Summary
Clinical evidence suggests a potentially causal interaction between sleep and affective brain function; nearly all mood disorders display co-occurring sleep abnormalities, commonly involving rapid-eye movement (REM) sleep [1–4]. Building on this clinical evidence, recent neurobiological frameworks have hypothesized a benefit of REM sleep in palliatively decreasing next-day brain reactivity to recent waking emotional experiences [5, 6]. Specifically, the marked suppression of central adrenergic neurotransmitters during REM (commonly implicated in arousal and stress), coupled with activation in amygdala-hippocampal networks that encode salient events, is proposed to (re)process and de-potentiate previous affective experiences, decreasing their emotional intensity [3]. In contrast, the failure of such adrenergic reduction during REM sleep has been described in anxiety disorders, indexed by persistent high-frequency electroencephalographic (EEG) activity (>30Hz) [7–10]; a candidate factor contributing to hyper-arousal and exaggerated amygdala reactivity [3, 11–13]. Despite these neurobiological frameworks, and their predictions, the proposed benefit of REM sleep physiology in de-potentiating neural and behavioral responsivity to prior emotional events remains unknown. Here, we demonstrate that REM sleep physiology is associated with an overnight dissipation of amygdala activity in response to previous emotional experiences, altering functional-connectivity and reducing next-day subjective emotionality.
Results and Discussion
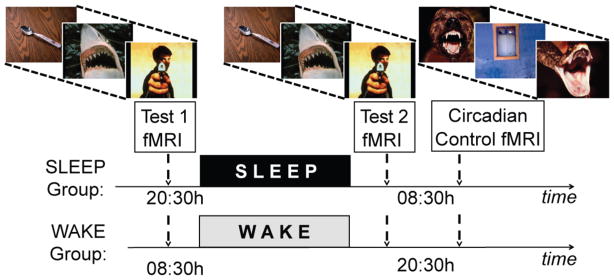
Building on the specific predictions of these neurobiological frameworks [3, 5, 6], and combining fMRI and EEG sleep recordings, we tested the hypothesis that (1) sleep decreases amygdala and behavioral reactivity in response to previously encountered emotional experiences, associated with re-established medial prefrontal cortex connectivity, and (2) that these brain and behavioral sleep benefits are proportional to the extent of decreased central adrenergic levels during REM sleep, as reflected by reduced gamma (30–40Hz) EEG activity; a validated proxy indexing reduced central adrenergic activity [7–10]. In short (and see Supplemental Experimental Procedures), thirty-four healthy adults (age: 18–30 years) were randomly assigned to one of two groups. Each performed two repeat fMRI tests (Test1, Test2), separated by 12hr containing a night of EEG-recorded sleep (Sleep-group, n=18, 10 females) or a waking day (Wake-group, n=16, 9 females; Figure 1). During each test, participants viewed and rated the subjective emotional intensity of 150 standardized affective pictures [14] on a 1–5 scale, corresponding to increasing intensity. Importantly, participants viewed the same stimuli at both test sessions, affording a measure of change in emotional reactivity to previously experienced affective stimuli (Test2 – Test1), following wake or sleep. Participants additionally performed a circadian control test at the second fMRI session, involving presentation of a novel set of affective stimuli (Supplemental Experimental Procedures). This control test allowed confirmation that behavioral and fMRI differences in reactivity identified following wake and sleep were independent of time-of-day (Supplemental Data).
Figure 1.
Experimental design: Both groups performed an emotion reactivity test twice inside the fMRI scanner; separated by 12hr, involving the rating and subsequent re-rating of the same standard set of 150 affective picture stimuli (three example images provided). The change in emotional reactivity following sleep (Sleep-group) or wake (Wake-group) was assessed by comparing data at Test1 (pre wake or sleep) with that at Test2 (post wake or sleep); Test2 – Test1. To examine possible time-of-day differences in emotional reactivity, independent of wake or sleep, an additional circadian control test was performed immediately after Test2 (morning in the Sleep-group, evening in the Wake-group). The circadian control test consisted of a novel set of 150 emotional images not seen before, matched in terms of arousal and valence to the original set used in Test1 and Test2 (sets used counterbalanced as either the experimental set or circadian control set).
