Abstract
The ability to expand hematopoietic stem and progenitor cells (HSPCs) in vitro will enhance the success of a wide range of transplant-related therapies. PTEN (phosphatase and tensin homologue deleted on chromosome 10) has been implicated as a regulator of murine HSPC self-renewal but little is understood about the role of PTEN in human HSPC regulation. We tested the impact of transient small interfering RNA (siRNA) - induced inhibition of PTEN expression in human CD34+ cells on their cell cycle profile, their susceptibility to retroviral transduction, and their ability to self-renew and repopulate non-obese diabetic (NOD)/severe combined immunodeficiency disease (SCID) with IL2 receptor gamma (G) chain deficiency mice (NSG mice). Reduced PTEN mRNA and protein levels were confirmed in PTEN siRNA treated CD34+ cells compared with control siRNA treated CD34+ cells. Transient silencing of PTEN in CD34+ cells promoted their entry into cell cycle, and increased their expansion in vitro compared with control siRNA treated CD34+ cells. When these cells were transduced with retroviral vectors, transduction efficiencies in the bulk CD34+ cells transfected with PTEN siRNA were significantly higher compared with CD34+ cells transfected with a control siRNA. Transient PTEN suppression in CD34+ cells also increased their proliferation and engraftment potential in NSG mice, and maintained their multilineage differentiation capacity in vivo. No mice developed myeloproliferative disorders or leukemias. Similar to findings with murine HSPC, PTEN may also promote quiescence of human HSPC. With optimization of technologies for transfer of siRNA in primary CD34+ cells, this approach may facilitate investigations into the mechanisms underlying HSPC self-renewal, and could find clinical applications in gene therapy protocols.
Keywords: Hematopoietic stem cells, siRNA, PTEN, gene therapy, cell cycle, NSG mice, retroviral vectors, expansion
INTRODUCTION
Ex vivo expansion of human HSPCs has been pursued for over two decades. Progress would have immediate positive implications for HSPC gene therapy, non-ablative stem cell transplantation, and permit use of stem cell grafts deemed unsuitable for transplantation purposes due to low HSPC content, as is often the case with cord blood collections. The ability to increase the number of HSPCs in culture would also facilitate investigations into the mechanisms underlying self-renewal.
A plethora of molecules have been implicated in HSPC self-renewal, including BMI-1, Hedgehog, Notch, WNT, and the transcription factors JUNB, c-myc, and ELF4 (1;2). PTEN has recently been added to the list of molecules with that function in murine HSPC self-renewal pathways (3;4), but little is known about the role of PTEN in human HSPC regulation. Class I phosphatidylinositol-3 kinase (PI3K) family members catalyze the conversion of phosphatidylinositol 4,5-bisphophate (PIP2) to phosphatidylinositol 3,4,5-trisphosphate (PIP3), a second messenger capable of recruiting a subset of proteins to cellular membranes, including the serine/threonine kinases AKT1, AKT2, AKT3, and PDK1 (5). Once positioned at cell membranes, AKT isoforms are activated by phosphorylation and promote cell proliferation and survival (5). PTEN negatively regulates the PI3K/AKT signaling pathway, inhibiting proliferation and survival.
The first lines of evidence demonstrating that PTEN plays a role in stem cell regulation came from studies of PTEN knock out in murine neuronal tissues. PTENnull neural stem/progenitor cells showed enhanced self-renewal capacity and G0–G1 cell cycle entry, as well as decreased growth factor dependency (6). More recently, permanent inactivation of PTEN in the murine hematopoietic system was found to result in excessive proliferation of HSPCs, resulting in their short-term expansion, but long-term exhaustion. PTEN-deficient mice developed a myeloproliferative disorder followed by acute leukemia in a multiple-hit leukemogenic process (7). Therefore, permanent inactivation of PTEN would not be desirable for HSPC expansion; however we hypothesized that transient inactivation of PTEN activity might allow HSPC expansion in vitro, without resulting in permanent HSPC exhaustion or increased susceptibility to leukemia. In this study, we report that transient silencing of PTEN expression in human CD34+ cells using siRNA resulted in enhanced G0–G1 transition, improved transduction efficiency with retroviral vectors, and increased engraftment in NSG mice.
METHODS
Selection of siRNA
Two PTEN siRNA duplexes (PTEN1 and PTEN2) were selected from nucleotide sequences more than 100 bp downstream of the AUG codon of the human PTEN gene (accession number NM_000314, Invitrogen, Carlsbad, CA, USA). These Stealth™ RNAi compounds are 25-mer dsRNA molecules containing chemical modifications that enhance nuclease stability, reduce off-target effects by limiting sense strand activity, and prevent induction of cellular stress response pathways (15). The sequences on PTEN targeted by both siRNAs were different and non-overlapping. Sequences were:
PTEN1: 5′-CCAAUGGCUAAGUGAAGAUGACAAU-3′;
PTEN2: 5′-CCACACGACGGGAAGACAAGUUCAU-3′
Control siRNA with medium guanine-cytosine content was designed to minimize sequence homology to any known vertebrate transcript. This was used to compare the results with PTEN siRNA and to exclude nonspecific effects of siRNA.
