SUMMARY
Fusobacterium nucleatum is a gram-negative oral anaerobe, capable of systemic dissemination causing infections and abscesses, often in mixed-species, at different body sites. We have shown previously that F. nucleatum adheres to and invades host epithelial and endothelial cells via a novel FadA adhesin. In this study, vascular endothelial (VE)-cadherin, a member of the cadherin family and a cell-cell junction molecule, was identified as the endothelial receptor for FadA, required for F. nucleatum binding to the cells. FadA co-localized with VE-cadherin on endothelial cells, causing relocation of VE-cadherin away from the cell-cell junctions. As a result, the endothelial permeability was increased, allowing the bacteria to cross the endothelium through loosened junctions. This crossing mechanism may explain why the organism is able to disseminate systemically to colonize in different body sites and even overcome the placental and blood-brain barriers. Co-incubation of F. nucleatum and E. coli enhanced penetration of the endothelial cells by the latter in the transwell assays, suggesting F. nucleatum may serve as an “enabler” for other microorganisms to spread systemically. This may explain why F. nucleatum is often found in mixed infections. This study reveals a possible novel dissemination mechanism utilized by pathogens.
INTRODUCTION
Fusobacterium nucleatum is a Gram-negative anaerobe ubiquitous in the oral cavity. It has been implicated in various forms of periodontal diseases (Moore & Moore, 1994), as well as in extra-oral infections such as brain, liver, spleen, and lung abscesses, septicemia related infections, pelvic inflammatory disease, and intrauterine infections (Cheung & Bellas, 2007b, Cheung & Bellas, 2007a, Cigarran et al., 2008, Han et al., 2010, Han et al., 2009, Kai et al., 2008, Koornstra et al., 1998, Saini et al., 2003). F. nucleatum infections often involve multiple species (Han et al., 2009). As one of the most prevalent species associated with intrauterine infections, F. nucleatum has received increasing attention in recent years as a model organism to study the implication of oral bacteria in pregnancy complications, a new frontier in reproductive infectious disease research (Fardini et al., 2010, Han et al., 2010, Han et al., 2004, Han et al., 2009, Ikegami et al., 2009, Liu et al., 2007).
As part of its virulence cycle, F. nucleatum has the ability to adhere to and invade different types of host cells including epithelial and endothelial cells (Han et al., 2000, Han et al., 2004). The surface adhesin FadA expressed by F. nucleatum has been shown to play a major role in the cell attachment process (Han et al., 2005, Xu et al., 2007). This adhesin is highly conserved in oral fusobacteria, such as F. nucleatum, whereas it is not found in non-oral fusobacterial species (Han et al., 2005). FadA exists in two forms, the non-secreted pre-FadA consisting of 129 amino acids and the secreted mature FadA (mFadA) consisting of 111 amino acids. The secreted mFadA is exposed on the bacterial surface while pre-FadA is anchored in the inner membrane (Xu et al., 2007). Together, mFadA and pre-FadA form a high molecular-weight complex (FadAc), required for attachment and invasion of the host cells. The crystal structure of mFadA reveals two antiparallel alpha-helical arms linked by a non-alpha-helical hairpin loop (Nithianantham et al., 2009). The mFadA monomers associate in a head-to-tail pattern via a novel leucine chain motif to form a filamentous structure (Nithianantham et al., 2009).
Hematogenous transmission seems to be the route used by bacteria to spread from the oral cavity to deeper organs with crossing of the endothelial barrier as a key step in the process (Fardini et al., 2010, Han et al., 2010). It has been demonstrated in pregnant mice that F. nucleatum colonizes specifically in the placenta once it enters the circulation. The bacteria then cause localized infection and inflammation leading to fetal demise either prematurely or at term (Han et al., 2004, Liu et al., 2007). Attachment and invasion of endothelial cells by F. nucleatum has been observed in infected mouse placentas (Han et al., 2004, Liu et al., 2007). A FadA mutant was shown to be defective in placental colonization, probably due to its inability to bind or invade endothelial cells (Ikegami et al., 2009).
The aim of the current study was to elucidate the endothelial cell receptor for FadA in order to better understand the molecular mechanism involved in systemic dissemination of F. nucleatum. We have identified vascular endothelial (VE)-cadherin, an endothelial-specific cell junction molecule, as a receptor for FadA. VE-cadherin is required for F. nucleatum binding to endothelial cells. Moreover, binding of FadA to endothelial cells increases the endothelial permeability and penetration by E. coli in vitro.
