Abstract
Aims
Whether the selective serotonin reuptake inhibitor sertraline at 200 mg/day delays relapse in recently abstinent cocaine dependent individuals
Design
12-week, double blind, placebo-controlled clinical trial with 2-week residential stay followed by 10-wk outpatient participation
Setting
Veterans Affairs residential unit and outpatient treatment research program
Participants
Cocaine-dependent volunteers (N=86) with depressive symptoms (Hamilton score > 15), but otherwise no major psychiatric or medical disorder or contraindication to sertraline.
Measurements
Participants were housed on a drug-free residential unit (wks 1–2) and randomized to receive sertraline or placebo. Participants then participated on an outpatient basis during weeks 3–12 while continuing to receive study medication. Patients participated in a day substance abuse day treatment program during weeks 1–3 and underwent weekly cognitive behavioral therapy during weeks 4–12. The primary outcome measure was thrice-weekly urine results and secondary measure was Hamilton Depression scores.
Findings
Pre hoc analyses were performed on those who participated beyond week 2. Generally no group differences in retention or baseline characteristics occurred. Sertraline patients showed a trend toward longer time before their first cocaine-positive urine (“lapse,” χ2=3.67, p=0.056), went significantly longer before having two consecutive urine samples positive for cocaine (“relapse,” χ2=4.03, p=0.04) and showed significantly more days to lapse (26.1±3.2±10.5; z = 2.89, p=0.004) and relapse (21.3±10.8 vs 32.3±14.9; z=2.25, p=0.02). Depression scores decreased over time (F=43.43, p<0.0001), but did not differ between groups (F =0.09, p = 0.77).
Conclusions
Sertraline delays time to relapse relative to placebo in cocaine dependent patients who initially achieve at least two weeks of abstinence.
Keywords: cocaine dependence, randomized clinical trial, placebo control, sertraline, relapse prevention, cognitive behavioral therapy
INTRODUCTION
Cocaine dependence is a major US public health problem.[1–3] Problems associated with cocaine use include psychiatric disorders,[4] injection use, high-risk sexual behavior,[5] HIV and HCV transmission,[5] crime and fetal drug exposure.[6] Despite these compelling factors, no robustly effective pharmacotherapy for cocaine dependence has been developed.
Depressive symptoms are common among cocaine abusers[7–13] and have been associated with greater severity of cocaine dependence and impairment [13–17] as well as poor treatment outcome.[18–21] In addition, a lifetime history of depression is associated with greater self-reported cocaine withdrawal.[22] Although most well-controlled trials have had disappointing results with antidepressants in unselected cocaine-dependent patients,[23–32] antidepressants have shown some efficacy in treating depressed subgroups of cocaine-dependent patients [31, 33–36] (for exception [37]). These findings suggested that depressed cocaine dependent patients would most likely benefit from antidepressants.
In order to select an appropriate antidepressant for this patient subgroup, we tried to identify a serotonin reuptake inhibitor (SSRI) that might block both dopamine and serotonin reuptake, since these appear to be the most critical neurotransmitters involved in both depression and stimulant dependence. For example, chronic cocaine use is associated with dopaminergic [38–44] as well as serotonergic [42, 45] deficits. Moreover, protracted cocaine withdrawal can include about two weeks of depressive symptoms [46–48] and cessation of repeated cocaine administration in rats alters prolactin response to certain serotonin agonists, reflecting an alteration in serotonin function similar to that observed in depression. [49] These deficits after chronic cocaine administration suggest a neurobiological link to depression, because the dopaminergic system has also been implicated in depression. [50, 51] Meanwhile, because serotonin stimulates dopamine release, [52, 53] a serotonergic deficit, suggested as one primary abnormality in depression, [54–56] could also lead to a dopaminergic deficit. Consequently, administration of antidepressants that inhibit both dopamine and serotonin reuptake would modulate serotonergic and/or dopaminergic neurotransmission. Thus, we examined the efficacy of the selective serotonin reuptake inhibitor (SSRI) sertraline, [57] which has putative dopamine reuptake inhibition properties [58, 59] as well as no norepinephrine reuptake inhibition and sigma receptor blockade, [60–63] to prolong abstinence in recently abstinent cocaine dependent patients with depressive symptoms. Because deficits in serotonergic functioning are thought to underlie both depression [64, 65]and the expression of cocaine withdrawal,[66, 67] which might impact relapse, a relapse paradigm was employed such that participants abstained from cocaine during weeks 1–2 and time to reinstatement of cocaine use was determined.
METHODS
Participants
Eighty-six male and female cocaine-dependent individuals (ages 18–52, including 33 females, 61 African-Americans, 5 Hispanics, 19 Caucasians, and 1 other) seeking treatment for cocaine dependence were recruited from the greater West Haven area from December of 1999 through November of 2003 after giving informed consent to participate in this randomized clinical trial approved by the Yale Human Investigations Committee and the VA Connecticut Human Studies Subcommittee. Participants met the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria for cocaine dependence, as determined from the Structured Clinical Interview for DSM-IV (SCID), had urine toxicologies positive for cocaine or benzoylecgonine during the month prior to study entry, had a history of cocaine use being a minimum of 70 dimes during the preceding 3 months and at least one previous episode of using at least 1 gram within a five day period, and had a score of >15 on the Hamilton Depression Scale. Exclusions included medical conditions contraindicating use of sertraline (e.g., pregnancy or breastfeeding, liver enzyme levels greater than three times normal, or medications that might have a major interaction with sertraline), a current diagnosis of drug (nicotine excluded) or alcohol physical dependence (i.e., reported withdrawal symptoms upon abrupt cessation or, when dependence is suspected, inability to produce negative alcohol breath tests with no symptoms over at least 3 consecutive days), a history of major psychiatric disorder (psychosis, schizophrenia, or bipolar), ill health (e.g., major cardiovascular, renal, endocrine, hepatic disorder), current suicidality and an inability to read and understand the consent form. Women of childbearing age were included provided they had a negative urine pregnancy test, agreed to use adequate contraception to prevent pregnancy during the study and agreed to monthly pregnancy tests.
Design and Procedure
Ninety-four participants were initially determined as being eligible to participate in this 12-week randomized, double blind, clinical trial through a 1-week, centralized recruiting/screening procedure. Participants were admitted to research beds in the Quarterway House (QWH) at the Veterans Affairs Connecticut Healthcare System during weeks 1–2 and randomly assigned to receive either sertraline or placebo. Then participants transferred to the Outpatient Treatment Research Program at the West Haven Veterans Affairs Healthcare System and continued to receive sertraline or placebo during weeks 3–12. The data manager performed the randomization using a computerized urn randomization program, [68] balancing groups on age, sex and race. Only the research pharmacist and data manager were aware of the medication condition.
Starting in week 1, participants attended the 3-week VA Substance Abuse Day Treatment Program. During weeks 4–12, subjects participated in weekly 1-hour, individual cognitive behavioral therapy,[69] manual-driven as a controlled element of the study. The session also provided an opportunity for subjects to review critical issues and problem areas. During weeks 3–12, participants attended the outpatient treatment research program at least 3 days/wk to attend the Day Treatment Program (week 3) complete study tasks, undergo counselling (weeks 4–12) and receive study medication. Compliance with study requirements during weeks 3–12 was facilitated through the use of contingency management procedures, whereby subjects are given monetary compensation for clinic attendance and for returning take-home bottles. Participants could earn up to $250 for attending all scheduled appointments and returning blister packs. Supervised urines, self-reported adverse effects, and vital signs were obtained thrice weekly; mood and drug use self-reports were obtained once weekly. At the end of 12 weeks, patients were tapered off the study medication and referred to an appropriate treatment program.
