Abstract
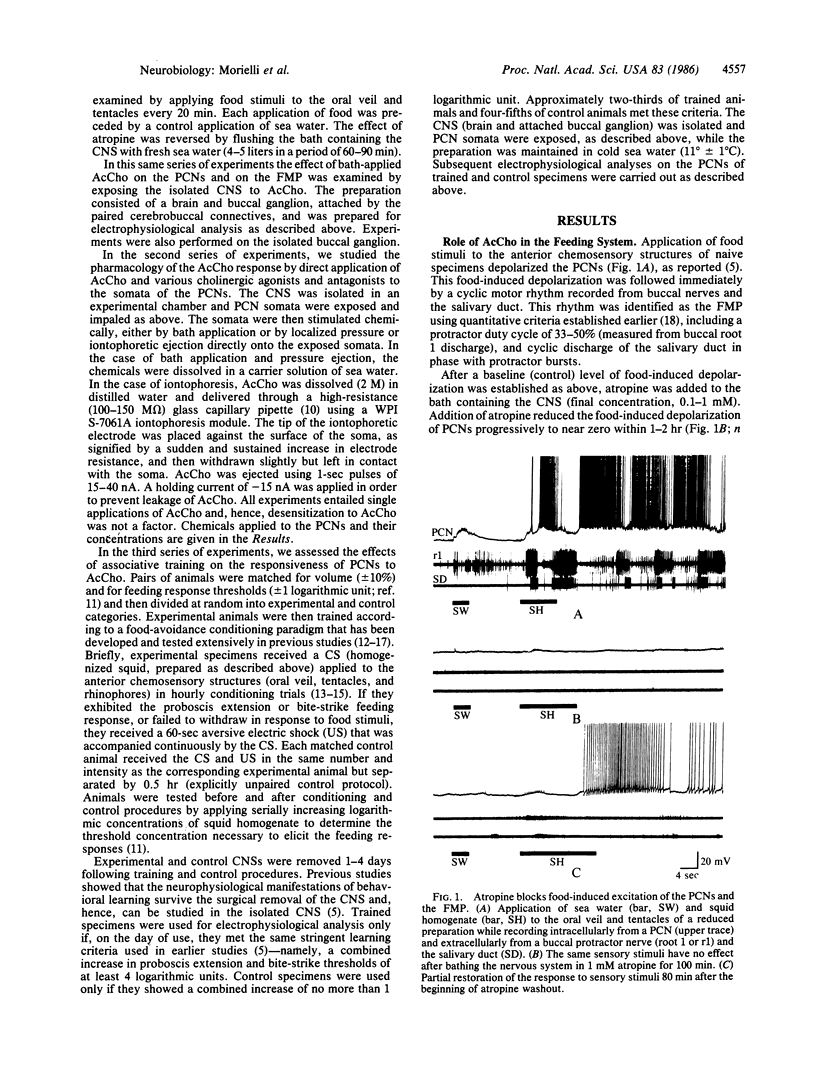
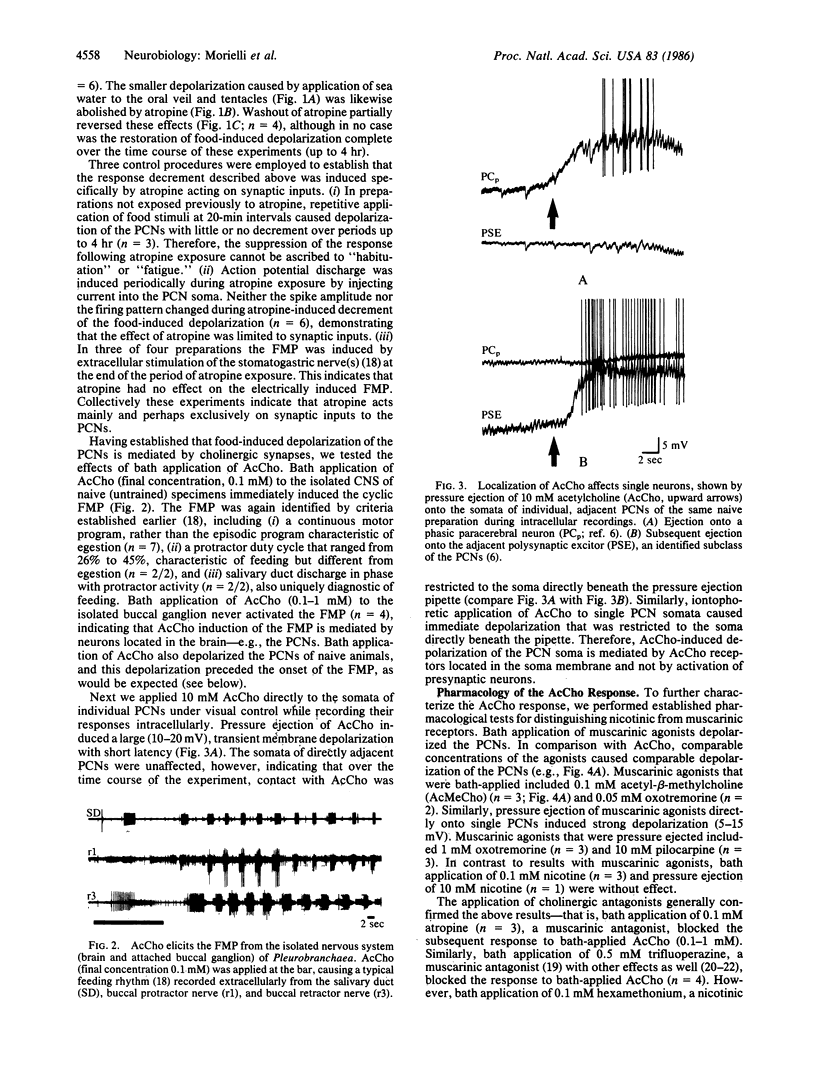
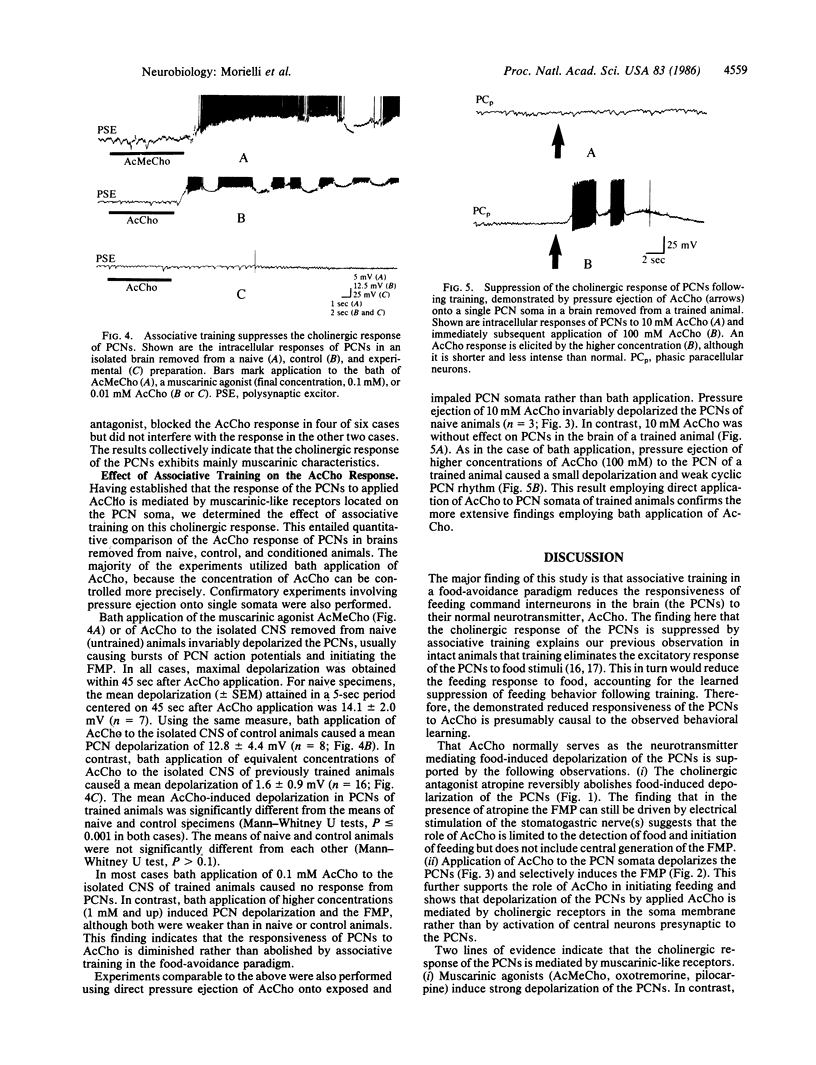
Food avoidance learning in the mollusc Pleurobranchaea entails reduction in the responsiveness of key brain interneurons in the feeding neural circuitry, the paracerebral feeding command interneurons (PCNs), to the neurotransmitter acetylcholine (AcCho). Food stimuli applied to the oral veil of an untrained animal depolarize the PCNs and induce the feeding motor program (FMP). Atropine (a muscarinic cholinergic antagonist) reversibly blocks the food-induced depolarization of the PCNs, implicating AcCho as the neurotransmitter mediating food detection. AcCho applied directly to PCN somata depolarizes them, indicating that the PCN soma membrane contains AcCho receptors and induces the FMP in the isolated central nervous system preparation. The AcCho response of the PCNs is mediated by muscarinic-like receptors, since comparable depolarization is induced by muscarinic agonists (acetyl-beta-methylcholine, oxotremorine, pilocarpine), but not nicotine, and blocked by muscarinic antagonists (atropine, trifluoperazine). The nicotinic antagonist hexamethonium, however, blocked the AcCho response in four of six cases. When specimens are trained to suppress feeding behavior using a conventional food-avoidance learning paradigm (conditionally paired food and shock), AcCho applied to PCNs in the same concentration as in untrained animals causes little or no depolarization and does not initiate the FMP. Increasing the concentration of AcCho 10-100 times, however, induces weak PCN depolarization in trained specimens, indicating that learning diminishes but does not fully abolish AcCho responsiveness of the PCNs. This study proposes a cellular mechanism of long-term associative learning--namely, postsynaptic modulation of neurotransmitter responsiveness in central neurons that could apply also to mammalian species.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkon D. L. Calcium-mediated reduction of ionic currents: a biophysical memory trace. Science. 1984 Nov 30;226(4678):1037–1045. doi: 10.1126/science.6093258. [DOI] [PubMed] [Google Scholar]