Differences in amygdala reactivity
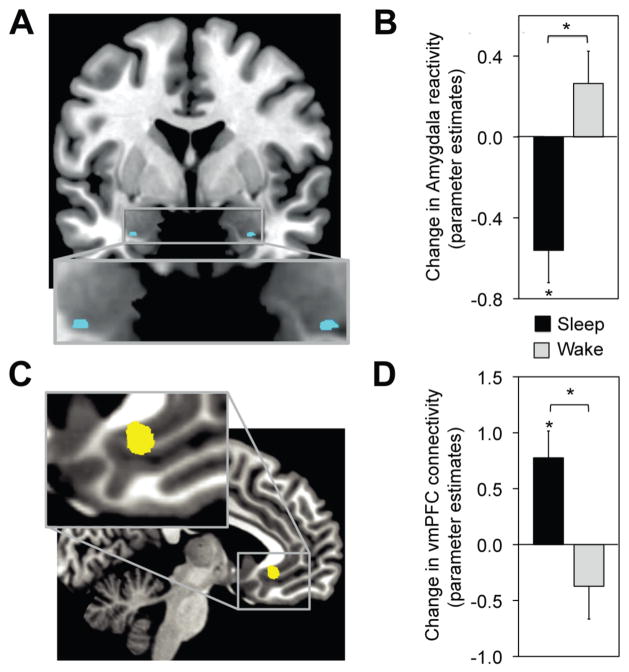
We first sought to determine the change in emotional brain reactivity following wake or sleep, focusing a priori on the amygdala [3, 5, 6]. Consistent with the experimental prediction, a significant Group (Wake, Sleep) × Test (Test1, Test2) interaction was observed in bilateral amygdala; revealing an overnight decrease in activity in the Sleep-group, yet increase across the day in the Wake-group (Figure 2a&b). Moreover, and consonant with the proposed function of top-down regulation [13, 15–21], these overnight reductions in amygdala activity were additionally associated with changes in ventromedial prefrontal cortex (vmPFC) functional connectivity. Specifically, a significant Group (Wake, Sleep) × Test (Test1, Test2) amygdala connectivity interaction was observed with the vmPFC (Figure 2c&d), expressing an overnight increase in the Sleep-group and converse decrease across the day in the Wake-group. Thus, a night of sleep decreased amygdala activity in response to previously encountered emotional stimuli. Furthermore, this overnight dissipation in amygdala activity was further associated with an increase in vmPFC connectivity.
Figure 2.
(A,B) fMRI differences in emotion reactivity: Group × Test session interaction in bilateral amygdala (blue), demonstrating a significant decrease in activity from Test1 to Test2 in the Sleep-group yet increase in the Wake-group (peak MNI coordinates [x, y, z]; left:−27, 0, −27; Z-score = 3.07; right: 27, 0, −27; Z-score = 3.14). (C,D) fMRI differences in functional connectivity: Group × Test session interaction in amygdala-vmPFC connectivity (yellow), demonstrating increased connectivity from Test1 to Test2 in the Sleep-group yet decreased coupling in the Wake-group (peak MNI coordinates [x, y, z]; −6, 30, −7; Z-score = 3.22). Differences in activation and connectivity thresholded at P < 0.05 family wise error (FWE) corrected for multiple comparisons. *P < 0.05, Error bars: S.E.M.
Change in subjective emotional reactivity
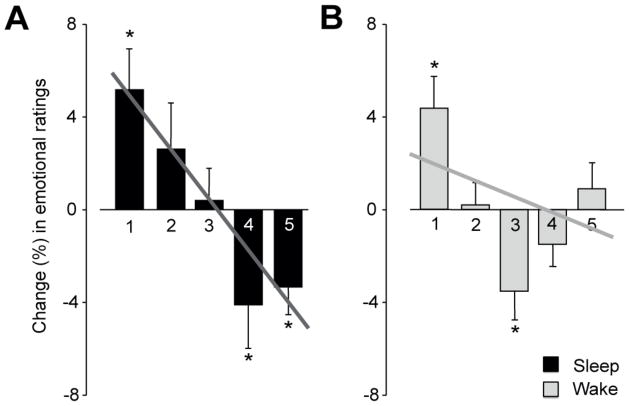
Next, we tested the prediction that these overnight decreases in amygdala responsivity were accompanied by a corresponding reduction in subjective emotional intensity ratings, specifically for the most intense emotional responses (5-ratings), where the greatest hypothesized benefit of sleep should occur. As with amygdala activity, a significant Group (Wake, Sleep) × Test (Test1, Test2) interaction was observed in intense emotional ratings (P < 0.05; Figure 3a&b), decreasing in the Sleep-group (P < 0.05) while increasing in the Wake-group. Indeed, within the Sleep-group, there was a significant linear shift in the profile of change across the 1–5 ratings (P < 0.001), with reductions in the most intense ratings (4’s and 5’s), and a progressive increase in neutral ratings (1’s and 2’s Figure 3a). In contrast, no significant linear trend or reductions in extreme emotional ratings (4’s and 5’s) were observed in the Wake-group (Figure 3b). Therefore, the overnight decrease in amygdala activity following sleep was additionally accompanied by a concomitant reduction in subjective emotional reactivity in response to these previously encountered affective stimuli.
