Abstract
BACKGROUND
The aim of this work was to analyze the diagnostic and prognostic value of serum human epididymis protein 4 (HE4) and Risk for Ovarian Malignancy Algorithm (ROMA) in epithelial ovarian cancer (EOC).
METHODS
Preoperative serum samples of 419 women (140 healthy controls, 131 ovarian benign cysts, 34 endometriosis, 114 EOC) were tested for CA125 and HE4 using fully automated methods (Abbott ARCHITECT) and validated cut-off values.
RESULTS
For the discrimination of benign masses from EOC, in pre-menopausal women the sensitivity and specificity were 92.3% and 59.4% for CA125, 84.6% and 94.2% for HE4, and 84.6% and 81.2% for ROMA while in post-menopausal women the sensitivity and specificity were 94.3% and 82.3% for CA125, 78.2% and 99.0% for HE4, 93.1% and 84.4% for ROMA.
In patients with EOC, elevated CA125, HE4 and ROMA levels were associated with advanced FIGO stage, sub-optimally debulking, ascites, positive cytology, lymph node involvement and advanced age (all p≤0.05). Elevated HE4 and ROMA (both p≤0.01), but not CA125 (p=0.0579), were associated with undifferentiated tumours. In multivariable analysis, elevated HE4 and ROMA (all p≤0.05) were independent prognostic factors for shorter overall survival, disease free survival and progression free survival.
CONCLUSIONS and IMPACT
This study underlines the high specificity of HE4 in discriminating endometriosis and ovarian benign cysts from EOC and the high sensitivity of CA125 in detecting EOC. We demonstrated HE4 and ROMA as independent prognostic factors. Multicenter studies are needed to draw firm conclusions about the applicability of HE4 and ROMA in clinical practice.
Keywords: Epithelial ovarian cancer, HE4, ROMA, diagnosis, prognosis
Introduction
Epithelial ovarian cancer (EOC) is the most frequent cause of death from gynaecological cancer. It has the highest fatality-to-case ratio of all gynaecological malignancies, being characterized by early widespread metastasis and high-grade malignancy at diagnosis. The five-year survival rate is about 80–90% for patients with stage I disease and only 30% for patients with stage III or IV. Although survival has improved with the use of maximal cytoreductive surgery along with platinum- and taxol-based chemotherapy, nearly 80% of ovarian cancers relapse and patients inevitably succumb to the development of chemotherapy-resistant disease (1).
At the moment, serum CA125 is the commonly used biomarker for EOC diagnosis. Jacobs and colleagues (2) developed the widely used Risk of Malignancy Index (RMI), an algorithm that uses ultrasound findings, architectural features of pelvic mass, CA125 levels and menopausal status to stratify patients into high- and low-risk groups. However, since CA125 is associated with a high false-positive rate among benign gynaecologic conditions, such as endometriosis that affects mainly women in pre-menopause, its use for EOC detection is almost exclusively reserved for post-menopausal cases (3–6). Furthermore, CA125 has low sensitivity in identifying patients with early EOC disease, being increased in only 50% of patients with stage I (7). CA125 is also used to monitor response to therapy and in early detection of ovarian cancer recurrence after treatment (8–11), but the value of preoperative CA125 is not associated with prognosis of EOC patients (12–13). Clinicopathological features known to be prognostic variables for EOC are age, surgical stage (FIGO stage), histological subtype, differentiation grade, ascites, lymph node involvement and residual tumour after cytoreductive surgery. According to the three-yearly analysis of the FIGO Annual Report on the Results of Treatment in Gynaecological Cancer, stage, grade and residual tumour have the greatest prognostic value (14). However, these factors provide an insufficient picture of EOC biologyand they are frequently interrelated.
Therefore, there is a pressing need to develop new methods for EOC diagnosis and prognosis. First, the preoperative diagnosis of EOC would refer patients to centers specialized in optimal tumor debulking and complete surgical staging, since it has been shown that optimal surgery treatment improves overall survival in EOC patients (15–20). Moreover, the prediction of disease outcome in EOC patients could be useful for developing individually tailored and possibly more effective post-surgical treatments.
Serum analysis is a low-cost, non-invasive technique and it is not subjected to operator variability, such as imaging analysis. Therefore, considerable efforts are underway to identify new serum biomarkers that alone or in combination with CA125 could improve EOC diagnosis (7, 21–36).
In the majority of studies, HE4 has emerged as one of the most promising new serum biomarker in EOC diagnosis. Previous reports evaluated the clinical utility of the HE4 and CA125 combination (ROMA algorithm) in order to assess the risk of EOC pathology in patients presenting with a pelvic mass (32, 37–42). However, some recent papers (39, 41–42) showed that diagnostic accuracy of ROMA compared to CA125 and HE4 alone is still controversial. At the present the prognostic value of HE4 has been investigated by only one study (43) in patients with advanced EOC. This report showed HE4 as an independent prognostic factor for progression free survival. However, in such study, the prognostic analysis was conducted in a small sample size, the comparison with prognostic value of CA125 was not evaluated and EOC patients were not dichotomized by median value of biomarker, as usually performed in prognostic studies.
The aim of this work was to analyze diagnostic and prognostic value of serum HE4, CA125 and ROMA in a large number of patients, using fully automated methods for biomarkers determination that guarantee a higher reproducibility and robustness of assay results(41).
Initially, we analyzed the diagnostic performance of HE4 compared to CA125 in discriminating among subjects with EOC, ovarian benign cysts, endometriosis and healthy controls. Then, we analyzed ROMA algorithm, compared to CA125 and HE4 alone, for the differential diagnosis between benign pelvic mass and EOC.
Finally, we investigated the role of HE4 and ROMA, in comparison with CA125 and established prognostic factors, in predicting overall survival (OS), disease-free survival (DFS), and progression free survival (PFS) in EOC patients.
Material and Methods
Patients’ characteristics
A total of 419 patients referred to the Gynaecologic Oncology Department of the University of Brescia from 2003 to 2010 were included in the study. All patients signed an informed consent approved by the Institutional Review Board. Patients with a past or concomitant history of malignancy were excluded from the study. Cohorts of patients in pre- and post-menopause were balanced as regards the number and age. Pre-menopausal women included 39 healthy controls (age: mean 39 years; range 21–53), 34 patients with endometriosis (age: mean 36.5 years; range 25–51), 35 patients with ovarian benign cysts (age mean: 41.5 years; range 18–59) and 26 patients with EOC (age mean: 44.7 years; range 33–54). Post-menopausal women included 101 healthy controls (age: mean 63.3 years; range 40–76), 96 patients with ovarian benign cysts (age mean: 64.0 years; range 46–89) and 87 patients with EOC (age mean: 66.3 years; range 46–87). One EOC patient had an unknown menopausal status and thus was included only in prognostic analysis. Women were considered in post-menopause if they reported no menstrual periods within the 12 months before blood collection.
EOC patients’ charts were reviewed to obtain all clinical and pathological features at the moment of the diagnosis treatment and during follow-up. Low malignant potential tumors were excluded from this study. Standard treatment for EOC consisted in complete pelvic surgery with cytoreductive surgery in advanved stages and platinum-based chemotherapy. Cytoreductive surgery included total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy and pelvic and periaortic lymph node sampling, with cytological evaluation of ascites or peritoneal washing. The staging procedure was performed according to the International Federation of Gynaecologists and Obstetricians (FIGO) system standards. Histological subtype and differentiation grade were assigned according to World Health Organization criteria.