- Bammer G. Pharmacological investigations of neurotransmitter involvement in passive avoidance responding: a review and some new results. Neurosci Biobehav Rev. 1982 Fall;6(3):247–296. doi: 10.1016/0149-7634(82)90041-0. [DOI] [PubMed] [Google Scholar]
- Castellucci V. F., Carew T. J., Kandel E. R. Cellular analysis of long-term habituation of the gill-withdrawal reflex of Aplysia californica. Science. 1978 Dec 22;202(4374):1306–1308. doi: 10.1126/science.214854. [DOI] [PubMed] [Google Scholar]
- Castellucci V. F., Kandel E. R. A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5004–5008. doi: 10.1073/pnas.71.12.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll R. P., Davis W. J., Kovac M. P. Neural mechanisms of motor program switching in the mollusc Pleurobranchaea. I. Central motor programs underlying ingestion, egestion, and the "neutral" rhythm(s). J Neurosci. 1985 Jan;5(1):48–55. doi: 10.1523/JNEUROSCI.05-01-00048.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll R. P., Kovac M. P., Davis W. J. Neural mechanisms of motor program switching in the mollusc Pleurobranchaea. II. Role of the ventral white cell, anterior ventral, and B3 buccal neurons. J Neurosci. 1985 Jan;5(1):56–63. doi: 10.1523/JNEUROSCI.05-01-00056.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis W. J., Gillette R., Kovac M. P., Croll R. P., Matera E. M. Organization of synaptic inputs to paracerebral feeding command interneurons of Pleurobranchaea californica. III. Modifications induced by experience. J Neurophysiol. 1983 Jun;49(6):1557–1572. doi: 10.1152/jn.1983.49.6.1557. [DOI] [PubMed] [Google Scholar]
- Davis W. J., Gillette R. Neural correlate of behavioral plasticity in command neurons of Pleurobranchaea. Science. 1978 Feb 17;199(4330):801–804. doi: 10.1126/science.622572. [DOI] [PubMed] [Google Scholar]
- Drachman D. A. Memory and cognitive function in man: does the cholinergic system have a specific role? Neurology. 1977 Aug;27(8):783–790. doi: 10.1212/wnl.27.8.783. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Peper K. Iontophoretic application of acetylcholine: advantages of high resistance micropipettes in connection with an electronic current pump. Pflugers Arch. 1974 Apr 22;348(3):263–272. doi: 10.1007/BF00587417. [DOI] [PubMed] [Google Scholar]
