Abstract
Rationale
Protein kinase Cα (PKCα) activity and protein level are induced during cardiac disease where it controls myocardial contractility and propensity to heart failure in mice and rats. For example, mice lacking the gene for PKCα have enhanced cardiac contractility and reduced susceptibility to heart failure following long-term pressure overload or after myocardial infarction injury. Pharmacologic inhibition of PKCα/β with Ro-32-0432, Ro-31-8220 or ruboxistaurin (LY333531) similarly enhances cardiac function and antagonizes heart failure in multiple models of disease in both mice and rats.
Objective
Large and small mammals differ in several key indexes of heart function and biochemistry, lending uncertainty as to how PKCα/β inhibition might affect or protect a large animal model of heart failure.
Methods and Results
Here we demonstrate that ruboxistaurin administration to a pig model of myocardial infarction-induced heart failure was protective. Twenty-kilogram pigs underwent left anterior descending artery (LAD) occlusion resulting in myocardial infarctions and were then divided into vehicle or ruboxistaurin feed groups, after which they were monitored monthly for the next 3 months. Ruboxistaurin administered pigs showed significantly better recovery of myocardial contractility 3 months after infarction injury, greater ejection fraction, and greater cardiac output compared with vehicle treated pigs.
Conclusions
These results provide additional evidence in a large animal model of disease that PKCα/β inhibition (with ruboxistaurin) represents a tenable and novel therapeutic approach for treating human heart failure.
Keywords: Heart failure, contractility, PKC, signaling, cardiomyopathy
Introduction
The protein kinase C family of Ca2+ and/or lipid-activated serine-threonine protein kinases are critical mediators of signal transduction in the heart where they regulate disease responsiveness.1,2 The PKC family is broadly classified by activation characteristics, such that the conventional PKC isozymes (PKCα, ßI/II, and γ) are Ca2+- and lipid-activated, while the novel isozymes (ε, θ, η, and δ) and atypical isozymes (ζ, and λ) are Ca2+ independent but activated by distinct lipids.1,2 PKCα is the predominant conventional PKC isoform expressed in the mouse, rabbit, and human heart,3-5 and many different disease causing stimuli result in its activation, including heart failure.6-13
Transgenic mice with greater PKCα activity showed decreased cardiac contractility, ventricular dilation, and secondary hypertrophy, suggesting that increased PKCα signaling is detrimental to the heart.14,15 Indeed, PKCα−/− mice are protected from insults or genetic mutations that would otherwise induce heart failure.14,16 By comparison, PKCβ/γ−/− mice showed more severe heart failure when stressed,17 suggesting that PKCα is the primary disease affecting isoform in the heart and the best candidate for inhibition. Transgenic mice with inducible expression of a dominant negative PKCα mutant in cardiomyocytes of the heart also showed reduced failure progression after myocardial infarction (MI) injury.16
Results in genetically modified animal models and in isolated adult myocytes clearly showed a cardioprotective effect with PKCα inhibition. Thus, we and others carefully examined the effects of cPKC inhibitors of the bisindolylmaleimide class, such as ruboxistaurin (LY333531), Ro-32-0432 or Ro-31-8220, in different rodent heart failure models. Short-term or long-term treatment with Ro-31-8220 in the Csrp3 null mouse model of heart failure augmented cardiac contractility and restored pump function.5 PKC inhibition with Ro-31-8220 or Ro-32-0432 also reduced mortality and cardiac contractile abnormalities in a mouse model of myotonic dystrophy type 1.18 Another inhibitor, ruboxistaurin, prevented death in wildtype mice throughout 10 weeks of pressure-overload stimulation, reduced ventricular dilation, enhanced ventricular performance, reduced fibrosis, and reduced pulmonary edema comparable to or better than metoprolol treatment.17 Ruboxistaurin was also administered to PKCβ/γ null mice subjected to pressure overload, resulting in less death and heart failure, strongly suggesting PKCα as the primary target of this drug in mitigating heart disease.17 In addition, Boyle et al. showed that ruboxistaurin reduced ventricular fibrosis and dysfunction following MI in rats.19 Ruboxistaurin treatment also significantly decreased infarct size and enhanced recovery of left ventricular function and reduced markers of cellular necrosis in mice subjected to 30 min of ischemia followed by 48 h of reperfusion.20 Connelly et al. demonstrated that ruboxistaurin attenuated diastolic dysfunction, myocyte hypertrophy, collagen deposition, and preserved cardiac contractility in a rat diabetic heart failure model.21 These results in rodents overwhelmingly support the contention that PKCα/β inhibition with ruboxistaurin, or related compounds, protects the heart from failure after injury. Hence, if ruboxistaurin is similarly protective in a large animal model of heart failure, there should be little resistance remaining towards initiating clinical trials in patients with heart failure, especially given the apparent safety of this compound in other human trials.22
