Abstract
Expansion of hematopoietic stem cells (HSCs) is beneficial in settings where HSC numbers are limited, such as cord blood transplantation. The human homeobox transcription factor HOXB4 has been shown to enhance stem cell expansion in several experimental models. We have shown previously that HOXB4 overexpression in monkey CD34+ cells has a dramatic effect on expansion and engraftment of short-term repopulating cells. Here, we wished to compare the effects of HOXB4 and another candidate gene, NUP98-HOXA10hd (NA10hd). We used a competitive repopulation assay in pigtailed macaques to study engraftment of CD34+ cells modified with gammaretroviral HOXB4YFP or NA10hdGFP. We found that HOXB4YFP contributed more to early hematopoiesis (<30 days), whereas NA10hdGFP contributed more to later hematopoiesis. In each case, we observed two distinct peaks in engraftment of NA10hd-transduced cells, one within 20 days post transplant and another after 5–6 months. Analysis of CD14+, CD3+, and CD20+ subsets confirmed that higher percentages of cells of each lineage were derived from NA10hdGFP+ progenitors than from HOXB4YFP+ progenitors. In conclusion, we show that HOXB4 and NA10hd both have a significant impact on hematopoietic reconstitution; however, these effects are differential and therefore may offer complementary strategies for HSC expansion.
Watts and colleagues compare the effects of HOXB4 and NUP98-HOXA10hd on the expansion and engraftment of CD34+ cells. A competitive repopulation assay using CD34+ cells modified with gammaretroviral vectors expressing HOXB4YFP or NA10hdGFP was performed in pigtailed macaques. HOXB4YFP contributed more to early hematopoiesis whereas NA10hdGFP had a more significant impact at later stages.
Introduction
Hematopoietic stem cell (HSC) transplantation is a standard therapy for a variety of diseases that are unresponsive to alternative treatment options. For a large percentage of the population, especially minorities, availability of appropriate donors for allogeneic HSC transplantation is limited (Johansen et al., 2008); for this reason, alternative sources of HSCs, such as umbilical cord blood, are under investigation. Hematopoietic transplantation using umbilical cord blood offers the advantages of rapid accessibility and a potentially lower risk of graft-versus-host disease (Ballen, 2005); however, progress in the field is limited by the low number of cells in a single cord blood unit. Due to the relatively low cell dose, there is a risk of delayed engraftment and associated infectious complications (Laughlin et al., 2004; Rocha et al., 2004). Ex vivo expansion of cord blood cells prior to transplantation can increase cell numbers and should minimize the duration of severe cytopenia following transplantation, thus alleviating a number of early transplant-related complications (McNiece, 2004). As an alternative, most centers now use double cord blood unit transplant strategies. Although this technique has helped to overcome cell dose limitations, there continues to be delayed engraftment and immune reconstitution, and it is typical to see a single unit emerge as the dominant source of long-term hematopoiesis (Ballen et al., 2007). However, the benefits of ex vivo expansion of stem cells reach beyond cord blood transplantation; other applications include increasing the number of gene-modified cells in gene therapy protocols and boosting cell numbers for transplant following nonmyeloablative conditioning.
Historically, ex vivo expansion techniques have fallen into three broad categories: those using cytokines (Dorrell et al., 2000; Gupta et al., 2000), those implementing coculture with stroma and other supportive cells (Chute et al., 2002; Kawano et al., 2003), and those involving genetic modification with stem cell self-renewal genes. A particular challenge of these approaches is to maximize HSC self-renewal capacity and repopulating potential, yet minimize differentiation and accumulation of lineage-committed cells. Among the techniques listed above, genetic modification has resulted in the most robust expansion of engrafting cells; in particular, overexpression of the human homeobox transcription factor HOXB4 has been reported to promote expansion and self-renewal of long-term, multilineage HSCs without compromising differentiation in a murine model (Antonchuk et al., 2002).
