Abstract
Background
Previous neuroimaging research indicates that brain atrophy in Huntington disease (HD) begins many years before movement abnormalities become severe enough to warrant diagnosis. Most clinical trials being planned for individuals in the prediagnostic stage of HD propose to use delay of disease onset as the primary outcome measure. Although formulae have been developed, based on age and CAG repeat length, to predict when HD motor onset will occur, it would be useful to have additional measures that can improve the accuracy of prediction of disease onset.
Methods
The current study examined MRI measures of striatum and white matter volume in 85 individuals prospectively followed from pre-HD stage through diagnosable motor onset (“incident cases”) and 85 individuals individually-matched with incident cases on CAG repeat length, sex, and age, who were not diagnosed with HD during the course of the study.
Results
Volumes of striatum and white matter were significantly smaller in individuals who would be diagnosed 1 to 4 years following the initial MRI scan, compared to those who would remain in the pre-HD stage. Putamen volume was the measure that best distinguished between the two groups.
Conclusions
Results suggest that MRI volumetric measures may be helpful in selecting individuals for future clinical trials in pre-HD where HD motor onset is the primary outcome measure. In planning for multisite clinical trials in pre-HD, investigators may also want to consider using more objective measures, such as MRI volumes, in addition to onset of diagnosable movement disorder, as major outcome measures.
Keywords: Huntington disease, MRI, onset, prediction, prospective, striatum
Introduction
Huntington disease (HD) is an autosomal dominant neurodegenerative disorder caused by a CAG repeat expansion in the HTT gene on chromosome 4 (p16.3) (1). HD diagnosis is based on the presence of extrapyramidal movement abnormalities, typically beginning in the patient’s 40s or 50s, but the features of the disease also include cognitive decline and psychiatric impairment (see Ross & Tabrizi (2) for a review of disease pathogenesis and characteristics). Discovery of the HD gene in 1993 made it possible to identify individuals who have the gene mutation prior to the onset of symptoms. Previous neuroimaging studies indicate that significant brain atrophy, particularly in the striatum, occurs many years before diagnosis of HD (3,4). Thus, treatment will likely be most effective if it is administered prior to diagnosis. Most clinical trials that are currently being planned for subjects in the pre-diagnostic stage of HD propose to use delay of onset of HD as the primary outcome measure.
Because CAG repeat length is inversely correlated with age of onset (5,6), it is possible to predict approximate age of onset of HD motor impairments for individuals who have the HD mutant allele. Several formulae have been derived from retrospective data to predict when diagnosable motor signs of HD will occur (3,7,8). As the relationship between age at onset and the length of the CAG repeat expansion accounts for only 47% (8) to 73% (9) of the variance, it would be useful to identify other measurements that could be used to improve the accuracy of predicting the age at which motor impairment will become severe enough to warrant diagnosis. The current study was designed to determine whether neuroimaging measures could be useful in distinguishing individuals prospectively diagnosed with HD (“incident cases”) from individuals of equivalent CAG and age who remain in the pre-diagnostic stage of disease (“pre-HD”). Improving prediction of age at diagnosis will be useful in designing clinical trials in pre-HD that rely on diagnosis of HD as the major outcome measure.
Methods and Materials
Participants
The analyses presented here are based on a sample of 170 participants from PREDICT-HD (10), an international multi-site study following a large sample of individuals who are at risk for HD by virtue of having a parent with HD. All participants for the current study tested positive for the HD gene mutation but were not diagnosed with HD at study enrollment (“pre-HD”). One half of these individuals were diagnosed with the disorder sometime during the course of the study (“incident cases”), and the other half (“non-diagnosed”) were selected as individual matches for the incident cases, based on sex, age (within 2 years), and CAG repeat length (within 1). All incident cases had MRI data at least one year prior to being diagnosed; for 42 of the incident cases, the MRI was obtained two years prior to diagnosis; for 3 incident cases the MRI was obtained three years prior to diagnosis; and for another 3 the MRI was obtained four years prior to diagnosis. All non-diagnosed participants were seen at least one year following the MRI visit and none had yet received a diagnosis of HD at the time of data analysis (23 had verification of non-diagnosed status 2 or more years after the MRI; 17 had verification 3 or more years after the MRI; and 11 had verification 4 or more years after the MRI). Participants for this study came from 28 sites, with 1 to 16 participants per site. It was not possible to match the incident cases and non-diagnosed participants on site. However, there was no systematic bias in the diagnostic status of participants coming from any given site. Table 1 provides demographic and clinical information on the two groups. All aspects of the study were approved by the Institutional Review Board at each participating institution, and all participants gave written informed consent.
Table 1.
Demographic Information and Structural Volumes for Incident Cases and Non-diagnosed Participants (Based on Clinician Judgment)
| Incident | Non-diagnosed | Statistic* (p-value) |
|
|---|---|---|---|
| N | 85 | 85 | |
| Gender | 61 F 24 M |
61 F 24 M |
|
| Mean CAG repeat length (s.d.) | 43.1 (2.4) | 43.1 (2.3) | 1.0 (p = .32) |
| Mean Age at MRI (s.d.) | 44.9 (9.2) | 44.9 (9.1) | 0.98 (p = .33) |
| Years between MRI visit and “verification” visit** | 1.75 (0.77) | 2.46 (1.40) | |
| Mean UHDRS Motor Score at MRI Visit (s.d.) | 11.4 (6.6) | 4.9 (5.5) | 7.00 (p < .0001) |
| Caudate Volume | |||
| Raw (cc) ± s.d. | 4.61 (1.38) | 5.17(1.28) | |
| Corrected*** ± s.d. | .35 (.10) | .40 (.10) | 5.39 (p < .0001) |
| Putamen Volume | |||
| Raw (cc) ± s.d. | 6.01 (1.24) | 5.17(1.28) | |
| Corrected*** ± s.d. | .46 (.08) | .40 (.10) | 3.55 (p = .0006) |
| Total Striatal Volume | |||
| Raw (cc) ± s.d. | 10.63 (2.47) | 11.95 (2.58) | |
| Corrected*** ± s.d. | .81 (.17) | .92 (.20) | 5.01 (p < .0001) |
| White Matter Volume | |||
| Raw (cc) ± s.d. | 342.39 (61.29) | 357.86 (63.48) | |
| Corrected*** ± s.d | 26.06 (3.31) | 27.42 (3.57) | 2.89 (p = .0049) |
matched sample t-tests, based on ICV-corrected volumes (region divided by ICV) for structural measures.