Methods
Animal studies and MI model
This study, using female Yorkshire pigs (~ 20 kg body weight) was approved by the Institutional Animal Care and Use Committee. All procedures were performed under propofol (2-10 mg/kg/h) anaesthesia. For MI generation, we introduced an 8F sheet into the femoral artery and cannulated the left anterior descending (LAD) coronary artery with an 8F hockey stick guiding catheter (Cordis Infiniti, Johnson & Johnson). After injecting 100 mg nitroglycerin and obtaining a baseline coronary angiogram, we placed a 5F balloon catheter (Cordis Infiniti, Johnson & Johnson) into the LAD after the first diagonal branch, thus occluding 2/3rds of the LAD tributary for 90 minutes. The resulting infarct size was approximately 15% of the left ventricle, determined by TTC staining. The 48-hr survival rate was 86% (n = 15). Thirteen animals were randomised to receive either control pig chow or pig chow enriched with Ruboxistaurin (Eli Lilly).
Oral treatment with ruboxistaurin
Pigs were given 10 mg/kg/day ruboxistaurin in separate doses twice a day starting immediately after MI until 12 weeks. Ruboxistaurin was administrated mixed with the regular animal diet. Four days of oral ruboxistaurin treatment (2 days at 5 mg/kg/day and 2 days at 10 mg/kg/day) produced plasma levels of 93 +/− 31 ng/ml of ruboxistaurin and 817 +/− 179 ng/ml of the primary metabolite.
Assessment of myocardial function and structure
We assessed myocardial function and structure at baseline (i.e. before MI generation), 48 hours, 1 month, 2 months, and 3 months after MI. We performed echocardiography with an iE33 ultrasound machine (Philips Medical Systems) equipped with an X3-1 and S8-3 transducer during end-expiratory breath-hold in an R-wave-trigged mode. Images were obtained in the standard LV apical and short axis views with a high frame rate (> 60 frames/s). QLab software (Philips) was used for analysis of strain rate. Two stable and well-defined consecutive cardiac cycles were acquired digitally for each measurement.
For hemodynamic catheterization, we accessed the femoral artery and vein with 7F sheets and placed a 6F Millar Micro-Tip catheter (Millar Instruments Inc.) into the aorta, the left ventricle, and the right ventricle. We determined the following parameters: systolic pressure, end-diastolic pressure, peak LV pressure rate of rise (dP/dt)max and Tau value (time constant of isovolumic relaxation); (dP/dt)max/P was calculated as (dP/dt)max/(systolic – end-diastolic pressure). The mean of at least 3 consecutive cardiac cycles was calculated for each measurement.
We performed coronary angiography on day 2, after one, and three months using an Integris H5000 single-plane fluoroscopy system (Philips Medical Systems). All images were acquired and analysed by an investigator blinded to the study arm. We euthanized pigs by i.v. injection of EuthasolR (pentobarbital, phenytoin, 1 ml/4.5 kg), removed the hearts, resected the right ventricle, and cut the left ventricle into 6 slices of the same thickness We visualised viable myocardium by staining 5 of these slices with TTC and quantified scar volume.
Western blot assessment of PKC target proteins
Hearts were collected at the end of the study for protein extraction and western blotting as previously described.5 Primary antibodies included PKCα, PKCβI, PKCβII, PKCγ, CaV1.2, RyR (Santa Cruz Biotechnology), GAPDH (Research Diagnostics), Phospho-PLN, Phospho-CaV1.2, Phospho-RyR2 (Badrilla) PLN (Pierce), Phospho-PKC, GRK2, TnI, Phospho-TnI (Cell Signaling), Phospho-PKC (Upstate), Phospho-TnI, TnT (Abcam), SERCA2 (Affinity Bioreagents), MyBP-C, and Phospho-MyBP-C (gift from Jeffrey Robbins, Cincinnati Children's Hospital, Cincinnati, OH). For analysis of TnT, GRK2 and SERCA2 phosphorylation, the Phos-Tag reagent (Wako Chemicals) was used at 30 μM. Chemifluorescent detection was performed with Vistra ECF reagent (Amersham Pharmacia Biotech) and scanned with a Gel-Doc XR (Bio-Rad).
