Abstract
A subset of children develops persistent insulin autoantibodies (IAA; almost always as the only islet autoantibody) without evidence of progression to diabetes. The aim of the current study was the development and characterization of the performance of a nonradioactive fluid phase IAA assay in relation to standard IAA radioassay. We developed a nonradioactive IAA assay where bivalent IAA cross-link two insulin moieties in a fluid phase. The serum samples positive for anti-islet autoantibodies from 150 newly diagnosed patients with diabetes (Barbara Davis Center plus Diabetes Autoantibody Standardization Program [DASP] workshop) and 70 prediabetic subjects who were followed to diabetes were studied. In addition, sequential samples from 64 nondiabetic subjects who were persistently IAA+ were analyzed. With 99th percentile of specificity, the new assay with the technology from Meso Scale Discovery Company (MSD-IAA) detects as positive 61% (61 of 100) of new-onset patients and 80% (56 of 70) of prediabetic patients compared with our current fluid phase micro-IAA radioassay (mIAA; 44 and 74%, respectively). In addition, MSD-IAA demonstrated better sensitivity than our mIAA from blinded DASP workshop (68 vs. 56% with the same 99% specificity). Of 64 IAA+ nondiabetic subjects, 25% (8 of 32) who had only IAA and thus the low risk for progression to diabetes were positive with MSD-IAA assay. In contrast, 100% (32 of 32) high-risk children (IAA plus other islet autoantibodies) were positive with MSD-IAA. The IAA detectable by radioassay, but not MSD-IAA, were usually of lower affinity compared with the IAA of the high-risk children. These data suggest that a subset of IAA with current radioassay (not MSD-IAA) represents biologic false positives in terms of autoimmunity leading to diabetes. We hypothesize that factors related to the mechanism of loss of tolerance leading to diabetes determine high affinity and MSD-IAA reactivity.
Insulin autoantibodies (IAA) are often the first autoantibody to appear before the development of type 1A diabetes in children prospectively followed from birth (1). IAA is one of four major islet autoantibody assays validated in Centers for Disease Control and Prevention (CDC)-sponsored DASP (Diabetes Autoantibody Standardization Program) workshops of the Immunology of Diabetes Society (2–4). Early Immunology of Diabetes Society workshops demonstrated that although multiple ELISA assays readily detected insulin antibodies after injection of subcutaneous human insulin, standard ELISA formats were unable to detect IAA of new-onset patients with diabetes or individuals progressing to type 1 diabetes (5–7). These standard ELISA assays bound insulin to plates and likely obscured a key insulin epitope with plate binding of insulin.
The IAA that predicted the development of type 1A diabetes were of high affinity and recognized restricted unique conformational epitopes of the insulin molecule (8–11). We have reported recently (12) that in contrast with the IAA of prediabetic patients, the IAA of the spontaneous animal model, the NOD mouse, can readily be detected in an ELISA format, confirming earlier reports (13). This difference between the detection of murine IAA and the human autoantibodies in ELISA format despite equivalent signals with fluid phase radioassays (utilizing human insulin for both) was quite striking and reinforced the concept that binding of insulin to plates obscured a critical epitope seen by the autoantibodies of most prediabetic patients (12).
In addition to the inability to develop ELISA assays for IAA, the current fluid phase IAA radioassays (mIAA) have proved to be difficult for many laboratories to implement (9,14). Although IAA are usually of high affinity, capacities are very low and signals for the majority of patients very low except for younger children developing diabetes. DASP workshops have demonstrated that although the majority of laboratories have good specificity and sensitivity when measuring GAD, IA-2, and ZnT8 autoantibodies, this has not been achieved for IAA. 125I-labeling of the insulin molecule could potentially be a problem interfering with antibody binding to insulin. Given the need for improved IAA assays and the hypothesis that the binding of insulin to solid phases obscures a key determinant for recognition by human autoantibodies, we attempted to immobilize insulin to a solid phase that preserved critical determinants. Insulin is a relatively small protein of only 51 amino acids with disulfide linked A and B chains, and thus it was not surprising that insulin directly bound to plates did not allow detection of prediabetic IAA (data not shown). Given prior studies in which we found that all diabetic patients positive for IAA reacted with proinsulin in fluid phase radioassay (6), we produced biotinylated and Sulfo-tagged proinsulin to develop a capture IAA assay. Streptavidin, but not avidin, was able to capture the biotinylated proinsulin with autoantibody bound proinsulin pairs (biotinylated and Sulfo-tagged proinsulin), and this allowed us to develop a plate capture nonradioactive assay for IAA. When we applied the MSD-IAA assay to prospective samples of the DAISY (Diabetes Auto-immunity Study in the Young) study we were surprised to discover that radioassay IAA+ children expressing multiple islet autoantibodies (high-risk children with IAA plus GAD, IA-2, and/or ZnT8 autoantibodies), but only a minority of those expressing only IAA (low risk) were positive with the new MSD-IAA assay, showing marked biologic specificity.