A group of 98 EOC patients was evaluated for survival analysis. The remaining 16 EOC patients were excluded from survival analysis because 2 refused chemotherapy, 4 were not eligible for primary surgery because of their poor medical conditions and 10 had incomplete follow-up. Patients were followed up from the date of surgery until death or November 30, 2010 (median follow-up: 19.5 months, range 1–85 months).
HE4 and CA125 immunoassays
Blood was drawn before any surgical or chemotherapeutic treatment and centrifuged within half an hour for serum collection. Serum samples were stored at −80°C until analysis. Levels of CA125 and HE4 were measured by chemiluminescent microparticle immunoassays (CMIA) on the fully automated ARCHITECT instrument (Abbott Diagnostics Division, Wiesbaden, Germany) at the III Laboratory Service, Spedali Civili di Brescia, Italy. According to the indications of the HE4 manufacturer, the normal ranges were ≤70 pmol/L for pre-menopausal state and ≤140 pmol/L in menopausal state.
Roma Algorithm
ROMA utilizes the results for HE4 and CA125 to generate a predictive index (PI) for EOC (32), calculated by these formulas:
Then, ROMA value is calculated as follow: ROMA value (%) = exp(PI) / [1+exp(PI)] *100. According to the indications of the HE4 manufacturer, indexes of at least 7.4% and 25.3% indicate a high risk for the presence of EOC in pre- or post-menopause, respectively.
Statistical analysis
The Wilcoxon-Mann-Whitney test and the Kruskal-Wallis test were used to compare biomarkers distributions across two and more than two subgroups of patients respectively. Differences between the proportions of patients with level of biomarkers above thresholds have been compared within the same subgroup of patients with Mc Nemar test.
Area under the receiver operating characteristic (ROC) curves were used to quantify each biomarker’s ability to discriminate between diagnostic groups. Areas under the ROC curves (AUCs) were compared with the method described by DeLong (44).
For survival analysis, three end-points (cancer relapse, cancer progression and cancer related death) were used to calculate DFS, PFS, OS, respectively. DFS was defined as the time interval between the date of surgery and the date of identification of disease recurrence, PFS was defined as the time interval between the date of surgery and the date of identification of progressive disease (disease not treatable with curative intent) and OS was defined as the time interval between the date of surgery and the date of death. For all three end-points the last date of follow-up was used for censored subjects.
Survival curves were calculated using Kaplan–Meier method, and differences in survival between subgroups of patients were tested using the log-rank test.
The effect of HE4, CA125 and ROMA serum levels on prognosis was evaluated categorizing them on the basis of the median values, computed on the whole cohort (Low; High).
Univariate Cox proportional hazard models were fitted to evaluate the role of CA125, HE4, ROMA and established prognostic factors on the considered outcomes. Multivariable Cox regression models were used to estimate the effect of biomarkers adjusted for FIGO stage, residual tumour and histological subtype, the most important established prognostic factors.
All P-values were two-sided. A P-value less than 0.05 was considered to indicate statistical significance. All the analyses were performed using STATA 11.0 software (Stata Corporation, College Station, Texas)
Results
CA125, HE4 and ROMA diagnostic performances
Comparison of CA125 and HE4 levels between pre- and post-menopausal healthy controls showed that CA125 is significantly higher in pre-menopausal than in post-menopausal status (14.9 U/ml vs 10.1 U/ml, p=0.0001), while HE4 is inversely significantly higher in post-menopausal than in pre-menopausal status (41.2 pM vs 35.2 pM, p=0.001). For this reason CA125 and HE4 diagnostic performances were analyzed separately in pre- and post-menopausal women. CA125 and HE4 values detected in healthy controls and in patients with endometriosis, ovarian benign cysts and EOCs are represented in Figure 1.
Figure 1. Serum CA125 and HE4 levels detected in healthy controls and in patients with endometriosis, ovarian cysts and epithelial ovarian cancer.
HC, healthy controls; End, patients with endometriosis; Cysts, patients with ovarian benign cysts; EOC, patients with epithelial ovarian cancer; HE4, human epididymis protein 4
The levels of HE4 and CA125 were significantly higher in EOC patients compared with healthy controls, endometriosis and ovarian benign cysts, independently from menopausal status (all p<0.0001). Both CA125 and HE4 levels were slightly higher in patients with ovarian cysts when compared with healthy controls, but these differences reached the statistical significance only in post-menopausal women (14.5 U/ml vs 10.1 U/ml, p<0.0001 for CA125; 43.8 pM vs 41.2 pM, p=0.0381 for HE4) and not in pre-menopausal ones (p=0.1561 for CA125; p=0.2718 for HE4). In pre-menopausal women, HE4 and CA125 showed different ability in discriminating endometriosis from healthy controls and ovarian benign cysts. CA125 was significantly higher in patients with endometriosis (49.1 U/ml) than in healthy controls (14.9 U/ml) and ovarian benign cysts (16.3 U/ml) (both p<0.0001). On the contrary, HE4 showed a marginally significant increase in endometriosis (39.1 pM) towards healthy controls (35.2 pM) (p=0.0447) and a not statistically significant increase in endometriosis towards ovarian benign cysts (36.2 pM) (p=0.5015).
In order to evaluate the differences in diagnostic abilities between CA125 and HE4, we used the reference value indicated by standard clinical use for CA125 (35 U/ml) or proposed by the manufacturer for HE4. In patients with EOC, CA125 and HE4 levels were above cut-off in 82/87 (94.3%) and in 68/87 (78.1%) post-menopausal patients, respectively, and in 24/26 (92.3%) and in 22/24 (84.6%) pre-menopausal patients. The difference between CA125 and HE4 was statistically significant only in post-menopausal women (p=0.0002). In patients with ovarian benign cysts, CA125 and HE4 levels were above the cut-off values in 17/96 (17.7%) and in 1/96 (1.0%) post-menopausal women, respectively (p=0.002), and in 3/35 (8.5%) and 2/35 (5.7%) pre-menopausal women, respectively (p=0.6547). In patients with endometriosis, all in pre-menopausal status, CA125 and HE4 were above cut-off in significantly (p=0.0001) different percentages: 25/34 (73.5%) and 2/34 (5.8%), respectively. Moreover, at these cut-offs, 2 out of 140 (1.4%) healthy controls were identified as positive by CA125, while no healthy control (0%) was positive for HE4. The overall abilities of CA125 and HE4 to discriminate among subjects belonging to the four cohorts were also evaluated by ROC curves (Table 1). In post-menopausal status, CA125 ROC-AUC was significantly higher than HE4 ROC-AUC when comparing EOCs vs healthy controls; in pre-menopausal status, CA125-AUCs were significantly higher than HE4-AUCs when comparing endometriosis vs ovarian benign cysts or healthy controls. Other differences between CA125-AUCs and HE4-AUCs did not reach statistical significance.
Table 1.