Preparation of human CD34+ cells and delivery of siRNA into CD34+ cells
Human CD34+ cells were collected from normal healthy volunteers who gave informed consent in accordance with the Declaration of Helsinki and National Institutes of Health Institutional Review Board–approved protocols. Donors received 5 days of G-CSF (Filgrastim; Amgen, Thousand Oaks, CA) 10 μg/Kg given as a single daily subcutaneous injection with leukapheresis initiated on the morning of day 5. Large volume (15L) leukapheresis procedures were performed with a model CS-3000 Plus continuous-flow apheresis device (Fenwal Division, Baxter, Deerfield, IL). The apheresis products were enriched for CD34+ cells by immunomagnetic beads affinity elution with a magnetic cell selection system (Isolex 300I, Nexell Therapeutics, Irvine, CA).
The siRNA were transfected in human CD34+ cells by nucleofection according to manufacturer’s instructions at a concentration of 3×106 cells/cuvette (Amaxa Biosystems, Gaithersburg, MD, USA). After transfection, cells were: 1) cultured in X-vivo medium supplemented with 1% human serum albumin (Baxter, Westlake Village, CA), SCF 100ng/mL, Flt3-L 100ng/mL, and TPO 100ng/mL for a period of 1 to 10 days before analysis; or 2) transplanted into NSG mice (see below). Untreated control cells were not subjected to electroporation.
Real time RT-PCR assay
The total cellular RNA from cells was isolated with the RNeasy kit (Qiagen, Valencia, CA, USA) and purified with DNase following the RNeasy cleanup protocol (Qiagen). An equal amount of purified total RNA (50 ng) from each sample was examined in each experiment. Real-time PCR was performed in 96-well plates using the ABI 7500 Real-Time PCR System (Applied Biosystems (ABI), Foster City, CA) in a final reaction volume of 25 μl. A nontemplate control (Rnase-free water) was included on every plate. The thermal cycler conditions were 2 min hold at 50°C (activation), 10 min hold at 95°C, followed by 45 cycles of 15 sec at 95°C (denaturation) and 1 min at 60°C (annealing/extension). PCR primers and fluorogenic probes for PTEN gene were purchased as Assays-On-Demand (ABI). The assays were supplied as a 20X mix of PCR primers and TaqMan 6-FAM dye labeled probes with a nonfluorescent quencher at the 3′ end. The assay number for PTEN was Hs00829813_s1. As the endogenous control in each RT-PCR reaction, human cellular GAPDH mRNA from Integrated DNA Technologies (Coralville, IA, USA) was used, and the sequences of the primer set were as follows:
GAPDH forward: 5′-GGCACCCAGGACAATGAAG-3′
GAPDH reverse: 5′-GCCGATCCACACGGAGTACT-3′
GAPDH probe: 5′-(JOE)CAAGATGATTGCTCCTCCTGAG-3′
Percent knockdown of the PTEN mRNA in each group was determined as a ratio based on PTEN mRNA of non-transfected cells.
Western blot analysis
Human CD34+ cells were cultured for up to 10 days, and protein extracts were quantified at different points using the Bradford method (Bradford reagent; Bio-Rad, Hercules, CA). Ten micrograms of each sample were separated using NuPAGE Bis-Tris gels (Invitrogen, Carlsbad, CA) and transferred onto nitrocellulose membranes (iBlot Gel Transfer Stacks; Invitrogen) using the iBlot dry blotting system (Invitrogen). The membrane was blocked with 5% milk, and then incubated overnight with a primary rabbit-anti-human PTEN antibody (1:1000 dilution, Cell Signaling, Danvers, MA). The secondary antibody was a horseradish peroxidase (HRP) labeled goat anti-rabbit immunoglobulin (1:10,000 dilution, Dako, Carpinteria, CA). Membranes were incubated with HRP substrate (Millipore Corporation, Billerica, MA) and images were acquired with the Fujifilm LAS-4000 image analyzer (Fujifilm life science) and Multi-Gauge V3.0 software (Fujifilm Life Science).
