Abstract
Acute promyelocytic leukemia (APL) results from a chromosomal translocation that gives rise to the leukemogenic fusion protein PML-RARα (promyelocytic leukemia–retinoic acid α receptor). Differentiation of leukemic cells and complete remission of APL are achieved by treatment of patients with pharmacological doses of all-trans retinoic acid (ATRA), making APL a model disease for differentiation therapy. However, because patients are resistant to further treatment with ATRA on relapse, it is necessary to develop alternative treatment strategies to specifically target APL. We therefore sought to develop a treatment strategy based on lentiviral vector-mediated delivery of small interfering RNA (siRNA) that specifically targets the breakpoint region of PML-RARα. Unlike treatment with ATRA, which resulted in differentiation of leukemic NB4 cells, delivery of siRNA targeting PML-RARα into NB4 cells resulted in both differentiation and apoptosis, consistent with the specific knockdown of PML-RARα. Intraperitoneal injection of NB4 cells transduced with lentiviral vectors delivering PML-RARα-specific siRNA but not control siRNA prevented development of disease in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice. Taken together, these results indicate that development of PML-RARα-specific siRNA may represent a promising treatment strategy for ATRA-resistant APL.
Ward and colleagues use lentiviral vector-mediated small interfering RNA to knock down expression of the leukemogenic fusion protein, promyelocytic leukemia–retinoic acid α receptor (PML-RARα). They demonstrate that this approach prevents the development of disease in a mouse model of acute promyelocytic leukemia.
Introduction
Acute promyelocytic leukemia (APL) represents approximately 10% of all acute myeloid leukemias in adults (Melnick and Licht, 1999; Zelent et al., 2001). The majority of APL cases are characterized by the presence of a chromosomal translocation, t(15;17), that results in the generation of the oncogenic fusion protein PML-RARα (promyelocytic leukemia–retinoic acid α receptor) (He et al., 1999; Melnick and Licht, 1999; Zelent et al., 2001). The two most abundant isoforms result from different breakpoints located in the PML gene on chromosome 15 and are referred to as the long and short isoforms of PML-RARα (Pandolfi et al., 1992a,b). A common therapy for APL is treatment with all-trans retinoic acid (ATRA), which results in differentiation of leukemic cells by relieving the transcriptional repressor function of the PML-RARα fusion protein (Lin et al., 1999, 2001). Thus, APL is considered a model disease for differentiation therapy. However, after relapse patients become resistant to further treatment with ATRA, necessitating alternative therapy including treatment with arsenic trioxide (As2O3), which is known to induce degradation of PML-RARα and apoptosis of leukemic cells (Chen et al., 1996). In addition, the most common variant APL fusion oncogene encoding PLZF-RARα responds neither to ATRA (Guidez et al., 1994) nor As2O3 (Kitamura et al., 2000). The importance of degradation of the oncogenic fusion protein in the therapy of APL has been demonstrated for both treatment with As2O3 and with ATRA (Nasr et al., 2008). Specifically, PML-RARα degradation was crucial for elimination of leukemia-initiating cells (LICs).
Small interfering RNA (siRNA) represents an alternative method to the degradation of proteins by knocking down protein expression as a result of mRNA degradation (Elbashir et al., 2001a,b; Brummelkamp et al., 2002). Lentiviral vector-mediated delivery of short hairpin RNA (shRNA) has been demonstrated to result in efficient knockdown of gene expression both in vitro and in vivo (Brummelkamp et al., 2002; Tiscornia et al., 2003). Because of the development of ATRA-resistant APL on relapse and the potential to apply siRNA-mediated knockdown of PML-RARα to the elimination of LICs, the present study describes the development of PML-RARα-specific siRNA, using a lentiviral vector approach in APL patient-derived NB4 cells (Lanotte et al., 1991).