RESEARCH DESIGN AND METHODS
Subjects.
Serum samples from both 100 newly diagnosed patients with diabetes (studied within two weeks of diagnosis at the Barbara Davis Center for Childhood Diabetes) and 70 prediabetic subjects who were followed to overt diabetes were selected on the basis of expressing one or more anti-islet autoantibody (of insulin, GAD65, IA-2, and ZnT8). One hundred age-matched normal control samples from DAISY participants (the same control population for mIAA assay) were also analyzed. Sequential serum samples were analyzed from 64 nondiabetic subjects from the DAISY study who were IAA+ with or without other autoantibodies and longitudinally followed. The prospectively studied DAISY children were selected to be either first degree relatives of patients with type 1 diabetes or general population children with high-risk HLA haplotypes (e.g., DR3: DRB1*0301, DQA1*0501, DQB1*0201 or DR4: DRB1*04, DQA1*0301, DQB1*0302). In addition, 150 de-identified blinded samples from DASP were provided by Dr. Patricia Mueller of the CDC. Signed written informed consents were obtained from participants, and the study was approved by the Institutional Review Board of the University of Colorado.
Sulfo-TAG labeling of proinsulin.
Proinsulin (kindly provided by Eli Lilly) in PBS was mixed with Sulfo-TAG (MSD) at 1:5 molar ratio and incubated at room temperature for 2 h with shielding from light. After incubation, the product was purified with a desalting spin column (Thermo Scientific) to remove unbounded Sulfo-TAG. The final product was evaluated for protein concentration using a bicinchoninic acid (BCA) assay kit (Sigma), and the amount of Sulfo-TAG labeled to proinsulin was determined by spectrophotometry at the wavelength of 450 nm.
Biotin labeling of proinsulin.
The labeling was performed with a biotinylation kit (Thermo Scientific). In brief, proinsulin (Eli Lilly) in PBS was mixed with biotin (from the kit) at 1:5 molar ratio and incubated at room temperature for 1 h. After incubation, the free biotin was removed with a desalting spin column provided in the kit. The protein concentration was determined with a BCA kit (Sigma), and the amount of biotin labeled to proinsulin by spectrophotometry was determined at the wavelength of 500 nm.
IAA plate capture assay (MSD-IAA).