Because cocaine abstinence must have been initiated and maintained during weeks 1–2, subjects had to submit urine samples negative for cocaine by the beginning of week 2 and were administratively discharged from the study if a urinalysis result became positive for cocaine or other drugs during this time. Subjects were discharged from the trial if they missed attending clinic to receive their weekly medication, missed three consecutive supervised urines or the investigator felt that subjects' health or wellbeing was threatened by continuation in the study. Subjects were withdrawn from the study and referred to an appropriate treatment program if pregnancy test was positive. Subjects were not discontinued for illicit drug use during the outpatient portion of the trial unless the investigators felt a safety issue for the subject was at hand. Subjects administratively discharged from the study were offered referral to a local treatment program.
Medication
During sertraline induction, patients received either placebo or an initial dose of 50 mg sertraline hydrochloride once daily via blue opaque capsules starting the day after admission to the residential unit (day 1 of week 1). This dose was gradually increased over a 3-week period until subjects received 200 mg once daily. [70] Riboflavin was added to the capsules to ascertain compliance. Subjects were then maintained on this dose for the duration of the trial. When subjects were transferred to the outpatient program, they were administered capsules once weekly, with take-home doses given in blister packs to take once a day for the rest of the week. Subjects were required to return take-home blister packs when they came in to receive their next weekly dose pack, for which they received monetary compensation. At the end of the 12 weeks, participants were tapered off sertraline or placebo over a five-day period.
Assessments
At intake, participants were interviewed using the Structured Clinical Interview for DSM-IV,[71] the Addiction Severity Index,[72] and the Cocaine Selective Severity Assessment (CSSA) [73, 74]. The clinician-rated version of the Hamilton Depression Scale (HAM-D) [75] was completed at screening, intake and weekly thereafter. After initial training by a physician investigator, experienced clinical research staff had to show 100% agreement on at least 3 co-rated interviews in order to administer the scale and participated in quarterly reviews to prevent drift. Supervised urine samples were obtained thrice weekly and tested for the presence of cocaine metabolite (benzoylecgonine) and other drugs using an Olympus AU 640 Emit system (Olympus America Inc., Melville, NY) with a cut-off concentration of 300 ng/ml. Self-report assessments of cocaine (e.g., days during which cocaine was used; dimes of cocaine used, with a dime roughly equivalent to $10 street worth or 100 mg of cocaine or crack cocaine) and other drug use were obtained on day1 of each week using analog scales and 7-day recall method instruments developed in previous studies.[76, 77] Participants were monitored at every visit for any adverse symptoms.
Compliance with taking medications was verified by inspecting a 15 ml aliquot of each urine sample under an ultraviolet light (long wave setting - 366 nm) in a dimly lit room for riboflavin fluorescence. The urine sample was considered positive (consistent with taking the study medication) if a bright fluorescence glow is observed while the sample is examined under the ultraviolet light.
Data Analyses
Because cocaine use beyond the residential portion of the trial was the primary study outcome and participants had to demonstrate two weeks of abstinence in order to continue in the study, only data from those participants who were retained in the study beyond the residential stay were used in the analyses (see figure 1). In addition, data from 8 participants were excluded from analyses (except for averse event reports) because their HAM-D scores did not meet inclusion criterion. Differences in baseline subject characteristics between treatment groups were determined using t test or its non-parametric analogue, Wilcoxon or Kruskal-Wallis test for continuous variables and Pearson Chi Square tests for categorical variables. All participants that dropped out of the study after entering the outpatient portion of the trial were considered treatment failures at the time they left the study even if they had submitted only cocaine-free urine samples during their study participation. Kaplan-Meier survival analysis was performed to test for differences in retention, time to lapse (i.e., first cocaine-positive urine sample) and time to relapse (two consecutive cocaine-positive urine samples) between treatment groups. Between-groups differences in number of days to lapse or relapse were determined using t-tests. Repeated measures mixed modelling was used to determine differential changes over time between groups in weekly self-reported alcohol (days) or cocaine (days, dimes) use and HAM-D score. For all analyses, p < 0.05 was used to infer statistical significance. SAS software (SAS System for Windows Version 9.2, SAS Institute Inc., Cary, NC, USA) was employed.
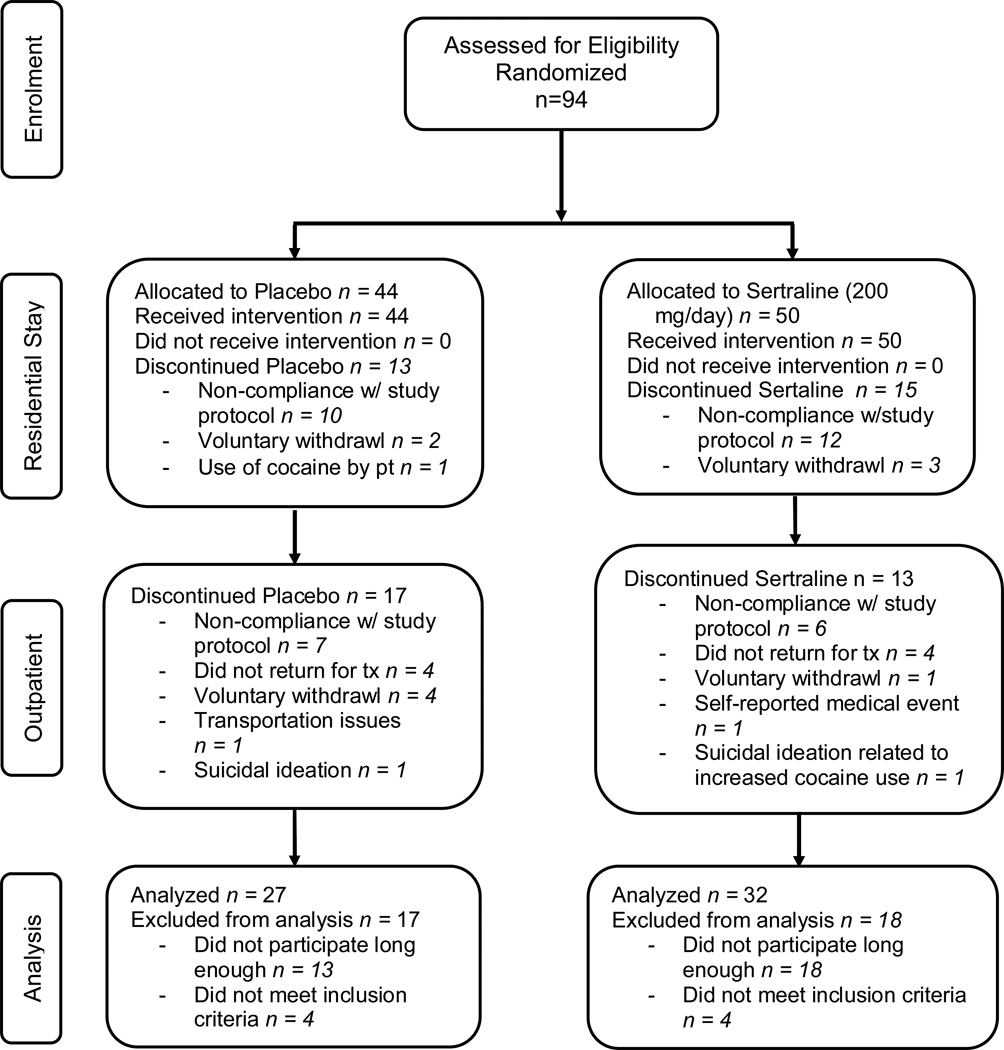
Figure 1.