Materials and Methods
Generation of lentiviral vectors
High-titer lentiviral vectors expressing green fluorescent protein (GFP) and delivering PML-RARα-specific siRNA were generated as previously described (Tiscornia et al., 2003, 2006), with shRNA expression driven by the mouse U6 promoter. The following siRNA sequences targeting the PML-RARα breakpoints were designed and expressed as shRNAs: siPML-RARα short 5′-GAAAGCCATTGAGACCCAGA-3′ and siPML-RARα long 5′-GGCAGCCATTGAGACCCAGA-3′. The control lentiviral vectors expressing GFP with or without an shGFP expression cassette were as previously described (Tiscornia et al., 2003). A lentiviral vector construct expressing the short isoform of PML-RARα was used to generate stable 293T cells as described below.
Cell culture and ATRA treatment
HEK 293T cells were maintained in high-glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM glutamine, and penicillin and streptomycin. NB4 cells (Lanotte et al., 1991) were cultured in RPMI supplemented with 10% (v/v) fetal bovine serum, 2 mM glutamine, and penicillin and streptomycin. Treatment of cells with ATRA (Sigma-Aldrich, St. Louis, MO) was carried out at 1 μM for 5 days.
Transient transfections and Western blot analysis
293T cells were plated in 6-well plates and transiently transfected with 0.5 μg of a retro- or lentiviral PML-RARα expression construct and 1.5 μg of siRNA lentiviral vector construct per well, using the calcium phosphate precipitation method (Graham and van der Eb, 1973). Whole cell extracts were prepared 72 hr posttransfection, using lysis buffer containing 20 mM HEPES (pH 7.9), 400 mM NaCl, 10 mM KCl, 1 mM EDTA (pH 8.0), 0.5% Nonidet P-40 (NP-40), 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM dithiothreitol (DTT), 1 mM Na3VO4, 5 mM NaF, 20% glycerol, and 1% (v/v) mammalian protease inhibitor (Sigma-Aldrich). Proteins were transferred to polyvinylidene difluoride (PVDF) membranes and probed with anti-PML (H-238; Santa Cruz Biotechnology, Santa Cruz, CA), anti-RARα (C-20; Santa Cruz Biotechnology), and anti-α-tubulin (DM1A; Sigma-Aldrich) antibodies. Detection was done with horseradish peroxidase-conjugated secondary antibodies, using an enhanced chemiluminescence (ECL) detection kit according to instructions provided by the manufacturer (GE Healthcare, Piscataway, NJ).
Transductions and flow cytometry
To generate 293T cells stably expressing the short isoform of PML-RARα, cells were transduced twice with 2 ml of viral vector supernatants. PML-RARα-expressing 293T cells were transduced with high-titer lentiviral vectors specifically targeting the short isoform at a multiplicity of infection (MOI) of 50 or with equivalent p24 of GFP control vectors. NB4 cells were transduced on two consecutive days at an MOI of 125 in a minimal volume of medium and allowed to expand, followed by determination of transduction efficiencies by flow cytometry and confirmation of specific PML-RARα knockdown by Western blotting. Using this transduction protocol, transduction efficiencies of 98% or greater were routinely achieved. For analysis of apoptosis and differentiation, cells were stained with 7-aminoactinomycin D (7-AAD; Sigma-Aldrich) and allophycocyanin-conjugated anti-CD11b (anti-CD11b–APC) or annexin V–APC (both from BD Biosciences, San Jose, CA), followed by flow cytometry 6 days after transduction. Data analysis was performed with CellQuest (BD Biosciences).
Intraperitoneal injection of transduced NB4 cells
Nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice (Jackson Laboratory, Bar Harbor, ME) were housed at the Salk Institute for Biological Studies (La Jolla, CA) animal facility under sterile conditions. Age-matched (∼8–9 weeks) female mice were injected intraperitoneally with 3×105 NB4 cells either left untransduced or transduced with lentiviral vectors expressing only GFP or expressing GFP and shRNA as described previously. NB4 cells were injected the day after transduction. Animals were monitored daily for development of disease, killed 180 days postinjection unless otherwise noted, and analyzed for the presence of intraperitoneal tumors and enlarged spleens. All animal experiments were approved by the Institutional Animal Care and Use Committee of the Salk Institute for Biological Studies.