We have shown previously that HOXB4 overexpression in primate CD34+ cells has a dramatic effect on expansion and engraftment of short-term repopulating cells (<7 weeks post transplant) and a significant, though less pronounced, effect on long-term repopulating cells (Zhang et al., 2006). More recently, it was reported that another gene in the HOX family, a nucleoporin 98–homeobox A10 fusion gene [NUP98-HOXA10 (NA10)] shows a much greater potency to expand mouse long-term repopulating cells than HOXB4. Moreover, this potent effect on HSC expansion (>1,000-fold in 7-day culture) was retained in a fusion in which the HOXA10 portion was restricted to only the 60-amino acid homeodomain portion (so-called NUP98-HOXA10hd or NA10hd). NA10hd-expanded HSC had full lymphomyeloid reconstituting capacity without any evidence of the lineage skewing associated with HOXB4 or the intact NUP98-HOXA10 fusions (Ohta et al., 2007). Based on these promising findings, in the current study we set out to directly compare the effects of HOXB4 and NA10hd in our nonhuman primate stem cell transplantation model.
Materials and Methods
Animals
Pigtailed macaques (Macaca nemestrina) were housed and cared for at the University of Washington Primate Research Center. All protocols were approved by the Institutional Animal Care and Use Committee of the Fred Hutchinson Cancer Research Center and the University of Washington. For 5 days, macaques were given human granulocyte colony-stimulating factor (G-CSF) once daily at 100 μg/kg, administered as a subcutaneous injection. After 5 days, bone marrow was harvested from the femora. All animals were conditioned with fractionated, myeloablative total body irradiation of 1,020 cGy delivered by linear accelerator. G-CSF was administered daily until the animals began to engraft, defined as absolute neutrophil count (ANC) of >1,000/μl for 3 consecutive days. Other standard supportive care included transfusions, fluid and electrolytes, and antibiotics. Hematopoietic recovery was monitored by daily complete blood counts. A total of three macaques were transplanted and followed for this study.
Retrovirus preparation
The generation of Phoenix GALV-pseudotyped MSCV-NA10hd-ires-GFP and MSCV-HOXB4-ires-YFP viral vectors has been described previously (Antonchuk et al., 2001; Pineault et al., 2004; Zhang et al., 2006). Virus titers were assayed on HT1080 cells, and titers were obtained in the range of 1×105 to 2×105 IU/ml. Vector supernatant was filtered through a 0.22-μm filter and frozen at −80°C until used for transduction. The use of two different markers, green fluorescent protein (GFP) and yellow fluorescent protein (YFP), allows for a competitive repopulation approach; cells from peripheral blood or bone marrow can easily be analyzed by flow cytometry for the presence of GFP+ and YFP+ cells.
Transduction and expansion of CD34+ cells
CD34+ cells were enriched from bone marrow using the 12.8 IgM anti-CD34 antibody and IgM microbeads (Miltenyi Biotec, Auburn, CA). After CD34 enrichment, the average purity was 87% (range: 80–90%) (Table 1). CD34+ cells were split into two equal fractions for gene transduction and ex vivo expansion. Cells were cultured in Iscove's modified Dulbecco's medium supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 100 ng/ml each of recombinant human stem cell factor, recombinant human Fms-like tyrosine kinase 3 ligand, thrombopoietin, and G-CSF for 2 days of prestimulation prior to transduction. Transduction was carried out on flasks coated with the CH-296 fragment of fibronectin (Retronectin, Takara, Shiga, Japan) and consisted of two 4-hr viral exposures (one each day for 2 consecutive days).
Table 1.