Figure 3.
Change in behavioral reactivity between Test1 and Test2 for (A) the Sleep-group, expressing a significant linear shift (P < 0.001), and significant decrease in the most intense emotional ratings (4’s, 5’s) and increase in non-emotional ratings, (B) the Wake-group, resulting in no significant linear profile shift or decrease in the most intense emotional ratings. *P < 0.05, Error bars: S.E.M.
Associations with REM sleep physiology
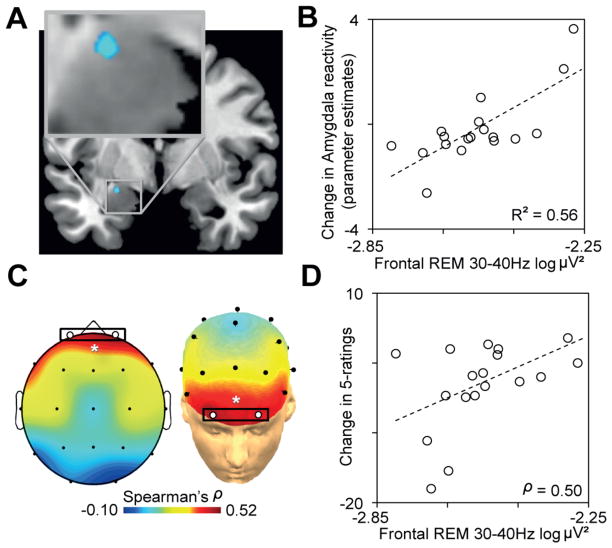
We finally sought to test the prediction that the overnight decreases in amygdala and behavioral reactivity in the Sleep-group were predicted by the extent of reduced REM sleep gamma EEG activity; a validated marker of decreased central adrenergic activity [7–10]. Analysis focused a priori on prefrontal EEG activity, based on this region’s dense adrenergic innervation [22, 23] and established role in emotion regulation [24, 25]. Consistent with this prediction, the extent of overnight decrease in both amygdala and behavioral reactivity was significantly correlated with the extent of reduced prefrontal gamma EEG activity during REM (Figure 4a–d), such that those with the lowest levels of REM-gamma (indicative of lowest central adrenergic activity [7–10]) expressed the largest overnight decrease in emotion reactivity. That this effect was unique to prefrontal REM-gamma was demonstrated by three additional analyses (Supplemental Data), describing specificity at the level of (1) topography – the strength of the predictive relationship between gamma activity and the change in both amygdala and behavioral reactivity decreased from anterior to posterior EEG derivations, (2) frequency – no other frequency band from these same prefrontal EEG derivations correlated with the change in amygdala activity or behavioral reactivity, and (3) brain-state – unlike REM sleep, no significant correlation was found between prefrontal gamma power during NREM sleep and the changes in emotional responsivity.
Figure 4.
(A) Pearson’s correlation between prefrontal EEG gamma power (average of Fp1–Fp2 EEG derivations) and the extent of overnight decrease in amygdala (blue) activity from Test1 to Test2 (peak MNI coordinates [x, y, z]; −22, −7, −17; Z-score = 3.55), and (B) corresponding scatter plot of this amygdala-gamma power relationship, with R2 noted only for descriptive purposes [46, 47], with less gamma activity predicting the degree of overnight decrease in emotional activity. (C) Topographical Spearman’s correlation (ρ) plot between REM gamma power and the change in emotional reactivity (5-ratings) demonstrating a significant prefrontal relationship (average of Fp1–Fp2, white circles), and (D) corresponding scatter plot and Spearman’s ρ value: the extent of reduced gamma EEG activity over prefrontal cortex was proportional to the overnight decrease in emotional reactivity. *P < 0.05.