- Fisher S. K., Klinger P. D., Agranoff B. W. Muscarinic agonist binding and phospholipid turnover in brain. J Biol Chem. 1983 Jun 25;258(12):7358–7363. [PubMed] [Google Scholar]
- Friedman E., Lerer B., Kuster J. Loss of cholinergic neurons in the rat neocortex produces deficits in passive avoidance learning. Pharmacol Biochem Behav. 1983 Aug;19(2):309–312. doi: 10.1016/0091-3057(83)90057-6. [DOI] [PubMed] [Google Scholar]
- Gillette R., Kovac M. P., Davis W. J. Command neurons in Pleurobranchaea receive synaptic feedback from the motor network they excite. Science. 1978 Feb 17;199(4330):798–801. doi: 10.1126/science.622571. [DOI] [PubMed] [Google Scholar]
- Gillette R., Kovac M. P., Davis W. J. Control of feeding motor output by paracerebral neurons in brain of Pleurobranchaea californica. J Neurophysiol. 1982 May;47(5):885–908. doi: 10.1152/jn.1982.47.5.885. [DOI] [PubMed] [Google Scholar]
- Hawkins R. D., Abrams T. W., Carew T. J., Kandel E. R. A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science. 1983 Jan 28;219(4583):400–405. doi: 10.1126/science.6294833. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Kehoe J. The physiological role of three acetylcholine receptors in synaptic transmission in Aplysia. J Physiol. 1972 Aug;225(1):147–172. doi: 10.1113/jphysiol.1972.sp009932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Three acetylcholine receptors in Aplysia neurones. J Physiol. 1972 Aug;225(1):115–146. doi: 10.1113/jphysiol.1972.sp009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovac M. P., Davis W. J., Matera E. M., Croll R. P. Organization of synaptic inputs to paracerebral feeding command interneurons of Pleurobranchaea californica. I. Excitatory inputs. J Neurophysiol. 1983 Jun;49(6):1517–1538. doi: 10.1152/jn.1983.49.6.1517. [DOI] [PubMed] [Google Scholar]
- Kovac M. P., Davis W. J., Matera E. M., Morielli A., Croll R. P. Learning: neural analysis in the isolated brain of a previously trained mollusc, Pleurobranchaea californica. Brain Res. 1985 Apr 8;331(2):275–284. doi: 10.1016/0006-8993(85)91553-7. [DOI] [PubMed] [Google Scholar]