the verification visit, which occurred 1 to 4 years after the MRI scan, is when a diagnosis of HD was made for the incident cases and the most recent visit at which the non-diagnosed participants were verified to still be free of diagnosable signs and symptoms
Corrected volumes = (structure volume/intracranial volume) * 100
PREDICT-HD participants are seen yearly by clinicians experienced in the evaluation of movement disorders and specifically trained for PREDICT-HD on administration of the Unified Huntington’s Disease Rating Scale (UHDRS) (11). Using this standardized scale that includes a series of specific assessments of HD-related motor movements, the clinician assigns a Motor Score, ranging from 0 to 124, and then assigns a score from 0 to 4 on the HD Diagnostic Rating Scale indicating the rater’s level of confidence that the motor abnormalities reflect the presence of HD. In accordance with clinical practice (12), HD diagnosis is operationally defined as a score of 4, indicating that the rater has ≥ 99% certainty that the participant shows “unequivocal presence of an otherwise unexplained extrapyramidal movement disorder.” Participants were excluded from the current study if they received a rating of 4 at baseline. All incident cases received a rating of 4 sometime during the course of the study; all non-diagnosed participants received scores of < 4 at all visits prior to the MRI scan and at least one year following the MRI scan.
Because of the large number of clinicians involved in this multi-site study and because it was not possible to individually match incident cases with non-diagnosed participants from the same sites, there was some concern about the consistency of criteria used for making diagnoses. A reanalysis was performed using groups that were based on UHDRS Motor score rather than on clinical diagnosis. The UHDRS Motor score of each incident case at the time of diagnosis was noted, along with the number of years between the scan and the diagnosis (“scan-to-diagnosis interval”). For each matched non-diagnosed case, we identified the UHDRS Motor score that was obtained at the same post-scan interval as the matched pair’s scan-to-diagnosis interval. A review of these UHDRS Motor scores revealed some overlap between the incident cases and non-diagnosed cases in the range of 9 (the lowest score for an incident case) to 26 (the highest score for a non-diagnosed participant). The UHDRS Motor score that best discriminated the incident cases from the non-diagnosed participants was 10.5. Thus, the groups were reformed, using this cut-off rather than actual clinical diagnosis. As a result, there were 13 of the original 85 pairs in which both members of the pair now fell in the same group. For 2 pairs the incident case had a UHDRS Motor Score < 10.5, and in 11 pairs the non-diagnosed participant had a UHDRS Motor Score > 10.5. Group analyses were then redone omitting these 13 pairs.
MRI Acquisition and Analysis
All MRI scans were obtained using a standard protocol that included an axial 3D volumetric spoiled gradient echo series and a dual echo proton density/T2 series. Scans were processed at The University of Iowa using AutoWorkup (13), an automated procedure implemented in BRAINS (14) and artificial neural networks (15). Volume measures were determined for caudate, putamen, total striatum (caudate + putamen), and “cortical” white matter volume (excluding white matter in the cerebellum, brainstem, and subcortical region). These regions were selected based on our previous research examining which brain regions showed the greatest volumetric differences between pre-HD and gene-negative individuals (10). After completion of AutoWorkup, all scans were individually inspected for correct realignment and coregistration, tissue classification, and accuracy of brain and subcortical structures. Participants were included in this study only if they had scans that passed inspection for all measures. Intracranial volume was also calculated to allow for correction of structural volumes for overall head size. (See Supplement for details on scan acquisition and analysis.)
Statistical Analysis
The sample consisted of 1-to-1 matched pairs. For continuous outcome variables, paired t-tests were conducted to examine group differences in MRI volumes (corrected for intracranial volume), based on either diagnostic status (incident vs. non-diagnosed) or UHDRS-Motor group (10 and under vs. 11 and above). For binary outcome variables, conditional logistic regression was performed to determine which imaging variables (corrected for ICV) best predicted group membership. Each imaging measure was standardized to have a mean of 0 and standard deviation (SD) of 1. Each of the standardized variables was analyzed separately using conditional logistic regression. All reported confidence intervals (CI) are two-sided and have confidence levels of 95%, unless specified otherwise. A significance level of 0.05 is used for all hypothesis testing. All statistical analyses were carried out with SAS v9.1 (Cary, NC).