Flow diagram of subject progress through the phases of the randomized clinical trial.
RESULTS
Retention, Adverse Events, Missing Data, Compliance
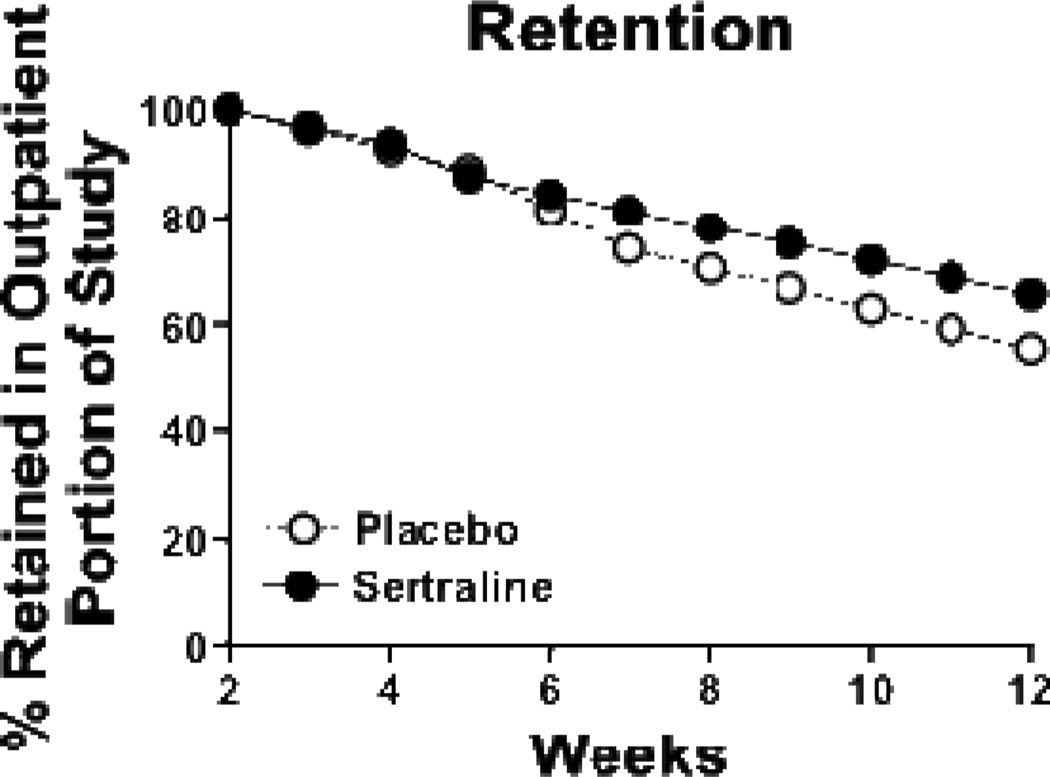
Of the 86 participants who were enrolled into the study proper and met inclusion criteria, 27 (31.4%) dropped out prior to the outpatient portion of the study irrespective of treatment group (χ2=0.04, df=1, p=0.84; figure 1). Those who dropped out had a significantly greater number of years of education than those who were retained through the residential stay (11.4 ± 2.9 yrs vs 8.7 ± 4.1 yrs; z=2.41, df=1, p=0.02), but no other significant baseline differences were observed. During the outpatient portion of the study, participant retention rates did not differ between treatment groups (χ2= 1.55, df=1, p=0.21; figure 2) with 34 (39.5% of intent-to-treat sample) participants completing the protocol. Fifty-two participants did not complete the protocol for the reasons specified in figure 1. Eight study-related adverse events occurred; that is, sexual dysfunction (N=2), symptoms suggestive of sertraline withdrawal during the taper (N=1), and suicidal ideation (N=5), 2 of which resulted in voluntary withdrawal from the study and admittance to a local treatment facility (figure 1).
Figure 2.
Weekly percentage of participants retained in each of the two treatment groups during the outpatient portion of the 12-week trial: placebo (open circles), sertraline (closed circles).
The mean number of missing urine samples during the outpatient portion of the trial out of the total possible (i.e., 30 urine samples) did not differ between placebo and sertraline groups (i.e., 6.9 (±9.8) versus 4.2 (±5.4); χ2=0.31, df=1, p=0.58). Essentially all urine samples showed some degree of fluorescence when inspected under UV light.
Baseline Characteristics
Of the 94 enrolled into the study proper, eight participants did not meet eligibility requirements and 27 did not participate beyond week 2 (Figure 1). Table 1 shows the demographic data for the 59 participants who were retained in the study beyond the residential portion of the trial. Groups generally did not differ in terms of subject characteristics, except for the number of days alcohol was ingested during the month prior to study entry; however, this did not appear to be clinically significant, particularly given that the prevalence of alcohol dependence did not differ across groups (table 1).
Table 1.
Summary of Group Characteristics
| Measure | Placebo (SD) |
Sertraline (SD) |
Test Statistic |
df | p |
|---|---|---|---|---|---|
| N | 27 | 32 | |||
| Age (yrs) | 38.8 (6.6) | 37.8 (5.2) | t=0.65 | 1 | 0.52 |
| Race (C/AA/H/O) | 6/20/0/1 | 10/19/3/0 | χ2=4.64 | 3 | 0.21 |
| Gender (% female) | 37.0 | 40.6 | χ2=0.08 | 1 | 0.78 |
| Education (years) | 8.5 (4.1) | 8.9 (4.2) | t=0.33 | 1 | 0.68 |
| Employment (%≥p/t)* | 44.4 | 43.8 | χ2=4.28 | 5 | 0.51 |
| Marital Status (% never married) | 63.0 | 62.5 | χ2=1.85 | 4 | 0.76 |
| HAM-D Score | 24.3 (6.3) | 24.3 (6.6) | t=0.00 | 1 | 1.00 |
| CSSA Score | 45.4 (14.2) | 42.4 (11.5) | t=0.88 | 1 | 0.38 |
| Current SCID Diagnoses (%) | |||||
| Cocaine Dependence | 100 | 100 | - | - | |
| Alcohol Dependence | 55.6 | 46.9 | χ2=0.51 | 2 | 0.60 |
| Depression** | 77.8 | 81.3 | χ2=0.11 | 2 | 0.74 |
| ASI Drug Use Data | |||||
| Cocaine Use (days/past 30d) | 21.0 (8.4) | 20.5 (8.2) | t=0.23 | 1 | 0.82 |
| Alcohol Use (days/past 30d) | 11.1 (9.3) | 7.0 (8.5) | t=1.77 | 1 | 0.03 |
Race: C, Caucasian; AA, African American; H, Hispanic; O, Other.
Employment: #≥p/t = number of subjects with part time or full time employment.
Several different categories of employment and marital status were analyzed; however, for simplicity only one category is presented.
Current major depressive disorder inclusive of substance-induced depressive disorder.
Bold p values highlight significant effect.