Statistical analysis
All data analysis was performed in a blinded manner. Data were presented as mean ± standard error of mean. Statistical analysis was performed with SPSS software (SPSS Inc.) using non-parametric Wilcoxon test.
Results and Discussion
Analysis of pig heart function after MI with or without ruboxistaurin treatment
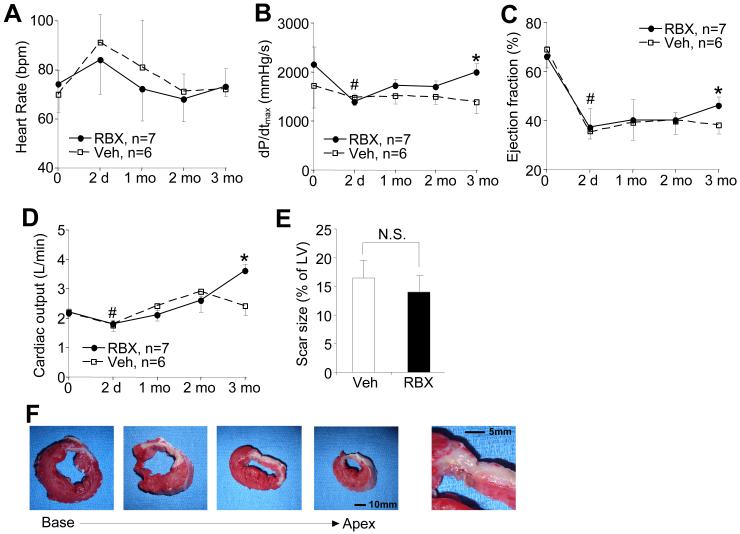
While ruboxistaurin was shown to dramatically attenuate heart failure in select mouse and rat models of disease, its applicability to large mammals with heart failure remains uncertain. Here we utilized 20 kg Yorkshire pigs to evaluate heart failure over a 3-month period after MI injury. The study was limited to 3 months to curtail costs and suffering, to conserve limited quantities of ruboxistaurin, to reduce the effect of rapid weight gain that typically occurs in juvenile pigs that would otherwise skew data interpretation, and because 3 months is sufficient time to uncover heart failure in control animals. Two days after LAD occlusion (MI injury) heart rate was significantly increased in both vehicle and ruboxistaurin treated pigs, which gradually dropped back to pre-MI levels by 2-3 months (Figure 1A). Vehicle and ruboxistaurin treated pigs showed a loss of cardiac contractility, ejection fraction, and cardiac output 2 days after MI injury (Figure 1B,C,D). This loss of cardiac performance remained depressed in vehicle treated pigs over the 3 month period, although ruboxistaurin treated pigs showed a significant recovery of cardiac contractility, ejection fraction, and cardiac output 3 months after MI injury (Figure 1B,C,D). There was no difference in infarct size normalized to the left ventricular area between the control and ruboxistaurin treated groups assessed by TTC staining at the end of the study (Figure 1E,F). No overt differences in cardiac histopathology were observed between the treated and control pigs (data not shown). Taken together, these results showed that ruboxistaurin treatment benefitted the heart and augmented myocardial recover following MI injury, suggesting a novel therapeutic approach for heart failure post MI in humans.
Figure 1.
Ruboxistaurin attenuates heart failure in pigs post MI. A, Millar catheter-based analysis of heart rate and B, contractility in vehicle or ruboxistaurin treated (10 mg/kg/day) pigs subjected to MI injury at the indicated times after injury (days or months). #P<0.05 versus 0 time point. *P<0.05 versus vehicle treated pigs at 3 months. C, left ventricular ejection fraction measured by ventriculography in vehicle or ruboxistaurin treated pigs after MI for the indicated periods of time (days or months). *P<0.05 versus vehicle at 3 months. D, Cardiac output measured with a Swan Ganz catheter in vehicle or ruboxistaurin treated pigs after MI for the indicated periods of time. *P<0.05 versus vehicle at 3 months. E, Quantitation of scar size after TTC staining to show area of injury between the 2 groups. N.S.=not significantly different. F, Pig heart slices after MI injury stained with TTC (white area is not stained by TTC and represents the area of infraction).