Preinfusion Data for All Animals Involved in Study
| |
Animal |
|
||
|---|---|---|---|---|
| M06067 | K07063 | M07053 | Average | |
| Vectors used | MSCV-NA10hd-ires-GFP | MSCV-NA10hd-ires-GFP | MSCV-NA10hd-ires-GFP | |
| MSCV-HOXB4-ires-YFP | MSCV-HOXB4-ires-YFP | MSCV-HOXB4-ires-YFP | ||
| % CD34 after enrichment | 90 | 80 | 90 | 87 |
| No. of CD34-enriched cells, day 0 | 4×107 | 4×107 | 4×107 | 4×107 |
| Transduction efficiency | 19% GFP+ | 13% GFP+ | 12% GFP+ | 15% GFP+ |
| 27% YFP+ | 22% YFP+ | 16% YFP+ | 22% YFP+ | |
| Fold expansion, GFP arm | 28 | 35 | 32 | 32 |
| Fold expansion, YFP arm | 26 | 35 | 28 | 30 |
| Weight at transplant (kg) | 3.0 | 3.3 | 3.0 | 3.1 |
| Cell dose per kg | 1.9×108 (GFP arm) | 2.1×108 (GFP arm) | 2.1×108 (GFP arm) | 2.0×108 (GFP arm) |
| 1.7×108 (YFP arm) | 2.1×108 (YFP arm) | 1.9×108 (YFP arm) | 1.9×108 (YFP arm) | |
| % GFP/YFP positive on day of transplant | 25% GFP+ | 16% GFP+ | 13% GFP+ | 18% GFP+ |
| 30% YFP+ | 24% YFP+ | 18% YFP+ | 24% YFP+ | |
| ANC<100/μl (days) | 7 | 0 | 1 | 2.7 |
| ANC<500/μl (days) | 10 | 2 | 2 | 4.7 |
For each animal, half of the cells were transduced with MSCV-NA10hd-ires-GFP using a 3-day transduction protocol and subsequently expanded for 6 additional days. The remaining cells were transduced with MSCV-HOXB4-ires-YFP and expanded for 6 additional days. On the day of transplant, these two fractions were pooled and infused into the recipient intravenously.
Colony-forming cell assays
Colony-forming cell assays were initiated in two-layer agarose in minimum essential medium, supplemented with 20% fetal bovine serum, 4 U/ml erythropoietin (Amgen, Thousand Oaks, CA), and 100 ng/ml each of stem cell factor, granulocyte-macrophage colony-stimulating factor, G-CSF, thrombopoietin, interleukin-3, and interleukin-6. After 12–14 days of incubation at 37°C, colonies of >50 cells were enumerated.
Flow cytometry
Flow cytometric analysis was used to detect the presence of GFP+ and YFP+ cells approximately twice per week. At least 20,000 events were analyzed per sample. Nontransduced cells from a control animal were used to determine gates for positive cells. Subset analysis was performed at intervals of 3 months and consisted of antibody labeling using phycoerythrin-conjugated anti-CD3, CD4, CD8, CD13, CD14, CD20, and CD34. All antibodies were purchased from Becton Dickinson (Becton, Dickinson and Company, San Jose, CA).
Taqman PCR analysis
Taqman quantitative real-time PCR analysis was used to confirm marking results obtained from flow cytometry. Genomic DNA was extracted using the QIAmp DNA blood kit (Qiagen, Valencia, CA). The primers and conditions used to identity GFP/YFP and actin have been described previously (Kurre et al., 2003). Negative controls consisted of DNA from normal, nontransduced cells as well as a reagent control. Positive controls consisted of serial dilutions of DNA, which were used to generate a standard curve.
Statistical analysis
Student's paired t tests were used for the analysis of experiments. For in vivo studies, we used days 1–30 to analyze early engraftment and days 31 onward to analyze mid to late engraftment.
Results
HOXB4-expanded cells contribute to early engraftment, whereas NA10hd-expanded cells contribute to later engraftment
Our objective was to directly compare the engraftment and gene marking potential of NA10hd- and HOXB4-expanded cells. We used a competitive repopulation assay in which each monkey received cells genetically modified with MSCV-NA10hd-ires-GFP and expanded for 6 days and cells genetically modified with MSCV-HOXB4-ires-YFP and expanded for 6 days. Expansion and transplantation data for these animals are summarized in Table 1. Of note, the table shows that there were no differences in the in vitro fold expansion of the GFP and YFP arms.