Cocaine and Alcohol Use During the Trial
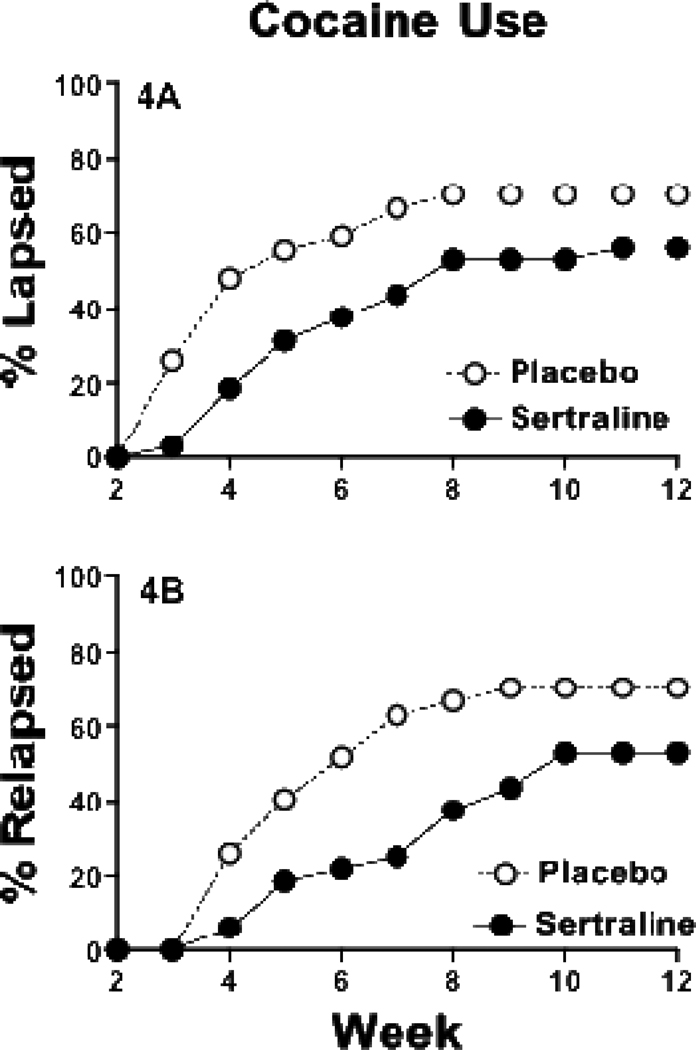
Nineteen (70.3%) and 18 (56.2%) participants experienced a lapse, while 19 (70.3%) and 17 (53.1%) experienced a relapse, in the placebo and sertraline groups, respectively (χ2=2.17, df=1, p=0.14 and χ2=3.23, df=1, p=0.07). Participants in the placebo group showed a trend toward lapsing more quickly over time (figure 3A) and relapsed (figure 3B) significantly more quickly over time relative to those receiving sertraline (χ2=3.67, df=1, p=0.056 and χ2=4.03, df=1, p=0.04, respectively). The mean time to lapse after leaving the residential treatment facility for the placebo group was 13.2 (±10.5) days compared to a mean time to lapse of 26.1 (±16.7) days for the sertraline group, a significant difference of almost two additional weeks (z = 2.89, df=1, p=0.004). There was also a significant difference between the placebo and sertraline groups in time to relapse (21.3±10.8 and 32.3±14.9 days, respectively; z=2.25, df=1, p=0.02).
Figure 3.
The percentage of participants who lapsed (i.e., first urine sample positive for cocaine; top panel) or relapsed (i.e., first two consecutive urine samples positive for cocaine; bottom panel) each week across the outpatient portion of the 12-week trial: placebo (open circles), sertraline (closed circles).
Self-reported cocaine use was quite low, with only 6 participants reporting having used cocaine, and no significant differences between groups were observed in terms of number dimes (F=0.79, df= 1, 53, p=0.38) or number of days used (F=0.58, df= 1, 53, p=0.45) during the previous week (data not shown). Additionally, there was no significant change over time in number of dimes used (F=1.23, df=9, 340, p = 0.28), or number of days used (F=1.13, df=9, 340, p=0.34) during the previous week (data not shown). Similarly, there was no significant difference between treatment groups in the self-reported number of days alcohol was used (F=3.06, df=1, 53, p=0.09; data not shown). Additionally, there was no significant change over time in self-reported number of days alcohol used (F=1.63, df= 1, 339, p=0.11; data not shown).
Depressive Symptoms
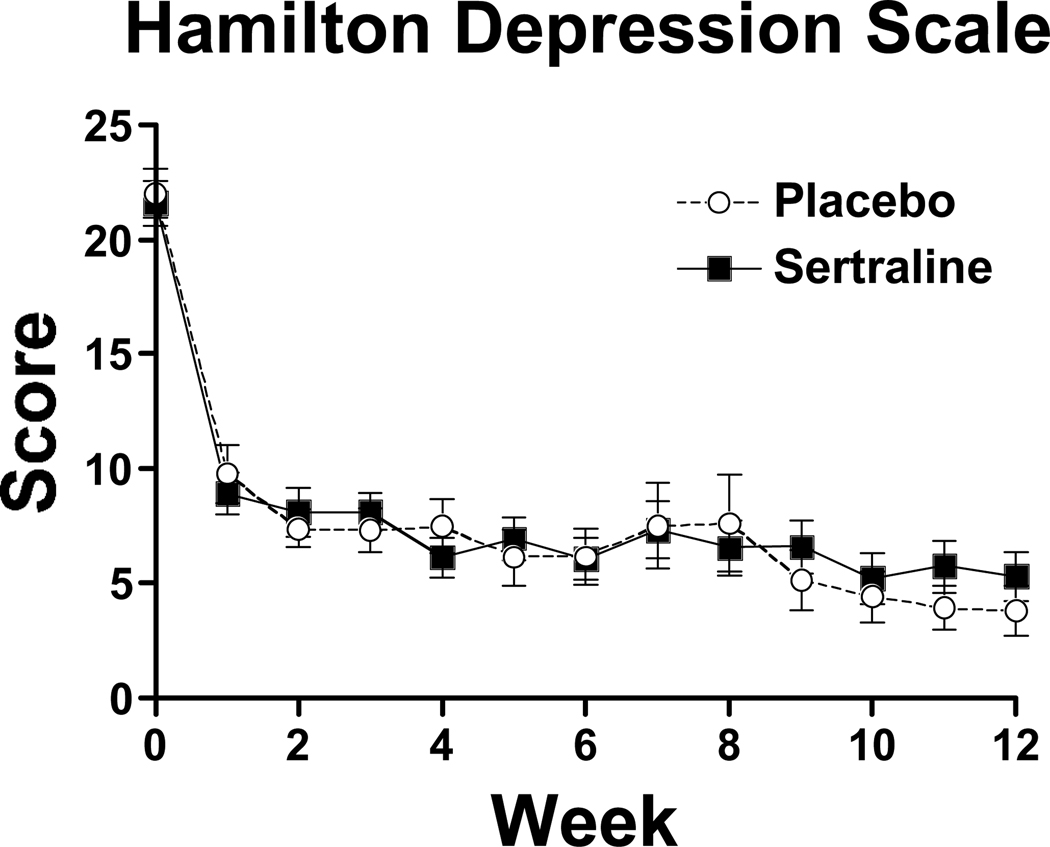
At baseline, severity of depressive symptoms did not differ between groups (table 1). Scores on the Hamilton Depression Scale significantly decreased over time during the 12-wk study (F = 43.43, df=12, 497, p<0.0001), but did not differ across groups (F =0.09, d=1, p = 0.77; figure 4). This decrease in depressive symptoms occurred during the two weeks of residential treatment, with depressive symptoms severity score remaining relatively consistent over time during the outpatient portion of the trial (F=1.49, df=10, 391, p=0.14).
Figure 4.