Cell Cycle Analysis
Seventy-two hours after transfection, siRNA treated CD34+ cells were washed and resuspended at a concentration of 1 × 106 cells/mL in HBSS buffer (Mediatech, Herndon, VA, USA) supplemented with 10% FCS (Quality Biological, Gaithersburg, MD, USA), 20 mM HEPES (Mediatech, Herndon, VA, USA) and 1g/L glucose (Mediatech, Herndon, VA, USA). Cells were stained for DNA and RNA content with Hoechst (Hst) 33342 (Molecular Probes, Eugene, OR, USA) and pyronin Y (Sigma, St. Louis, MO, USA) respectively. Hst dye was added to the resuspended cells at a concentration of 6.5 μg/mL and then incubated at 37 °C for 45 minutes. At the end of the incubation, Pyronin Y was added at a concentration of 1 μg/mL and the cells were incubated at 37°C for an additional 45 minutes. This protocol was adapted from Shapiro (16). Cell cycle analysis was done on a Becton-Dickinson LSRII flow cytometer and analyzed by using FACS Diva Software (Becton Dickinson, Franklin Lakes, NJ, USA).
Gammaretroviral transduction
The gammaretroviral vector MND.MFG.YFP c10 was used to test the efficiency of gene transduction in human CD34+ cells after siRNA treatment. This vector encodes an enhanced yellow fluorescence protein (YFP) under the control of the 5′ modified viral long terminal repeat. Gibbon ape leukemia virus (GALV)-pseudotyped gamma-retrovirus stocks were produced harvesting virally conditioned medium from Phoenix-GALV-MND.MFG.YFP c10 packaging cells as described previously (17;18). The medium containing viruses was harvested every 24 hours, filtered through a 0.45-mM filter, and stored at −80°C. Virus harvests had titers of approximately 1.5 × 105 IU/mL as determined by transduction of K562 cells. After transfection with siRNA, the CD34+ cells were prestimulated for 48 hours in X-VIVO/2% HSA supplemented with rhuSCF, rhuFLT-3, TPO and 4 μg/mL protamine sulfate (Sigma, St Louis, Missouri). For transduction, CD34+ cells were transferred to 6-well plates previously coated with the CH-296 fragment of fibronectin (Retronectin®, TaKaRa, Shiga, Japan) per manufacturer’s instructions and preloaded twice with virus-containing medium using a spinoculation procedure (MOI = 0.3) (19). Cells were incubated at 37°C for 48 hours in a 5% CO2 humidified incubator until examined for YFP expression on a Coulter FC500 analyzer (Beckman Coulter, Fullerton, CA, USA).
NSG mouse repopulation assay
Sublethally irradiated (300 cGy) 10–13 week old NSG mice were transplanted by tail-vein injection according to standard protocols (20) with 3×106 CD34+ cells previously transfected with PTEN1, PTEN2, or control siRNAs. Cells were transplanted immediately after transfection. Control (untreated) mice were transplanted with 1.5×106 human CD34+ cells not subjected to electroporation. At week 8 post-transplantation, mice were sacrificed and BM mononuclear cells were harvested from both femurs and tibias. Red blood cells were lysed and aliquots containing at least 5 × 105 cells were stained with anti-human CD45 antibodies (CD45-PE, Becton Dickinson, Franklin Lakes, NJ, USA) for detection of human cell engraftment. For lineage engraftment analyses, cells were stained with anti-human CD3-PE, CD15-PE, CD20-PE and CD34-PE (Becton Dickinson, Franklin Lakes, NJ, USA), and analyzed on a Coulter FC500 analyzer.