- Kubanis P., Zornetzer S. F., Freund G. Memory and postsynaptic cholinergic receptors in aging mice. Pharmacol Biochem Behav. 1982 Aug;17(2):313–322. doi: 10.1016/0091-3057(82)90086-7. [DOI] [PubMed] [Google Scholar]
- Lo Conte G., Bartolini L., Casamenti F., Marconcini-Pepeu I., Pepeu G. Lesions of cholinergic forebrain nuclei: changes in avoidance behavior and scopolamine actions. Pharmacol Biochem Behav. 1982 Nov;17(5):933–937. doi: 10.1016/0091-3057(82)90475-0. [DOI] [PubMed] [Google Scholar]
- Mason S. T. The neurochemistry and pharmacology of extinction behavior. Neurosci Biobehav Rev. 1983 Fall;7(3):325–347. doi: 10.1016/0149-7634(83)90036-2. [DOI] [PubMed] [Google Scholar]
- Mazzei G. J., Schatzman R. C., Turner R. S., Vogler W. R., Kuo J. F. Phospholipid-sensitive Ca2+-dependent protein kinase inhibition by R-24571, a calmodulin antagonist. Biochem Pharmacol. 1984 Jan 1;33(1):125–130. doi: 10.1016/0006-2952(84)90379-4. [DOI] [PubMed] [Google Scholar]
- Mpitsos G. J., Collins S. D. Learning: rapid aversive conditioning in the gastropod mollusk Pleurobranchaea. Science. 1975 May 30;188(4191):954–957. doi: 10.1126/science.1138366. [DOI] [PubMed] [Google Scholar]
- Mpitsos G. J., Collins S. D., McClellan A. D. Learning: a model system for physiological studies. Science. 1978 Feb 3;199(4328):497–506. doi: 10.1126/science.622551. [DOI] [PubMed] [Google Scholar]
- Mpitsos G. J., Davis W. J. Learning: classical and avoidance conditioning the mollusk Pleurobranchaea. Science. 1973 Apr 20;180(4083):317–320. doi: 10.1126/science.180.4083.317. [DOI] [PubMed] [Google Scholar]
- Richelson E. Antipsychotics block muscarinic acetylcholine receptor-mediated cyclic GMP formation in cultured mouse neuroblastoma cells. Nature. 1977 Mar 24;266(5600):371–373. doi: 10.1038/266371a0. [DOI] [PubMed] [Google Scholar]
- Seeman P., Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975 Jun 20;188(4194):1217–1219. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- Slater N. T., Hall A. F., Carpenter D. O. Trifluoperazine and calcium antagonists accelerate cholinergic desensitization in Aplysia neurons. Brain Res. 1985 Mar 11;329(1-2):275–279. doi: 10.1016/0006-8993(85)90533-5. [DOI] [PubMed] [Google Scholar]