Weekly scores on the Hamilton Depression Scale during the 12-week trial: placebo (open circles), sertraline (closed circles). Each point represents the mean score across all participants for a given week. Bars represent standard deviation of the mean.
DISCUSSION
The findings of the present study suggest that sertraline delays relapse relative to placebo in recently abstinent cocaine-dependent individuals presenting with depressive symptoms. These results are consistent with those of a previous outpatient randomized, placebo-controlled clinical trial, in which the SSRI citalopram reduced cocaine use in cocaine dependent individuals.[78] In addition, preliminary data with the SSRI paroxetine suggested that it may reduce craving,[79] although data from well-controlled clinical trials are lacking. Sertraline showed initial promise to decrease craving in an open label pilot study[29]. However, sertraline administered at one-half the dose used in the present study did not reduce cocaine use relative to placebo in a CREST trial for cocaine dependence[80] or methamphetamine use relative to placebo in methamphetamine dependent individuals.[81] Overall, these findings suggest that sertraline at doses higher than 100 mg/day may be necessary for a therapeutic effect.
It has been suggested that SSRI’s are not efficacious for treating psychostimulant dependence. This appears to be mainly due to results of clinical trials with the SSRI fluoxetine that, although initially promising,[82–84] were not replicated under rigorous, double-blind, placebo controlled conditions,[24, 25, 28] even in depressed drug dependent populations.[37, 85, 86] The reasons for these mixed results are unclear, but may be due to methodological differences such as in study design. The present study used a relapse paradigm in which all participants were abstinent from cocaine for two weeks. Thus, medication was not initially administered during concomitant cocaine use. Indeed, Schmitz et al.[37] hypothesized that, because chronic cocaine use reduces serotonergic function, the efficacy of SSRI’s are compromised in active cocaine users; however, serotonergic function is restored after cessation of cocaine use. Thus, SSRI’s may have utility in recently abstinent cocaine dependent individuals.
Another reason for the mixed results could be the differing affinities of the SSRI’s for other receptor sites besides the serotonin transporter.[87] For instance, fluoxetine, a more potent inhibitor of 5-HT2C receptors than other serotonin reuptake inhibitors,[88] has activating properties that may produce insomnia and agitation in anxious patients[89, 90] through modulation of NE and DA systems.[91, 92] In contrast, citalopram is probably the most selective 5-HT reuptake inhibitor among the SSRIs with minimal activity at other receptors, except histamine H1 receptors.[87, 93] Meanwhile, sertraline has activity as a DA reuptake inhibitor in addition to its potent serotonin reuptake inhibitor properties.[58] Rothman et al.[94] recently postulated that agents with both serotonergic and dopaminergic based on evidence of a dual deficit in DA and 5-HT function during withdrawal from chronic cocaine abuse. Thus, sertraline, particularly in the context of recent abstinence from cocaine abuse, may have the type of pharmacological profile that would be efficacious in treating cocaine dependence.
On the other hand, there was no significant difference in the percentage of participants still completely abstinent through the last two weeks of the trial (44% in sertraline vs. 30% in placebo), which has been suggested as a Food and Drug Administration criterion for efficacy. However, a power calculation based on the difference in these two percentages indicated that we would have required a sample size only about 10 subjects larger in order to attain sufficient statistical power for our difference to be significant. Therefore, future studies of this medication might modestly increase the sample size, since a difference in sustained abstinence that is 47% greater than placebo has substantial clinical significance that begs for a sufficient sample size to establish its statistical significance to FDA standards
Sertraline’s effects on drug use did not appear to be related to alleviating depressive symptoms, with symptoms in both groups sharply declining within the first week of the study. This is consistent with results of the fluoxetine clinical trial in depressed cocaine abusers [37] as well as results in cocaine abusers entering non-medicated outpatient treatment,[95] suggesting that psychiatric symptoms at study entry may be more indicative of an acute rather than chronic state. Indeed, HAM-D scores were significantly correlated with CSSA scores at study entry (Pearson r=0.30, p=0.02), suggesting that depressive symptoms may reflect cocaine withdrawal severity. Nevertheless, whether co-occurring psychiatric disorders actually arise independent of substance use disorders has been questioned.[96] Given the report by Schmitz et al.[37] that those with lower depression ratings during the fluoxetine study were more likely to have cocaine-free urine samples, sertraline may have efficacy for cocaine dependence regardless of depression status.
Self-reported cocaine use results were inconsistent with urinalysis results. This is likely due to participants not reporting or under-reporting their use, with only one-sixth of those with positive cocaine urines reporting having used. In our experience, underreporting use is common in this population. Given that participants were abstinent prior to the outpatient phase, urine results do appear to be a more accurate measure for determining first use of cocaine.
Sertraline was generally well tolerated in this population with few adverse effects, which is consistent with recent reports of sertraline trials for cocaine dependence [80, 97]. There did appear to be a greater incidence of suicidal ideation (5.3%) than expected in the overall sample. These findings are in contrast with a more recently completed sertraline trial in cocaine dependent participants with depressive symptoms whereby no suicidal ideation occurred [97]. This result may therefore reflect some sort of sampling bias.
Almost one-third of participants dropped out during the residential stay, so the lack of outcome data with these individuals cannot inform our overall results. The majority of these dropouts occurred during the first part of the study when procedures with the residential milieu were still being clarified; however, no compensation was available during this portion of the study and, given the general difficulty retaining stimulant users, using some form of contingency for attendance may have increased retention during this time. Nevertheless, that only data from those who participated beyond week 2 were analyzed limits the generalizability of these findings to individuals who are abstinent and receiving sertraline for at least two weeks.
Of those who were retained past the residential stay, more than one-third maintained abstinence for the entire 10-wk outpatient phase. This is a striking finding suggesting that initiating two weeks of abstinence followed by manual-guided CBT can facilitate delayed relapse in such a difficult-to-treat population. More research is needed to determine the reproducibility of these findings. Nevertheless, the results of this study suggest that sertraline should be investigated further as a relapse prevention agent for cocaine dependence.
ACKNOWLEDGMENTS
This work was supported by grant P50-DA12762 and K05-DA00454 (TRK) from the National Institute on Drug Abuse.
The authors thank Nicole Carter and Lucas Moore for their assistance with the preparation of this manuscript.
Footnotes
Declaration of Interest:
Dr. Kosten is a consultant for Reckitt Benckizer, Catalyst Pharma, and Alkermes. There are no other disclosures.
Preliminary reports of this work were presented at the 2002 Annual Meeting of the College on Problems of Drug Dependence in Quebec City, Quebec, the 2003 Annual Meeting of the College on Problems of Drug Dependence in Bal Harbour, FL and 2005 Annual Meeting of the College on Problems of Drug Dependence in Orlando, FL.
The authors have no conflict of interest with the subject of this manuscript. The corresponding author takes full responsibility for the integrity of the data and the accuracy of the data analysis, and ascertains that she had full access to all of the data in the study.