RESULTS
Efficient PTEN silencing in human CD34+ cells by siRNA
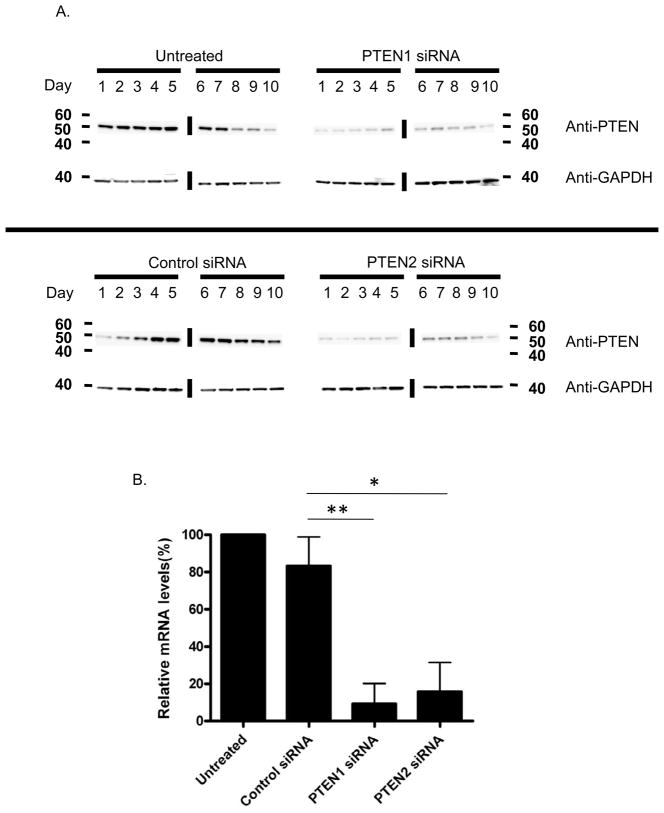
Two siRNA duplexes (PTEN1 and PTEN2) were used to knockdown PTEN expression in human CD34+ cells and non-silencing control siRNA was used to compare and exclude nonspecific effect of siRNA. We first established that nucleofection of 3.7 ug PTEN siRNA in human CD34+ cells is required for optimal silencing, as determined by western blot (data not shown), and this amount was used in all subsequent experiments. We next performed a time course study to determine the time of maximum PTEN silencing after transfection. Human CD34+ cells were cultured and collected daily for 10 days after transfection with PTEN or control siRNAs, and PTEN expression was assessed by western blot (Figure 1A). Compared to the control siRNA and untreated groups, the maximum stable decrease in PTEN expression was observed between day 1 and day 4 after transfection, followed by a partial recovery of PTEN expression thereafter, indicating a transient effect of PTEN siRNA. Hence, all subsequent analyses were performed within 4 days of transfection. In five independent experiments, PTEN expression was reduced by 73–100% (PTEN1) and 64–97% (PTEN2) relative to PTEN mRNA levels in untreated CD34+ cells 2 days after transfection (Figure 1B). In contrast, mRNA levels in samples transfected with control siRNA were only minimally reduced.
Figure 1. Transient silencing of PTEN expression in human CD34+ cells by siRNA.
(A) Western blot analysis of human CD34+ cell protein extracts 1 to 10 days following PTEN or control siRNA transfection. Untreated CD34+ cells served as a positive control. GAPDH was used as a control for equal loading of samples. The vertical lines indicate grouping of images from different gels. (B) Real-time PCR analysis of untreated human CD34+ cells, and CD34+ cells transfected with PTEN or control siRNA 48hours after transfection. Data are shown as mean percentages (± SD, n=5, t test) relative to untreated cells. mRNA levels were normalized to GAPDH expression. *p<0.01; **p<0.0001
Transient silencing of PTEN increases entry of human CD34+ cells into cell cycle
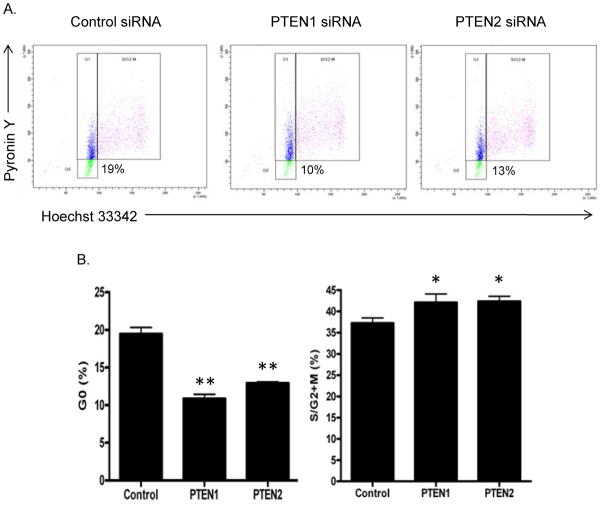
PTEN has been shown to control cell cycle entry in neural stem cells (6) and murine HSPC (3). We examined the cell cycle status of human CD34+ cells 72 hrs after transfection of PTEN or control siRNA. A representative analysis is shown in Figure 2A. In 3 independent experiments, a 1.5 to 1.8-fold decrease in the percentages of cells in the G0 phase of the cell cycle and a concomitant increase in the proportion of cells in S/G2-M were observed in PTEN siRNA transfected CD34+ cells compared with CD34+ cells transfected with control siRNA (Figure 2B). These results indicate that PTEN promotes quiescence of human CD34+ cells, and that transient silencing of PTEN increases entry of these cells into cell cycle.
Figure 2. Transient silencing of PTEN increases entry of human CD34+ cells into cell cycle.