Comparisons of the ROC-AUCs for CA125 and HE4 across the groups enrolled in this study
| CA125 ROC-AUC (95% CI) | HE4 ROC-AUC (95% CI) | p-value | ||
|---|---|---|---|---|
| Post-menopause | EOC vs HC | 0.9940 (0.9866–1.0000) | 0.9576 (0.9279–0.9873) | 0.0147 |
| EOC vs Cysts | 0.9602 (0.9353–0.9852) | 0.9400 (0.9039–0.97599) | 0.2439 | |
| Cysts vs HC | 0.6671 (0.5898–0.7444) | 0.5856 (0.5055–0.6656) | 0.1420 | |
| Pre-menopause | EOC vs HC | 0.9773 (0.9457–1.0000) | 0.9177 (0.8279–1.0000) | 0.1233 |
| EOC vs Cysts | 0.9549 (0.9053–1.0000) | 0.9016 (0.8068–0.9965) | 0.1867 | |
| EOC vs End | 0.8473 (0.7397–0.9549) | 0.8925 (0.7907–0.9944) | 0.3211 | |
| Cysts vs HC | 0.5960 (0.4644–0.7275) | 0.5744 (0.4390–0.7097) | 0.8280 | |
| End vs HC | 0.8982 (0.8183–0.9781) | 0.6369 (0.5055–0.7682) | 0.0011 | |
| End vs Cysts | 0.8361 (0.7307–0.9416) | 0.5471 (0.4075–0.6867) | 0.0019 | |
ROC-AUC, area under the receiver operating characteristic curve; HE4, human epididymis protein 4; CI, confidence interval; HC, healthy controls; Cysts, patients with ovarian benign cysts; End, patients with endometriosis; EOC, patients with epithelial ovarian cancer
ROMA algorithm was calculated in 278 patients presenting with pelvic mass (endometriosis, ovarian benign cysts and EOC). Distribution of patients with EOC, endometriosis and ovarian benign cysts according to their positivity and negativity for CA125, HE4, ROMA and diagnostic performances of the three serum markers are reported in Table 2.
Table 2.
Distribution of patients with epithelial ovarian cancer, endometriosis and ovarian benign cysts according to their positivity for CA125, HE4 and ROMA and diagnostic performances
| Pre-menopause | Post-menopause | |||
|---|---|---|---|---|
| EOC (N=26) | Cysts and Endometriosis (N=69) | EOC (N=87) | Cysts (N=96) | |
| CA125 | ||||
| positive | 24 | 28 | 82 | 17 |
| negative | 2 | 41 | 5 | 79 |
| Accuracy (95% CI) | 68.4 (58.1–77.6) | 88.0 (82.4–92.3) | ||
| Sensitivity (95% CI) | 92.3 (74.9–99.1) | 94.3 (87.1–98.1) | ||
| Specificity (95% CI) | 59.4 (46.9–71.1) | 82.3 (73.2–89.3) | ||
| HE4 | ||||
| positive | 22 | 4 | 68 | 1 |
| negative | 4 | 65 | 19 | 95 |
| Accuracy (95% CI) | 91.6 (84.1–96.3) | 89.1 (83.6–93.2) | ||
| Sensitivity (95% CI) | 84.6 (65.1–95.6) | 78.2 (68.0–86.3) | ||
| Specificity (95% CI) | 94.2 (85.8–98.4) | 99.0 (94.3–100.0) | ||
| ROMA | ||||
| positive | 22 | 4 | 81 | 15 |
| negative | 13 | 56 | 6 | 81 |
| Accuracy (95% CI) | 82.1 (72.9–89.2) | 88.5 (83.0–92.8) | ||
| Sensitivity (95% CI) | 84.6 (65.1–95.6) | 93.1 (85.6–97.4) | ||
| Specificity (95% CI) | 81.2 (69.9–89.6) | 84.4 (75.5–91.0) | ||
HE4, human epididymis protein 4; ROMA, Risk for Ovarian Malignancy Algorithm; EOC, patients with epithelial ovarian; positive, above cut-off; negative, under cut-off; CA125 cut-off value: 35 U/ml; HE4 cut-off values: ≤70 pmol/L for pre-menopausal state and ≤140 pmol/L in menopausal state; ROMA cut-off values: ≤7.4% for pre-menopausal state and ≤25.3% menopausal state
Of note, CA125, HE4 and ROMA detected 15 (6 in pre- and 9 in post-menopause), 11 (4 in pre-and 7 post-menopause) and 14 (4 in pre- and 10 post-menopause) of 21 EOC patients with stage I of disease.
CA125, HE4, ROMA serum levels and clinicopathological features of EOC patients
Relationships between CA125, HE4 and ROMA levels and clinicopathological characteristics were illustrated in Table 3. Elevated CA125, HE4 and ROMA levels were associated with advanced FIGO stage, suboptimally debulked tumor, ascites, positive cytology, lymph node involvement and advanced age (all p≤0.05). Elevated HE4 and ROMA levels (p≤0.01), but not CA125 levels (p=0.0579), were associated with undifferentiated tumours. Finally, CA125, HE4 and ROMA levels were associated with histological subtypes (all p≤0.0001). Indeed serous papillary, undifferentiated and mixed type showed higher levels of CA125, HE4, and ROMA, than endometrioid, clear cell and mucinous subtypes.
Table 3.
Association between serum CA125, HE4 and ROMA levels and clinicopathological characteristics of epithelial ovarian cancer patients
| N | CA125 Median | p-value | HE4 Median | p-value | ROMA Median | p-value | |
|---|---|---|---|---|---|---|---|
| Stage | |||||||
| Early stages (I–II) | 33 | 88.3 | <0.0001 | 116.5 | <0.0001 | 47.6 | <0.0001 |
| Late stages (III–IV) | 80 | 658.3 | 482.1 | 97.0 | |||
| Unknown | 1 | ||||||
| Grade | |||||||
| G1+G2 | 20 | 133.9 | 0.0579 | 101.7 | 0.0019 | 47.0 | 0.0101 |
| G3 | 83 | 542.9 | 438.0 | 94.6 | |||
| Unknown | 11 | ||||||
| Residual tumor (TR) | |||||||
| TR=0 cm | 46 | 129.7 | <0.0001 | 167.1 | <0.0001 | 73.5 | <0.0001 |
| TR >0 cm | 44 | 1040.3 | 629.9 | 97.2 | |||
| Unknown | 24 | ||||||
| Istological subtype | |||||||
| Serous papillary | 51 | 769.7 | <0.0001 | 488.1 | <0.0001 | 97.4 | <0.0001 |
| Endometrioid | 17 | 122.6 | 168.2 | 73.5 | |||
| Clear cell | 10 | 228.6 | 200.2 | 80.0 | |||
| Mucinous | 8 | 73.1 | 66.3 | 20.6 | |||
| Undifferentiated | 8 | 1553.2 | 476.25 | 95.6 | |||
| Mixed | 15 | 746.7 | 491.50 | 90.2 | |||
| Unknown | 5 | ||||||
| Presence of ascites | |||||||
| No | 44 | 112.9 | <0.0001 | 154.2 | <0.0001 | 64.2 | <0.0001 |
| Yes | 58 | 1110.8 | 760.8 | 98.3 | |||
| Unknown | 12 | ||||||
| Cytology | |||||||
| Negative | 19 | 141.7 | 0.0001 | 168.2 | 0.0042 | 73.5 | 0.0005 |
| Positive | 63 | 961.2 | 488.1 | 97.0 | |||
| Unknown | 32 | ||||||
| Lymph nodal involvement | |||||||
| Negative | 35 | 171.0 | 0.0009 | 169.4 | 0.0040 | 73.5 | 0.0004 |
| Positive | 34 | 764.1 | 460.6 | 96.4 | |||
| Unknown | 45 | ||||||
| Age | |||||||
| <50 | 27 | 171.5 | 0.0311 | 169.3 | 0.0022 | 74.2 | 0.002 |
| ≥50 | 87 | 563.8 | 438.0 | 95.3 | |||
HE4, human epididymis protein 4; ROMA, Risk for Ovarian Malignancy Algorithm; SD, standad deviation
CA125, HE4 and ROMA prognostic performances
At the time of the last follow-up, 42 (42.8%) patients were alive without evidence of disease, 11 (11.2%) patients were alive with disease, 33 (33.7%) patients died of disease (median OS, 46 months, 95% CI=32-n.e.) and 12 patients were alive with unknown status. The number of events for OS, DFS and PFS were 33, 47 and 38, respectively.