The assay protocol is summarized in Fig. 1. To optimize reagent concentrations and ratio of biotinylated to Sulfo-TAG proinsulin, various conditions for the assay were tested in a series of experiments varying the concentration of Sulfo-TAG–labeled proinsulin, biotin-labeled proinsulin and ratio of these two labeled proinsulin, the serum volume, and serum dilution. After preliminary optimization, we surprisingly found that binding of insulin antibodies, either a mouse monoclonal insulin antibody-125 (kindly provided by Dr. Tom Thomas of Vanderbilt) or IAA of patients, was inhibited by normal human serum for the MSD-IAA assay, but not for the radioassay. To decrease the inhibition, a step of acid treatment of serum was introduced into the assay. In brief, 15 μL of patient serum were mixed with 18 μL of 500 mmol/L of acetic acid. After incubation for 45 min at room temperature, 25 μL of the acid-treated solution was transferred to a freshly prepared antigen/neutralization solution consisting of 8.3 μL of 1 M Tris-HCl (pH 9.0) and 35 μL of labeled proinsulin (Sulfo-TAG–labeled proinsulin at concentration of 200 ng/mL and biotin-labeled proinsulin of 100 ng/mL) in PBS with 5% BSA. The mixture was incubated at room temperature for 2 h with shaking on a plate shaker followed by incubation at 4°C overnight (>16 h). The same day, 96-well streptavidin-coated MSD plates were blocked with 150 μL of 3% Blocker A (MSD) per well overnight at 4°C. The next day, the blocked MSD plate was washed with PBST (PBS with 0.05% Tween-20) three times followed by the addition of the overnight-incubated serum mixture into the MSD plate. After incubation at room temperature for 1 h, the plate was washed three times with PBST to remove uncaptured proinsulin. Finally, 150 μL/well of 2× Read buffer (MSD) were added and the plate was counted on a MSD Sector Imager 2400. The intra-assay coefficient variation was 5.2% (n = 8), and interassay coefficient variation was 9.2% (n = 6). A mouse monoclonal insulin antibody-125 was used as the assay internal standard positive control, and the result was expressed as an index (index = [Signalsample − SignalNegativeControl] / [SignalPositiveControl − SignalNegativeControl]).
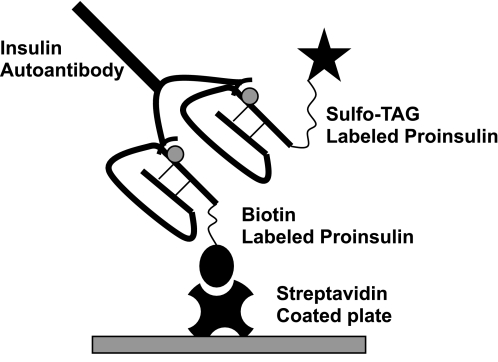
FIG. 1.
Illustration of the bivalent plate capture MSD-IAA assay. The IAA in serum will link the Sulfo-tagged proinsulin to the biotinylated proinsulin, which will be captured on the solid phase of the streptavidin-coated plate. Detection of plate-captured Sulfo-tagged proinsulin is accomplished with electrochemiluminescence.
RESULTS
Assay description.
A number of Sulfo-tagged fluid phase assays have been developed that depend upon complexing a biotinylated “bait” molecule with a Sulfo-tagged ruthenium-labeled protein. The biotin allows capture on streptavidin solid phase while the Sulfo-TAG provides electrochemical light emission for detection of the captured complex using an MSD electrochemiluminescent instrument. We initially tested both biotinylated and Sulfo-tagged proinsulin as competitors in our standard fluid phase IAA radioassay and demonstrated that both modified molecules were able to compete with 125I-insulin for binding to patients’ IAA (Fig. 2). The acid treatment of serum was introduced into assay protocol to decrease inhibition of signal by serum. After acid treatment, the MSD-IAA assay signal for both mouse monoclonal antibody and patient autoantibodies were greatly improved (see Fig. 3). Acid treatment of serum samples before the assay had no effect on our mIAA radioassay with 20 samples studied in parallel (data not shown). To test whether the IAA detected in MSD proinsulin assay reacted with insulin, 40 MSD-IAA+ samples at different levels (20 new onsets and 20 prediabetic samples) were randomly selected and all 40 MSD-IAA+ samples were completely absorbed by unlabeled insulin (data not shown).
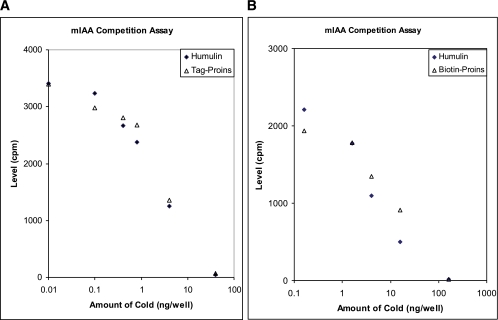
FIG. 2.
Both Sulfo-TAG and biotin-labeled proinsulin (Proins) were used as competitors in our standard IAA radioassay. Sulfo-TAG–labeled proinsulin (in A) and biotin-labeled proinsulin (in B) competed for binding 125I-insulin as well as unmodified human insulin (Humulin from Eli Lilly). cpm, counts per minute. (A high-quality color representation of this figure is available in the online issue.)