Results and Discussion
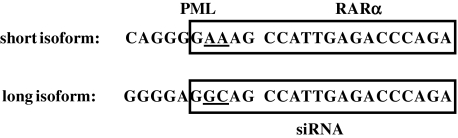
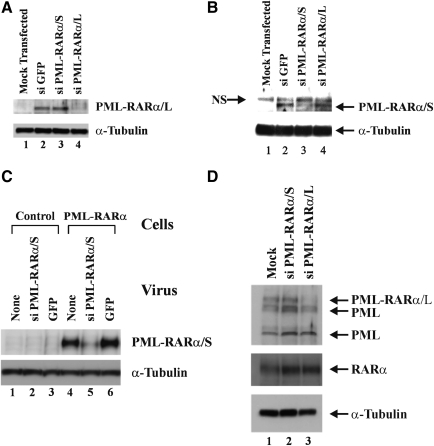
In an effort to develop an siRNA-based gene therapy approach for APL, lentiviral vectors containing shRNA sequences inserted in the U3 region were generated (Tiscornia et al., 2003) that specifically target the short and long isoforms of the oncogenic PML-RARα fusion protein. Because of identical breakpoint regions on chromosome 17 within the RARA gene (Kakizuka et al., 1991; Matsuoka et al., 1993), the siRNA molecules targeting the long and short PML-RARα isoforms differ by only two nucleotides in the 5′ region corresponding to sequence contributed by the PML portion of the fusions (Fig. 1). For lentiviral vectors containing siRNA targeting the long isoform of PML-RARα, specific knockdown was confirmed by cotransfection of 293T cells (Fig. 2A). A ratio of 1:3 (see Materials and Methods) was chosen for the plasmid expressing the target for knockdown and the siRNA-containing lentiviral vector, comparable to the range of previously described ratios that allow for specific siRNA screening and selection (Tiscornia et al., 2003, 2006, 2008). Specific knockdown of the long isoform of PML-RARα was seen only on cotransfection with lentiviral vectors delivering siRNAs that target the long isoform (Fig. 2A, lane 4), but not with lentiviral vectors delivering siRNAs that target the short isoform of PML-RARα (Fig. 2A, lane 3) or GFP (Fig. 2A, lane 2). The isoform specificity of siRNA sequences targeting PML-RARα was further confirmed in reciprocal cotransfection experiments of a construct expressing the short isoform of PML-RARα with siRNA-containing lentiviral vector constructs targeting the long and short isoforms (Fig. 2B). Expression of the short isoform of PML-RARα was knocked down only by siRNA targeting PML-RARα/S (Fig. 2B, lane 3), not by siRNA targeting PML-RARα/L (Fig. 2B, lane 4) or GFP (Fig. 2B, lane 2). These results indicate that the two-nucleotide difference in the 5′ region of the siRNAs conferred specificity of targeting, consistent with results demonstrating the general importance of the 5′ region of siRNA and microRNA molecules in specific knockdown (Lai, 2002; Lewis et al., 2003; Rajewsky and Socci, 2004).
FIG. 1.
Sequences of small interfering RNA (siRNA) specifically targeting the long and short isoforms of PML-RARα. Sequences at the breakpoint region are shown, with sequences contributed by PML and RARα indicated at the top. siRNA sequences are boxed. The breakpoint junction is indicated by the gap in the sequences; differences between siRNA sequences are underlined.
FIG. 2.