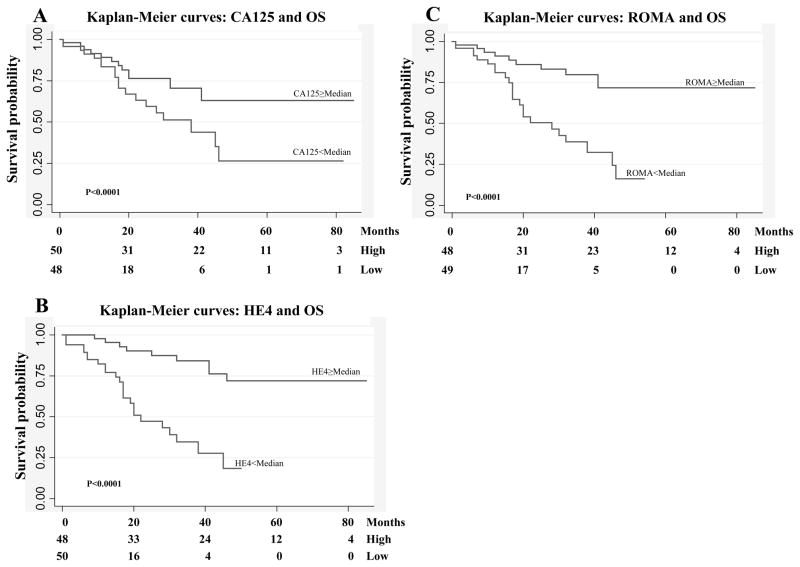
Survival analyses of OS, DFS and PFS on the basis of high vs low CA125, HE4, ROMA levels were significantly different (all p≤0.0001). The 2-yrs OS, DFS and PFS for EOC patients with low CA125 levels were 76.3% (95%CI: 60.3%–86.6%), 65.0% (95%CI: 47.9%–77.7%) and 71.1% (95%CI: 54.6%–82.5%) respectively, and decreased to 63.2% (95%CI: 44.4%–77.2%), 13.9% (95%CI: 4.0%–29.6%) and 44.9% (95%CI: 27.3%–61.1%) for patients with high levels. The 2-yrs OS, DFS and PFS for EOC patients with low HE4 levels were 90.1% (95%CI: 75.8%–96.2%), 71.3% (95%CI: 54.0%–83.1%) and 82.4% (95%CI: 66.5%–91.3%) respectively, and decreased to 47.3% (95%CI: 29.6%–63.2%), 8.9% (95%CI: 1.8%–23.5%) and 32.6% (95%CI: 1.7%–49.1%) for patients with high levels. The 2-yrs OS, DFS and PFS for EOC patients with low ROMA levels were 85.9% (95%CI: 0.71.3%–93.5%), 69.2% (95%CI: 51.9%–81.3%) and 77.9% (95%CI: 61.6%–87.9%) respectively, and decreased to 50.4% (95%CI: 32.0%–66.2%), 8.4% (95%CI: 1.6%–22.6%) and 35.7% (95%CI: 19.4%–52.4%) for patients with high levels. The Kaplan-Meier curves for OS on the basis of high vs low CA125, HE4 and ROMA levels were shown in Figure 2.
Figure 2. Kaplan-Meier survival curves in relation to serum CA125, HE4 and ROMA levels for epithelial ovarian cancer patients.
Serum human epididymis protein 4 (HE4) levels and overall survival (OS) (A), serum CA125 levels and overall survival (OS) (B), Risk for Ovarian Malignancy Algorithm (ROMA) levels and OS (C),
Univariate and multivariable analyses for survival were reported in Table 4. We couldn’t analyze the prognostic impact of tumor grade, due to extremely unbalanced number of events available for the analyses in the three subgroups (G1, G2, G3). As expected, clinicopathological features known to be prognostic variables for EOC such as FIGO stage, residual tumour after cytoreductive surgery, histological subtype, presence of ascites, positive cytology, lymph node involvement and age showed a statistically significant association with OS, DFS and PFS in univariate analyses, proving the validity of the patients cohort recruited in this study (all p≤0.02). In addition, univariate analyses demonstrated that elevated levels of CA125, HE4 and ROMA were significantly associated with shorter OS, DFS and PFS (all p≤0.028).
Table 4.