(A) Representative cell cycle analysis comparing G0, G1, and S/G2-M phases between PTEN1, PTEN2, and control siRNA treated CD34+ cells 3 days after transfection. (B) Mean percentages (± SD, n=3, t-test) of control or PTEN siRNA-treated CD34+ cells in G0 and S/G2+M phases of the cell cycles 3 days after transfection. *p<0.05; **p<0.01
Human CD34+ cells proliferate in vitro after transient PTEN silencing
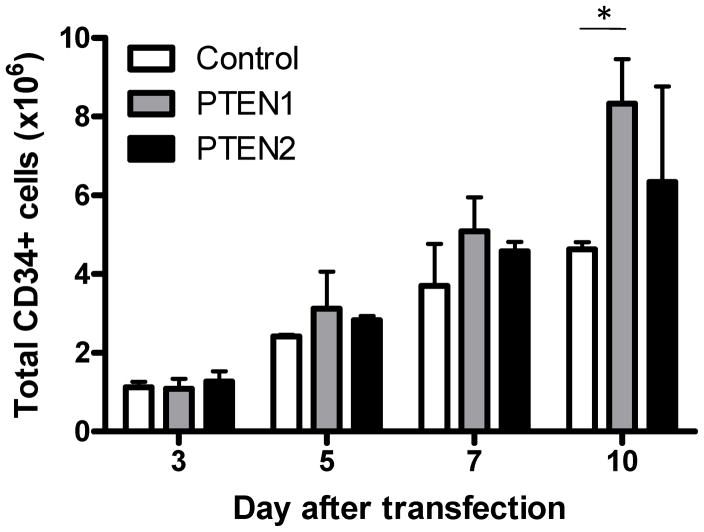
To evaluate the impact of PTEN suppression on in vitro proliferation, human CD34+ cells were maintained in culture up to 10 days after transfection with PTEN or control siRNA (n=3). After 3 days in culture with cytokines, the total number of cells had decreased to less than half the original number (3.0 × 106) of cells transfected in both the PTEN and control siRNA groups (Figure 3). However, 10 days after nucleofection, the mean total cell counts were higher in the PTEN1 (8.33 × 106) and PTEN2 (6.35 × 106) siRNA groups, compared with the control (4.63 × 106) siRNA group (Figure 3), suggesting that transient inactivation of PTEN activity results in HSPC expansion in vitro.
Figure 3. In vitro proliferation of human CD34+ cells after transient PTEN silencing.
At baseline (day 0), 3×106 CD34+ cells were transfected with control, PTEN1, or PTEN2 siRNAs. Cells were maintained in culture for 10 days in the presence of cytokines (SCF, Flt3-L and TPO) and the absolute numbers of CD34+ cells were counted at day 3, 5, 7, and 10 for each group (± SD, n=3, t-test). *p<0.005
Transient silencing of PTEN increases the efficiency of retroviral gene transduction in human CD34+ cells
We hypothesized that the increased proliferation and proportion of cycling CD34+ cells induced by PTEN siRNA may enhance their susceptibility to retroviral transduction for gene therapy applications. Human CD34+ cells were transfected with PTEN or control siRNAs, prestimulated for 2 days with cytokines and transduced for an additional 2 days with MND.MFG.YFP c10 retroviral vectors (MOI = 0.3). Viral transduction efficiencies in the bulk CD34+ cells transfected with PTEN1 or PTEN2 siRNAs were significantly higher compared with the control siRNA group, as measured by detection of yellow YFP by flow cytometry. A representative experiment is shown in figures 4A and 4B. The mean percentages of YFP+ cells from eight independent transduction experiments were 41.7%, 38.9%, and 29.4% for the PTEN1, PTEN2, and control groups, respectively (Figure 4C).
Figure 4. Transient silencing of PTEN enhances the efficiency of retroviral gene transduction in human CD34+ cells.