Lentiviral vector-mediated delivery of siRNA targeting PML-RARα results in specific knockdown of expression. (A) The indicated lentiviral siRNA expression constructs were cotransfected into 293T cells with an expression construct for the long isoform of PML-RARα. siPML-RARα/S and siPML-RARα/L target the breakpoint regions of the short and long PML-RARα isoforms, respectively. si GFP, irrelevant control siRNA. Immunoblotting of whole cell extracts was performed with anti-PML antibody to detect PML-RARα (right). An anti-α-tubulin antibody was used to confirm equal protein loading (bottom). (B) The short isoform of PML-RARα was cotransfected with the indicated lentiviral siRNA expression constructs, followed by Western blotting of whole cell extracts using anti-PML and anti-α-tubulin antibodies. NS, nonspecific band. (C) Control 293T cells or 293T cells stably expressing the short isoform of PML-RARα were left untransduced (lanes 1 and 4), or were transduced with a lentiviral vector expressing siRNA specific for the short PML-RARα isoform (lanes 2 and 5) or with control lentiviral vector expressing only GFP (lanes 3 and 6). Whole cell extracts were prepared 5 days posttransduction and Western blotting was performed with anti-RARα antibody to detect PML-RARα and anti-α-tubulin antibody. (D) NB4 cells were mock transduced or transduced with the indicated lentiviral vectors delivering siRNA against the breakpoint regions of the short or long PML-RARα isoform. Whole cell extracts were prepared 5 days posttransduction and immunoblotting was performed with anti-PML, anti-RARα, and anti-α-tubulin antibodies.
The ability to achieve specific knockdown of PML-RARα expression was further evaluated in cells that stably express either the short or long isoform of PML-RARα (Fig. 2C and D). Transduction of 293T cells stably expressing the short isoform of PML-RARα with lentiviral vectors delivering siRNA targeting the short PML-RARα isoform resulted in knockdown of expression (Fig. 2C, lane 5). By contrast, transduction with GFP control vectors had no effect on expression (Fig. 2C, lane 6). We next examined the effect of siRNA targeting the long and short isoforms of PML-RARα by transduction of NB4 cells that express the long PML-RARα isoform (Lanotte et al., 1991). Initial transduction experiments to evaluate the relative ease of NB4 cell transduction were carried out with GFP-expressing vectors at an MOI of 50. The resulting low transduction efficiencies with an estimated maximum of not more than 50% (data not shown) were inadequate to assess specific gene knockdown. We therefore conducted all subsequent experiments by sequential transduction at an MOI of 125 to achieve robust transduction efficiencies that allowed for the evaluation of specific knockdown of the long isoform of PML-RARα. As shown in Fig. 2D, transduction of NB4 cells under these conditions with lentiviral vectors targeting PML-RARα confirmed knockdown only with siRNA specific for the long isoform (Fig. 2D, lane 3) but not the short isoform (Fig. 2D, lane 2). Importantly, neither expression of any of several PML isoforms (Jensen et al., 2001) detected in NB4 cells nor the levels of RARα that is expressed from the nontranslocated copy of the RARA gene were affected by delivery of PML-RARα-specific siRNA, despite the 17-nucleotide homology between the siRNA and RARα sequences (Fig. 1).
The long-term effect of specific siRNA expression on NB4 cells was determined next. NB4 cells were transduced with lentiviral vectors delivering specific siRNA targeting the long isoform of PML-RARα or irrelevant control siRNA and observed for viability (Fig. 3). At 12 days posttransduction, cells that received control siRNA and cells that received specific siRNA appeared in abundance, similar to control cells that had received no siRNA (Fig. 3A, top). However, at 19 days posttransduction, nearly all cells that had received specific siRNA targeting PML-RARα had died, in contrast to the abundance of cells that had received control siRNA (Fig. 3A, bottom). Similarly, on transduction of NB4 cells with lentiviral vectors targeting the long or short isoform of PML-RARα, only specific siRNA targeting the long isoform resulted in loss of transduced GFP-positive cells by day 14 posttransduction (Fig. 3B, bottom). By contrast, comparable numbers of GFP-positive cells were present for both specific and nonspecific control siRNA at 5 days posttransduction as seen by both fluorescence microscopy and flow cytometry (Fig. 3B, top; and data not shown). These results indicate that specific targeting of PML-RARα results in cell death as a result of knockdown of the oncogenic fusion protein. Our results further indicate that expression of a nonspecific siRNA does not have measurable effects on cell viability, a prerequisite for use in a gene therapy setting.