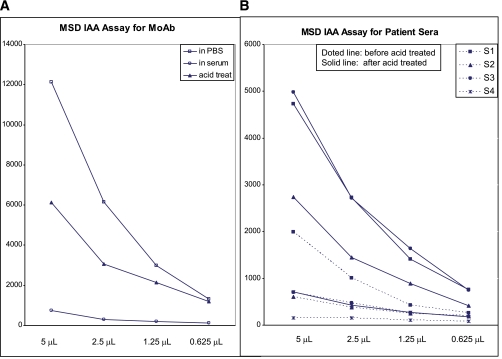
FIG. 3.
Illustration of normal human serum blocking MSD-IAA signal. The MSD-IAA radioassay signals with an insulin monoclonal antibody (MoAb; A) are decreased by addition of normal human serum, compared with equal volume of PBS with partial restoration of signal with acid treatment (acid treat) of human serum. B: Illustration of MSD-IAA assay signals of 4 patient sera with and without acid treatment. (A high-quality color representation of this figure is available in the online issue.)
Assay sensitivity/specificity.
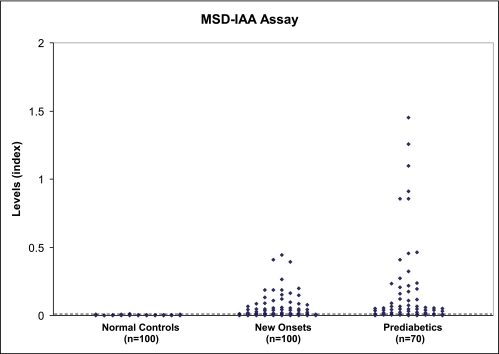
After a series of optimization steps, the MSD-IAA assay protocol described in research design and methods was used for all experiments. Figure 4 illustrates the IAA levels from our MSD-IAA assay for a total of 170 patient samples (100 newly diagnosed patients with diabetes and 70 prediabetic subjects) and 100 age-matched healthy control subjects. With upper limit of normal range set at 99th percentile (index 0.006) from 100 control subjects, 116 of 170 (68%) of new-onset and prediabetic patients were positive.
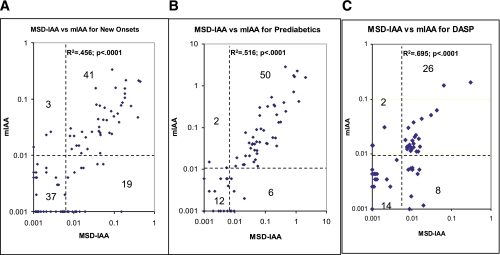
FIG. 4.
MSD-IAA index of sera from 100 normal control subjects, 100 newly diagnosed patients with type 1 diabetes, and 70 prediabetic patients who were followed to overt diabetes. With cutoff value of index 0.007 set at 99th percentile of 100 healthy control samples, 65% (110 of 170) of the patients were positive. (A high-quality color representation of this figure is available in the online issue.)
The positivity and levels of the IAA from MSD-IAA and our current radioassay (mIAA) were compared (Fig. 5). With 99th percentile of specificity set for both assays, 60% (60 of 100) of new-onset patients (Fig. 5A) and 80% (56 of 70) of prediabetic patients (Fig. 5B), in total 68% (116 of 170), were positive for MSD-IAA compared with mIAA (44 of 100, 44% for new-onset patients, and 52 of 70, 74% for prediabetic patients, in total 96 of 170, 56% [P = 0.03]). The IAA levels of the two assays were correlated for both new-onset patients (R2 = 0.456; P < 0.0001) and prediabetic patients (R2 = 0.516, P < 0.0001). In addition, a blinded DASP workshop set of 100 control subjects and 50 new-onset patients was tested for MSD-IAA assay, and IAA levels were also well correlated with our mIAA radioassay (R2 = 0.695; P < 0.0001) while MSD-IAA had a higher sensitivity of 68 vs. 56% in the radioassay (Fig. 5C) with the same specificity of 99% for both assays. Of 19 new-onset subjects who were MSD-IAA+ and mIAA radioassay-negative (Fig. 5A), 13 were children and all 19 subjects were positive for at least two other islet autoantibodies (GAD65, IA-2, and ZnT8). Of eight new onsets from DASP set who were MSD-IAA+ and mIAA radioassay-negative (Fig. 5C), all were positive for other islet autoantibodies, six positive for at least two other islet autoantibodies, and two positive for one other islet autoantibody (GAD65).