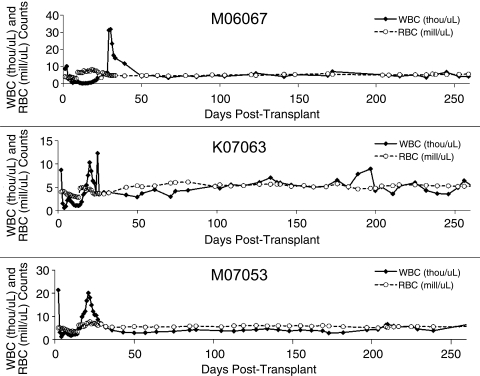
White blood cell (WBC) and red blood cell (RBC) counts were analyzed daily (Fig. 1). These data illustrate the kinetics of hematopoietic reconstitution following stem cell transplant. As the plots show, WBC and RBC counts stabilize by ∼6 weeks post transplant. Sharp drops in the WBC count characterize the plots from all three animals within the first few days. These nadirs are a result of the fractionated total body irradiation doses given during the 2 days prior to transplant. In addition, all three animals show occasional peaks in the WBC count during the first 30 days; these are due to administration of the colony-stimulating hormone G-CSF. In each animal, G-CSF was discontinued by day 30, because engraftment (defined as ANC >1,000/μl) had occurred.
FIG. 1.
WBC (thousand per microliter) and RBC (million per microliter) counts over time. In all three animals, WBC and RBC counts stabilize after ∼6 weeks post transplant. The initial sharp drop in WBC count is a result of the total body irradiation, whereas the occasional peaks in WBC count during the first 30 days are due to G-CSF administration. G-CSF was discontinued by day 30 in each animal.
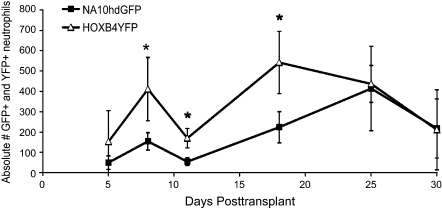
The average of the absolute number of GFP+ and YFP+ neutrophils in each macaque over the first 30 days following transplant was analyzed as well (Fig. 2). The absolute number of GFP+ and YFP+ cells was determined by multiplying the absolute neutrophil count on a specific day by the percentage of GFP+ or YFP+ cells on that same day, as determined by FACS. From these graphs, it can be concluded that HOXB4-expanded cells appear to make a significantly higher (p<0.04) contribution to early neutrophil engraftment than NA10hd-expanded cells.
FIG. 2.
Absolute number of gene-marked neutrophils in transplanted macaques. Absolute numbers of GFP+ cells and YFP+ cells were averaged at each time point for each animal (n=3). HOXB4YFP-expanded cells have a significant effect on early neutrophil engraftment, as compared with NA10hdGFP-expanded cells (*p<0.04).
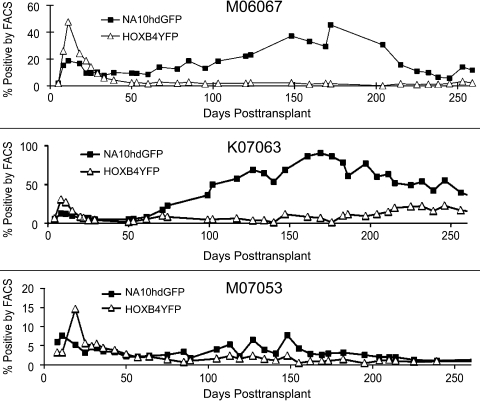
Illustrated in Fig. 3 are the overall marking levels in each of the three animals over the first 260 days of the study. A consistent trend is apparent among the three animals. In each case, HOXB4-expanded cells contribute to a significantly greater extent to early engraftment (<30 days) (p=0.01), whereas NA10hd-expanded cells show a significantly higher contribution to repopulation later after transplant (p=0.007). In each case, NA10hd-expanded cells contribute to two separate peaks in engraftment: one within the first 20 days, and a second around 5–6 months post transplant. Results from Taqman PCR (data not shown) confirm these results.
FIG. 3.
Percentage of NA10hdGFP+ and HOXB4YFP+ granulocytes. HOXB4YFP-expanded cells play a significantly greater role early after transplant (<30 days) (p=0.01), whereas NA10hdGFP-expanded cells play a significantly greater role later after transplant (p=0.007). In each case, NA10hdGFP-expanded cells contribute to two separate spikes in engraftment: one within the first 20 days, and a second around 5–6 months post transplant.