REFERENCES
- 1.SAMHSA. Rockville, MD: Department of Health and Human Services, Office of Applied Studies; 2007. Drug and Alcohol Services Information System (DASIS). Primary Cocaine Treatment Admissions: 2007. [Google Scholar]
- 2.SAMHSA. Rockville, MD: Department of Health and Human Services, Office of Applied Studies; 2007. Results from the 2006 National Survey on Drug Use and Health: National Findings. [Google Scholar]
- 3.NACO. The cocaine epidemic in America, in: The National Association of Counties. 2005
- 4.USDHHS. U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Mental Health Services, NIH, NIMH; 1999. Mental Health: A report from the surgeon general. [Google Scholar]
- 5.Perez CM, Suarez E, Torres EA. Epidemiology of hepatitis C infection and its public health implications in Puerto Rico. P R Health Sci J. 2004;23(2 Suppl):11–28. [PubMed] [Google Scholar]
- 6.Higgins ST, et al. A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatry. 1991;148(9):1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- 7.Compton WM, et al. Substance dependence and other psychiatric disorders among drug dependent subjects: Race and gender correlates. Amer J Addict. 2000;9:113–125. doi: 10.1080/10550490050173181. [DOI] [PubMed] [Google Scholar]
- 8.Kidorf M, et al. Prevalence of psychiatric and substance use disorders in opioid abusers in a community syringe exchange program. Drug Alcohol Depend. 2004;74(2):115–122. doi: 10.1016/j.drugalcdep.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Falck RS, et al. The prevalence and correlates of depressive symptomatology among a community sample of crack-cocaine smokers. J Psychoactive Drugs. 2002;34(3):281–288. doi: 10.1080/02791072.2002.10399964. [DOI] [PubMed] [Google Scholar]
- 10.Myers JK, et al. Six-month prevalence of psychiatric disorders in three communities 1980 to 1982. Arch Gen Psychiatry. 1984;41(10):959–967. doi: 10.1001/archpsyc.1984.01790210041006. [DOI] [PubMed] [Google Scholar]
- 11.Rounsaville BJ, et al. Psychiatric diagnoses of treatment-seeking cocaine abusers. Arch Gen Psychiatry. 1991;48(1):43–51. doi: 10.1001/archpsyc.1991.01810250045005. [DOI] [PubMed] [Google Scholar]
- 12.Wild TC, et al. Comorbid depression among untreated illicit opiate users: results from a multisite Canadian study. Can J Psychiatry. 2005;50(9):512–518. doi: 10.1177/070674370505000903. [DOI] [PubMed] [Google Scholar]
- 13.Booth BM, et al. Characteristics of cocaine users presenting to an emergency department chest pain observation unit. Acad Emerg Med. 2005;12(4):329–337. doi: 10.1197/j.aem.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 14.Conner KR, Pinquart M, Holbrook AP. Meta-analysis of depression and substance use and impairment among cocaine users. Drug Alcohol Depend. 2008;98(1–2):13–23. doi: 10.1016/j.drugalcdep.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilbey MM, Breslau N, Andreski P. Cocaine use and dependence in young adults: associated psychiatric disorders and personality traits. Drug Alcohol Depend. 1992;29(3):283–290. doi: 10.1016/0376-8716(92)90103-j. [DOI] [PubMed] [Google Scholar]
- 16.Kasarabada ND, et al. Variations in psychosocial functioning associated with patterns of progression in cocaine-dependent men. Addict Behav. 1998;23(2):179–189. doi: 10.1016/s0306-4603(97)00078-6. [DOI] [PubMed] [Google Scholar]
- 17.Leventhal AM, et al. Using addiction severity profiles to differentiate cocaine-dependent patients with and without comorbid major depression. Am J Addict. 2006;15(5):362–369. doi: 10.1080/10550490600860148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alterman AI, et al. Baseline prediction of 7-month cocaine abstinence for cocaine dependence patients. Drug Alcohol Depend. 2000;59(3):215–221. doi: 10.1016/s0376-8716(99)00124-6. [DOI] [PubMed] [Google Scholar]
- 19.Carroll KM, Rounsaville BJ, Bryant KJ. Alcoholism in treatment-seeking cocaine abusers: clinical and prognostic significance. J Stud Alcohol. 1993;54(2):199–208. doi: 10.15288/jsa.1993.54.199. [DOI] [PubMed] [Google Scholar]
- 20.McKay JR, et al. Relation of depression diagnoses to 2-year outcomes in cocaine-dependent patients in a randomized continuing care study. Psychol Addict Behav. 2002;16(3):225–235. [PubMed] [Google Scholar]
- 21.Schmitz JM, et al. Cocaine dependence with and without comorbid depression: a comparison of patient characteristics. Drug Alcohol Depend. 2000;60(2):189–198. doi: 10.1016/s0376-8716(99)00157-x. [DOI] [PubMed] [Google Scholar]
- 22.Helmus TC, et al. The relationship between self-reported cocaine withdrawal symptoms and history of depression. Addict Behav. 2001;26(3):461–467. doi: 10.1016/s0306-4603(00)00105-2. [DOI] [PubMed] [Google Scholar]
- 23.Arndt IO, et al. Desipramine treatment of cocaine dependence in methadone-maintained patients. Arch Gen Psychiatry. 1992;49(11):888–893. doi: 10.1001/archpsyc.1992.01820110052008. [DOI] [PubMed] [Google Scholar]
- 24.Batki SL, et al. A controlled trial of fluoxetine in crack cocaine dependence. Drug Alcohol Depend. 1996;41(2):137–142. doi: 10.1016/0376-8716(96)01233-1. [DOI] [PubMed] [Google Scholar]
- 25.Covi L, et al. Effects of combined fluoxetine and counseling in the outpatient treatment of cocaine abusers. Am J Drug Alcohol Abuse. 1995;21(3):327–344. doi: 10.3109/00952999509002701. [DOI] [PubMed] [Google Scholar]
- 26.Galloway GP, et al. Imipramine for the treatment of cocaine and methamphetamine dependence. J Addict Dis. 1994;13(4):201–216. doi: 10.1300/j069v13n04_08. [DOI] [PubMed] [Google Scholar]
- 27.Gawin FH, et al. Desipramine facilitation of initial cocaine abstinence. Arch Gen Psychiatry. 1989;46(2):117–121. doi: 10.1001/archpsyc.1989.01810020019004. [DOI] [PubMed] [Google Scholar]
- 28.Grabowski J, et al. Fluoxetine is ineffective for treatment of cocaine dependence or concurrent opiate and cocaine dependence: two placebo-controlled double-blind trials. J Clin Psychopharmacol. 1995;15(3):163–174. doi: 10.1097/00004714-199506000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Kosten TA, et al. An Open Trial of Sertraline for Cocaine Abuse. American Journal on Addictions. 1992;1(4):349–353. [Google Scholar]
- 30.Margolin A, et al. A multicenter trial of bupropion for cocaine dependence in methadone-maintained patients. Drug Alcohol Depend. 1995;40(2):125–131. doi: 10.1016/0376-8716(95)01198-6. [DOI] [PubMed] [Google Scholar]
- 31.Nunes EV, et al. Imipramine treatment of cocaine abuse: possible boundaries of efficacy. Drug Alcohol Depend. 1995;39(3):185–195. doi: 10.1016/0376-8716(95)01161-6. [DOI] [PubMed] [Google Scholar]
- 32.Weddington WW, Jr, et al. Comparison of amantadine and desipramine combined with psychotherapy for treatment of cocaine dependence. Am J Drug Alcohol Abuse. 1991;17(2):137–152. doi: 10.3109/00952999108992817. [DOI] [PubMed] [Google Scholar]
