superfamily, and evidence suggests they promote the early stages of adipogenesis, with pretreatment of cells with either BMP-4 or BMP-7 being sufficient to enhance adipogenic differentiation (17,44–46). In preliminary experiments where SGBS cells were treated with BMP-4 only before induction of differentiation, we did not detect any effects of BMP-4 treatment on commitment or differentiation. However, we did observe clear activation of canonical BMP signaling (Smad1/5/8 phosphorylation), demonstrating that the cells were responsive to BMP-4. Although the reason for the absence of any proadipogenic effect is unclear, it is noteworthy that the majority of the literature describing such proadipogenic effects comes from experiments on cells derived from bone marrow (17,44,45), leading us to suggest that cell type–specific differences may exist. Regardless, prolonged maintenance of SGBS cells in BMP-4 resulted in a marked, dose-dependent increase in adipogenesis. BAMBI knockdown decreased sensitivity to the proadipogenic actions of BMP-4, and this correlated with reduced longevity of BMP-4–stimulated Smad-1/5/8 phosphorylation. These findings suggest that BAMBI may act to preserve canonical BMP and TGF-β signaling pathways in preadipocytes. Further studies are required to elaborate this apparent paradox.
Collectively, the above findings (summarized in Fig. 8B) indicate that BAMBI is likely to play a central role in the modulation and integration of signaling from multiple autocrine/paracrine adipogenic factors that represent therapeutic antiobesity targets (18,19). In support of a potential physiological role for altered BAMBI expression during periods of adipose tissue remodeling involving adipogenesis (33), we found BAMBI expression was significantly reduced in adipose tissue from HFD-induced obese mice. Whether changes in BAMBI expression are also associated with and contribute to the pathophysiology of obesity-related diseases, such as type 2 diabetes, remains to be determined. BAMBI−/− mice have been generated and show no overt phenotype (47,48). Future studies are required to characterize adipose tissues from these mice and determine whether they exhibit adipocyte hyperplasia and improved metabolism relative to wild-type littermates when challenged with an HFD.
In summary, this work establishes BAMBI as a potent negative regulator of adipogenesis that affects commitment and subsequent differentiation of human preadipocytes. Furthermore, BAMBI acts as a signaling nexus to modulate the effects of several key autocrine/paracrine adipogenic regulators (Wnt, TGF-β, and BMP). Further investigations are required to determine whether manipulating BAMBI expression and/or function may represent an attractive way to promote adipose tissue remodeling and improve metabolic function.
ACKNOWLEDGMENTS
This work was supported by the Australian National Health and Medical Research Council, the Australian Heart Foundation, Diabetes Australia, and the National Natural Science Foundation of China (30871787).
No potential conflicts of interest relevant to this article were reported.
X.L. researched data, interpreted data, and wrote the manuscript. L.J.H. designed the study, interpreted data, and edited the manuscript. J.A.W., Y.-H.K., D.-F.L., F.S.N., C.H.W., A.B., and N.T. researched data. C.S.-P. researched data and edited the manuscript. J.B.P. designed the study, interpreted data, and edited the manuscript. G.-S.Y. designed the study and interpreted data. J.P.W. designed the study, interpreted data, wrote the manuscript, and is the guarantor of the article.
The authors thank the volunteers and surgeons at Princess Alexandra Hospital and Mater Health Services for providing tissue.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0998/-/DC1.
REFERENCES
- 1.Kersten S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep 2001;2:282–286 10.1093/embo-reports/kve071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prins JB, O’Rahilly S. Regulation of adipose cell number in man. Clin Sci (Lond) 1997;92:3–11 [DOI] [PubMed] [Google Scholar]
- 3.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature 2008;453:783–787 10.1038/nature06902 [DOI] [PubMed] [Google Scholar]
- 4.Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int J Obes Relat Metab Disord 2004;28(Suppl 4):S12–S21 10.1038/sj.ijo.0802853 [DOI] [PubMed] [Google Scholar]