Univariate and multivariable survival analyses in relation to serum CA125, HE4, ROMA levels and clinicopathological prognostic variables for epithelial ovarian cancer patients
| OS | DFS | PFS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Univariate analyses | |||||||||
|
CA125 ≥ median vs <median |
2.20 | 1.09–4.45 | 0.028 | 3.57 | 1.87–6.81 | <0.001 | 2.43 | 1.24–4.76 | 0.010 |
|
HE4 ≥ median vs <median |
5.69 | 2.55–12.67 | <0.001 | 4.73 | 2.40–9.33 | <0.001 | 5.80 | 2.66–12.64 | <0.001 |
|
ROMA ≥ median vs <median |
4.21 | 1.96–9.04 | <0.001 | 4.76 | 2.43–9.31 | <0.001 | 4.58 | 2.16–9.68 | <0.001 |
|
FIGO Stage Late vs Early |
5.28 | 1.81–15.36 | 0.002 | 6.66 | 2.53–17.5 | <0.001 | 6.49 | 2.18–19.35 | 0.001 |
|
Residual tumor (TR) ≥ 0 vs 0 |
4.13 | 1.84–9.25 | 0.001 | 5.97 | 2.92–12.21 | <0.001 | 5.27 | 2.41–11.5 | <0.001 |
|
Histological-type Serous vs non-serous |
2.43 | 1.15–5.12 | 0.020 | 2.57 | 1.40–4.72 | 0.002 | 2.50 | 1.25–5.01 | 0.009 |
|
Presence of ascites yes vs no |
3.99 | 1.77–8.97 | 0.001 | 5.03 | 2.48–10.20 | <0.001 | 4.70 | 2.11–10.45 | <0.001 |
|
Cytology positive vs negative |
8.48 | 1.97–36.47 | 0.004 | 7.52 | 2.28–24.77 | 0.001 | 8.87 | 2.07–38.02 | 0.003 |
|
Lymph nodal involvement yes vs no |
10.33 | 2.22–47.89 | 0.003 | 5.67 | 2.2–14.58 | <0.001 | 6.96 | 2.19–22.15 | 0.001 |
|
Age ≥50 vs <50 |
1.05 | 1.02–1.08 | 0.001 | 1.03 | 1.00–1.05 | 0.016 | 1.04 | 1.01–1.06 | 0.006 |
| Multivariable analysis (adjusted for FIGO stage, residual tumour and histological-type) | |||||||||
|
CA125 ≥ median vs <median |
1.40 | 0.61–3.21 | 0.424 | 1.88 | 0.92–3.85 | 0.085 | 1.35 | 0.64–2.88 | 0.433 |
|
HE4 ≥ median vs <median |
3.98 | 1.35–11.75 | 0.012 | 2.46 | 1.09–5.56 | 0.030 | 2.77 | 1.12–6.85 | 0.028 |
|
ROMA ≥ median vs <median |
3.22 | 1.21–8.57 | 0.019 | 2.67 | 1.25–5.72 | 0.011 | 2.76 | 1.15–6.59 | 0.022 |
OS, overall survival; DFS, disease-free survival; PFS, progression free survival; HE4, human epididymis protein 4; ROMA, Risk for Ovarian Malignancy Algorithm
Multivariable analysis was performed to estimate the effect of biomarkers adjusted for FIGO stage, residual tumour and histological subtype, the most important established prognostic factors. Interestingly, among the three biomarkers analyzed, we found that HE4 and ROMA (all p≤0.039), but not CA125 (all p≥0.082), are independent prognostic factors for OS, DFS and PFS. About the established prognostic factors only residual tumour has been found as independent prognostic factor for DFS (HR=3.05, 95%CI=1.29–7.21, p=0.011) and for PFS (HR=3.00, 95%CI=1.15–7.83, p=0.025).
Discussion
Epithelial ovarian cancer is often detected at an advanced stage and is characterized by poor survival. As a result it is the most frequent cause of death from gynaecological cancer. At the moment, transvaginal ultrasonography and CA125 assay are mainly used for EOC diagnosis. Two longitudinal randomized screening trials, PLCO and UKCTOCS, are ongoing to determine their potential role in early detection of EOC. However, transvaginal ultrasonography requires specialized knowledge to reduce inter-operator interpretation variability and, like CA125, it is only partially effective in discriminating benign and malignant lesions. Therefore, there is a pressing need for clinical practice to develop new, easy, low cost and standardized serum analysis methods for an accurate differential diagnosis between benign and malignant lesions. In fact, it has been demonstrated that discrimination between malignant and benign pelvic masses improves EOC patients care and outcome (15–20). Moore (32) proposed ROMA algorithm for the differential diagnosis between benign and malignant lesions and Nolen and colleagues (33), among 65 biomarkers tested, reaffirmed the superiority of the CA125/HE4 combination. Moreover, Moore (37) showed that ROMA algorithm has better diagnostic performance than the widely used RMI. However, diagnostic accuracy of ROMA compared to CA125 and HE4 alone is still controversial. This is mainly due to the fact that different assays for CA125 and HE4, different thresholds for HE4 and ROMA and different patient selection criteria were used in each study to compare diagnostic performances of HE4, CA125 and ROMA (32, 37–42). In the present study, in order to report consistent results about HE4, CA125 and ROMA diagnostic performances, we enrolled a large number (419) of women, selecting only invasive epithelial ovarian cancer as malignant ovarian pathology. Moreover, we analyzed HE4 and CA125 employing fully automated methods and using biomarkers’ thresholds largely validated by manufactures or by clinical practice. With these thresholds, CA125 classified correctly 98.6% of healthy controls, while HE4 classified correctly 100% of healthy controls; consequently, these thresholds seemed adequate for our study.
Our analysis performed on healthy controls, revealed that HE4 and CA125 are differentially expressed in pre- and post-menopause, in agreement with the literature (3, 45). Being the expression of the markers and the risk of developing benign and malignant pathologies correlated to the menopausal status, we analyzed separately pre- and post-menopausal women.
The biological function of HE4 has not been identified yet. HE4 is a member of the whey acidic protein (WAP) four-disulfide core gene cluster that harbors 15 small serine protease inhibitor genes. Comparative genomic hybridization studies showed that WAP gene cluster is among the most frequent amplified chromosomal regions in EOC (46–49), suggesting the potential presence of oncogenes in this region.
This study, according to previous reports (5–6, 39, 50), proved the high specificity of HE4 in differential diagnosis between lesions of malignant and benign nature, as it is less frequently increased in patients with benign cysts or endometriosis. In our experience, 17.7 % of post-menopausal patients with ovarian benign cysts and 73.5% of patients with endometriosis exceeded the threshold value for CA125, while only 1.0% of the same post-menopausal patients with ovarian benign cysts and 5.8% of patients with endometriosis exceeded the threshold value for HE4 (CA125 vs HE4: p≤0.002). According to the fact that CA125 is frequently increased in patients with endometriosis, when we compare endometriosis vs healthy controls we observed that CA125 ROC-AUC was significantly higher than HE4-AUC (0.8982 vs 0.6369: p= 0.0011).
There are conflicting results about CA125 sensitivity. Prior studies (23, 50–51) showed that HE4 is more sensitive than CA125 in detecting EOC, being elevated in patients with EOC that do not express CA125. However, the current study, in agreement with other observations (38, 52, 53) showed that CA125 has greater sensitivity than HE4 in detecting EOC. Indeed, in postmenopausal EOC, CA125 exceeded the threshold value in 94.2% of patients while HE4 exceeded the threshold value in only 78.1% of patients (94.2% vs 78.1% p=0.0002). Notably, we didn’t find any EOC patient positive for HE4 and negative for CA125, while 16 (including 5 stages I) EOC patients were positive only for CA125. Being CA125 more sensitive than HE4 in detecting patients with EOC, when we compare EOCs vs healthy controls by ROC curves, CA125-AUC was significantly higher than HE4-AUC (0.9940 vs 0.9576, p=0.0147).
Among pre-menopausal women, we observed that CA125 is more sensitive in detecting EOCs, while HE4 is more specific in discriminating ovarian cysts from EOCs. However, this analysis didn’t reach statistical significance, possibly because of the small sample size.
Concerning ROMA, some previous reports showed that CA125/HE4 combination yielded a higher diagnostic accuracy compared to single markers (23, 32), while other papers didn’t reach the same conclusions (38–39). In our experience, ROMA inherits the strengths and the weakness of CA125 and HE4 alone. Indeed, ROMA is more sensitive than HE4 but less sensitive than CA125 and it is more specific than CA125 but less specific than HE4. Unfortunately, because of the heterogeneity of previous studies, it is not possible to pool data and to perform a meta-analysis in order to obtain univocal indications about the clinical application of this algorithm in EOC diagnosis.