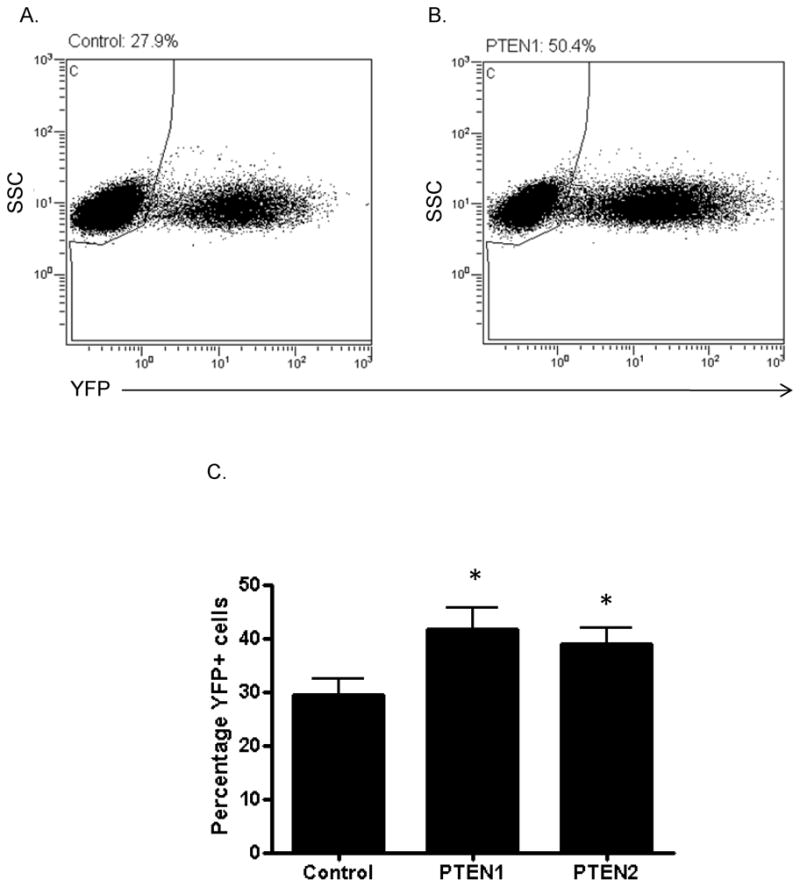
(A–B) Representative flow cytometry analysis of YFP expression after retroviral (MND.MFG.YFP c10) transduction of human CD34+ cells treated with control (A) or PTEN1 (B) siRNAs. (C) Mean percentages (± SD, n=8, t-test) of YFP+ cells after transduction of human CD34+ cells treated with control, PTEN1, or PTEN2 siRNAs. *p < 0.01
Transient silencing of PTEN expression enhances human cell engraftment in NSG mice
To evaluate whether transient PTEN silencing in human CD34+ cells enhances their proliferative and engraftment potential in vivo, we transplanted NSG mice with CD34+ cells immediately after transfection with PTEN or control siRNAs. Human cell engraftment was determined by flow cytometry using a human specific CD45 antibody two months after transplantation. The mean percentages of CD45+ human cells in the mouse bone marrow were 37.6%, 28.7%, 19.7%, and 19.2% for the PTEN1, PTEN2, control, and untreated groups respectively (Figure 5). NSG mice transplanted with PTEN1 or PTEN2 siRNA-treated CD34+ cells showed no significant differences in human myeloid, T cell, and B cell differentiation compared to control mice (data not shown). No mice developed myeloproliferative disorders or leukemias over the two months of follow-up. These data indicate that transient PTEN suppression increases the proliferative and engraftment potential of human repopulating stem/progenitor cells and maintains their multilineage differentiation capacity.
Figure 5. Comparison of human cell engraftment in the BM of NSG mice transplanted with human CD34+ cells transfected with control or PTEN1 or PTEN2 siRNA.
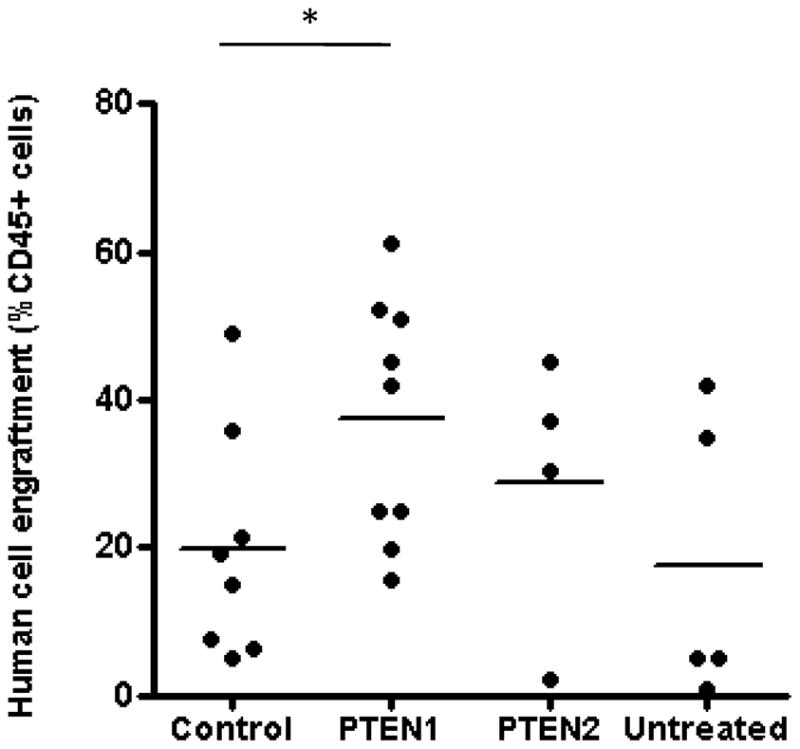
Percentages of human cell engraftment in the bone marrow of NSG mice as determined by flow cytometry using a human-specific CD45 antibody two months after transplantation. Horizontal bars represent means. Each dot represents an individual mouse. *p<0.05
DISCUSSION
PTEN is a non-redundant phosphatase that functions as a negative regulator of the PI3K-AKT pathway, which has important roles in cell proliferation, survival and differentiation (5). PTEN deficiency leads to accumulation of PIP3 which in turn activates several signaling molecules. Among them, Akt has been best characterized and its overexpression results in enhanced self-renewal of HSPC (9). A number of substrates have been identified for Akt including the proapoptotic factors BAD, caspase 3, and 9 (10). Phosphorylation of these molecules leads to changes in their subcellular localization, activities, or half lives, which in turn controls cell metabolism, cell death, cell cycle progression, and cell differentiation. Permanent inactivation of PTEN showed short-term expansion of HSPCs, but long-term decline as determined by their impaired ability to sustain hematopoietic reconstitution. Mice with PTEN-deficient BM had abnormal lineage fate determination and eventually developed myeloproliferative disorders (3).