- 33.Carroll KM, Rounsaville BJ. Psychosocial treatments for substance dependence. In: Oldham JM, Riba MB, editors. American Psychiatric Press Review of Psychiatry. Washington: American Psychiatric Press, Inc.; 1995. [Google Scholar]
- 34.Nunes EV, et al. Imipramine treatment of opiate-dependent patients with depressive disorders. A placebo-controlled trial. Arch Gen Psychiatry. 1998;55(2):153–160. doi: 10.1001/archpsyc.55.2.153. [DOI] [PubMed] [Google Scholar]
- 35.Nunes EV, et al. Imipramine treatment of methadone maintenance patients with affective disorder and illicit drug use. Am J Psychiatry. 1991;148(5):667–669. doi: 10.1176/ajp.148.5.667. [DOI] [PubMed] [Google Scholar]
- 36.Ziedonis DM, Kosten TR. Depression as a prognostic factor for pharmacological treatment of cocaine dependence. Psychopharmacol Bull. 1991;27(3):337–343. [PubMed] [Google Scholar]
- 37.Schmitz JM, et al. Fluoxetine treatment of cocaine-dependent patients with major depressive disorder. Drug Alcohol Depend. 2001;63(3):207–214. doi: 10.1016/s0376-8716(00)00208-8. [DOI] [PubMed] [Google Scholar]
- 38.Best SE, et al. SPECT imaging of the dopamine transporter in cocaine abstinence: Preliminary studies using [123-l]b-CIT. In: Harris LS, editor. Problems of Drug Dependence. Washington, DC: National Institute on Drug Abuse Research Monograph; 1994. [Google Scholar]
- 39.Malison RT, et al. Euphorigenic doses of cocaine reduce [123I]beta-CIT SPECT measures of dopamine transporter availability in human cocaine addicts. Psychopharmacology (Berl) 1995;122(4):358–362. doi: 10.1007/BF02246266. [DOI] [PubMed] [Google Scholar]
- 40.Volkow ND, et al. Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry. 1990;147(6):719–724. doi: 10.1176/ajp.147.6.719. [DOI] [PubMed] [Google Scholar]
- 41.Parsons LH, Smith AD, Justice JB., Jr Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine. Synapse. 1991;9(1):60–65. doi: 10.1002/syn.890090109. [DOI] [PubMed] [Google Scholar]
- 42.Parsons LH, Koob GF, Weiss F. Serotonin dysfunction in the nucleus accumbens of rats during withdrawal after unlimited access to intravenous cocaine. J Pharmacol Exp Ther. 1995;274(3):1182–1191. [PubMed] [Google Scholar]
- 43.Rossetti ZL, et al. Dramatic depletion of mesolimbic extracellular dopamine after withdrawal from morphine, alcohol or cocaine: a common neurochemical substrate for drug dependence. Ann N Y Acad Sci. 1992;654:513–516. doi: 10.1111/j.1749-6632.1992.tb26016.x. [DOI] [PubMed] [Google Scholar]
- 44.Weiss F, et al. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res. 1992;593(2):314–318. doi: 10.1016/0006-8993(92)91327-b. [DOI] [PubMed] [Google Scholar]
- 45.Cunningham KA. Modulation of serotonin function by acute and chronic cocaine: Neurophysiological analyses. In: Hammer R, editor. Molecular Neurobiology of Cocaine. Boca Raton, FL: CRC press; 1995. pp. 121–143. [Google Scholar]
- 46.Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43(2):107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- 47.Satel SL, et al. Clinical phenomenology and neurobiology of cocaine abstinence: a prospective inpatient study. Am J Psychiatry. 1991;148(12):1712–1716. doi: 10.1176/ajp.148.12.1712. [DOI] [PubMed] [Google Scholar]
- 48.Weddington WW, et al. Changes in mood, craving, and sleep during short-term abstinence reported by male cocaine addicts. A controlled, residential study. Arch Gen Psychiatry. 1990;47(9):861–868. doi: 10.1001/archpsyc.1990.01810210069010. [DOI] [PubMed] [Google Scholar]
- 49.Baumann MH, Rothman RB. Alterations in serotonergic responsiveness during cocaine withdrawal in rats: similarities to major depression in humans. Biol Psychiatry. 1998;44(7):578–591. doi: 10.1016/s0006-3223(98)00123-1. [DOI] [PubMed] [Google Scholar]
- 50.Jimerson DC. Role of dopamine mechanisms in the affective disorders. In: Meltzer HY, editor. Psychopharmacology: The third generation of progress. New York: Raven Press; 1987. pp. 505–511. [Google Scholar]
- 51.Willner P, et al. Reversal of stress-induced anhedonia by the dopamine receptor agonist, pramipexole. Psychopharmacology (Berl) 1994;115(4):454–462. doi: 10.1007/BF02245568. [DOI] [PubMed] [Google Scholar]
- 52.Benloucif S, Keegan MJ, Galloway MP. Serotonin-facilitated dopamine release in vivo: pharmacological characterization. J Pharmacol Exp Ther. 1993;265(1):373–377. [PubMed] [Google Scholar]
- 53.Parsons LH, Justice JB., Jr Perfusate serotonin increases extracellular dopamine in the nucleus accumbens as measured by in vivo microdialysis. Brain Res. 1993;606(2):195–199. doi: 10.1016/0006-8993(93)90984-u. [DOI] [PubMed] [Google Scholar]
- 54.Briley M, Moret C. Neurobiological mechanisms involved in antidepressant therapies. Clin Neuropharmacol. 1993;16(5):387–400. doi: 10.1097/00002826-199310000-00002. [DOI] [PubMed] [Google Scholar]
- 55.Cowen PJ. Serotonin receptor subtypes in depression: evidence from studies in neuroendocrine regulation. Clin Neuropharmacol. 1993;16 Suppl 3:S6–S18. [PubMed] [Google Scholar]
- 56.Meltzer HY, Lowry MT. The serotonin hypothesis of depression. In: Meltzer HY, editor. Psychopharmacology: The third generation of progress. New York: Raven Press; 1987. pp. 513–526. [Google Scholar]
- 57.Koe BK, et al. Sertraline, 1S,4S-N-methyl-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-1-naphthylamine, a new uptake inhibitor with selectivity for serotonin. J Pharmacol Exp Ther. 1983;226(3):686–700. [PubMed] [Google Scholar]
- 58.Goodnick PJ, Goldstein BJ. Selective serotonin reuptake inhibitors in affective disorders--I. Basic pharmacology. J Psychopharmacol. 1998;12(3 Suppl B):S5–S20. doi: 10.1177/0269881198012003021. [DOI] [PubMed] [Google Scholar]
- 59.Tatsumi M, et al. Pharmacological profile of neuroleptics at human monoamine transporters. Eur J Pharmacol. 1999;368(2–3):277–283. doi: 10.1016/s0014-2999(99)00005-9. [DOI] [PubMed] [Google Scholar]
- 60.Bolden-Watson C, Richelson E. Blockade by newly-developed antidepressants of biogenic amine uptake into rat brain synaptosomes. Life Sci. 1993;52(12):1023–1029. doi: 10.1016/0024-3205(93)90194-8. [DOI] [PubMed] [Google Scholar]
- 61.Guthrie SK. Sertraline: a new specific serotonin reuptake blocker. DICP. 1991;25(9):952–961. doi: 10.1177/106002809102500910. [DOI] [PubMed] [Google Scholar]
- 62.Richelson E. Pharmacology of antidepressants--characteristics of the ideal drug. Mayo Clin Proc. 1994;69(11):1069–1081. doi: 10.1016/s0025-6196(12)61375-5. [DOI] [PubMed] [Google Scholar]
- 63.Stahl SM. Not so selective serotonin reuptake inhibitors. J Clin Psychiatry. 1998;59(7):343–344. [PubMed] [Google Scholar]
- 64.Jans LAW, et al. Serotonergic Vulnerability and depression: assumptions, experimental evidence and implications. Molecular Psychiatry. 2007;12:522–543. doi: 10.1038/sj.mp.4001920. [DOI] [PubMed] [Google Scholar]