FIG. 3.
RNA interference (RNAi) specifically targeting PML-RARα results in loss of transduced cells. (A) RNAi against PML-RARα results in leukemic cell death. NB4 cells were transduced with the indicated lentiviral vectors delivering siRNAs. Cells were examined by bright-field microscopy 12 and 19 days posttransduction. (B) NB4 cells transduced with the indicated lentiviral vectors expressing siRNAs and GFP as described in Fig. 2 were examined by bright-field (top) and fluorescence microscopy (bottom) on days 5 and 14 posttransduction. Color images available online at www.liebertonline.com/hum
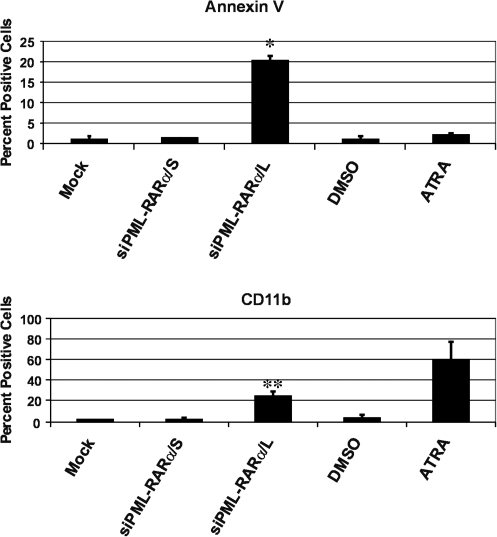
Because treatment of leukemic cells with ATRA or As2O3 has been reported to result in cellular differentiation and apoptosis, respectively (Chen et al., 1996), the mechanism of NB4 cell death in response to specific siRNA targeting PML-RARα was investigated next. Six days after transduction with lentiviral vectors delivering specific or control siRNA, viable 7-AAD-negative NB4 cells were analyzed for apoptosis and differentiation by staining with annexin V or anti-CD11b antibody, respectively, followed by flow cytometry (Fig. 4). Lentiviral vector-mediated delivery of specific siRNA targeting the long isoform of PML-RARα resulted in both apoptosis (Fig. 4, top) and differentiation (Fig. 4, bottom). By contrast, siRNA targeting the short PML-RARα isoform had no effect, as indicated by staining comparable to mock-transduced cells (Fig. 4). Furthermore, treatment of cells with ATRA resulted in differentiation (Fig. 4, bottom), but no significant apoptosis (Fig. 4, top). These results indicate that ATRA and siRNA-mediated knockdown of PML-RARα exert their potential therapeutic effects through different mechanisms. Because we were able to detect only a few cells (approximately 1% or less) staining positive for both CD11b and annexin V after siRNA-mediated knockdown of the long isoform of PML-RARα present in NB4 cells (data not shown), it is tempting to speculate that differentiation and apoptosis resulting from specific siRNA occur by distinct, as yet unknown molecular mechanisms. However, we cannot at present exclude the possibility of sequential events leading from siRNA-induced differentiation to apoptosis.
FIG. 4.
siRNA specifically targeting the long isoform of PML-RARα results in differentiation and apoptosis of leukemic cells. NB4 cells were mock transduced or transduced with the indicated lentiviral vectors as described in Fig. 2. Six days posttransduction cells were stained with annexin V–APC or anti-CD11b–APC antibody, followed by fluorescence-activated cell-sorting (FACS) analysis of 7-AAD-negative and GFP-positive transduced cells. Treatment with all-trans retinoic acid (ATRA) was included for the purpose of comparison; treatment with dimethyl sulfoxide (DMSO) served as the ATRA vehicle control. For mock-transduced cells and DMSO- and ATRA-treated cells, values shown represent the percentage of positive cells relative to the entire 7AAD-negative cell population. Results shown are the average with standard deviation of two independent experiments. *p<0.005 by Student t test for siPML-RARα/L relative to all other annexin V-stained cells; **p<0.05 for siPML-RARα/L relative to all other CD11b-stained cells except ATRA-treated cells.