In the present study, we sought to determine whether siRNA-based transient silencing of PTEN in human CD34+ cells could promote HSPC proliferative potential in vitro and improve human cell engraftment in NSG mice. siRNA are relatively unstable in blood and serum, as they are degraded by endo- and exonucleases, so their action is transient (11). They have been directly delivered into mammalian cells via nucleofection or, alternatively, using engineered viral vectors. Viral strategies are time consuming, require special safety precautions and, unless viral vectors are modified to eliminate their innate ability to integrate in the genome, their silencing effects are permanent. In this study, we directly transferred siRNA via nucleofection to achieve the desired transient silencing of PTEN. The reported (12) and our observed cell survival rates after nucleofection were approximately 50%. Less toxic methodologies for introducing the siRNA molecules into the target human HPSCs must be developed, for instance non-integrating lentiviral vectors, before this approach could be applied clinically.
Consistent with findings in the mouse, suppression of PTEN in human CD34+ cells led to their proliferation in vitro and progression from quiescence into active cycle. We hypothesized that the increased proportion of cycling CD34+ cells by transient PTEN suppression may enhance their susceptibility to retroviral transduction and may improve results in gene therapy protocols. Transduction efficiencies in the bulk CD34+ cells transfected with PTEN1 and PTEN2 siRNAs were significantly higher compared with CD34+ cells transfected with a control siRNA. Others have reported that reducing the levels of both TGF-β with antibodies and p27kip-1 with antisense oligonucleotides were required to stimulate cell cycle entry and increase gene transduction efficiencies in human hematopoietic cells (13). In contrast, the siRNA approach used in this study provides a relatively simple method for ex vivo manipulation and gene transduction of human CD34+ cells. Transplantation studies in NSG mice will be required to determine if improved transduction of cells with in vivo repopulating potential is achieved.
In mice, permanent deletion of PTEN increased HSPC proliferation and led to their depletion by inhibition of self-renewal (3;4). PTEN was also found to have a role in controlling hematopoietic lineage fate, as evidenced by an increased representation of myeloid and T-lymphoid lineages, and the decline in B-lineage numbers in PTEN mutants (3). We investigated the impact of transient PTEN silencing on the proliferative and engraftment potential of human CD34+ cells after transplantation into NSG mice. Mice were transplanted with PTEN or control siRNA treated CD34+ cells immediately after nucleofection. An increase in the number of human CD45+ cells was found in mice transplanted with CD34+ cells treated with PTEN1 siRNA compared with mice transplanted with control siRNA treated CD34+ cells. Both control and PTEN silenced groups showed similar multilineage differentiation, with equivalent percentages of myeloid and lymphoid human progeny cells. In addition, while PTEN mutant mice developed myeloproliferative diseases within days and transplantable leukemias (4), transient PTEN depletion in human CD34+ cells did not lead to leukemogenesis, although follow-up was short, and the xenograft model may not be adequate for testing the leukemic potential of perturbations in human hematopoietic cells.
Overall, our data suggest that the transient suppression of PTEN in CD34+ cells results in increased proliferation of HSPCs in vitro and increased self-renewal of cells capable of in vivo reconstitution in NSG mice, without an appreciable change in their capacity for multipotential differentiation. Optimization of technologies for transfer of siRNA in primary CD34+ cells will be required to allow improved cell survival and permit clinical applications in transplant-related therapies, including gene therapy protocols and adult cord blood stem cell transplantation where the number of HSPCs is limited.
