Abstract
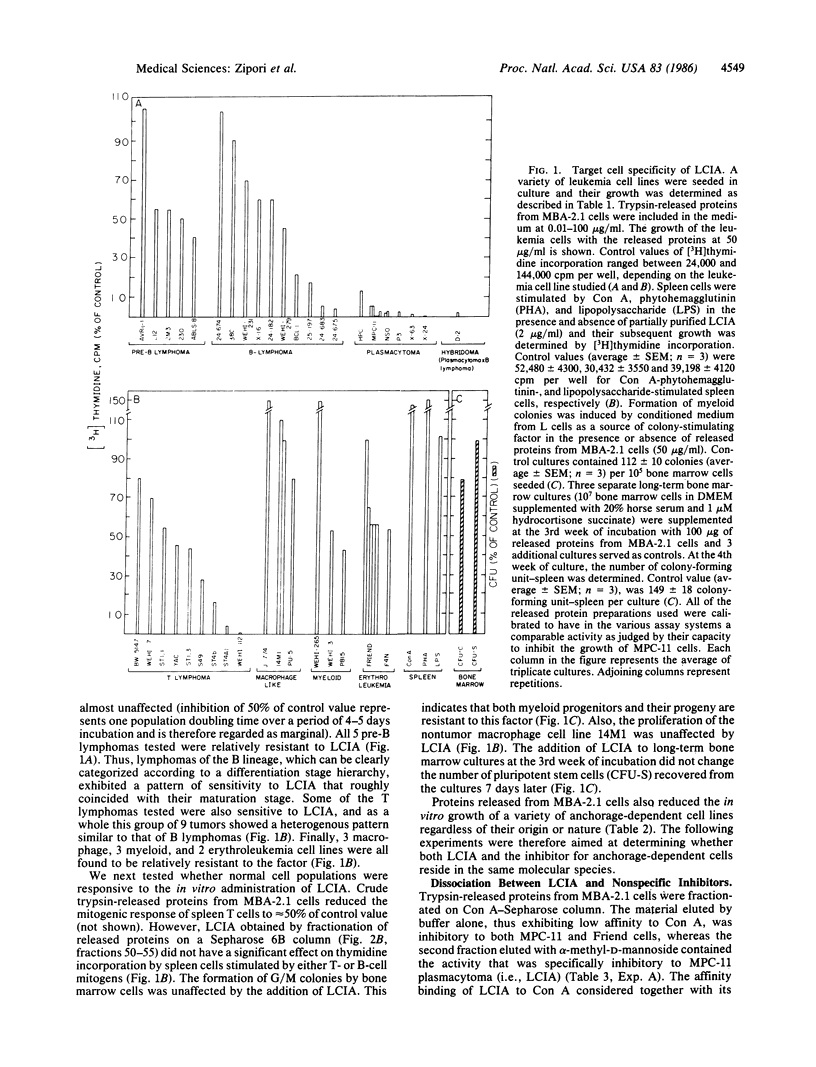
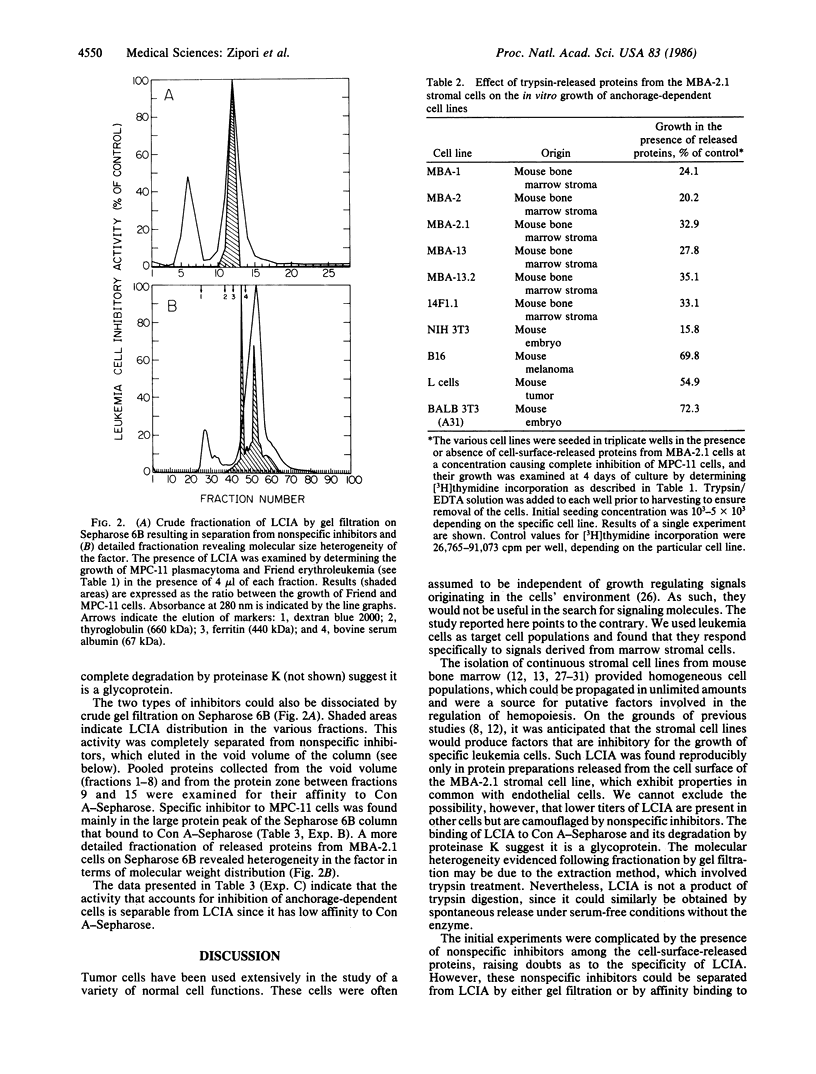
We have examined the mechanism by which stromal cells from the microenvironment of the bone marrow restricted the in vitro growth of certain hemopoietic tumors. A series of leukemia cell lines was used to monitor biological activities of stromal cell lines representing five distinct subtypes. Only an endothelial-like clone derived from mouse stroma (MBA-2.1) was consistently found to produce a cell-surface-associated glycoprotein that selectively inhibited the growth of plasmacytomas. The factor, designated leukemia cell inhibitory activity (LCIA), was not detected in anchorage-dependent cells of nonhemopoietic origin. Tumors of the lymphoid lineage and plasmacytomas in particular were the most sensitive to LCIA. Myeloid, macrophage, and erythroleukemia tumors were resistant to the factor, as were normal hemopoietic target cells including pluripotent stem cells, myeloid progenitor cells, and mitogen-stimulated spleen cells. Fractionation of trypsin-released proteins from MBA-2.1 cells by gel filtration and affinity binding to concanavalin A-Sepharose revealed two types of inhibitors; one was the specific leukemia cell inhibitor (i.e., LCIA); the other, present at a lower titer, was non-target-cell specific. The high sensitivity of plasmacytomas to LCIA versus the resistance of normal stem cells may be utilized for selective elimination of plasma cell tumors from bone marrow inocula.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess A. W., Metcalf D. The nature and action of granulocyte-macrophage colony stimulating factors. Blood. 1980 Dec;56(6):947–958. [PubMed] [Google Scholar]
- Greenberger J. S., Sakakeeny M. A., Davis L. M., Moloney W. C., Reid D. Biologic properties of factor-independent nonadherent hematopoietic and adherent preadipocyte cell lines derived from continuous bone marrow culture. Leuk Res. 1984;8(3):363–375. doi: 10.1016/0145-2126(84)90076-6. [DOI] [PubMed] [Google Scholar]
- Harigaya K., Cronkite E. P., Miller M. E., Shadduck R. K. Murine bone marrow cell line producing colony-stimulating factor. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6963–6966. doi: 10.1073/pnas.78.11.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harigaya K., Handa H. Generation of functional clonal cell lines from human bone marrow stroma. Proc Natl Acad Sci U S A. 1985 May;82(10):3477–3480. doi: 10.1073/pnas.82.10.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. W., Sephton R. G. Transferrin promotion of 67Ga and 59Fe uptake by cultured mouse myeloma cells. Cancer Res. 1977 Oct;37(10):3634–3638. [PubMed] [Google Scholar]