Ovarian cancer management could also be improved through the development of new methods for EOC prognosis. Therefore, the need for additional prognostic data to calibrate therapeutic tools on an individual basis in women with EOC seems obvious. In order to find out how CA125, HE4 and ROMA influence EOC biology, we analyzed the association between the biomarkers and the clinicopathological characteristics of the patients. Remarkably, elevated HE4 and ROMA levels (both p≤0.01), but not CA125 levels (p=0.0579), were associated with undifferentiated tumors, suggesting that they are correlated with cancer aggressiveness. Moreover, since elevated levels of CA125, HE4 and ROMA were found in patients suboptimally debulked, we could suppose that these biomarkers, associated with others parameters (i.e. like imaging analysis), would be useful in preoperative assessment of residual disease and, eventually, in the evaluation of a neoadiuvant treatment. Univariate analyses showed that CA125, HE4 and ROMA, as well as established clinicopathological prognostic variables for EOC, were significantly associated with shorter OS, DFS, and PFS (all p≤0.05). However, when multivariable analysis was performed, we found that only HE4 and ROMA were independent prognostic factors for OS, DFS and PFS. Moreover, multivariable analysis proved that HE4 and ROMA appear to be better prognostic factors than FIGO stage, residual tumor and histological subtype for OS, DFS, and PFS. These data suggest that HE4 and ROMA could reflect intrinsic tumor aggressiveness that established prognostic factors were not able to identify in our cohort of EOCs. Thus, elevated HE4 and ROMA levels, in association with the traditional prognostic factors, could be clinically useful in identifying high-risk EOC patients for a more aggressive tailored therapy, consisting in consolidation of treatment with chemotherapeutic drugs or novel biologic agents. To date, we are the first group that demonstrated HE4 and ROMA as independent prognostic factors for OS, DFS and PFS in EOC. Differently, the Peak’ study showed that HE4 is an independent prognostic factor only for PFS and only in patients with advanced (IIIc-IV) stage EOC. This could be explained by the fact that this latter analysis has been carried out in a small group of women and the effect of HE4 serum levels on prognosis was evaluated non categorizing HE4 on the basis of the median values, but on the basis of cut-off value proposed by Moore (23) for diagnostic purpose.
Recently, our group has reported the prognostic value of serum HE4 levels in poorly differentiated endometrial carcinoma patients. Higher serum HE4 levels were correlated with a more aggressive tumor phenotype and worse outcome (54) and similar results have been shown for breast and lung cancer (55–56). Moreover, it has already been demonstrated that Leukocyte Protease 1 (SLPI), one of the best-studied member of the WAP protein family, contrary to what expected for a protease inhibitor, promotes malignant potential of cancer cells (57). On the basis of the similarity of HE4 and SLPI, it is tempting to speculate that HE4, like SLP1, could directly enhance malignant potential of tumor.
In conclusion, this study confirms the diagnostic role of HE4 and ROMA already suggested by other reports, but it adds a clinically relevant information, as for the first time we showed that high levels of HE4 and ROMA are promising prognostic factors in EOC, identifying a subgroup of patients with poor survival and at higher risk of death and, subsequently, directing to a more aggressive adjuvant therapy. Further multicenter studies with homogeneous laboratory procedures for HE4 and CA125 assays, as well as patients’ selection criteria, are needed to draw firm conclusions about the applicability of HE4 and ROMA in clinical practice.
Acknowledgments
We wish to thank all the nurses working in the Department of Obstetrics and Gynaecology of the University of Brescia, and especially Ms. Margherita Franzoni, for the assistance in collecting blood samples. We are also grateful to Ms. Michela Faustini for her essential technical contribution in performing the ARCHITECT assays.
Financial support
Supported in part by grants from the Nocivelli, Foundation, Brescia, Italy, by grants from the Istituto Superiore di Sanità (Progetto Oncoproteomica, Programma Italia-USA “Farmacogenomica Oncologica”, convenzione 527/B4/4), Rome, Italy and by grants from the Ministero dell’Istruzione, dell’Università e della Ricerca (PRIN project, prot2008AZJM9E) Rome, Italy. This investigation was also supported by grants from NIH R01 CA122728-01A2 and R01 CA154460-01A1 to ADS, grants 501/A3/3 and 0027557 from the Italian Institute of Health (ISS) to ADS and by NIH Research Grant CA-16359 from the National Cancer Institute.
Footnotes
Conflict of interest statement
Dr. Claudio Galli is currently employed by Abbott Diagnostics as the Scientific Affairs Manager, Italy
References
- 1.Holschneider CH, Berek JS. Ovarian cancer: epidemiology, biology, and prognostic factors. Semin Surg Oncol. 2000;19(1):3–10. doi: 10.1002/1098-2388(200007/08)19:1<3::aid-ssu2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs I, Oram D, Fairbanks J, Turner J, Frost C, Grudzinskas JG. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br J Obstet Gynaecol. 1990;97(10):922–9. doi: 10.1111/j.1471-0528.1990.tb02448.x. [DOI] [PubMed] [Google Scholar]
- 3.Malkasian GD, Jr, Knapp RC, Lavin PT, Zurawski VR, Jr, Podratz KC, Stanhope CR, et al. Preoperative evaluation of serum CA 125 levels in premenopausal and postmenopausal patients with pelvic masses: discrimination of benign from malignant disease. Am J Obstet Gynecol. 1988;159(2):341–6. doi: 10.1016/s0002-9378(88)80081-4. [DOI] [PubMed] [Google Scholar]
- 4.Rustin GJ, Bast RC, Jr, Kelloff GJ, Barrett JC, Carter SK, Nisen PD, et al. Use of CA-125 in clinical trial evaluation of new therapeutic drugs for ovarian cancer. Clin Cancer Res. 2004;10(11):3919–26. doi: 10.1158/1078-0432.CCR-03-0787. [DOI] [PubMed] [Google Scholar]
- 5.Huhtinen K, Suvitie P, Hiissa J, Junnila J, Huvila J, Kujari H, et al. Serum HE4 concentration differentiates malignant ovarian tumours from ovarian endometriotic cysts. Br J Cancer. 2009;100(8):1315–9. doi: 10.1038/sj.bjc.6605011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bordin L, Fiore C, Donà G, Andrisani A, Ambrosini G, Faggian D, et al. Evaluation of erythrocyte band 3 phosphotyrosine level, glutathione content, CA-125, and human epididymal secretory protein E4 as combined parameters in endometriosis. Fertil Steril. 2010;94(5):1616–21. doi: 10.1016/j.fertnstert.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 7.Bast RC, Jr, Badgwell D, Lu Z, Marquez R, Rosen D, Liu J, et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 2005;15 (Suppl 3):274–81. doi: 10.1111/j.1525-1438.2005.00441.x. [DOI] [PubMed] [Google Scholar]