Effects of NA10hd and HOXB4 overexpression on hematopoietic subpopulations
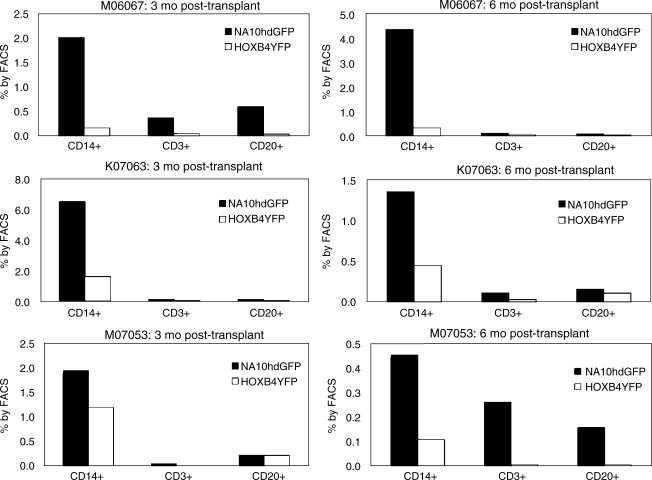
We speculated that NA10hd and HOXB4 may have a differential effect on the development of stem cells into specific hematopoietic subsets. Therefore, at 3 and 6 months post transplant, FACS subset analysis was performed to determine the percentage of NA10hdGFP+ and HOXB4YFP+ cells among CD14+ monocytes, CD3+ T cells, and CD20+ B cells (Fig. 4). The subset population percentages were normalized to CD34+ percentage at each time point to reflect differences in engraftment level. At 3 months and 6 months post transplant, GFP+ and YFP+ cells were present in cells of each of these lineages, except for the 3-month time point for macaque M07053, which showed 0% YFP+CD3+ cells. Flow cytometric analysis of these three phenotypes in peripheral blood show that NA10hd-expanded cells contribute to a greater degree than HOXB4-expanded cells among all three phenotypes, although in some cases these differences were minor.
FIG. 4.
Differential effects of NA10hd and HOXB4 overexpression on myeloid and lymphoid engraftment. Flow cytometric analysis of CD14+, CD3+, and CD20+ cells in peripheral blood at 3 and 6 months post transplant shows that NA10hd contributes to a greater degree in cells of all three phenotypes. Values have been indexed to CD34 percentage to indicate engraftment level.
The percentage of gene-marked CD34+ cells in the bone marrow was also analyzed (data not shown). Here, the percentage of CD34+ cells that were NA10hdGFP+ was higher than the percentage of CD34+ cells that were HOXB4YFP+ in all three animals at both time points (3 months and 6 months). At the 3-month time point, GFP+CD34+ percentages ranged from 1% to 12% (average 7.0%) and YFP+CD34+ percentages ranged from 1% to 10% (average 5.7%). Furthermore, the percentages of both GFP+CD34+ and YFP+CD34+ increased in the bone marrow between the 3-month time point and the 6-month time point, which coincided with the increase in marked granulocytes over time (Fig. 3). At 6 months post transplant, GFP+CD34+ percentages ranged from 11% to 27% (average 18.7%) and YFP+CD34+ percentages ranged from 4% to 12% (average 8.0%).
Effects on multilineage hematopoietic reconstitution
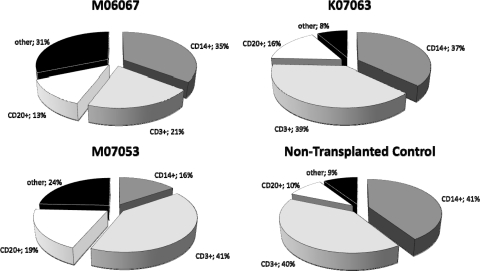
We compared the contributions of CD14+, CD3+, and CD20+ cells to overall hematopoiesis in M06067, K07063, and M07053 at 6 months post transplant and compared these with a nontransplanted control animal (Fig. 5). Results showed that, overall, the relative contributions of these subsets appeared to be reasonably normal. We observed that K07063 demonstrated the hematopoietic profile that most closely matched that of a normal macaque, with each subpopulation being within 6% of normal. In regard to the CD14+ population, M07053 appeared to be most divergent from normal, with only 16% of cells staining CD14+ (versus 41% in the control). Conversely, in terms of CD3+ percentage, M06067 was most aberrant, with 21% CD3+ cells (compared with 40% CD3+ in control). CD19 percentages were relatively consistent among all animals (between 10% and 19%).