Acknowledgments
We thank Dr. David Stroncek and the department of transfusion medicine staff for the apheresis and CD34+ cell selection from healthy donors. The gammaretrovirus vectors used in this study, MND.MFG.YFP c10 were a gift from Dr. Hans-Peter Kiem. We are also grateful to Keyvan Keyvanfar from the Hematology Branch flow facility for his assistance with flow sorting and analysis. This work was supported by the intramural research programs of the NHLBI (CED) of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST DISCLOSURE
The authors have declared that no conflict of interest exists
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huang X, Cho S, Spangrude GJ. Hematopoietic stem cells: generation and self-renewal. @Cell Death Differ. 2007;14:1851–1859. doi: 10.1038/sj.cdd.4402225. [DOI] [PubMed] [Google Scholar]
- 2.Blank U, Karlsson G, Karlsson S. Signaling pathways governing stem-cell fate. Blood. 2008;111:492–503. doi: 10.1182/blood-2007-07-075168. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Grindley JC, Yin T, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz OH, Valdez R, Theisen BK, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 5.Stiles B, Groszer M, Wang S, Jiao J, Wu H. PTENless means more. Dev Biol. 2006;273:175–184. doi: 10.1016/j.ydbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Groszer M, Erickson R, Scripture-Adams DD, et al. PTEN negatively regulates neural stem cell self-renewal by modulating G0–G1 cell cycle entry. Proc Natl Acad Sci U S A. 2006;103:111–116. doi: 10.1073/pnas.0509939103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo W, Lasky JL, Chang CJ, et al. Multi-genetic events collaboratively contribute to Pten-null leukaemia stem-cell formation. Nature. 2008;453:529–533. doi: 10.1038/nature06933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Ross AH. Why is PTEN an important tumor suppressor? J Cell Biochem. 2007;102:1368–1374. doi: 10.1002/jcb.21593. [DOI] [PubMed] [Google Scholar]
- 9.Sinor AD, Lillien L. Akt-1 expression level regulates CNS precursors. J Neurosci. 2004;24:8531–8541. doi: 10.1523/JNEUROSCI.1470-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stambolic V, Suzuki A, de la Pompa JL, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1009;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 11.Akhtar S, Benter IF. Nonviral delivery of synthetic siRNAs in vivo. J Clin Invest. 2007;117:3623–3632. doi: 10.1172/JCI33494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Von LG, Spanholtz J, Beckmann J, et al. Nucleofection, an efficient nonviral method to transfer genes into human hematopoietic stem and progenitor cells. Stem Cells Dev. 2006;15:278–285. doi: 10.1089/scd.2006.15.278. [DOI] [PubMed] [Google Scholar]
- 13.Dao MA, Taylor N, Nolta JA. Reduction in levels of the cyclin-dependent kinase inhibitor p27(kip-1) coupled with transforming growth factor beta neutralization induces cell-cycle entry and increases retroviral transduction of primitive human hematopoietic cells. Proc Natl Acad Sci U S A. 1998;95:13006–13011. doi: 10.1073/pnas.95.22.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gresch O, Engel FB, Nesic D, et al. New non-viral method for gene transfer into primary cells. Methods. 2004;33:151–163. doi: 10.1016/j.ymeth.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Klatt AR, Klinger G, Zech D, et al. RNAi in primary human chondrocytes: efficiencies, kinetics, and non-specific effects of siRNA-mediated gene suppression. Biologicals. 2007;35:321–328. doi: 10.1016/j.biologicals.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro HM. Flow cytometric estimation of DNA and RNA content in intact cells stained with Hoechst 33342 and pyronin Y. Cytometry. 1981;2:143–150. doi: 10.1002/cyto.990020302. [DOI] [PubMed] [Google Scholar]
- 17.Kiem HP, Heyward S, Winkler A, et al. Gene transfer into marrow repopulating cells: comparison between amphotropic and gibbon ape leukemia virus pseudotyped retroviral vectors in a competitive repopulation assay in baboons. Blood. 1997;90:4638–4645. [PubMed] [Google Scholar]
- 18.Horn PA, Topp MS, Morris JC, Riddell SR, Kiem HP. Highly efficient gene transfer into baboon repopulating cells using GALV-pseudotyped oncoretroviral vectors produced by human packaging cells. Blood. 2002;100:3960–3967. doi: 10.1182/blood-2002-05-1359. [DOI] [PubMed] [Google Scholar]
- 19.Hanenberg H, Xiao XL, Dilloo D, Hashino K, Kato I, Williams DA. Colocalization of retrovirus and target cells on specific fibronectin fragments increases genetic transduction of mammalian cells. Nat Med. 1996;2:876–882. doi: 10.1038/nm0896-876. [DOI] [PubMed] [Google Scholar]
- 20.Larochelle A, Vormoor J, Hanenberg H, et al. Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: implications for gene therapy. Nat Med. 1996;2:1329–1337. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]