- Haustein D., Marchalonis J. J., Harris A. W. Immunoglobulin of T lymphoma cells. Biosynthesis, surface representation, and partial characterization. Biochemistry. 1975 May 6;14(9):1826–1834. doi: 10.1021/bi00680a004. [DOI] [PubMed] [Google Scholar]
- Hyman R., Stallings V. Complementation patterns of Thy-1 variants and evidence that antigen loss variants "pre-exist" in the parental population. J Natl Cancer Inst. 1974 Feb;52(2):429–436. doi: 10.1093/jnci/52.2.429. [DOI] [PubMed] [Google Scholar]
- Kadouri A., Bohak Z. Factors affecting the production of plasminogen activator in cultures of normal fibroblasts. Dev Biol Stand. 1985;60:431–437. [PubMed] [Google Scholar]
- Kodama H. A., Amagai Y., Koyama H., Kasai S. A new preadipose cell line derived from newborn mouse calvaria can promote the proliferation of pluripotent hemopoietic stem cells in vitro. J Cell Physiol. 1982 Jul;112(1):89–95. doi: 10.1002/jcp.1041120114. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Lanotte M., Scott D., Dexter T. M., Allen T. D. Clonal preadipocyte cell lines with different phenotypes derived from murine marrow stroma: factors influencing growth and adipogenesis in vitro. J Cell Physiol. 1982 May;111(2):177–186. doi: 10.1002/jcp.1041110209. [DOI] [PubMed] [Google Scholar]
- Laskov R., Scharff M. D. Synthesis, assembly, and secretion of gamma globulin by mouse myeloma cells. I. Adaptation of the Merwin plasma cell tumor-11 to culture, cloning, and characterization of gamma globulin subunits. J Exp Med. 1970 Mar 1;131(3):515–541. doi: 10.1084/jem.131.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Genetic dissection of the control of normal differentiation in myeloid leukemic cells. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5554–5558. doi: 10.1073/pnas.74.12.5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCULLOCH E. A., TILL J. E. PROLIFERATION OF HEMOPOIETIC COLONY-FORMING CELLS TRANSPLANTED INTO IRRADIATED MICE. Radiat Res. 1964 Jun;22:383–397. [PubMed] [Google Scholar]
- Martin G. R. Teratocarcinomas and mammalian embryogenesis. Science. 1980 Aug 15;209(4458):768–776. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A. Autoinduction of differentiation in WEHI-3B leukemia cells. Int J Cancer. 1982 Dec 15;30(6):773–780. doi: 10.1002/ijc.2910300616. [DOI] [PubMed] [Google Scholar]
- Mintz B., Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski L., Reich E. Changes in malignant phenotype of a human carcinoma conditioned by growth environment. Cell. 1983 Jun;33(2):323–333. doi: 10.1016/0092-8674(83)90414-2. [DOI] [PubMed] [Google Scholar]