FIG. 5.
IAA levels from MSD-IAA assay and our current radioassay (mIAA) were compared among 100 newly diagnosed patients with type 1 diabetes (A), 70 prediabetic patients (B), and 50 blinded DASP workshop patient samples (C). The two assays were correlated (P < 0.0001), but MSD-IAA assay had higher sensitivity for all 3 groups with both assays set at 99% specificity. (A high-quality color representation of this figure is available in the online issue.)
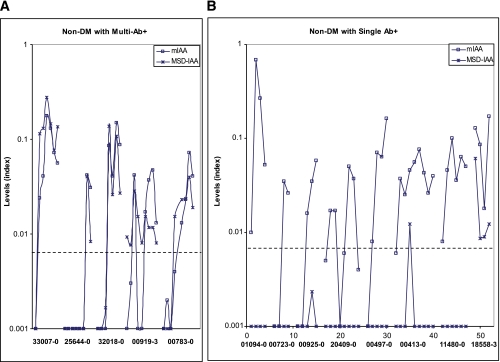
Analysis prospectively followed children of the DAISY study.
A group of sequential samples from 64 nondiabetic children from the DAISY study followed from birth with persistent mIAA positivity was tested for MSD-IAA. Of those with multiple autoantibodies 100% (32 of 32) were positive in the MSD-IAA assay, and for subjects expressing only mIAA 25% (8 of 32) were MSD-IAA assay-positive (P < 0.0001). Of note, positivity of the MSD-IAA for 11 children with ≥2 Abs preceded mIAA radioassay positivity. Figure 6 illustrates the pattern of autoantibody expression for the initial 13 DAISY children studied; five children were multiple anti-islet autoantibody-positive (≥2 Abs; IAA plus GAD65, IA-2, and/or ZnT8 autoantibodies), and eight were consistently mIAA+ only (single Ab). Surprisingly, multiple samples from subjects with ≥2 Abs were all positive (5 of 5) for MSD-IAA (panel A), whereas samples from subjects with only a single Ab (mIAA) on multiple samples were often MSD-IAA− (Fig. 6B) although positive with the radioassay mIAA.
FIG. 6.
Sera from children in the DAISY study persistently expressing IAA as a single autoantibody or with other islet autoantibodies were analyzed with the MSD-IAA assay. A: MSD-IAA were well correlated with radioassay mIAA for all five subjects (multiple follow-up positive for both assays) who were multiple islet autoantibody-positive. B: Seven and eight subjects with single islet autoantibody (mIAA only) were consistently MSD-IAA−. DM, diabetic subjects. (A high-quality color representation of this figure is available in the online issue.)
Avidity analysis.
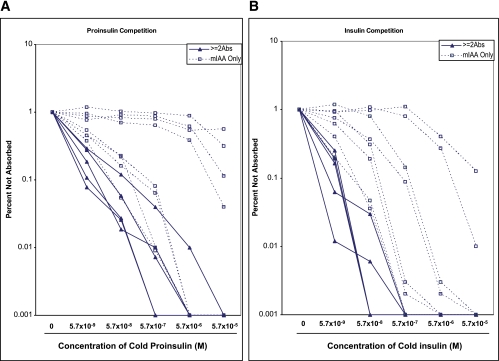
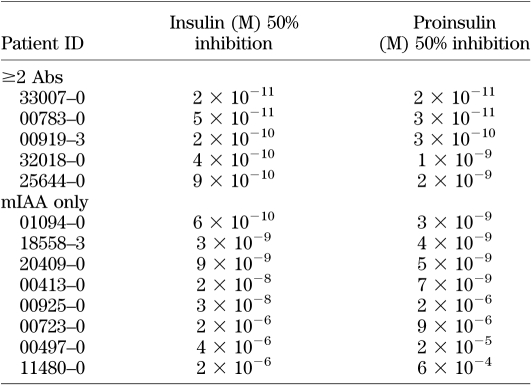
To identify characteristics associated with the IAA from these two different groups of subjects, we did competition analysis for the samples of the initial 13 DAISY children studied with different concentrations of insulin and proinsulin (Fig. 7A for proinsulin absorption curve and Fig. 7B for insulin absorption curve). We found that the IAA from the majority of the subjects with the single Ab required a higher concentration of insulin and proinsulin for 50% inhibition of binding (Table 1). Four of the single antibody IAA were minimally inhibited by competition with proinsulin, suggesting recognition of a different epitope. The 50% inhibition by insulin and proinsulin of the MSD-IAA+ samples were correlated (R2 = 0.9814; P < 0.0001).