- 8.Gadducci A, Landoni F, Maggino T, Sartori E, Zola P, Ferdeghini M, et al. Serum CA125 assay at the time of relapse has no prognostic relevance in patients undergoing chemotherapy for recurrent ovarian cancer: a multicenter Italian study. Int J Gynecol Cancer. 1997;7(1):78–83. doi: 10.1046/j.1525-1438.1997.00424.x. [DOI] [PubMed] [Google Scholar]
- 9.Gadducci A, Cosio S, Zola P, Landoni F, Maggino T, Sartori E. Surveillance procedures for patients treated for epithelial ovarian cancer: a review of the literature. Int J Gynecol Cancer. 2007;17(1):21–31. doi: 10.1111/j.1525-1438.2007.00826.x. [DOI] [PubMed] [Google Scholar]
- 10.Bhoola S, Hoskins WJ. Diagnosis and management of epithelial ovarian cancer. Obstet Gynecol. 2006;107(6):1399–410. doi: 10.1097/01.AOG.0000220516.34053.48. [DOI] [PubMed] [Google Scholar]
- 11.Aebi S, Castiglione M ESMO Guidelines Working Group. Newly and relapsed epithelial ovarian carcinoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl 4):21–3. doi: 10.1093/annonc/mdp117. [DOI] [PubMed] [Google Scholar]
- 12.Høgdall EV, Christensen L, Kjaer SK, Blaakaer J, Kjaerbye-Thygesen A, Gayther S, et al. CA125 expression pattern, prognosis and correlation with serum CA125 in ovarian tumor patients. From The Danish “MALOVA” Ovarian Cancer Study. Gynecol Oncol. 2007;104(3):508–15. doi: 10.1016/j.ygyno.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 13.Markmann S, Gerber B, Briese V. Prognostic value of Ca 125 levels during primary therapy. Anticancer Res. 2007;27(4A):1837–9. [PubMed] [Google Scholar]
- 14.FIGO (International Federation of Gynecology and Obstetrics) 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95 (Suppl 1):S1–257. doi: 10.1016/S0020-7292(06)60025-8. [DOI] [PubMed] [Google Scholar]
- 15.Hunter RW, Alexander ND, Soutter WP. Meta-analysis of surgery in advanced ovarian carcinoma: is maximum cytoreductive surgery an independent determinant of prognosis? Am J Obstet Gynecol. 1992;166(2):504–11. doi: 10.1016/0002-9378(92)91658-w. [DOI] [PubMed] [Google Scholar]
- 16.Bristow RE, Gossett DR, Shook DR, Zahurak ML, Tomacruz RS, Armstrong DK, et al. Micropapillary serous ovarian carcinoma: surgical management and clinical outcome. Gynecol Oncol. 2002;86(2):163–70. doi: 10.1006/gyno.2002.6736. [DOI] [PubMed] [Google Scholar]
- 17.Giede KC, Kieser K, Dodge J, Rosen B. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol. 2005;99(2):447–61. doi: 10.1016/j.ygyno.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Earle CC, Schrag D, Neville BA, Yabroff KR, Topor M, Fahey A, et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst. 2006;98(3):172–80. doi: 10.1093/jnci/djj019. [DOI] [PubMed] [Google Scholar]
- 19.Paulsen T, Kjaerheim K, Kaern J, Tretli S, Tropé C. Improved short-term survival for advanced ovarian, tubal, and peritoneal cancer patients operated at teaching hospitals. Int J Gynecol Cancer. 2006;16 (Suppl 1):11–7. doi: 10.1111/j.1525-1438.2006.00319.x. [DOI] [PubMed] [Google Scholar]
- 20.Engelen MJ, Kos HE, Willemse PH, Aalders JG, de Vries EG, Schaapveld M, et al. Surgery by consultant gynecologic oncologists improves survival in patients with ovarian carcinoma. Cancer. 2006;106(3):589–98. doi: 10.1002/cncr.21616. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Yu Y, Xu F, Berchuck A, van Haaften-Day C, Havrilesky LJn, et al. Combining multiple serum tumor markers improves detection of stage I epithelial ovarian cancer. Gynecol Oncol. 2007;107(3):526–31. doi: 10.1016/j.ygyno.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McIntosh MW, Liu Y, Drescher C, Urban N, Diamandis EP. Validation and characterization of human kallikrein 11 as a serum marker for diagnosis of ovarian carcinoma. Clin Cancer Res. 2007;13(15 Pt 1):4422–8. doi: 10.1158/1078-0432.CCR-06-2224. [DOI] [PubMed] [Google Scholar]
- 23.Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008;108(2):402–8. doi: 10.1016/j.ygyno.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Havrilesky LJ, Whitehead CM, Rubatt JM, Cheek RL, Groelke J, He Q, et al. Evaluation of biomarker panels for early stage ovarian cancer detection and monitoring for disease recurrence. Gynecol Oncol. 2008;110(3):374–82. doi: 10.1016/j.ygyno.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 25.Bertenshaw GP, Yip P, Seshaiah P, Zhao J, Chen TH, Wiggins WS, et al. Multianalyte profiling of serum antigens and autoimmune and infectious disease molecules to identify biomarkers dysregulated in epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2872–81. doi: 10.1158/1055-9965.EPI-08-0464. [DOI] [PubMed] [Google Scholar]
- 26.Visintin I, Feng Z, Longton G, Ward DC, Alvero AB, Lai Y, et al. Diagnostic markers for early detection of ovarian cancer. Clin Cancer Res. 2008;14(4):1065–72. doi: 10.1158/1078-0432.CCR-07-1569. [DOI] [PubMed] [Google Scholar]
- 27.Palmer C, Duan X, Hawley S, Scholler N, Thorpe JD, Sahota RA, et al. Systematic evaluation of candidate blood markers for detecting ovarian cancer. PLoS One. 2008;3(7):e2633. doi: 10.1371/journal.pone.0002633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henic E, Borgfeldt C, Christensen IJ, Casslén B, Høyer-Hansen G. Cleaved forms of the urokinase plasminogen activator receptor in plasma have diagnostic potential and predict postoperative survival in patients with ovarian cancer. Clin Cancer Res. 2008;14(18):5785–93. doi: 10.1158/1078-0432.CCR-08-0096. [DOI] [PubMed] [Google Scholar]
- 29.Nolen B, Marrangoni A, Velikokhatnaya L, Prosser D, Winans M, Gorelik E, et al. A serum based analysis of ovarian epithelial tumorigenesis. Gynecol Oncol. 2009;112(1):47–54. doi: 10.1016/j.ygyno.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nosov V, Su F, Amneus M, Birrer M, Robins T, Kotlerman J, et al. Validation of serum biomarkers for detection of early-stage ovarian cancer. Am J Obstet Gynecol. 2009;200(6):639.e1–5. doi: 10.1016/j.ajog.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 31.Amonkar SD, Bertenshaw GP, Chen TH, Bergstrom KJ, Zhao J, Seshaiah P, et al. Development and preliminary evaluation of a multivariate index assay for ovarian cancer. PLoS One. 2009;4(2):e4599. doi: 10.1371/journal.pone.0004599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore RG, McMeekin DS, Brown AK, DiSilvestro P, Miller MC, Allard WJ, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40–6. doi: 10.1016/j.ygyno.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nolen B, Velikokhatnaya L, Marrangoni A, De Geest K, Lomakin A, Bast RC, Jr, et al. Serum biomarker panels for the discrimination of benign from malignant cases in patients with an adnexal mass. Gynecol Oncol. 2010;117(3):440–5. doi: 10.1016/j.ygyno.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yurkovetsky Z, Skates S, Lomakin A, Nolen B, Pulsipher T, Modugno F, et al. Development of a multimarker assay for early detection of ovarian cancer. J Clin Oncol. 2010;28(13):2159–66. doi: 10.1200/JCO.2008.19.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdel-Azeez HA, Labib HA, Sharaf SM, Refai AN. HE4 and mesothelin: novel biomarkers of ovarian carcinoma in patients with pelvic masses. Asian Pac J Cancer Prev. 2010;11(1):111–6. [PubMed] [Google Scholar]