Taken together, these findings describe an overnight de-potentiation of neural (amygdala) and behavioral (subjective) responsivity to previously encountered affective stimuli [3, 5, 6]. Moreover, the success of this de-potentiation was predicted by REM sleep gamma EEG activity, a surrogate marker indexing central adrenergic activity [7–10]. Our data can be interpreted within a recently proposed homeostatic model of REM sleep involving the reduction of emotional tone originally associated with waking salient experiences, orchestrated by the marked reduction in adrenergic activity during REM sleep [3, 5]. Alternatively, or in addition, such findings may be explained by the recognized benefit of REM on emotional memory consolidation [3, 26–28], associated with theta EEG activity [29, 30], thereby decreasing post-sleep stimulus novelty and hence emotion reactivity. That the changes in neural and behavioral reactivity reported in the current study correlated with REM gamma activity and not theta activity suggests that each component (de-potentiation, consolidation), while potential constituents of a broader function of REM [3], are conceivably distinct. Nevertheless, either mechanism independently, or their combination, may account for our findings, and represents a clear future target for experimental investigation.
Guided by recent neurobiological models [3, 5], the current study focused on the sleep-dependent differences in emotion reactivity within the central nervous system (specifically the brain). However, these models predict similar downstream adaptive reductions in reactivity with the peripheral nervous system. The consequential impact of such altered central nervous system processing on peripheral nervous system reactivity has potentially important implications, especially considering their respective efferent—afferent interactions known to supporting symbiotic emotional homeostasis [31].
Translationally, our results may afford mechanistic insights into a collection of affective disorders where amplified emotion reactivity and sleep disruption are highly co-morbid, particularly the anxiety disorders [1, 2, 4]. Of special relevance in this context is the condition of PTSD, characterized by REM abnormalities [11, 32–35], hyper-arousal [36–40] and exaggerated amygdala reactivity [41–43]. Indeed, the current findings offer a putative neurobiological explanation for the recent pharmacological treatment success involving nighttime suppression of adrenergic activity in PTSD, restoring REM sleep features and improving clinical symptomatology [12, 44, 45].
Supplementary Material
Highlights.
Sleep decreases amygdala activity to prior waking emotional experiences
The amygdala decrease is associated with re-established prefrontal connectivity
These neural changes are accompanied by overnight reductions in subjective reactivity
Reductions in both brain and behavioral reactivity are associated with REM physiology
Acknowledgments
This work was supported by National Institutes of Health: National Institute on Aging (RO1AG031164) and National Institute of Mental Health (R01MH093537). We thank Dr. Matthew Brett for helpful advice on fMRI analyses, and the research assistants involved in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49:651–670. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- 2.Benca RM, Okawa M, Uchiyama M, Ozaki S, Nakajima T, Shibui K, Obermeyer WH. Sleep and mood disorders. Sleep Med Rev. 1997;1:45–56. doi: 10.1016/s1087-0792(97)90005-8. [DOI] [PubMed] [Google Scholar]
- 3.Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135:731–748. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harvey AG. Sleep and circadian rhythms in bipolar disorder: seeking synchrony, harmony, and regulation. Am J Psychiatry. 2008;165:820–829. doi: 10.1176/appi.ajp.2008.08010098. [DOI] [PubMed] [Google Scholar]
- 5.Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- 6.Levin R, Nielsen TA. Disturbed dreaming, posttraumatic stress disorder, and affect distress: a review and neurocognitive model. Psychol Bull. 2007;133:482–528. doi: 10.1037/0033-2909.133.3.482. [DOI] [PubMed] [Google Scholar]
- 7.Maloney KJ, Cape EG, Gotman J, Jones BE. High-frequency gamma electroencephalogram activity in association with sleep-wake states and spontaneous behaviors in the rat. Neuroscience. 1997;76:541–555. doi: 10.1016/s0306-4522(96)00298-9. [DOI] [PubMed] [Google Scholar]
- 8.Cape EG, Jones BE. Differential modulation of high-frequency gamma-electroencephalogram activity and sleep-wake state by noradrenaline and serotonin microinjections into the region of cholinergic basalis neurons. J Neurosci. 1998;18:2653–2666. doi: 10.1523/JNEUROSCI.18-07-02653.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berridge CW, Foote SL. Effects of locus coeruleus activation on electroencephalographic activity in neocortex and hippocampus. J Neurosci. 1991;11:3135–3145. doi: 10.1523/JNEUROSCI.11-10-03135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keane PE, Candy JM, Bradley PB. The role of endogenous catecholamines in the regulation of electrocortical activity in the encephale isole cat. Electroencephalogr Clin Neurophysiol. 1976;41:561–570. doi: 10.1016/0013-4694(76)90001-8. [DOI] [PubMed] [Google Scholar]