FIG. 5.
Percentage of lymphoid (CD3+ and CD20+) and myeloid (CD14+) cells in transplanted animals compared with nontransplanted control. Data were obtained from peripheral blood stains of M06067, K07063, and M07053 at 6 months post transplant.
Discussion
Here we show that HOXB4- and NA10hd-expanded repopulating cells have distinct hematopoietic engraftment kinetics following transplantation, with HOXB4-expanded cells contributing more to early engraftment (1–4 weeks) and NA10hd-expanded cells contributing more to later-term engraftment (3–6 months). Furthermore, two distinct peaks in marking levels define the contribution of NA10hd-overexpressing cells; the first is evident within the first 3 weeks following transplant, and the second rise in gene marking appears around 5–6 months post transplant. In addition, we showed that NA10hd-expanded cells contribute more significantly to hematopoietic subsets.
At 3 months and 6 months post transplant, we observed the presence of GFP+ and YFP+ cells in each of the three subpopulations studied: CD14+, CD3+, and CD20+. The only circumstance in which gene-marked cells were not observed was in the YFP arm of the CD3 population of M07053 at the 3-month time point, although it is likely that these cells were present, only at a level too low to be detected with our instruments. Analysis of these three phenotypes in peripheral blood showed that NA10hd-expanded cells contribute to a greater degree than HOXB4-expanded cells among all three phenotypes. Furthermore, analyses of the percentage of GFP+ and YFP+ cells in bone marrow CD34+ cells were in agreement with the trends seen in overall gene marking (Fig. 3) and marking in subsets (Fig. 4). Our data showed that a higher percentage of CD34+ cells were NA10hdGFP+ than HOXB4YFP+ at both time points; in addition, the percentage of gene-marked cells in both arms increased over time with a direct correlation to overall gene marking, thus indicating that true repopulating cells were transduced. Analysis of the hematopoietic composition of each of the three animals compared with a control, nontransplanted macaque illustrated that the percentages of CD3+, CD20+, and CD14+ cells were relatively similar to normal, particularly in K07063. We suspect that variations in the percent contribution of different subsets is most likely due to individual variability, as it is not unexpected to see inherent differences in hematopoietic makeup particularly within the first 6–12 months after transplant as hematopoiesis is returning to baseline levels.
Our results comparing HOXB4 and NA10hd in the nonhuman primate model vary from those published by Ohta and colleagues (Ohta et al., 2007) in the murine transplantation model. This group reported a dramatic increase in short-term repopulating potential of NA10hd compared with HOXB4. They observed a >2,000-fold increase in competitive repopulating units (CRU) among NA10hd-overexpressing cells compared with only an 80-fold increase in CRU among HOXB4-overexpressing cells in 10-day cultures. In contrast, we found comparable short-term expansion potential of these two genes, as shown in Fig. 2. In addition, to achieve equivalent levels of reconstitution, 100-fold higher doses of HOXB4-overexpressing cells were required in the murine model. On the other hand, we found that transplantation of comparable doses of HOXB4- and NA10hd-overexpressing cells yielded higher short-term engraftment of HOXB4 cells. NA10hd-overexpressing cells certainly played an important role, but not until later after transplantation (after the first month). Furthermore, Ohta et al. reported that NA10 overexpression (compared with HOXB4 overexpression) appeared to block terminal differentiation, resulting in sustained production of cells with the primitive phenotype. However, we show that a greater proportion of lineage-committed cells (CD14+, CD3+, and CD20+) originate from NA10hd-transduced cells as compared with HOXB4-transduced cells (Fig. 4).
Our results are particularly relevant because our model is of high clinical significance. Inherent differences in the biology of murine and primate stem cells may limit the translational potential of murine studies (Mestas and Hughes, 2004). In addition, the shorter life span of mice prevents long-term follow-up of these animals. As we have shown, the engraftment contribution of NA10hd-transduced cells and HOXB4-transduced cells varies over time; thus, studies such as these in murine models would likely not be possible. It is also relevant to mention that the data presented here are in agreement with our previous findings with HOXB4 on short- and long-term nonhuman primate repopulating cells (Zhang et al., 2006).