- 36.Cramer DW, Bast RC, Jr, Berg CD, Diamandis EP, Godwin AK, Hartge P, et al. Ovarian cancer biomarker performance in prostate, lung, colorectal, and ovarian cancer screening trial specimens. Cancer Prev Res (Phila) 2011;4(3):365–74. doi: 10.1158/1940-6207.CAPR-10-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore RG, Jabre-Raughley M, Brown AK, Robison KM, Miller MC, Allard WJ, et al. Comparison of a novel multiple marker assay vs the Risk of Malignancy Index for the prediction of epithelial ovarian cancer in patients with a pelvic mass. Am J Obstet Gynecol. 2010;203(3):228.e1–6. doi: 10.1016/j.ajog.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Gorp T, Cadron I, Despierre E, Daemen A, Leunen K, Amant F, et al. HE4 and CA125 as a diagnostic test in ovarian cancer: prospective validation of the Risk of Ovarian Malignancy Algorithm. Br J Cancer. 2011;104(5):863–70. doi: 10.1038/sj.bjc.6606092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montagnana M, Danese E, Ruzzenente O, Bresciani V, Nuzzo T, Gelati M. The ROMA (Risk of Ovarian Malignancy Algorithm) for estimating the risk of epithelial ovarian cancer in women presenting with pelvic mass: is it really useful? Clin Chem Lab Med. 2011;49(3):521–5. doi: 10.1515/CCLM.2011.075. [DOI] [PubMed] [Google Scholar]
- 40.Kim YM, Whang DH, Park J, Kim SH, Lee SW, Park HA, et al. Evaluation of the accuracy of serum human epididymis protein 4 in combination with CA125 for detecting ovarian cancer: a prospective case-control study in a Korean population. Clin Chem Lab Med. 2011;49(3):527–34. doi: 10.1515/CCLM.2011.085. [DOI] [PubMed] [Google Scholar]
- 41.Ruggeri G, Bandiera E, Zanotti L, Belloli S, Ravaggi A, Romani C, et al. HE4 and epithelial ovarian cancer: Comparison and clinical evaluation of two immunoassays and a combination algorithm. Clin Chim Acta. 2011;412(15–16):1447–53. doi: 10.1016/j.cca.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 42.Jacob F, Meier M, Caduff R, Goldstein D, Pochechueva T, Hacker N, et al. No benefit from combining HE4 and CA125 as ovarian tumor markers in a clinical setting. Gynecol Oncol. 2011;121(3):487–91. doi: 10.1016/j.ygyno.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 43.Paek J, Lee SH, Yim GW, Lee M, Kim YJ, Nam EJ, et al. Prognostic significance of human epididymis protein 4 in epithelial ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2011 doi: 10.1016/j.ejogrb.2011.05.021. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 44.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. [PubMed] [Google Scholar]
- 45.Bon GG, Kenemans P, Verstraeten R, van Kamp GJ, Hilgers J. Serum tumor marker immunoassays in gynecologic oncology: establishment of reference values. Am J Obstet Gynecol. 1996;174(1 Pt 1):107–14. doi: 10.1016/s0002-9378(96)70381-2. [DOI] [PubMed] [Google Scholar]
- 46.Iwabuchi H, Sakamoto M, Sakunaga H, Ma YY, Carcangiu ML, Pinkel D, et al. Genetic analysis of benign, low-grade, and high-grade ovarian tumors. Cancer Res. 1995;55(24):6172–80. [PubMed] [Google Scholar]
- 47.Sonoda G, Palazzo J, du Manoir S, Godwin AK, Feder M, Yakushiji M, et al. Comparative genomic hybridization detects frequent overrepresentation of chromosomal material from 3q26, 8q24, and 20q13 in human ovarian carcinomas. Genes Chromosomes Cancer. 1997;20(4):320–8. [PubMed] [Google Scholar]
- 48.Kiechle M, Jacobsen A, Schwarz-Boeger U, Hedderich J, Pfisterer J, Arnold N. Comparative genomic hybridization detects genetic imbalances in primary ovarian carcinomas as correlated with grade of differentiation. Cancer. 2001;91(3):534–40. [PubMed] [Google Scholar]
- 49.Ferreira Z, Hurle B, Rocha J, Seixas S. Differing evolutionary histories of WFDC8 (short-term balancing) in Europeans and SPINT4 (incomplete selective sweep) in Africans. Mol Biol Evol. 2011 doi: 10.1093/molbev/msr106. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Holcomb K, Vucetic Z, Miller MC, Knapp RC. Human epididymis protein 4 offers superior specificity in the differentiation of benign and malignant adnexal masses in premenopausal women. Am J Obstet Gynecol. 2011 doi: 10.1016/j.ajog.2011.05.017. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 51.Anastasi E, Marchei GG, Viggiani V, Gennarini G, Frati L, Reale MG. HE4: a new potential early biomarker for the recurrence of ovarian cancer. Tumour Biol. 2010;31(2):113–9. doi: 10.1007/s13277-009-0015-y. [DOI] [PubMed] [Google Scholar]
- 52.Andersen MR, Goff BA, Lowe KA, Scholler N, Bergan L, Drescher CW, et al. Use of a Symptom Index, CA125, and HE4 to predict ovarian cancer. Gynecol Oncol. 2010;116(3):378–83. doi: 10.1016/j.ygyno.2009.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park Y, Lee JH, Hong DJ, Lee EY, Kim HS. Diagnostic performances of HE4 and CA125 for the detection of ovarian cancer from patients with various gynecologic and non-gynecologic diseases. Clin Biochem. 2011 doi: 10.1016/j.clinbiochem.2011.04.011. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 54.Bignotti E, Ragnoli M, Zanotti L, Calza S, Falchetti M, Lonardi S, et al. Diagnostic and prognostic impact of serum HE4 detection in endometrial carcinoma patients. Br J Cancer. 2011;104(9):1418–25. doi: 10.1038/bjc.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamashita S, Tokuishi K, Hashimoto T, Moroga T, Kamei M, Ono K, et al. Prognostic significance of HE4 expression in pulmonary adenocarcinoma. Tumour Biol. 2011;32(2):265–71. doi: 10.1007/s13277-010-0118-5. [DOI] [PubMed] [Google Scholar]
- 56.Kamei M, Yamashita S, Tokuishi K, Hashioto T, Moroga T, Suehiro S, et al. HE4 expression can be associated with lymph node metastases and disease-free survival in breast cancer. Anticancer Res. 2010;30(11):4779–83. [PubMed] [Google Scholar]
- 57.Devoogdt N, Hassanzadeh Ghassabeh G, Zhang J, Brys L, De Baetselier P, Revets H. Secretory leukocyte protease inhibitor promotes the tumorigenic and metastatic potential of cancer cells. Proc Natl Acad Sci U S A. 2003;100(10):5778–82. doi: 10.1073/pnas.1037154100. [DOI] [PMC free article] [PubMed] [Google Scholar]