The effect of PML-RARα-specific siRNA was examined in vivo after intraperitoneal injection of transduced NB4 cells into NOD/SCID mice under the conditions established in vitro that resulted in efficient and specific knockdown of the long isoform of PML-RARα. Similar to results shown for in vitro experiments, transduction of NB4 cells used for transplantations routinely resulted in transduction efficiencies of at least 98%. On injection of mice with control cells that had been left untransduced, all animals developed disease as evidenced by intraperitoneal tumor formation and death at 73–76 days postinjection (Table 1). Similar results were seen on transplantation of cells transduced with a GFP control vector without an shRNA expression cassette (data not shown). By contrast, no animals injected with NB4 cells that had been transduced with lentiviral vectors delivering specific siRNA targeting the long isoform of PML-RARα developed any clinical signs of disease by 180 days postinjection (Table 1). Consistent with the specific effect of the siRNA targeting the long isoform of PML-RARα, two of four animals injected with NB4 cells transduced with lentiviral vectors targeting the short isoform of PML-RARα displayed obvious signs of disease on visual inspection by 180 days postinjection, including ruffled fur and hunched posture (Table 1). Examination of the intraperitoneal cavity and spleen on necropsy indicated the presence of tumors or approximately 4- to 5-fold enlarged spleens (0.45–0.48 g) in three of four animals, consistent with the presence of leukemic cells. It is tempting to speculate that in contrast to mock transduction or transduction with GFP control vectors, siRNA targeting the short isoform may have contributed to delayed onset of disease, indicative of residual targeting ability in vivo despite the specificity of siRNA targeting the long and short isoforms observed in vitro. Thus, considerations of specificity must be borne in mind when designing siRNA for in vivo applications and therapeutic purposes, although the precise mechanisms underlying our in vivo observations are currently unknown.
Table 1.
Effect of Small Interfering RNA Specifically Targeting PML-RARα on Development of Disease after Intraperitoneal Injection of NB4 Cellsa
| |
Cells transplanted |
||
|---|---|---|---|
| No siRNA | siPML-RARα/S | siPML-RARα/L | |
| Mice with disease/mice per group | 3/3 | 3/4 | 0/5 |
NOD/SCID, nonobese diabetic/severe combined immunodeficient; siRNA, small interfering RNA; siPML-RARα/L, siRNA targeting the promyelocytic leukemia–retinoic acid α receptor fusion protein (long isoform); siPML-RARα/S, siRNA targeting PML-RARα (short isoform).
NB4 cells (3×105) transduced with lentiviral vectors delivering siRNA as described in Fig. 2 or left untransduced were injected into NOD/SCID mice by the intraperitoneal route. Animals in the control group that received cells not expressing siRNA were analyzed on death 73–76 days postinjection; remaining mice were killed 180 days postinjection, at which time two of the four animals in the siPML-RARα/S group had developed signs of disease on visual inspection and three of four animals displayed disease on necropsy. No animals in the siPML-RARα/L group had developed signs of disease on either visual inspection or on necropsy.
Taken together, the results described herein indicate that development of PML-RARα-specific siRNA may represent a promising treatment strategy for ATRA-resistant APL and targeting of LICs (Nasr et al., 2008). Although treatment of PML-RARα-positive leukemic cells with As2O3 results primarily in apoptosis at high concentrations of treatment, partial differentiation is known to occur in response to low concentrations of As2O3 (Chen et al., 1996, 1997; Nasr et al., 2009). By contrast, the therapeutic effect of treatment with ATRA results from terminal differentiation of APL cells thought to be mediated primarily through the binding of retinoic acid (RA) to the ligand-binding domain of the RARα portion of the PML-RARα fusion protein and subsequent release of transcriptional repression (Lin et al., 1999, 2001; Nasr et al., 2009). Consistent with this mechanism of ATRA action, we did not detect apoptosis in response to treatment of NB4 cells with ATRA, as shown in Fig. 4. Furthermore, our results concerning both differentiation and apoptosis resulting from siRNA-mediated knockdown of PML-RARα expression are reminiscent of the mechanism of action seen for treatment with As2O3. By contrast, ribozyme-mediated degradation of PML-RARα resulted in apoptosis in the absence of differentiation (Nason-Burchenal et al., 1998b).