It is important to recognize that, in the current study, we did not include a “control” cell population transduced with a reporter gene but lacking a gene with a competitive advantage. We intentionally selected this experimental design because we have previously compared the effects of a HOXB4-expanded cell population versus a control population (Zhang et al., 2006). In this previous study, we showed that HOXB4 overexpression in primate CD34+ cells has a significant effect on expansion and engraftment of short-term repopulating cells (<7 weeks post transplant) and a less pronounced effect on long-term repopulating cells. Thus, it was our intent to expand upon our previous studies and compare HOXB4 and NA10hd. We would also like to emphasize that it was not our intention to determine whether or not HOXB4 or NA10hd treatment shortened the time to neutrophil or platelet recovery. This question was more closely addressed in our 2006 work (Zhang et al., 2006) described above. Comparing the recovery kinetics of HOXB4-modified cells, NA10hd-modified cells, and control cells would involve a much more complicated three-arm competitive repopulation study, which was outside the scope of our current study. Here, it was our objective to examine the effects on hematopoietic repopulating cells, and not the kinetics of engraftment.
We recently showed a predisposition to leukemia in a large-animal model after gene modification using a HOXB4-expressing retroviral vector (Zhang et al., 2008). Based on our previous studies, as well as those of others (Hacein-Bey-Abina et al., 2008), we recognize and understand that there are inherent risks in using retrovirally-transduced cells in a clinical setting. However, our intent here was to compare the effects of HOXB4 and NA10hd and, for technical reasons, we selected retroviral-mediated gene transfer as the means by which to accomplish this goal. In the future, it is our hope that similar experiments can be conducted using a nonintegrating approach, such as HOXB4 or NA10 protein-mediated expansion or adenoviral vector-mediated expansion. In short, we stress that it is our objective to present a model, and by no means do we suggest that this paradigm is ready for direct clinical translation. More thorough, long-term follow-up would certainly be required before such a step could be taken.
In conclusion, we have shown that HOXB4-mediated expansion of CD34+ cells contributes to short-term engraftment, whereas NA10hd-mediated expansion contributes to mid-term engraftment. However, the percentage of NA10hdGFP+ cells appears to decline after 6 months post transplant, implying that the influence of NA10hd decreases with time. This indicates that neither HOXB4 nor NA10hd efficiently transduces long-term repopulating cells but, rather, both improve short-term engraftment and thus provide temporary support until long-term repopulating cells take over. Consequently, HOXB4-expanded cells may play an important role in preventing severe neutropenia after myeloablative stem cell transplantation, and NA10hd-expanded cells may enhance mid-term engraftment at 3–6 months after transplantation. This work offers potential in settings involving limited number of cells, such as cord blood transplantation. Given that clinical cord blood transplantation is characterized by delayed engraftment, HOXB4 and NA10hd may play complementary roles during hematopoietic recovery. Furthermore, this strategy can also be used to boost cell numbers in gene therapy protocols and in regimens involving nonmyeloablative conditioning.
Acknowledgments
We would like to thank Veronica Nelson and the staff at the University of Washington National Primate Research Center for assistance with the animals used in this study. We would also like to thank Bonnie Larson and Helen Crawford for their help in preparing the manuscript. This work was supported by the following grants: Canadian Institute of Health Research Team Grant in Stem Cell Expansion CTP79856, National Institutes of Health (NIH) grant R01HL084345 (H.P.K.), and the Core Center of Excellence in Hematology NIH grant P30DK056465 (H.P.K.). Hans-Peter Kiem is a Markey Molecular Medicine Investigator and the recipient of the José Carreras/E. Donnall Thomas Endowed Chair for Cancer Research.