Results
Volumes of caudate, putamen, striatum, and white matter (corrected for intracranial volume) were significantly smaller for incident cases than for the non-diagnosed participants (see Table 1). Using conditional logistic regression, the results for caudate show that for any two matched subjects with the same age, sex, and CAG length, if the case subject’s caudate was 1 SD unit less than the control (non-diagnosed) subject, the odds of HD diagnosis for the case subject had a multiplicative increase of 1.9, which was equivalent to a 47.0% increase in the odds that the case subject would be in the diagnosed group, as compared to the control subject (95% CI = 21.5%–64.3%). For putamen, a 1 SD unit lower score for a case subject was accompanied by a multiplicative increase in the odds of HD diagnosis of 3.1, which was a 68.1% increase in odds (95% CI = 44.9%–81.6%). For total striatum, the increase in the odds was 2.7 (63.5% increase) (95% CI = 44.9%–81.6%), and for white matter the increase in odds was 1.7 (41.4% increase) (95% CI = 12.7%–60.3%). Multivariate logistic regression was then performed to determine which imaging variable had the highest predictive value of diagnostic status (incident vs. non-diagnosed). When the caudate, putamen, and total white matter volume were all included in the model, multivariate conditional logistic regression showed that the putamen was the only variable which was statistically significant, χ2(1) = 7.9, p = .0049.
When the data were reanalyzed based on the UHDRS-Motor score groups (10 and under vs. 11 and above), group differences became somewhat more robust (see Table 2). Multivariate logistic regression continued to demonstrate that putamen was the only variable which was statically significant in predicting group membership, χ2(1) = 9.1, p = .0025.
Table 2.
Demographic Information and Structural Volumes for Participants Categorized by UHDRS Motor Score
| UHDRS Motor Score 11 and over |
UHDRS Motor Score 10 and under |
Statistic* (p-value) |
|
|---|---|---|---|
| N | 72 | 72 | |
| Gender | 49 F 23 M |
49 F 23 M |
|
| Mean CAG repeat length (s.d.) | 43.1 (2.4) | 43.0 (2.2) | .70 (p = .48) |
| Mean Age at MRI (s.d.) | 44.4 (8.7) | 44.4 (8.6) | .77 (p = .44) |
| Years between MRI visit and “verification” visit** | 1.7 (.73) | 2.5 (1.4) | |
| Mean UHDRS Motor Score at MRI Visit (s.d.) | 11.8 (6.4) | 3.6 (3.5) | 9.49 (p < .0001) |
| Caudate Volume | |||
| Raw (cc) ± s.d. | 4.7 (1.4) | 5.3 (1.3) | |
| Corrected*** ± s.d. | .35 (.10) | .41 (.10) | 3.71 (p = .0004) |
| Putamen Volume | |||
| Raw (cc) ± s.d. | 6.1 (1.3) | 7.0 (1.4) | |
| Corrected*** ± s.d. | .46 (.09) | .54 (.11) | 6.25 (p < .0001) |
| Total Striatal Volume | |||
| Raw (cc) ± s.d. | 10.8 (2.6) | 12.3 (2.5) | |
| Corrected*** ± s.d. | .81 (.17) | .94 (.19) | 5.59 (p < .0001) |
| White Matter Volume | |||
| Raw (cc) ± s.d. | 348.3 (62.4) | 364.3 (63.2) | |
| Corrected*** ± s.d | 26.10 (3.2) | 27.87 (3.3) | 3.48 (p = .0009) |
matched sample t-tests, based on ICV-corrected volumes (region divided by ICV) for structural measures.
the verification visit, which occurred 1 to 4 years after the MRI scan, is when a diagnosis of HD was made for the cases with higher UHDRS Motor Scores and the most recent visit at which those with lower UHDRS Motor Scores were verified to still be free of diagnosable signs and symptoms
Corrected volumes = (structure volume/intracranial volume) * 100
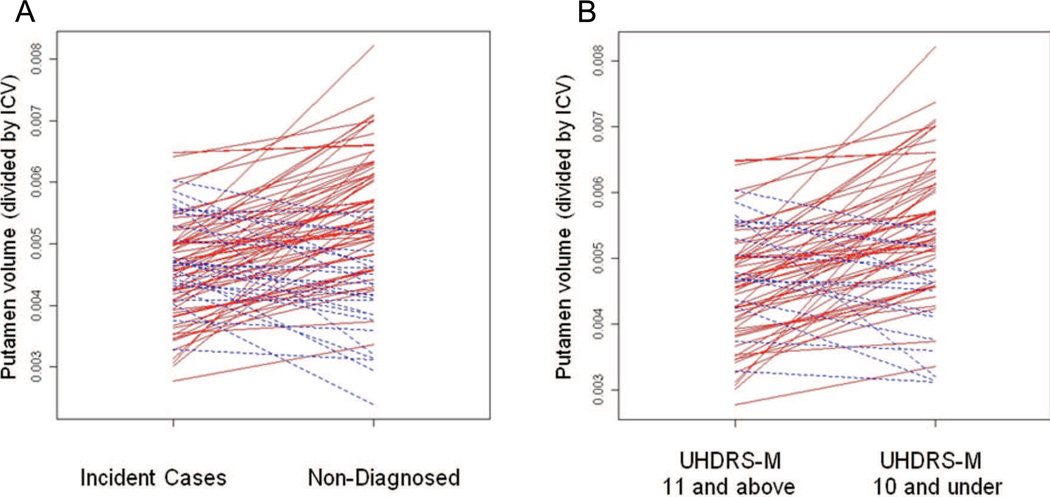
Although the group analysis showed that MRI measures were significantly related to future diagnosis, it was not the case that all incident cases had smaller striatal volumes (corrected for ICV) 1 to 4 years prior to diagnosis as compared with age- and CAG-matched participants who remained non-diagnosed (Figure 1). For the majority of matched pairs, however, the relevant structure volumes were larger for the non-diagnosed participant than for the matched incident case. The number of matched pairs with this pattern (i.e., larger volume of any magnitude for the non-diagnosed participant) was 56 (65.88%) for caudate, 61 (71.76%) for putamen, 62 (72.94%) for total striatum, and 50 (58.82%) for white matter. For the UHDRS-Motor groupings, the number of matched pairs (out of 72) in which the member with the lower UHDRS-Motor score had a larger volume than the member with the higher score was 48 (66%) for caudate, 56 (77%) for putamen, 54 (75%) for total striatum, and 45 (63%) for white matter.