Resistance to treatment with RA including ATRA is associated with point mutations in the RARα ligand-binding domain of the PML-RARα fusion protein (Roussel and Lanotte, 2001). Because the RARα ligand-binding domain lies far C-terminal to the PML-RARα breakpoint region targeted by siRNA described herein (Lin et al., 2001; Zelent et al., 2001), it is tempting to predict that ATRA-resistant APL should be sensitive to siRNA-mediated therapeutic effects similar to ribozyme-mediated targeting of PML-RARα (Nason-Burchenal et al., 1998a). In conclusion, although the main limitation in the application of siRNA as a treatment in a clinical setting lies in the need for effective delivery methods, targeting the breakpoint of a fusion oncogene is feasible and effective in the model system employed in our study.
Acknowledgments
The authors thank Dr. Inder M. Verma for generous support throughout this study and Dr. Oded Singer for technical advice. S.V.W. was supported in part by National Research Service Award F32CA115276 from the National Cancer Institute, National Institutes of Health, U.S. Public Health Service.
Author Disclosure Statement
No competing financial interests exist.
References
- Brummelkamp T.R. Bernards R. Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Chen G.Q. Zhu J. Shi X.G., et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RARα/PML proteins. Blood. 1996;88:1052–1061. [PubMed] [Google Scholar]
- Chen G.Q. Shi X.G. Tang W. Xiong S.M., et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL). I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- Elbashir S.M. Harborth J. Lendeckel W., et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001a;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Elbashir S.M. Lendeckel W. Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001b;15:188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F.L. van der Eb A.J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guidez F. Huang W. Tong J.H., et al. Poor response to all-trans retinoic acid therapy in a t(11;17) PLZF/RARα patient. Leukemia. 1994;8:312–317. [PubMed] [Google Scholar]
- He L.Z. Merghoub T. Pandolfi P.P. In vivo analysis of the molecular pathogenesis of acute promyelocytic leukemia in the mouse and its therapeutic implications. Oncogene. 1999;18:5278–5292. doi: 10.1038/sj.onc.1203088. [DOI] [PubMed] [Google Scholar]
- Jensen K. Shiels C. Freemont P.S. PML protein isoforms and the RBCC/TRIM motif. Oncogene. 2001;20:7223–7233. doi: 10.1038/sj.onc.1204765. [DOI] [PubMed] [Google Scholar]
- Kakizuka A. Miller W.H., Jr. Umesono K., et al. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RARα with a novel putative transcription factor, PML. Cell. 1991;66:663–674. doi: 10.1016/0092-8674(91)90112-c. [DOI] [PubMed] [Google Scholar]
- Kitamura K. Hoshi S. Koike M., et al. Histone deacetylase inhibitor but not arsenic trioxide differentiates acute promyelocytic leukaemia cells with t(11;17) in combination with all-trans retinoic acid. Br. J. Haematol. 2000;108:696–702. doi: 10.1046/j.1365-2141.2000.01933.x. [DOI] [PubMed] [Google Scholar]
- Lai E.C. Micro RNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat. Genet. 2002;30:363–364. doi: 10.1038/ng865. [DOI] [PubMed] [Google Scholar]
- Lanotte M. Martin-Thouvenin V. Najman S., et al. NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3) Blood. 1991;77:1080–1086. [PubMed] [Google Scholar]