FIG. 7.
Sera with IAA from 13 subjects in Fig. 6 with single or multiple islet autoantibodies were incubated with different concentrations of either proinsulin (A) or insulin (B) and analyzed with our standard mIAA radioassay. IAA from subjects with single autoantibody (dotted line), compared with subjects with multiple autoantibodies (solid line), required higher concentrations of proinsulin and insulin for one-half maximal inhibition. Results were expressed as percent of signal not absorbed. (A high-quality color representation of this figure is available in the online issue.)
TABLE 1.
The estimated concentrations of insulin and proinsulin causing 50% inhibition of IAA signals in radioassay (mIAA) for 13 children from the DAISY study, 5 with multiple islet autoantibodies (≥2 Abs), and 8 with a single autoantibody (mIAA only)
DISCUSSION
In 1974, Bottazzo et al. (15) reported the presence of autoantibodies in sera of patients with diabetes and polyendocrine autoimmunity reacting with frozen sections of human pancreas. Although this assay is still used, it has been largely replaced by the measurement of autoantibodies reacting with defined islet autoantigens, in particular insulin, GAD65, IA-2, and ZnT8 (4,16–18). The least reproducible (between laboratories) of the biochemical assays for islet autoantibodies has been the assay for IAA in DASP workshops. This is a particularly important assay in that IAA are often the first autoantibody to appear in prediabetic children followed from birth, and when they first appear they are already of high affinity (1). Low-affinity IAA are less associated with progression to diabetes, and assays have been developed that use competition with a set concentration of unlabeled insulin to help identify lower risk IAA (11).
There already exist both a GAD65 and IA-2 plate capture autoantibody ELISA assays that have performed well in DASP workshops (4). Despite the importance of IAA no validated (in CDC-sponsored DASP workshops) nonradioactive assay is available. We believe we have solved the difficulty of developing a solid phase capture nonradioactive IAA assay using biotinylation of proinsulin (rather than using insulin as bait) and streptavidin rather than avidin for capture. Based on not using radioactivity and semiautomated high throughput assay format (although there is an added step for acid treatment of serum samples), the MSD-IAA assay should be suitable for general application. Acidification of serum is usually applied in assays to disassociate pre-existing bound complexes. Given lack of effect of charcoal absorption of free insulin on the MSD assay and the high concentration of unlabeled insulin required to inhibit MSD-IAA assay we do not believe endogenous insulin in serum is causing the inhibition of MSD-IAA signal. The mechanism of MSD-IAA signal inhibited by normal serum and released by acidification of serum is unknown at present, but it has been used in other MSD-based assays (19–21).