Figure 1.
Lines connect corrected putamen volumes (putamen divided by intracranial volume) of matched pairs: (A) Volume for incident case on the left connected to volume for matched non-diagnosed pair member on the right (N = 85 pairs); (B) Volume for member with higher UHDRS-Motor score (11 and above) on the left connected to volume for matched pair member with the lower score on the right (10 and under). Red lines indicate pairs for which the incident case (or member with the higher Motor score) had a smaller caudate volume than the matched non-diagnosed participant (or member with the lower Motor score); blue lines indiate pairs for which the reverse was true.
Discussion
Results from this study demonstrate that volumetric MRI measures can aid in the prediction of diagnosis of HD in individuals, 1 to 4 years prior to disease onset. All of these regions were significantly smaller in those individuals who would be diagnosed 1–4 years later, as compared to those whose signs and symptoms would not be severe enough to warrant a diagnosis, even though the groups were well-matched on age, sex, and CAG repeat length.
Among the regions studied, putamen contributed most to prediction of diagnosis. As diagnosis is based on motor signs (and not cognitive or psychiatric impairment), it is possible that this reflects a stronger association between putamen and motor signs than between caudate and motor signs, as suggested by some early research in HD (16) and lesion studies (17). Cross-sectional studies by our group and others have suggested that putamen volume reduction may be slightly greater than caudate volume reduction at all pre-HD stages (4) and in early stages of manifest HD (18,19), and that the correlation with estimated years to onset may be slightly higher for putamen volume than for caudate volume (20). Longitudinal studies, however, suggest that there is somewhat greater change over time in caudate than in putamen volume, both in pre-HD (21) and manifest HD (22,23) and that the rate of atrophy may become significantly greater than zero earlier in the pre-HD period for caudate than for putamen (24). Our longitudinal findings are not consistent, however, with longitudinal functional imaging studies using PET (positron emission tomography) that show a slightly greater change over time for putamen than caudate (25,26). While white matter volume did not predict group status (incident vs. nondiagnosed) as well as striatal volume, it is possible that other methods of assessing white matter, such as diffusion tensor imaging would yield different results.
Strengths of this study include the relatively large sample of individuals who have been followed prospectively from non-diagnosed to diagnosed status, as well as very close individual matching with participants who would remain non-diagnosed. Although many of the non-diagnosed participants had some motor signs and were likely approaching HD diagnosis (as reflected by confidence ratings of 3 on the HD Diagnostic Rating Scale in the non-diagnosed group at the follow-up “verification” visit), they continued to be considered to not meet criteria for motor onset at least one year following the MRI that was used for the current data analysis. Since diagnosis is based on motor signs, it is not surprising that initial motor scores were higher in participants later given the diagnosis, and we did not attempt to incorporate both motor and imaging variables in the analysis.
An unavoidable weakness of the study is the potential lack of consistency among raters regarding the criteria they use to make a diagnosis. Because of the rarity of HD, it would not be possible to conduct this study at a single site, and it was not possible to individually match subjects from the same sites. Because criteria for making a diagnosis are subjective (the rater must have ≥ 99% certainty that the participant shows “unequivocal presence of an otherwise unexplained extrapyramidal movement disorder”), some variability is expected in raters’ judgment of the severity of motor signs necessary to warrant a diagnosis. More robust group differences were observed for the reanalysis involving groupings based on UHDRS-Motor score cutoffs, whereby cases were omitted when the diagnosis was based on a relatively low UHDRS-Motor score (10 or less) and (more often), when a diagnosis had not been made despite a relatively high score (11 or greater). Of course, the assignment of the UHDRS-Motor score, like diagnosis, involves some subjective judgment by the clinician, and necessarily influences the decision to make a diagnosis. It must be noted as well that our cut-off of 10.5 for the UHDRS-Motor groupings is based only on data from this sample and should not be considered as a recommendation for a diagnostic threshold. Because it was not possible to recruit the matched pairs from the same site, another limitation of the study was that the members of each pair were not scanned on the same scanner.
Results of this study suggest that structural MRI volumes, especially of putamen, can be useful in identifying those individuals who can be expected to be diagnosed within a relatively short timeframe. However, because of the lack of 100% predictive validity, it is not recommended that these measures be used clinically to predict for individual patients when diagnosable symptoms will occur. These measures could, however, be quite useful in selecting participants for clinical trials in which diagnosis of HD is a primary outcome measure. In planning for multisite clinical trials in pre-HD, investigators may also want to consider using more objective measures, such as MRI volumes, in addition to onset of diagnosable movement disorder, as a major outcome measure.
Supplementary Material
Acknowledgments
This research is supported by the National Institutes for Health, National Institute of Neurological Disorders and Stroke (NS40068) and CHDI Foundation, Inc.
We thank the PREDICT-HD sites, the study participants, and the National Research Roster for Huntington Disease Patients and Families.