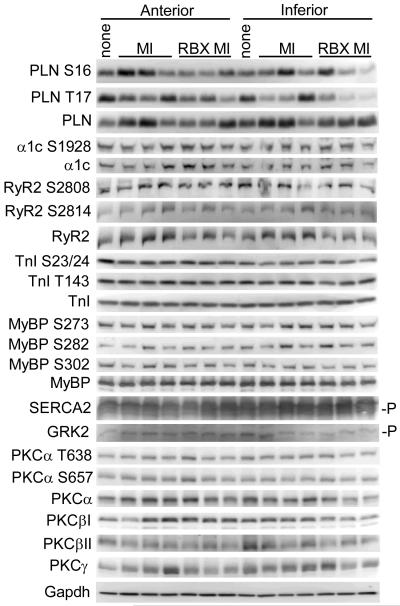
The mechanism whereby ruboxistaurin treatment protected the pig heart post MI is uncertain. Ventricular dilation post MI injury over the 3 months of the study remained the same between vehicle and ruboxistaurin treated groups (data not shown) and infarct and scar size was not different (Figure 1D,E), suggesting that ventricular remodeling was not altered by PKCα/β inhibition, or that not enough time had passed to permit accurate assessment of beneficial changes in ventricular geometry with ruboxistaurin treatment. We also failed to observe a change in ventricular remodeling in transgenic mice expressing a dominant negative PKCα protein in the heart after MI injury, which might suggest that inhibition of PKCα protects the heart through a unique mechanism of action that is independent of structural remodeling.21 Indeed, we have previously proposed that the overwhelming beneficial effect associated with PKCα inhibition/deletion on the heart was due to an increase in cardiac contractility or the efficiency of myofilament function.23 However, attempts to identify a direct PKCα/β phosphorylation target that might underlie an alteration in cardiac contractility in ruboxistaurin treated pig hearts were unsuccessful (Figure 2). Quantitation of these blots showed no significant changes when anterior and inferior regions were combined (Table 1). Similarly, we also failed to identify a change in phosphorylation of these same nodal control proteins in the hearts of mice treated with the PKCα/β inhibitors Ro-31-8220 or Ro-32-0432.5 Thus, while we previously demonstrated that PKCα−/− mice have increased sarcoplasmic reticulum calcium levels due to changes in inhibitor-1 phosphorylation and the subsequent phosphorylation of phospholamban, PKCα/β inhibitory drugs may not be “potent” enough at the physiologic doses employed to show the same effect. However, acute administration of Ro-31-8220, Ro-32-0432 or ruboxistaurin to mice or rats in vivo, or in an isolated work performing heart preparation, did significantly enhanced cardiac contractility (within minutes), suggesting that the same regulatory mechanisms are in place and likely being affected. Despite these observations, the employed PKCα/β antagonists might only partially inhibit phosphorylation of downstream PKCα targets over an integrated period of time, and at any single time-point the effect is too subtle to detect. Regardless of this issue, our results strongly support the contention that inhibition of PKCα/β activity with ruboxistaurin has a significant beneficial effect on the pig heart under conditions that would otherwise induce failure. Taken together with similar beneficial effects observed in mice and rats in heart failure, a unified front emerges with overwhelming data that support the contention that PKCα inhibitory drugs should be translated to the heart failure clinic in appropriate patients, especially since drugs like ruboxistaurin are apparently safe and have already been used in large late phase clinical trials.22
Figure 2.
Western blot analysis of calcium handling proteins, myofilament proteins, and PKCα/β/γ for the native protein or for the indicated specific phosphorylation site from pig hearts after 3 months of ruboxistaurin treatment or no treatment after myocardial infarction injury. One non-infarcted control is shown. The indicated proteins or phosphoproteins were analyzed from pieces of anterior or inferior portions of the left ventricle. The “-P” designation represents specialized gel electrophoresis conditions that separate proteins with differential phosphorylation.