- Ralph P., Moore M. A., Nilsson K. Lysozyme synthesis by established human and murine histiocytic lymphoma cell lines. J Exp Med. 1976 Jun 1;143(6):1528–1533. doi: 10.1084/jem.143.6.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar M. D., Shevach E. M., Green I., Potter M. Transplantation and preliminary characterisation of lymphocyte surface markers of Abelson virus-induced lymphomas. Nature. 1975 Feb 13;253(5492):550–552. doi: 10.1038/253550a0. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Warner N. L., Moore M. A., Metcalf D. A transplantable myelomonocytic leukemia in BALB-c mice: cytology, karyotype, and muramidase content. J Natl Cancer Inst. 1969 Oct;43(4):963–982. [PubMed] [Google Scholar]
- Webb C. G., Gootwine E., Sachs L. Developmental potential of myeloid leukemia cells injected into midgestation embryos. Dev Biol. 1984 Jan;101(1):221–224. doi: 10.1016/0012-1606(84)90132-5. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Baltimore D. Preparation of syngeneic tumor regressor serum reactive with the unique determinants of the Abelson murine leukemia virus-encoded P120 protein at the cell surface. J Virol. 1979 Sep;31(3):776–784. doi: 10.1128/jvi.31.3.776-784.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipori D., Bol S. The role of fibroblastoid cells and macrophages from mouse bone marrow in the in vitro growth promotion of haemopoietic tumour cells. Exp Hematol. 1979 Apr;7(4):206–218. [PubMed] [Google Scholar]
- Zipori D. Cell interactions in the bone marrow microenvironment: role of endogenous colony-stimulating activity. J Supramol Struct Cell Biochem. 1981;17(4):347–357. doi: 10.1002/jsscb.380170406. [DOI] [PubMed] [Google Scholar]
- Zipori D. Conditions required for the inhibition of in vitro growth of a mouse myeloma cell line by adherent bone-marrow cells. Cell Tissue Kinet. 1981 Sep;14(5):479–488. doi: 10.1111/j.1365-2184.1981.tb00554.x. [DOI] [PubMed] [Google Scholar]
- Zipori D., Duksin D., Tamir M., Argaman A., Toledo J., Malik Z. Cultured mouse marrow stromal cell lines. II. Distinct subtypes differing in morphology, collagen types, myelopoietic factors, and leukemic cell growth modulating activities. J Cell Physiol. 1985 Jan;122(1):81–90. doi: 10.1002/jcp.1041220113. [DOI] [PubMed] [Google Scholar]
- Zipori D., Friedman A., Tamir M., Silverberg D., Malik Z. Cultured mouse marrow cell lines: interactions between fibroblastoid cells and monocytes. J Cell Physiol. 1984 Feb;118(2):143–152. doi: 10.1002/jcp.1041180206. [DOI] [PubMed] [Google Scholar]
- Zipori D. In vitro proliferation of mouse lymphoblastoid cell lines: growth modulation by various populations of adherent cells. Cell Tissue Kinet. 1980 May;13(3):287–298. doi: 10.1111/j.1365-2184.1980.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Zipori D., Toledo J., von der Mark K. Phenotypic heterogeneity among stromal cell lines from mouse bone marrow disclosed in their extracellular matrix composition and interactions with normal and leukemic cells. Blood. 1985 Aug;66(2):447–455. [PubMed] [Google Scholar]