PREDICT-HD Investigators, Coordinators, Motor Raters, Cognitive Raters
Active: September 2009 – August 2010
Thomas Wassink, MD, Stephen Cross, BA, Nicholas Doucette, BA, Mycah Kimble, BA, Patricia Ryan, MSW, LISW, MA, Jessica Wood, MD, PhD, Eric A. Epping, MD, PhD, and Leigh J. Beglinger, PhD (University of Iowa, Iowa City, Iowa, USA);
Edmond Chiu, MD, Olga Yastrubetskaya, PhD, Joy Preston, Anita Goh, D.Psych, Chathushka Fonseka, and Liz Ronsisvalle (St. Vincent’s Hospital, The University of Melbourne, Kew, Victoria, Australia);
Phyllis Chua, MD, and Angela Komiti, BS, MA (The University of Melbourne, Royal Melbourne Hospital, Melbourne, Australia)
Lynn Raymond, MD, PhD, Rachelle Dar Santos, BSc, and Joji Decolongon, MSC, CCRP (University of British Columbia, Vancouver, British Columbia, Canada);
Adam Rosenblatt, MD, Christopher A. Ross, MD, PhD, Barnett Shpritz, BS, MA, OD, and Claire Welsh (Johns Hopkins University, Baltimore, Maryland, USA);
William M. Mallonee, MD, Greg Suter, BA, and Judy Addison (Hereditary Neurological Disease Centre, Wichita, Kansas, USA);
Ali Samii, MD, and Alma Macaraeg, BS (University of Washington and VA Puget Sound Health Care System, Seattle, Washington, USA);
Randi Jones, PhD, Cathy Wood-Siverio, MS, Stewart A. Factor, DO, and Claudia Testa, MD, PhD (Emory University School of Medicine, Atlanta, Georgia, USA);
Roger A. Barker, BA, MBBS, MRCP, Sarah Mason, BSC, Anna Goodman, PhD, Rachel Swain, BA, and Anna DiPietro (Cambridge Centre for Brain Repair, Cambridge, UK);
Elizabeth McCusker, MD, Jane Griffith, RN, Clement Loy, MD, David Gunn, BS, and Linda Stewart, RN (Westmead Hospital, Sydney, Australia);
Bernhard G. Landwehrmeyer, MD, Michael Orth MD, PhD, Sigurd Süβmuth, MD, RN, Katrin Barth, RN, and Sonja Trautmann, RN (University of Ulm, Ulm, Germany);
Kimberly Quaid, PhD, Melissa Wesson, MS, and Joanne Wojcieszek, MD (Indiana University School of Medicine, Indianapolis, IN);
Mark Guttman, MD, Alanna Sheinberg, BA, and Irita Karmalkar, BSc (Centre for Addiction and Mental Health, University of Toronto, Markham, Ontario, Canada);
Susan Perlman, MD and Arik Johnson, PsyD (University of California, Los Angeles Medical Center, Los Angeles, California, USA);
Michael D. Geschwind, MD, PhD, Jon Gooblar, BA, and Gail Kang, MD (University of California San Francisco, California, USA);
Tom Warner, MD, PhD, Maggie Burrows, RN, BA, Marianne Novak, MD, Thomasin Andrews, MD, BSC, MRCP, Elisabeth Rosser, MBBS, FRCP, and Sarah Tabrizi, MD, PhD (National Hospital for Neurology and Neurosurgery, London, UK);
Anne Rosser, MD, PhD, MRCP, Kathy Price, RN, and Sarah Hunt, BSc (Cardiff University, Cardiff, Wales, UK);
Frederick Marshall, MD, Amy Chesire, LCSW-R, MSG, Mary Wodarski, BA, and Charlyne Hickey, RN, MS (University of Rochester, Rochester, New York, USA);
Oksana Suchowersky, MD, FRCPC, Sarah Furtado, MD, PhD, FRCPC, and Mary Lou Klimek, RN, BN, MA (University of Calgary, Calgary, Alberta, Canada);
Peter Panegyres, MB, BS, PhD, Elizabeth Vuletich, BSC, Steve Andrew, and Rachel Zombor, MPSYC (Neurosciences Unit, Graylands, Selby-Lemnos & Special Care Health Services, Perth, Australia);
Joel Perlmutter, MD, Stacey Barton, MSW, LCSW, and Amy Schmidt (Washington University, St. Louis, Missouri, USA);
Zosia Miedzybrodzka, MD, PhD, Sheila A. Simpson, MD, Daniela Rae, RN, and Mariella D’Alessandro, PhD (Clinical Genetics Centre, Aberdeen, Scotland, UK);
David Craufurd, MD, Ruth Fullam, BSC, and Elizabeth Howard, MD (University of Manchester, Manchester, UK);
Pietro Mazzoni, MD, PhD, Karen Marder, MD, MPH, and Paula Wasserman, MA (Columbia University Medical Center, New York, New York, USA);
Rajeev Kumar, MD and Diane Erickson, RN (Colorado Neurological Institute, Englewood, Colorado, USA);
Vicki Wheelock, MD, Terry Tempkin, RNC, MSN, Nicole Mans, BA, MS, and Kathleen Baynes, PhD (University of California Davis, Sacramento, California, USA);
Joseph Jankovic, MD, Christine Hunter, RN, CCRC, and William Ondo, MD (Baylor College of Medicine, Houston, Texas, USA);
Justo Garcia de Yebenes, MD, Monica Bascunana Garde, Marta Fatas, BA, and Asuncion Martinez-Descales (Hospital Ramón y Cajal, Madrid, Spain);
Wayne Martin, MD, Pamela King, BScN, RN, and Satwinder Sran, BSC (University of Alberta, Edmonton, Alberta, Canada);
Anwar Ahmed, PhD, Stephen Rao, PhD, Christine Reece, BS, Janice Zimbelman, PhD, PT, Alexandra Bea, BA, Emily Newman, BA, and Alex Bura, BA (Cleveland Clinic Foundation, Cleveland, Ohio, USA).