- 5.Kim JY, van de Wall E, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 2007;117:2621–2637 10.1172/JCI31021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang MY, Grayburn P, Chen S, Ravazzola M, Orci L, Unger RH. Adipogenic capacity and the susceptibility to type 2 diabetes and metabolic syndrome. Proc Natl Acad Sci USA 2008;105:6139–6144 10.1073/pnas.0801981105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Tienen FH, van der Kallen CJ, Lindsey PJ, Wanders RJ, van Greevenbroek MM, Smeets HJ. Preadipocytes of type 2 diabetes subjects display an intrinsic gene expression profile of decreased differentiation capacity. Int J Obes (Lond) 2011;35:1154–1164 [DOI] [PubMed] [Google Scholar]
- 8.Hutley L, Shurety W, Newell F, et al. Fibroblast growth factor 1: a key regulator of human adipogenesis. Diabetes 2004;53:3097–3106 10.2337/diabetes.53.12.3097 [DOI] [PubMed] [Google Scholar]
- 9.Hutley LJ, Herington AC, Shurety W, et al. Human adipose tissue endothelial cells promote preadipocyte proliferation. Am J Physiol Endocrinol Metab 2001;281:E1037–E1044 [DOI] [PubMed] [Google Scholar]
- 10.Newell FS, Su H, Tornqvist H, Whitehead JP, Prins JB, Hutley LJ. Characterization of the transcriptional and functional effects of fibroblast growth factor-1 on human preadipocyte differentiation. FASEB J 2006;20:2615–2617 10.1096/fj.05-5710fje [DOI] [PubMed] [Google Scholar]
- 11.Widberg CH, Newell FS, Bachmann AW, et al. Fibroblast growth factor receptor 1 is a key regulator of early adipogenic events in human preadipocytes. Am J Physiol Endocrinol Metab 2009;296:E121–E131 10.1152/ajpendo.90602.2008 [DOI] [PubMed] [Google Scholar]
- 12.Onichtchouk D, Chen YG, Dosch R, et al. Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature 1999;401:480–485 10.1038/46794 [DOI] [PubMed] [Google Scholar]
- 13.Yan X, Lin Z, Chen F, et al. Human BAMBI cooperates with Smad7 to inhibit transforming growth factor-beta signaling. J Biol Chem 2009;284:30097–30104 10.1074/jbc.M109.049304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Z, Gao C, Ning Y, He X, Wu W, Chen YG. The pseudoreceptor BMP and activin membrane-bound inhibitor positively modulates Wnt/beta-catenin signaling. J Biol Chem 2008;283:33053–33058 10.1074/jbc.M804039200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science 2000;289:950–953 10.1126/science.289.5481.950 [DOI] [PubMed] [Google Scholar]
- 16.Choy L, Skillington J, Derynck R. Roles of autocrine TGF-beta receptor and Smad signaling in adipocyte differentiation. J Cell Biol 2000;149:667–682 10.1083/jcb.149.3.667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowers RR, Lane MD. A role for bone morphogenetic protein-4 in adipocyte development. Cell Cycle 2007;6:385–389 10.4161/cc.6.4.3804 [DOI] [PubMed] [Google Scholar]
- 18.Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A. Adipogenesis and WNT signalling. Trends Endocrinol Metab 2009;20:16–24 10.1016/j.tem.2008.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zamani N, Brown CW. Emerging roles for the transforming growth factor-beta superfamily in regulating adiposity and energy expenditure. Endocr Rev 2011;32:387–403 10.1210/er.2010-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wabitsch M, Brenner RE, Melzner I, et al. Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. Int J Obes Relat Metab Disord 2001;25:8–15 10.1038/sj.ijo.0801520 [DOI] [PubMed] [Google Scholar]
- 21.Arner E, Westermark PO, Spalding KL, et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes 2010;59:105–109 10.2337/db09-0942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitehead JP, Simpson F, Hill MM, et al. Insulin and oleate promote translocation of inosine-5′ monophosphate dehydrogenase to lipid bodies. Traffic 2004;5:739–749 10.1111/j.1600-0854.2004.00217.x [DOI] [PubMed] [Google Scholar]
- 23.Raddatz K, Turner N, Frangioudakis G, et al. Time-dependent effects of Prkce deletion on glucose homeostasis and hepatic lipid metabolism on dietary lipid oversupply in mice. Diabetologia 2011;54:1447–1456 10.1007/s00125-011-2073-0 [DOI] [PubMed] [Google Scholar]
- 24.Rose FJ, Webster J, Barry JB, Phillips LK, Richards AA, Whitehead JP. Synergistic effects of ascorbic acid and thiazolidinedione on secretion of high molecular weight adiponectin from human adipocytes. Diabetes Obes Metab 2010;12:1084–1089 10.1111/j.1463-1326.2010.01297.x [DOI] [PubMed] [Google Scholar]
- 25.Prusty D, Park BH, Davis KE, Farmer SR. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma ) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes. J Biol Chem 2002;277:46226–46232 10.1074/jbc.M207776200 [DOI] [PubMed] [Google Scholar]
- 26.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev 2005;16:139–149 10.1016/j.cytogfr.2005.01.001 [DOI] [PubMed] [Google Scholar]
- 27.Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev 2005;16:233–247 10.1016/j.cytogfr.2005.01.007 [DOI] [PubMed] [Google Scholar]