Table 1.
Quantitation of western blotting from Figure 2*.
| Control MI | ±SE | N | RBX MI | ±SE | N | |
|---|---|---|---|---|---|---|
| P-PLN S16 | 1 | 0.1484 | 6 | 0.6989 | 0.1323 | 6 |
| P-PLN T17 | 1 | 0.1686 | 6 | 0.6179 | 0.1808 | 6 |
| P-CaV1.2 S1928 | 1 | 0.06157 | 6 | 0.9453 | 0.07016 | 6 |
| P-RyR2 S2808 | 1 | 0.07453 | 6 | 1.007 | 0.04664 | 6 |
| P-RyR2 S2814 | 1 | 0.09668 | 6 | 1.174 | 0.04865 | 6 |
| P-TnI S23/24 | 1 | 0.07356 | 6 | 1.02 | 0.09644 | 6 |
| P-TnI T143 | 1 | 0.03434 | 6 | 0.9662 | 0.04292 | 6 |
| P-MyBP-C S273 | 1 | 0.06311 | 6 | 1.012 | 0.08399 | 6 |
| P-MyBP-C S282 | 1 | 0.1388 | 6 | 1.185 | 0.1676 | 6 |
| P-MyBP-C S302 | 1 | 0.07872 | 6 | 0.9289 | 0.06183 | 6 |
| P-PKCα/β/γ T638 | 1 | 0.02391 | 6 | 0.9056 | 0.08258 | 6 |
| P-PKCα S657 | 1 | 0.06466 | 6 | 1.056 | 0.08495 | 6 |
| PKCα | 1 | 0.04457 | 6 | 0.8684 | 0.07415 | 6 |
| PKCβI | 1 | 0.09436 | 6 | 0.949 | 0.1308 | 6 |
| PKCβII | 1 | 0.07333 | 6 | 0.8084 | 0.1281 | 6 |
| PKCγ | 1 | 0.08483 | 6 | 0.7911 | 0.1233 | 6 |
None of the values were significantly different between treated and untreated controls. N=6 ventricular samples from 3 different animals (2 samples each).
The dosage of ruboxistaurin used here was 10 mg/kg/day, which achieved a blood level of 93 ng/ml with an active metabolite level of 817 ng/ml. These concentrations are similar to that achieved by us previously with mice receiving 120 mg/kg/day,17 and similar to a lower end of what has been achieved in human patients receiving a 32 mg dosage.24 However, considering the half life of ruboxistaurin in humans of 6-12 hours, a dosage of approximately 30-60 mg bid should be considered for future application in heart failure patients. Another issue is that ruboxistaurin appears to have a vascular protective effect, hence its prior use in human clinical trials for diabetic retinopathy.22 These results suggest that ruboxistaurin might also protect the heart by preserving endothelial cell function and microvascular integrity, in addition to an effect on contractility or diminution of reactive signaling in myocytes and possibly fibroblasts.
Novelty and Significance.
What is known
Genetic or pharmacologic inhibition of PKCα in mouse protects from heart failure
Pharmacologic inhibition of PKCα in rats protects from heart failure
PKCα activity is increased in heart failure
What new information does this article contribute
We provide the first proof that pharmacologic inhibition of PKCα/β reduces heart failure in a large animal model
Ruboxistaurin promotes recovery of myocardial function in a pig model of MI-induced heart failure
This study was designed to examine if inhibition of PKCα/β with ruboxistaurin would be efficacious in a large animal model of heart failure following myocardial infarction injury. The significance is that if this drug is effective in the pig in reducing heart failure, it will suggest a clinical therapy to try in humans with heart failure. Ruboxistaurin also appears to be relatively safe in previous clinical trials, so it could be rapidly evaluated in heart failure patients.
Acknowledgments
Sources of funding
This work was supported by the National Institutes of Health (J.D.M., R.J.H.) a grant from the Fondation Leducq (J.D.M., R.J.H), and the Howard Hughes Medical Institute (J.D.M.). R.N.C was supported a grant from the National Heart, Lung, and Blood Institute. D.L. received support from the German Research Foundation (DFG). We would like to thank Jeffrey W. Cramer (Scientist at Eli Lilly) for technical excellence in measuring ruboxistaurin and its metabolite in blood from the pigs in the study.