Steering Committee
Jane Paulsen, PhD, Principal Investigator, Eric A. Epping, MD, PhD, Hans Johnson, PhD, Megan Smith, PhD, Janet Williams, PhD, RN, FAAN, Leigh Beglinger, PhD, James Mills, MS (University of Iowa Hospitals and Clinics, Iowa City, IA); Elizabeth Aylward, PhD (Seattle Children's Research Institute, WA); Kevin Biglan, MD (University of Rochester, Rochester, NY); Blair Leavitt, MD (University of British Columbia, Vancouver, BC, Canada); Marcy MacDonald, PhD (Massachusetts General Hospital); Martha Nance, MD (Hennepin County Medical Center, Minneapolis, MN); and Cheryl Erwin, JD, PhD (University of Texas Medical School at Houston).
Scientific Sections
Bio Markers: Blair Leavitt, MDCM, FRCPC (Chair) and Michael Hayden, PhD (University of British Columbia); Stefano DiDonato, MD (Neurological Institute “C. Besta,” Italy); Ken Evans, PhD (Ontario Cancer Biomarker Network); Wayne Matson, PhD (VA Medical Center, Bedford, MA); Asa Peterson, MD, PhD (Lund University, Sweden), Sarah Tabrizi, MD, PhD (National Hospital for Neurology and Neurology and Neurosurgery, London); Beth Borowsky, PhD (CHDI); Andrew Juhl, BS, James Mills, MS, Kai Wang, PhD (University of Iowa); and David Weir, BSc (University of British Columbia).
Brain: Jean Paul Vonsattell, PhD (Chair), and Carol Moskowitz, ANP, MS (Columbia University Medical Center); Anne Leserman, MSW, LISW, Lynn Schaul, BA, and Stacie Vik, BA (University of Iowa).
Cognitive: Deborah Harrington, PhD (Chair), Gabriel Castillo, BS, Jessica Morison, BS, and Jason Reed, BS (University of California, San Diego), Michael Diaz, PhD, Ian Dobbins, PhD, Tamara Hershey, PhD, Erin Foster, OTD, and Deborah Moore, BA (Washington University Cognitive Science Battery Development); Holly Westervelt, PhD (Chair, Quality Control and Training, Alpert Medical School of Brown University), Jennifer Davis, PhD, and Geoff Tremont, PhD, MS (Scientific Consultants, Alpert Medical School of Brown University); Megan Smith, PhD (Chair, Administration), David J. Moser, PhD, Leigh J. Beglinger, PhD, Kelly Rowe, and Danielle Theriault, BS (University of Iowa); Carissa Gehl, PhD (VA Medical Center, Iowa City, IA); Kirsty Matheson (University of Aberdeen); Karen Siedlecki, PhD (Fordham University); Marleen Van Walsem (EHDN); Susan Bonner, BA, Greg Elias, BA, and Melanie Faust, BS (Rhode Island Hospital); Beth Borowski, PhD (CHDI); Noelle Carlozzi (University of Michigan); Kevin Duff, PhD (University of Utah); Nellie Georgiou-Karistianis (St. Vincent’s Hospital, The University of Melbourne, Australia); Julie Stout, PhD (Monash University, Melbourne, Australia); Herwig Lange (Air-Rahazentrum); and Kate Papp (University of Connecticut).
Functional: Janet Williams, PhD (Chair), Leigh J. Beglinger, PhD, Anne Leserman, MSW, LISW, Eunyoe Ro, MA, Lee Anna Clark, Nancy Downing, Joan Laing, PhD, Kristine Rees, BA, and Stacie Vik, BA (University of Iowa); Rebecca Ready, PhD (University of Massachusetts); Anthony Vaccarino, PhD (Ontario Cancer Biomarker Network); Sarah Farias, PhD (University of California, Davis); Noelle Carlozzi, PhD (University of Michigan); and Carissa Gehl, PhD (VA Medical Center, Iowa City, IA).
Genetics: Marcy MacDonald, PhD (Co-Chair), Jim Gusella, PhD, and Rick Myers, PhD (Massachusetts General Hospital); Michael Hayden, PhD (University of British Columbia); Tom Wassink, MD (Co-Chair) Eric A. Epping, MD, PhD, Andrew Juhl, BA, James Mills, MS, and Kai Wang, PhD (University of Iowa); Zosia Miedzybrodzka, MD, PhD (University of Aberdeen); and Christopher Ross, MD, PhD (Johns Hopkins University).
Imaging:
Administrative: Ron Pierson, PhD (Chair), Kathy Jones, BS, Jacquie Marietta, BS, William McDowell, AA, Greg Harris, BS, Eun Young Kim, MS, Hans Johnson, PhD, and Thomas Wassink, MD (University of Iowa); John Ashburner, PhD (Functional Imaging Lab, London); Steve Potkin, MD (University of California, Irvine); and Arthur Toga, PhD (University of California, Los Angeles).
Striatal: Elizabeth Aylward, PhD (Chair, Seattle Children's Research Institute).
Surface Analysis: Peg Nopoulos, MD (Chair), and Eric Axelson, BSE (University of Iowa).
Shape Analysis: Christopher A. Ross (Chair), MD, PhD, Michael Miller, PhD, and Sarah Reading, MD (Johns Hopkins University); Mirza Faisal Beg, PhD (Simon Fraser University).