- 28.Aubin D, Gagnon A, Sorisky A. Phosphoinositide 3-kinase is required for human adipocyte differentiation in culture. Int J Obes (Lond) 2005;29:1006–1009 10.1038/sj.ijo.0802961 [DOI] [PubMed] [Google Scholar]
- 29.Patel NG, Holder JC, Smith SA, Kumar S, Eggo MC. Differential regulation of lipogenesis and leptin production by independent signaling pathways and rosiglitazone during human adipocyte differentiation. Diabetes 2003;52:43–50 10.2337/diabetes.52.1.43 [DOI] [PubMed] [Google Scholar]
- 30.Hutley LJ, Newell FS, Kim YH, et al. A putative role for endogenous FGF-2 in FGF-1 mediated differentiation of human preadipocytes. Mol Cell Endocrinol 2011;339:165–171 10.1016/j.mce.2011.04.012 [DOI] [PubMed] [Google Scholar]
- 31.Kennell JA, MacDougald OA. Wnt signaling inhibits adipogenesis through beta-catenin-dependent and -independent mechanisms. J Biol Chem 2005;280:24004–24010 10.1074/jbc.M501080200 [DOI] [PubMed] [Google Scholar]
- 32.Choy L, Derynck R. Transforming growth factor-beta inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J Biol Chem 2003;278:9609–9619 10.1074/jbc.M212259200 [DOI] [PubMed] [Google Scholar]
- 33.Strissel KJ, Stancheva Z, Miyoshi H, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 2007;56:2910–2918 10.2337/db07-0767 [DOI] [PubMed] [Google Scholar]
- 34.Pils D, Wittinger M, Petz M, et al. BAMBI is overexpressed in ovarian cancer and co-translocates with Smads into the nucleus upon TGF-beta treatment. Gynecol Oncol 2010;117:189–197 10.1016/j.ygyno.2009.12.034 [DOI] [PubMed] [Google Scholar]
- 35.Khin SS, Kitazawa R, Win N, et al. BAMBI gene is epigenetically silenced in subset of high-grade bladder cancer. Int J Cancer 2009;125:328–338 10.1002/ijc.24318 [DOI] [PubMed] [Google Scholar]
- 36.Togo N, Ohwada S, Sakurai S, et al. Prognostic significance of BMP and activin membrane-bound inhibitor in colorectal cancer. World J Gastroenterol 2008;14:4880–4888 10.3748/wjg.14.4880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carethers JM. Intersection of transforming growth factor-beta and Wnt signaling pathways in colorectal cancer and metastasis. Gastroenterology 2009;137:33–36 10.1053/j.gastro.2009.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol 2002;250:231–250 10.1006/dbio.2002.0779 [DOI] [PubMed] [Google Scholar]
- 39.Paulsen M, Legewie S, Eils R, Karaulanov E, Niehrs C. Negative feedback in the bone morphogenetic protein 4 (BMP4) synexpression group governs its dynamic signaling range and canalizes development. Proc Natl Acad Sci U S A 2011;108:10202–10207 [DOI] [PMC free article] [PubMed]
- 40.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 2009;17:9–26 10.1016/j.devcel.2009.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sekiya T, Adachi S, Kohu K, et al. Identification of BMP and activin membrane-bound inhibitor (BAMBI), an inhibitor of transforming growth factor-beta signaling, as a target of the beta-catenin pathway in colorectal tumor cells. J Biol Chem 2004;279:6840–6846 10.1074/jbc.M310876200 [DOI] [PubMed] [Google Scholar]
- 42.Sekiya T, Oda T, Matsuura K, Akiyama T. Transcriptional regulation of the TGF-beta pseudoreceptor BAMBI by TGF-beta signaling. Biochem Biophys Res Commun 2004;320:680–684 10.1016/j.bbrc.2004.06.017 [DOI] [PubMed] [Google Scholar]
- 43.Karaulanov E, Knöchel W, Niehrs C. Transcriptional regulation of BMP4 synexpression in transgenic Xenopus. EMBO J 2004;23:844–856 10.1038/sj.emboj.7600101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang QQ, Otto TC, Lane MD. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci USA 2004;101:9607–9611 10.1073/pnas.0403100101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bowers RR, Kim JW, Otto TC, Lane MD. Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: role of the BMP-4 gene. Proc Natl Acad Sci USA 2006;103:13022–13027 10.1073/pnas.0605789103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neumann K, Endres M, Ringe J, et al. BMP7 promotes adipogenic but not osteo-/chondrogenic differentiation of adult human bone marrow-derived stem cells in high-density micro-mass culture. J Cell Biochem 2007;102:626–637 10.1002/jcb.21319 [DOI] [PubMed] [Google Scholar]
- 47.Chen J, Bush JO, Ovitt CE, Lan Y, Jiang R. The TGF-beta pseudoreceptor gene Bambi is dispensable for mouse embryonic development and postnatal survival. Genesis 2007;45:482–486 10.1002/dvg.20320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tramullas M, Lantero A, Díaz A, et al. BAMBI (bone morphogenetic protein and activin membrane-bound inhibitor) reveals the involvement of the transforming growth factor-beta family in pain modulation. J Neurosci 2010;30:1502–1511 10.1523/JNEUROSCI.2584-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]