- 11.Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev. 2008;12:169–184. doi: 10.1016/j.smrv.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D, Shofer J, O’Connell J, Taylor F, Gross C, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928–934. doi: 10.1016/j.biopsych.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 13.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang PJ. International Affective Picture System (IAPS): Technical manual and affective ratings. Gainesville, FL: University of Florida; 1997. [Google Scholar]
- 15.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 16.Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- 17.Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- 19.Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- 20.Sierra-Mercado D, Jr, Corcoran KA, Lebron-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci. 2006;24:1751–1758. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- 21.Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 24.Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows A coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58:275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 25.Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Wagner U, Hallschmid M, Rasch B, Born J. Brief sleep after learning keeps emotional memories alive for years. Biol Psychiatry. 2006;60:788–790. doi: 10.1016/j.biopsych.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 27.Wagner U, Gais S, Born J. Emotional Memory Formation Is Enhanced across Sleep Intervals with High Amounts of Rapid Eye Movement Sleep. Learn Mem. 2001;8:112–119. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Payne JD, Stickgold R, Swanberg K, Kensinger EA. Sleep preferentially enhances memory for emotional components of scenes. Psychol Sci. 2008;19:781–788. doi: 10.1111/j.1467-9280.2008.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popa D, Duvarci S, Popescu AT, Lena C, Pare D. Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proc Natl Acad Sci U S A. 2010;107:6516–6519. doi: 10.1073/pnas.0913016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishida, Pearsall, Buckner, Walker REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009;19:1158–1166. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- 32.Breslau N, Roth T, Burduvali E, Kapke A, Schultz L, Roehrs T. Sleep in lifetime posttraumatic stress disorder: a community-based polysomnographic study. Arch Gen Psychiatry. 2004;61:508–516. doi: 10.1001/archpsyc.61.5.508. [DOI] [PubMed] [Google Scholar]
- 33.Habukawa M, Uchimura N, Maeda M, Kotorii N, Maeda H. Sleep findings in young adult patients with posttraumatic stress disorder. Biol Psychiatry. 2007;62:1179–1182. doi: 10.1016/j.biopsych.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Germain A, Buysse DJ, Nofzinger E. Sleep-specific mechanisms underlying posttraumatic stress disorder: integrative review and neurobiological hypotheses. Sleep Med Rev. 2008;12:185–195. doi: 10.1016/j.smrv.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yetkin S, Aydin H, Ozgen F. Polysomnography in patients with post-traumatic stress disorder. Psychiatry Clin Neurosci. 2010;64:309–317. doi: 10.1111/j.1440-1819.2010.02084.x. [DOI] [PubMed] [Google Scholar]
- 36.Nutt DJ. The psychobiology of posttraumatic stress disorder. J Clin Psychiatry. 2000;61(Suppl 5):24–29. discussion 30–22. [PubMed] [Google Scholar]
- 37.O’Donnell T, Hegadoren KM, Coupland NC. Noradrenergic mechanisms in the pathophysiology of post-traumatic stress disorder. Neuropsychobiology. 2004;50:273–283. doi: 10.1159/000080952. [DOI] [PubMed] [Google Scholar]
- 38.Krystal JH, Neumeister A. Noradrenergic and serotonergic mechanisms in the neurobiology of posttraumatic stress disorder and resilience. Brain Res. 2009;1293:13–23. doi: 10.1016/j.brainres.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strawn JR, Geracioti TD., Jr Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25:260–271. doi: 10.1002/da.20292. [DOI] [PubMed] [Google Scholar]
- 40.Frewen PA, Lanius RA. Toward a psychobiology of posttraumatic self-dysregulation: reexperiencing, hyperarousal, dissociation, and emotional numbing. Ann N Y Acad Sci. 2006;1071:110–124. doi: 10.1196/annals.1364.010. [DOI] [PubMed] [Google Scholar]
- 41.Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 42.Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto A, Gordon E, Bryant RA. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage. 2006;29:347–357. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 43.Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A, Gordon E, Williams LM. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Hum Brain Mapp. 2008;29:517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, Dobie DJ, Hoff D, Rein RJ, Straits-Troster K, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160:371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- 45.Shad MU, Suris AM, North CS. Novel combination strategy to optimize treatment for PTSD. Hum Psychopharmacol. 2011 doi: 10.1002/hup.1171. [DOI] [PubMed] [Google Scholar]
- 46.Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition (the paper formerly known as Voodoo Correlations in Social Neuroscience) Perspect Psychol Sci. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- 47.Poldrack RA, Mumford JA. Independence in ROI analysis: where is the voodoo? Soc Cogn Affect Neurosci. 2009;4:208–213. doi: 10.1093/scan/nsp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.