DTI: Vincent A. Magnotta, PhD (Chair, University of Iowa); Karl Helmer, PhD (Massachusetts General Hospital); Kelvin Lim, MD (University of Ulm, Germany); Mark Lowe, PhD (Cleveland Clinic); Sasumu Mori, PhD (Johns Hopkins University); Allen Song, PhD (Duke University); and Jessica Turner, PhD (University of California, Irvine).
fMRI: Steve Rao, PhD (Chair), Erik Beall, PhD, Katherine Koenig, PhD, Michael Phillips, MD, Christine Reece, BS, and Jan Zimbelman, PhD, PT (Cleveland Clinic); and April Bryant (University of Iowa).
Motor: Kevin Biglan, MD (University of Rochester), Karen Marder, MD (Columbia University), and Jody Corey-Bloom, MD, PhD (University of California, San Diego) all Co-Chairs; Michael Geschwind, MD, PhD (University of California, San Francisco); Ralf Reilmann, MD and Zerka Unds (Muenster, Germany); and Andrew Juhl, BS (University of Iowa).
Psychiatric: Eric A. Epping, MD, PhD (Chair), Nancy Downing, RN, MSN, Jess Fiedorowicz, MD, Robert Robinson, MD, Megan Smith, PhD, Leigh Beglinger, PhD, James Mills, MS, Kristine Rees, BA, Adam Ruggle, Stacie Vik, BA, Janet Williams, PhD, Dawei Liu, PhD, David Moser, PhD, and Kelly Rowe (University of Iowa); Karen Anderson, MD (University of Maryland); David Craufurd, MD (University of Manchester); Mark Groves, MD (Columbia University); Anthony Vaccarino, PhD and Ken Evans, PhD (Ontario Cancer Biomarker Network); Hugh Rickards, MD (Queen Elizabeth Psychiatric Hospital); Eric van Duijn, MD (Leiden University Medical Center, Netherlands); Irina Antonijevic, MD, PhD, and Joseph Giuliano (CHDI); Phyllis Chua (The University of Melbourne, Royal Melbourne Hospital); and Kimberly Quaid, PhD (Indiana University School of Medicine).
Core Sections
Statistics: James Mills, MEd, MS, Dawei Liu, PhD, Jeffrey Long, PhD, Wenjing Lu, Kai Wang, PhD, and Ying Zhang, PhD (University of Iowa).
Recruitment/Retention: Martha Nance, MD (Chair, University of Minnesota); Anne Leserman, MSW, LISW, Nicholas Doucette, BA, Mycah Kimble, BA, Patricia Ryan, MSW, LISW, MA, Kelli Thumma, BA, Elijah Waterman, BA, and Jeremy Hinkel, BA (University of Iowa).
Ethics: Cheryl Erwin, JD, PhD, (Chair, McGovern Center for Health, Humanities and the Human Spirit); Eric A. Epping, MD, PhD Janet Williams, PhD, Nicholas Doucette, BA, Anne Leserman, MSW, LISW, James Mills, MS, Lynn Schaul, BA, and Stacie Vik, BA (University of Iowa); Martha Nance, MD (University of Minnesota); and Lisa Hughes, MEd (University of Texas Medical School at Houston).
IT/Management: Hans Johnson, PhD (Chair), R.J. Connell, BS, Karen Pease, BS, Ben Rogers, BA, BSCS, Jim Smith, AS, Shuhua Wu, MCS, Roland Zschiegner, Erin Carney, Bill McKirgan, Mark Scully, and Ryan Wyse (University of Iowa); Jeremy Bockholt (AMBIGroup).
Program Management
Administrative: Chris Werling-Witkoske (Chair), Karla Anderson, BS, Kristine Bjork, BA, Ann Dudler, Jamy Schumacher, Sean Thompson, BA, Leann Davis, Machelle Henneberry, Greg Ennis, MA, and Stacie Vik, BA (University of Iowa).
Financial: Steve Blanchard, MSHA, Kelsey Montross, BA, and Phil Danzer (University of Iowa).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Data were presented as part of a talk at the International Neuropsychology Society in Boston, Feb 2–5, 2011. The title of the talk was “Neuroimaging in Pre-HD: Results from PREDICT-HD.”