Perhaps the most striking finding of the current study is the markedly divergent results for the standard IAA radioassay and the new MSD-IAA assay comparing children expressing only IAA (at low risk of progression to diabetes) with those children expressing IAA and the other islet autoantibodies. It has been known for more than a decade that individuals expressing only a single islet autoantibody are at low risk, and for those relatives expressing only IAA from the Diabetes Prevention Trial study there was essentially no risk of progression to diabetes (0 of 407) (22). One hypothesis for the very low risk of those with only a single islet autoantibody (e.g., insulin) is that spreading of autoimmunity to other autoantigens is needed to increase risk or marks a stage closer to overt diabetes. Our data are consistent with an alternative explanation for those with only IAA that it is likely related to prior studies of IAA affinity (11). Namely, the IAA of those with isolated IAA is often qualitatively different from the IAA with other islet autoantibodies (of GAD65, ZnT8, and IA-2 autoantibodies). DAISY subjects were selected to be at increased risk for diabetes (first-degree relatives or general population children with high-risk HLA DR3 and/or DR4), and thus the majority of IAA+ children had DR3 and/or DR4. We speculate that the mechanism of immunization may relate to expressing radioassay-positive, lower affinity MSD-IAA− IAA. The simplest hypothesis would be that the lower risk IAA often result from immunization with a cross-reactive molecule, whereas higher affinity, higher risk IAA result from immunization with insulin/proinsulin itself. Although both forms of IAA can be competed with insulin and thus are not a biochemical false positive, in terms of biologic relevance those antibodies detected with only the radioassay appear to be predominantly false positives relative to predicting disease status. There is already data that insulin antibodies induced after subcutaneous human insulin therapy and the insulin autoimmune syndrome differ from prediabetic insulin autoantibodies (23), and this study indicates that nonpredictive IAA can usually be distinguished by failure to give signal with the bivalent MSD-IAA assay. A caveat is that given long enough follow-up some individuals with only radioassay-positive IAA (perhaps as adults) may develop additional autoantibodies or progress to diabetes, and thus both longer follow-up and studies of additional populations with the described MSD-IAA assay are needed.
ACKNOWLEDGMENTS
This study was funded by National Institutes of Health Grant DK32083, Diabetes Autoimmunity Study in the Young Grant DK32493, Diabetes Endocrine Research Center Grant P30 DK57516, the Immune Tolerance Network (AI15416), the Juvenile Diabetes Research Foundation Grant 11-2005-15, the Children’s Diabetes Foundation, and the Brehm Coalition Diabetes and Endocrinology Research Center Clinical Core (DK57516).
No potential conflicts of interest relevant to this article were reported.
L.Y. and G.S.E. researched data and wrote the manuscript. D.M., L.S., K.J., and M.R. researched data and reviewed and edited the manuscript. G.S.E. is the guarantor of this article.
The authors thank Dr. Tom Thomas of Vanderbilt for the gift of an insulin monoclonal antibody, Eli Lilly Company for supplying proinsulin, and MSD Company for helpful discussions during assay development.
REFERENCES
- 1.Achenbach P, Koczwara K, Knopff A, Naserke H, Ziegler AG, Bonifacio E. Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest 2004;114:589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu E, Eisenbarth GS. Accepting clocks that tell time poorly: fluid-phase versus standard ELISA autoantibody assays. Clin Immunol 2007;125:120–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams AJK, Bingley PJ, Bonifacio E, Palmer JP, Gale EAM. A novel micro-assay for insulin autoantibodies. J Autoimmun 1997;10:473–478 [DOI] [PubMed] [Google Scholar]
- 4.Törn C, Mueller PW, Schlosser M, Bonifacio E, Bingley PJ; Participating Laboratories Diabetes Antibody Standardization Program: evaluation of assays for autoantibodies to glutamic acid decarboxylase and islet antigen-2. Diabetologia 2008;51:846–852 [DOI] [PubMed] [Google Scholar]