- Lewis B.P. Shih I.H. Jones-Rhoades M.W., et al. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- Lin R.J. Egan D.A. Evans R.M. Molecular genetics of acute promyelocytic leukemia. Trends Genet. 1999;15:179–184. doi: 10.1016/s0168-9525(99)01710-2. [DOI] [PubMed] [Google Scholar]
- Lin R.J. Sternsdorf T. Tini M. Evans R.M. Transcriptional regulation in acute promyelocytic leukemia. Oncogene. 2001;20:7204–7215. doi: 10.1038/sj.onc.1204853. [DOI] [PubMed] [Google Scholar]
- Matsuoka A. Miyamura K. Emi N., et al. Unexpected heterogeneity of PML/RARα fused mRNA detected by nested polymerase chain reaction in acute promyelocytic leukemia. Leukemia. 1993;7:1151–1155. [PubMed] [Google Scholar]
- Melnick A. Licht J.D. Deconstructing a disease: RARα, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood. 1999;93:3167–3215. [PubMed] [Google Scholar]
- Nason-Burchenal K. Allopenna J. Begue A., et al. Targeting of PML/RARα is lethal to retinoic acid-resistant promyelocytic leukemia cells. Blood. 1998a;92:1758–1767. [PubMed] [Google Scholar]
- Nason-Burchenal K. Takle G. Pace U., et al. Targeting the PML/RARα translocation product triggers apoptosis in promyelocytic leukemia cells. Oncogene. 1998b;17:1759–1768. doi: 10.1038/sj.onc.1202075. [DOI] [PubMed] [Google Scholar]
- Nasr R. Guillemin M.C. Ferhi O., et al. Eradication of acute promyelocytic leukemia-initiating cells through PML-RARA degradation. Nat. Med. 2008;14:1333–1342. doi: 10.1038/nm.1891. [DOI] [PubMed] [Google Scholar]
- Nasr R. Lallemand-Breitenbach V. Zhu J., et al. Therapy-induced PML/RARA proteolysis and acute promyelocytic leukemia cure. Clin. Cancer Res. 2009;15:6321–6326. doi: 10.1158/1078-0432.CCR-09-0209. [DOI] [PubMed] [Google Scholar]
- Pandolfi P.P. Alcalay M. Fagioli M., et al. Genomic variability and alternative splicing generate multiple PML/RARα transcripts that encode aberrant PML proteins and PML/RARα isoforms in acute promyelocytic leukaemia. EMBO J. 1992a;11:1397–1407. doi: 10.1002/j.1460-2075.1992.tb05185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandolfi P.P. Alcalay M. Longo L., et al. Molecular genetics of the t(15;17) of acute promyelocytic leukemia (APPL) Leukemia. 1992b;6(Suppl. 3):120S–122S. [PubMed] [Google Scholar]
- Rajewsky N. Socci N.D. Computational identification of microRNA targets. Dev. Biol. 2004;267:529–535. doi: 10.1016/j.ydbio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Roussel M.J. Lanotte M. Maturation sensitive and resistant t(15;17) NB4 cell lines as tools for APL physiopathology: Nomenclature of cells and repertory of their known genetic alterations and phenotypes. Oncogene. 2001;20:7287–7291. doi: 10.1038/sj.onc.1204863. [DOI] [PubMed] [Google Scholar]
- Tiscornia G. Singer O. Ikawa M. Verma I.M. A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNA. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1844–1848. doi: 10.1073/pnas.0437912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiscornia G. Singer O. Verma I.M. Design and cloning of lentiviral vectors expressing small interfering RNAs. Nat. Protoc. 2006;1:234–240. doi: 10.1038/nprot.2006.36. [DOI] [PubMed] [Google Scholar]
- Tiscornia G. Singer O. Verma I.M. Design and cloning of an shRNA into a lentiviral silencing vector: Version A. CSH Protoc. 20082008 doi: 10.1101/pdb.prot5010. pdb.prot5009. [DOI] [PubMed] [Google Scholar]
- Zelent A. Guidez F. Melnick A., et al. Translocations of the RARα gene in acute promyelocytic leukemia. Oncogene. 2001;20:7186–7203. doi: 10.1038/sj.onc.1204766. [DOI] [PubMed] [Google Scholar]