Glossary
Non-Standard Abbreviations
- LAD
left anterior descending
- MI
myocardial infarction
- PKC
protein kinase C
Footnotes
Disclosures:
The ruboxistaurin compound was obtained from Eli Lilly under an MTA.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Churchill E, Budas G, Vallentin A, Koyanagi T, Mochly-Rosen D. PKC isozymes in chronic cardiac disease: possible therapeutic targets? Annu Rev Pharmacol Toxicol. 2008;48:569–599. doi: 10.1146/annurev.pharmtox.48.121806.154902. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg SF. Structural basis of protein kinase C isoform function. Physiol Rev. 2008;88:1341–1378. doi: 10.1152/physrev.00034.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pass JM, Gao J, Jones WK, Wead WB, Wu X, Zhang J, Baines CP, Bolli R, Zheng YT, Joshua IG, Ping P. Enhanced PKC beta II translocation and PKC beta II-RACK1 interactions in PKC epsilon-induced heart failure: a role for RACK1. Am J Physiol Heart Circ Physiol. 2001;281:H2500–H2510. doi: 10.1152/ajpheart.2001.281.6.H2500. [DOI] [PubMed] [Google Scholar]
- 4.Ping P, Zhang J, Qiu Y, Tang XL, Manchikalapudi S, Cao X, Bolli R. Ischemic preconditioning induces selective translocation of protein kinase C isoforms epsilon and eta in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res. 1997;81:404–414. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- 5.Hambleton M, Hahn H, Pleger ST, Kuhn MC, Klevitsky R, Carr AN, Kimball TF, Hewett TE, Dorn GW, 2nd, Koch WJ, Molkentin JD. Pharmacological- and gene therapy-based inhibition of protein kinase Calpha/beta enhances cardiac contractility and attenuates heart failure. Circulation. 2006;114:574–582. doi: 10.1161/CIRCULATIONAHA.105.592550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Windt LJ, Lim HW, Haq S, Force T, Molkentin JD. Calcineurin promotes protein kinase C and c-Jun NH2-terminal kinase activation in the heart. Cross-talk between cardiac hypertrophic signaling pathways. J Biol Chem. 2000;275:13571–13579. doi: 10.1074/jbc.275.18.13571. [DOI] [PubMed] [Google Scholar]
- 7.Gu X, Bishop SP. Increased protein kinase C and isozyme redistribution in pressure-overload cardiac hypertrophy in the rat. Circ Res. 1994;75:926–931. doi: 10.1161/01.res.75.5.926. [DOI] [PubMed] [Google Scholar]
- 8.Jalili T, Takeishi Y, Song G, Ball NA, Howles G, Walsh RA. PKC translocation without changes in Galphaq and PLC-beta protein abundance in cardiac hypertrophy and failure. Am J Physiol. 1999;277:H2298–H2304. doi: 10.1152/ajpheart.1999.277.6.H2298. [DOI] [PubMed] [Google Scholar]
- 9.Takeishi Y, Bhagwat A, Ball NA, Kirkpatrick DL, Periasamy M, Walsh RA. Effect of angiotensin-converting enzyme inhibition on protein kinase C and SR proteins in heart failure. Am J Physiol. 1999;276:H53–H62. doi: 10.1152/ajpheart.1999.276.1.H53. [DOI] [PubMed] [Google Scholar]
- 10.Bayer AL, Heidkamp MC, Patel N, Porter M, Engman S, Samarel AM. Alterations in protein kinase C isoenzyme expression and autophosphorylation during progression of pressure overload-induced left ventricular hypertrophy. Mol Cell Biochem. 2003;242:145–152. [PubMed] [Google Scholar]
- 11.Wang J, Liu X, Sentex E, Takeda N, Dhalla NS. Increased expression of protein kinase C isoforms in heart failure due to myocardial infarction. Am J Physiol. Heart Circ. Physiol. 2003;284:H2277–H2287. doi: 10.1152/ajpheart.00142.2002. [DOI] [PubMed] [Google Scholar]