- 65.Carver CS, Johnson SL, Joorman J. Serotonergic function: Two-mode models of self-regulation, and vulnerability to depression: What depression has in common with impulsive aggression. Psychological Bulletin. 2008;134(6):912–943. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Filip M, et al. The serotonergic system and its role in cocaine addiction. Pharmacological Reports. 2005;57:685–700. [PubMed] [Google Scholar]
- 67.Filip M, et al. Behavioral evidence for the significance of serotoninergic (5-HT) receptors in cocaine addiction. Addict Biol. 2010;15(3):227–249. doi: 10.1111/j.1369-1600.2010.00214.x. [DOI] [PubMed] [Google Scholar]
- 68.Wei LJ, Lachin JM. Properties of the urn randomization in clinical trials. Control Clin Trials. 1988;9(4):345–364. doi: 10.1016/0197-2456(88)90048-7. [DOI] [PubMed] [Google Scholar]
- 69.Carroll KM. Rockville, MD: National Institute on Drug Abuse; 1998. A cognitive-behavioral approach: treating cocaine addiction; pp. 98–4308. [Google Scholar]
- 70.Preskorn SH, Lane RM. Sertraline 50 mg daily: the optimal dose in the treatment of depression. Int Clin Psychopharmacol. 1995;10(3):129–141. doi: 10.1097/00004850-199510030-00001. [DOI] [PubMed] [Google Scholar]
- 71.First MB, et al. Structured Clinical Interview for DSM-IV, patient ed. Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- 72.McLellan AT, et al. An improved diagnostic instrument for substance abuse subjects: The addiction severity index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 73.Kampman KM, et al. Reliability and validity of the Cocaine Selective Severity Assessment. Addict Behav. 1998;23(4):449–461. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 74.Mulvaney FD, et al. Cocaine abstinence symptomatology and treatment attrition. J Subst Abuse Treat. 1999;16(2):129–135. doi: 10.1016/s0740-5472(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 75.Hamilton MA. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–82. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kosten T, et al. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug Alcohol Depend. 2003;70(3):315–325. doi: 10.1016/s0376-8716(03)00032-2. [DOI] [PubMed] [Google Scholar]
- 77.Oliveto A, et al. Efficacy of dose and contingency management procedures in LAAM-maintained cocaine-dependent patients. Drug Alcohol Depend. 2005;79(2):157–165. doi: 10.1016/j.drugalcdep.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 78.Moeller FG, et al. Citalopram combined with behavioral therapy reduces cocaine use: a double-blind, placebo-controlled trial. Am J Drug Alcohol Abuse. 2007;33(3):367–378. doi: 10.1080/00952990701313686. [DOI] [PubMed] [Google Scholar]
- 79.Piasecki MP, et al. An exploratory study: the use of paroxetine for methamphetamine craving. J Psychoactive Drugs. 2002;34(3):301–304. doi: 10.1080/02791072.2002.10399967. [DOI] [PubMed] [Google Scholar]
- 80.Winhusen TM, et al. A placebo-controlled screening trial of tiagabine, sertraline and donepezil as cocaine dependence treatments. Addiction. 2005;100 Suppl 1:68–77. doi: 10.1111/j.1360-0443.2005.00992.x. [DOI] [PubMed] [Google Scholar]
- 81.Shoptaw S, et al. Randomized, placebo-controlled trial of sertraline and contingency management for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2006;85(1):12–18. doi: 10.1016/j.drugalcdep.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 82.Batki SL, et al. Fluoxetine for cocaine dependence in methadone maintenance: quantitative plasma and urine cocaine/benzoylecgonine concentrations. J Clin Psychopharmacol. 1993;13(4):243–250. [PubMed] [Google Scholar]
- 83.Pollack MH, Rosenbaum JF. Fluoxetine treatment of cocaine abuse in heroin addicts. J Clin Psychiatry. 1991;52(1):31–33. [PubMed] [Google Scholar]
- 84.Schmitz JM, et al. Medication take-home doses and contingency management. Exp Clin Psychopharmacol. 1998;6(2):162–168. doi: 10.1037//1064-1297.6.2.162. [DOI] [PubMed] [Google Scholar]
- 85.Cornelius JR, et al. Fluoxetine versus placebo in depressed alcoholic cocaine abusers. Psychopharmacol Bull. 1998;34(1):117–121. [PubMed] [Google Scholar]
- 86.Petrakis I, et al. Fluoxetine treatment of depressive disorders in methadone-maintained opioid addicts. Drug Alcohol Depend. 1998;50(3):221–226. doi: 10.1016/s0376-8716(98)00032-5. [DOI] [PubMed] [Google Scholar]
- 87.Carrasco JL, Sandner C. Clinical effects of pharmacological variations in selective serotonin reuptake inhibitors: an overview. Int J Clin Pract. 2005;59(12):1428–1434. doi: 10.1111/j.1368-5031.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- 88.Palvimaki EP, et al. Differential effects of fluoxetine and citalopram treatments on serotonin 5-HT(2C) receptor occupancy in rat brain. Int J Neuropsychopharmacol. 1999;2(2):95–99. doi: 10.1017/S1461145799001406. [DOI] [PubMed] [Google Scholar]
- 89.Buchman N, Strous RD, Baruch Y. Side effects of long-term treatment with fluoxetine. Clin Neuropharmacol. 2002;25(1):55–57. doi: 10.1097/00002826-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 90.Thase ME. Treatment issues related to sleep and depression. J Clin Psychiatry. 2000;61 Suppl 11:46–50. [PubMed] [Google Scholar]
- 91.Brymer C, Winograd CH. Fluoxetine in elderly patients: is there cause for concern? J Am Geriatr Soc. 1992;40(9):902–905. doi: 10.1111/j.1532-5415.1992.tb01987.x. [DOI] [PubMed] [Google Scholar]
- 92.Millan MJ, Dekeyne A, Gobert A. Serotonin (5-HT)2C receptors tonically inhibit dopamine (DA) and noradrenaline (NA), but not 5-HT, release in the frontal cortex in vivo. Neuropharmacology. 1998;37(7):953–955. doi: 10.1016/s0028-3908(98)00078-1. [DOI] [PubMed] [Google Scholar]
- 93.Owens MJ, Knight DL, Nemeroff CB. Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol Psychiatry. 2001;50(5):345–350. doi: 10.1016/s0006-3223(01)01145-3. [DOI] [PubMed] [Google Scholar]
- 94.Rothman RB, Blough BE, Baumann MH. Dual dopamine/serotonin releasers: potential treatment agents for stimulant addiction. Exp Clin Psychopharmacol. 2008;16(6):458–474. doi: 10.1037/a0014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Husband SD, et al. Decline in self-reported dysphoria after treatment entry in inner-city cocaine addicts. Journal of Consulting and Clinical Psychology. 1996;64(1):221–224. doi: 10.1037//0022-006x.64.1.221. [DOI] [PubMed] [Google Scholar]
- 96.Kadden RM, Kranzler HR, Rounsaville BJ. Validity of the Distinction Between “Substance-Induced” and “Independent” Depression and Anxiety Disorders [Google Scholar]
- 97.Mancino MJ, et al. A randomized placebo-controlled trial of sertraline and sertraline plus gabapentin in depressed, recently abstinent cocaine-dependent patients. 2009 Annual Meeting of the College on Problems of Drug Dependence; Reno/Sparks, NV. 2009. [Google Scholar]