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- Antonchuk J. Sauvageau G. Humphries R.K. HOXB4 overexpression mediates very rapid stem cell regeneration and competitive hematopoietic repopulation. Exp. Hematol. 2001;29:1125–1134. doi: 10.1016/s0301-472x(01)00681-6. [DOI] [PubMed] [Google Scholar]
- Antonchuk J. Sauvageau G. Humphries R.K. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell. 2002;109:39–45. doi: 10.1016/s0092-8674(02)00697-9. [DOI] [PubMed] [Google Scholar]
- Ballen K.K. New trends in umbilical cord blood transplantation. Blood. 2005;105:3786–3792. doi: 10.1182/blood-2004-10-4125. [DOI] [PubMed] [Google Scholar]
- Ballen K.K. Spitzer T.R. Yeap B.Y., et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol. Blood Marrow Transplant. 2007;13:82–89. doi: 10.1016/j.bbmt.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chute J.P. Saini A.A. Chute D.J., et al. Ex vivo culture with human brain endothelial cells increases the SCID-repopulating capacity of adult human bone marrow. Blood. 2002;100:4433–4439. doi: 10.1182/blood-2002-04-1238. [DOI] [PubMed] [Google Scholar]
- Dorrell C. Gan O.I. Pereira D.S., et al. Expansion of human cord blood CD34+CD38− cells in ex vivo culture during retroviral transduction without a corresponding increase in SCID repopulating cell (SRC) frequency: dissociation of SRC phenotype and function. Blood. 2000;95:102–110. [PubMed] [Google Scholar]
- Gupta P. Oegema T.R.J. Brazil J.J., et al. Human LTC-IC can be maintained for at least 5 weeks in vitro when interleukin-3 and a single chemokine are combined with O-sulfated heparan sulfates: requirement for optimal binding interactions of heparan sulfate with early-acting cytokines and matrix proteins. Blood. 2000;95:147–155. [PubMed] [Google Scholar]
- Hacein-Bey-Abina S. Garrigue A. Wang G.P., et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen K.A. Schneider J.F. McCaffree M.A. Woods G.L. Efforts of the United States' National Marrow Donor Program and Registry to improve utilization and representation of minority donors (Review) Transfus. Med. 2008;18:250–259. doi: 10.1111/j.1365-3148.2008.00865.x. [DOI] [PubMed] [Google Scholar]
- Kawano Y. Kobune M. Yamaguchi M., et al. Ex vivo expansion of human umbilical cord hematopoietic progenitor cells using a coculture system with human telomerase catalytic subunit (hTERT)-transfected human stromal cells. Blood. 2003;101:532–540. doi: 10.1182/blood-2002-04-1268. [DOI] [PubMed] [Google Scholar]
- Kurre P. Morris J. Thomasson B., et al. Scaffold attachment region-containing retrovirus vectors improve long-term proviral expression after transplantation of GFP-modified CD34+ baboon repopulating cells. Blood. 2003;102:3117–3119. doi: 10.1182/blood-2003-03-0962. [DOI] [PubMed] [Google Scholar]
- Laughlin M.J. Eapen M. Rubinstein P., et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N. Engl. J. Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- McNiece I. Ex vivo expansion of hematopoietic cells. Exp. Hematol. 2004;32:409–410. doi: 10.1016/j.exphem.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Mestas J. Hughes C.C. Of mice and not men: differences between mouse and human immunology (Review) J. Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Ohta H. Sekulovic S. Bakovic S., et al. Near-maximal expansions of hematopoietic stem cells in culture using NUP98-HOX fusions. Exp. Hematol. 2007;35:817–830. doi: 10.1016/j.exphem.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineault N. Abramovich C. Ohta H. Humphries R.K. Differential and common leukemogenic potentials of multiple NUP98-Hox fusion proteins alone or with Meis1. Mol. Cell. Biol. 2004;24:1907–1917. doi: 10.1128/MCB.24.5.1907-1917.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha V. Labopin M. Sanz G., et al. Acute Leukemia Working Party of European Blood Marrow Transplant Group; Eurocord-Netcord Registry. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N. Engl. J. Med. 2004;351:2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- Zhang X.-B. Beard B.C. Beebe K., et al. Differential effects of HOXB4 on nonhuman primate short- and long-term repopulating cells. PLoS Med. 2006;3:e173. doi: 10.1371/journal.pmed.0030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.-B. Beard B.C. Trobridge G.D., et al. High incidence of leukemia in large animals after stem cell gene therapy with a HOXB4-expressing retroviral vector. J. Clin. Invest. 2008;118:1502–1510. doi: 10.1172/JCI34371. [DOI] [PMC free article] [PubMed] [Google Scholar]