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.The Huntington's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 2.Ross CA, Tabrizi SJ. Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10:83–98. doi: 10.1016/S1474-4422(10)70245-3. [DOI] [PubMed] [Google Scholar]
- 3.Aylward EH, Codori AM, Barta PE, Pearlson GD, Harris GJ, Brandt J. Basal ganglia volume and proximity to onset in presymptomatic Huntington disease. Arch Neurol. 1996;53:1293–1296. doi: 10.1001/archneur.1996.00550120105023. [DOI] [PubMed] [Google Scholar]
- 4.Paulsen JS, Nopoulos PC, Aylward E, Ross CA, Johnson H, Magnotta VA, Juhl A, et al. Striatal and white matter predictors of estimated diagnosis for Huntington disease. Brain Res Bull. 2010;82:201–207. doi: 10.1016/j.brainresbull.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duyao M, Ambrose C, Myers R, Novelletto A, Persichetti F, Frontali M, Folstein S, et al. Trinucleotide repeat length instability and age of onset in Huntington's disease. Nat Genet. 1993;4:387–392. doi: 10.1038/ng0893-387. [DOI] [PubMed] [Google Scholar]
- 6.Stine OC, Pleasant N, Franz ML, Abbott MH, Folstein SE, Ross CA. Correlation between the onset age of Huntington's disease and length of the trinucleotide repeat in IT-15. Hum Mol Genet. 1993;2:1547–1549. doi: 10.1093/hmg/2.10.1547. [DOI] [PubMed] [Google Scholar]
- 7.Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington's disease based on CAG length. Clin Genet. 2004;65:267–277. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 8.Ranen NG, Stine OC, Abbott MH, Sherr M, Codori AM, Franz ML, Chao NI, et al. Anticipation and instability of IT-15 (CAG)n repeats in parent-offspring pairs with Huntington disease. Am J Hum Genet. 1995;57:593–602. [PMC free article] [PubMed] [Google Scholar]
- 9.Brinkman RR, Mezei MM, Theilmann J, Almqvist E, Hayden MR. The likelihood of being affected with Huntington disease by a particular age, for a specific CAG size. Am J Hum Genet. 1997;60:1202–1210. [PMC free article] [PubMed] [Google Scholar]
- 10.Paulsen JS, Hayden M, Stout JC, Langbehn DR, Aylward E, Ross CA, Guttman M, et al. Preparing for preventive clinical trials: the Predict-HD study. Arch Neurol. 2006;63:883–890. doi: 10.1001/archneur.63.6.883. [DOI] [PubMed] [Google Scholar]
- 11.Huntington Study Group. Unified Huntington's Disease Rating Scale: reliability and consistency. Mov Disord. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 12.Rosenblatt A Huntington's Disease Society of America (New York N.Y.), Foundation for the Care and Cure of Huntington's Disease. A physician's guide to the management of Huntington's disease. 2nd ed. New York: Huntington's Disease Society of America; 1999. [Google Scholar]
- 13.Pierson R, Johnson H, Harris G, Keefe H, Paulsen JS, Andreasen NC, Magnotta VA. Fully automated analysis using BRAINS: AutoWorkup. Neuroimage. 2011;54:328–336. doi: 10.1016/j.neuroimage.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magnotta VA, Harris G, Andreasen NC, O'Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 15.Powell S, Magnotta VA, Johnson H, Jammalamadaka VK, Pierson R, Andreasen NC. Registration and machine learning-based automated segmentation of subcortical and cerebellar brain structures. Neuroimage. 2008;39:238–247. doi: 10.1016/j.neuroimage.2007.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starkstein SE, Brandt J, Folstein S, Strauss M, Berthier ML, Pearlson GD, Wong D, et al. Neuropsychological and neuroradiological correlates in Huntington's disease. J Neurol Neurosurg Psychiatry. 1988;51:1259–1263. doi: 10.1136/jnnp.51.10.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatia KP, Marsden CD. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain. 1994;117(Pt 4):859–876. doi: 10.1093/brain/117.4.859. [DOI] [PubMed] [Google Scholar]
- 18.Rosas HD, Koroshetz WJ, Chen YI, Skeuse C, Vangel M, Cudkowicz ME, Caplan K, et al. Evidence for more widespread cerebral pathology in early HD: an MRI-based morphometric analysis. Neurology. 2003;60:1615–1620. doi: 10.1212/01.wnl.0000065888.88988.6e. [DOI] [PubMed] [Google Scholar]
- 19.Harris GJ, Pearlson GD, Peyser CE, Aylward EH, Roberts J, Barta PE, Chase GA, et al. Putamen volume reduction on magnetic resonance imaging exceeds caudate changes in mild Huntington's disease. Ann Neurol. 1992;31:69–75. doi: 10.1002/ana.410310113. [DOI] [PubMed] [Google Scholar]
- 20.Harris GJ, Codori AM, Lewis RF, Schmidt E, Bedi A, Brandt J. Reduced basal ganglia blood flow and volume in pre-symptomatic, gene-tested persons at-risk for Huntington's disease. Brain. 1999;122(Pt 9):1667–1678. doi: 10.1093/brain/122.9.1667. [DOI] [PubMed] [Google Scholar]
- 21.Aylward EH, Nopoulos PC, Ross CA, Langbehn DR, Pierson RK, Mills JA, Johnson HJ, et al. Longitudinal change in regional brain volumes in pre-HDHuntington disease. J Neurol Neurosurg Psychiatry. 2011;82:405–410. doi: 10.1136/jnnp.2010.208264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandenberghe W, Demaerel P, Dom R, Maes F. Diffusion-weighted versus volumetric imaging of the striatum in early symptomatic Huntington disease. J Neurol. 2009;256:109–114. doi: 10.1007/s00415-009-0086-0. [DOI] [PubMed] [Google Scholar]
- 23.Aylward EH, Li Q, Stine OC, Ranen N, Sherr M, Barta PE, Bylsma FW, et al. Longitudinal change in basal ganglia volume in patients with Huntington's disease. Neurology. 1997;48:394–399. doi: 10.1212/wnl.48.2.394. [DOI] [PubMed] [Google Scholar]
- 24.Aylward EH, Sparks BF, Field KM, Yallapragada V, Shpritz BD, Rosenblatt A, Brandt J, et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004;63:66–72. doi: 10.1212/01.wnl.0000132965.14653.d1. [DOI] [PubMed] [Google Scholar]
- 25.Feigin A, Tang C, Ma Y, Mattis P, Zgaljardic D, Guttman M, Paulsen JS, et al. Thalamic metabolism and symptom onset in preclinical Huntington's disease. Brain. 2007;130:2858–2867. doi: 10.1093/brain/awm217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciarmiello A, Cannella M, Lastoria S, Simonelli M, Frati L, Rubinsztein DC, Squitieri F. Brain white-matter volume loss and glucose hypometabolism precede the clnical symptoms of Huntington's disease. J Nucl Med. 2006;47:215–222. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.