- 5.Greenbaum CJ, Palmer JP, Kuglin B, Kolb H. Insulin autoantibodies measured by radioimmunoassay methodology are more related to insulin-dependent diabetes mellitus than those measured by enzyme-linked immunosorbent assay: results of the Fourth International Workshop on the Standardization of Insulin Autoantibody Measurement. J Clin Endocrinol Metab 1992;74:1040–1044 [DOI] [PubMed] [Google Scholar]
- 6.Castaño L, Ziegler AG, Ziegler R, Shoelson S, Eisenbarth GS. Characterization of insulin autoantibodies in relatives of patients with type I diabetes. Diabetes 1993;42:1202–1209 [DOI] [PubMed] [Google Scholar]
- 7.Greenbaum CJ, Wilkin TJ, Palmer JP. Fifth international serum exchange workshop for insulin autoantibody (IAA) standardization. Diabetologia 1992;35:798–800 [DOI] [PubMed] [Google Scholar]
- 8.Palmer JP, Asplin CM, Clemons P, et al. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science 1983;222:1337–1339 [DOI] [PubMed] [Google Scholar]
- 9.Achenbach P, Schlosser M, Williams AJ, et al. Combined testing of antibody titer and affinity improves insulin autoantibody measurement: Diabetes Antibody Standardization Program. Clin Immunol 2007;122:85–90 [DOI] [PubMed] [Google Scholar]
- 10.Koczwara K, Muller D, Achenbach P, Ziegler AG, Bonifacio E. Identification of insulin autoantibodies of IgA isotype that preferentially target non-human insulin. Clin Immunol 2007;124:77–82 [DOI] [PubMed] [Google Scholar]
- 11.Schlosser M, Koczwara K, Kenk H, et al. In insulin-autoantibody-positive children from the general population, antibody affinity identifies those at high and low risk. Diabetologia 2005;48:1830–1832 [DOI] [PubMed] [Google Scholar]
- 12.Babaya N, Liu E, Miao D, Li M, Yu L, Eisenbarth GS. Murine high specificity/sensitivity competitive europium insulin autoantibody assay. Diabetes Technol Ther 2009;11:227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu L, Eisenbarth G, Bonifacio E, Thomas J, Atkinson M, Wasserfall C. The second murine autoantibody workshop: remarkable interlaboratory concordance for radiobinding assays to identify insulin autoantibodies in nonobese diabetic mice. Ann N Y Acad Sci 2003;1005:1–12 [DOI] [PubMed] [Google Scholar]
- 14.Schlosser M, Mueller PW, Törn C, Bonifacio E, Bingley PJ; Participating Laboratories Diabetes Antibody Standardization Program: evaluation of assays for insulin autoantibodies. Diabetologia 2010;53:2611–2620 [DOI] [PubMed] [Google Scholar]
- 15.Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet 1974;2:1279–1283 [DOI] [PubMed] [Google Scholar]
- 16.Achenbach P, Warncke K, Reiter J, et al. Type 1 diabetes risk assessment: improvement by follow-up measurements in young islet autoantibody-positive relatives. Diabetologia 2006;49:2969–2976 [DOI] [PubMed] [Google Scholar]
- 17.Bingley PJ, Gale EA; European Nicotinamide Diabetes Intervention Trial (ENDIT) Group Progression to type 1 diabetes in islet cell antibody-positive relatives in the European Nicotinamide Diabetes Intervention Trial: the role of additional immune, genetic and metabolic markers of risk. Diabetologia 2006;49:881–890 [DOI] [PubMed] [Google Scholar]
- 18.Wenzlau JM, Moua O, Sarkar SA, et al. SlC30A8 is a major target of humoral autoimmunity in type 1 diabetes and a predictive marker in prediabetes. Ann N Y Acad Sci 2008;1150:256–259 [DOI] [PubMed] [Google Scholar]
- 19.Myler HA, McVay S, Kratzsch J. Troubleshooting PEG-hGH detection supporting pharmacokinetic evaluation in growth hormone deficient patients. J Pharmacol Toxicol Methods 2010;61:92–97 [DOI] [PubMed] [Google Scholar]
- 20.Zhong ZD, Dinnogen S, Hokom M, et al. Identification and inhibition of drug target interference in immunogenicity assays. J Immunol Methods 2010;355:21–28 [DOI] [PubMed] [Google Scholar]
- 21.Lofgren JA, Dhandapani S, Pennucci JJ, et al. Comparing ELISA and surface plasmon resonance for assessing clinical immunogenicity of panitumumab. J Immunol 2007;178:7467–7472 [DOI] [PubMed] [Google Scholar]
- 22.Orban T, Sosenko JM, Cuthbertson D, et al. ; Diabetes Prevention Trial-Type 1 Study Group Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2009;32:2269–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirata Y. Methimazole and insulin autoimmune syndrome with hypoglycemia. Lancet 1983;2:1037–1038 [DOI] [PubMed] [Google Scholar]