- 12.Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL, Mintze K, Pickard T, Roden R, Bristow MR, Sabbah HN, Mizrahi JL, Gromo G, King GL, Vlahos CJ. Increased protein kinase C activity and expression of calcium-sensitive isoforms in the failing human heart. Circulation. 1999;99:384–391. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- 13.Simonis G, Briem SK, Schoen SP, Bock M, Marquetant R, Strasser RH. Protein kinase C in the human heart: differential regulation of the isoforms in aortic stenosis or dilated cardiomyopathy. Mol Cell Biochem. 2007;305:103–111. doi: 10.1007/s11010-007-9533-3. [DOI] [PubMed] [Google Scholar]
- 14.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Iodi B, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. PKCa regulates cardiac contractility and propensity towards heart failure. Nat Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 15.Hahn HS, Marreez Y, Odley A, Sterbling A, Yussman MG, Hilty KC, Bodi I, Liggett SB, Schwartz A, Dorn GW., 2nd. Protein kinase Calpha negatively regulates systolic and diastolic function in pathological hypertrophy. Circ Res. 2003;93:1111–1119. doi: 10.1161/01.RES.0000105087.79373.17. [DOI] [PubMed] [Google Scholar]
- 16.Hambleton M, York A, Sargent MA, Kaiser RA, Lorenz JN, Robbins J, Molkentin JD. Inducible and myocyte-specific inhibition of PKCalpha enhances cardiac contractility and protects against infarction-induced heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H3768–71. doi: 10.1152/ajpheart.00486.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Q, Chen X, Macdonnell SM, Kranias EG, Lorenz JN, Leitges M, Houser SR, Molkentin JD. Protein kinase C{alpha}, but not PKC{beta} or PKC{gamma}, regulates contractility and heart failure susceptibility: implications for ruboxistaurin as a novel therapeutic approach. Circ Res. 2009;105:194–200. doi: 10.1161/CIRCRESAHA.109.195313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang GS, Kuyumcu-Martinez MN, Sarma S, Mathur N, Wehrens XH, Cooper TA. PKC inhibition ameliorates the cardiac phenotype in a mouse model of myotonic dystrophy type 1. J Clin Invest. 2009;119:3797–806. doi: 10.1172/JCI37976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyle AJ, Kelly DJ, Zhang Y, Cox AJ, Gow RM, Way K, Itescu S, Krum H, Gilbert RE. Inhibition of protein kinase C reduces left ventricular fibrosis and dysfunction following myocardial infarction. J Mol Cell Cardiol. 2005;39:213–21. doi: 10.1016/j.yjmcc.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Kong L, Andrassy M, Chang JS, Huang C, Asai T, Szabolcs MJ, Homma S, Liu R, Zou YS, Leitges M, Yan SD, Ramasamy R, Schmidt AM, Yan SF. PKCbeta modulates ischemia-reperfusion injury in the heart. Am J Physiol Heart Circ Physiol. 2008;294:H1862–H1870. doi: 10.1152/ajpheart.01346.2007. [DOI] [PubMed] [Google Scholar]
- 21.Connelly KA, Kelly DJ, Zhang Y, Prior DL, Advani A, Cox AJ, Thai K, Krum H, Gilbert RE. Inhibition of protein kinase C-beta by ruboxistaurin preserves cardiac function and reduces extracellular matrix production in diabetic cardiomyopathy. Circ Heart Fail. 2009;2:129–37. doi: 10.1161/CIRCHEARTFAILURE.108.765750. [DOI] [PubMed] [Google Scholar]
- 22.The PKC-DRS Study Group Effect of ruboxistaurin in patients with diabetic macular edema: thirty-month results of the randomized PKC-DMES clinical trial. Arch Ophthalmol. 2007;125:318–24. doi: 10.1001/archopht.125.3.318. [DOI] [PubMed] [Google Scholar]
- 23.Liu Q, Molkentin JD. Protein kinase Cα as a heart failure therapeutic target. J Mol Cell Cardiol. 2011 doi: 10.1016/j.yjmcc.2010.10.004. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wise S, Yuen E, Chan C, Poo Y-K, Teng L, Lau T, Voelker J. Effects of chronic renal failure on the pharmacokinetics of ruboxistaurine and its active metabolite 338522. Clin Pharmacokinet. 2006;45:297–303. doi: 10.2165/00003088-200645030-00005. [DOI] [PubMed] [Google Scholar]