RESULTS
Identification of VE-cadherin as a potential FadA receptor by yeast-two-hybrid (Y2H) screening
The coding sequence for full-length fadA, expressing both pre-FadA and mFadA thus constituting FadAc, was used as a bait to screen a random-primed human placenta cDNA library. From this screening, a total of 20 different open reading frames (ORFs) were identified, among which 14 had in frame human placental DNA insertions without stop codons. Among the 14 ORFs, two members of the cadherin family, vascular endothelial (VE)-cadherin (CDH5), a cell-cell junction molecule unique to the endothelial cells (Vestweber, 2008), and a non classical protocadherin (PCDH1), were identified. Similar to the other classical cadherins, VE-cadherin’s 784 amino acid residues are divided into 5 extracellular (EC) domains, a single transmembrane domain, and an intracellular conserved domain (Vestweber, 2008). The smallest FadA-interacting region identified by Y2H, VE-cadherin415–534, corresponds to the second half of the EC4 domain and the first half of the EC5 domain (Figure 1). The potential FadA interacting region of PCDH1 from residue 417 to 527 spanned from EC4 to EC6 domains of the 7 composing the protein. ClustalW alignment revealed 32% identity shared by the corresponding PCDH1 domain and the VE-cadherin domain with multiple gaps. We chose to further analyze VE-cadherin due to its relevance to F. nucleatum interaction with the endothelial cells.
Figure 1. Diagram of VE-cadherin structure and main interacting proteins.
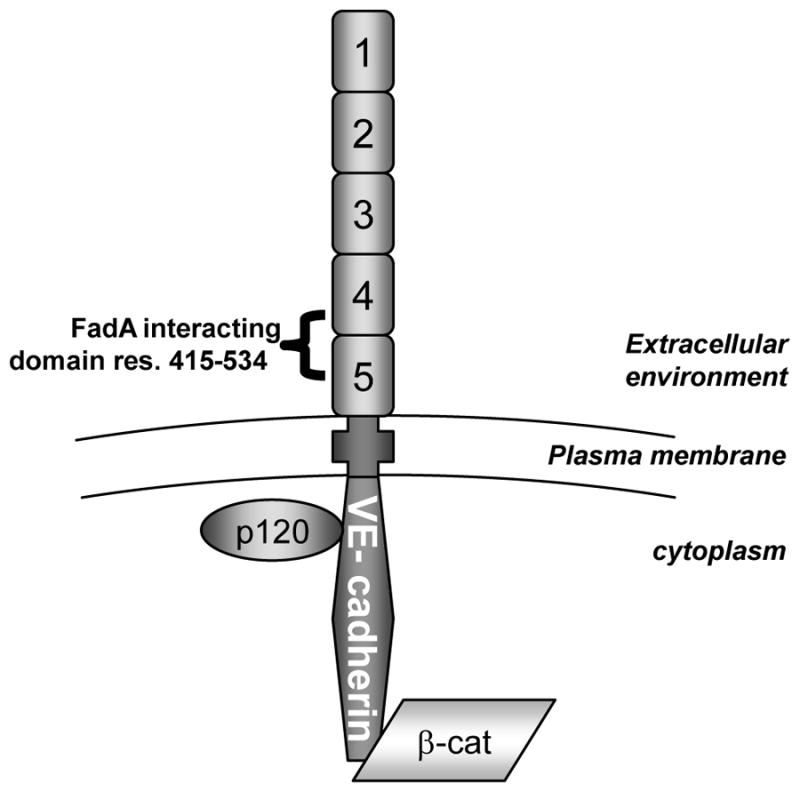
VE-cadherin is a cell surface exposed protein composed of 5 extracellular domains (EC), a unique transmembrane domain, and an intracellular domain interacting with catenins among which are p120 and β-catenin (β-cat). The cadherin-catenin complex is involved in the maintenance of cell-cell adherent junction and barrier integrity of the endothelium. The region bound by FadA identified by Y2H is a sequence of 119 residues composed of the 2nd half of EC4 and the 1st half of EC5.
FadAc binds directly to VE-cadherin415–534 region
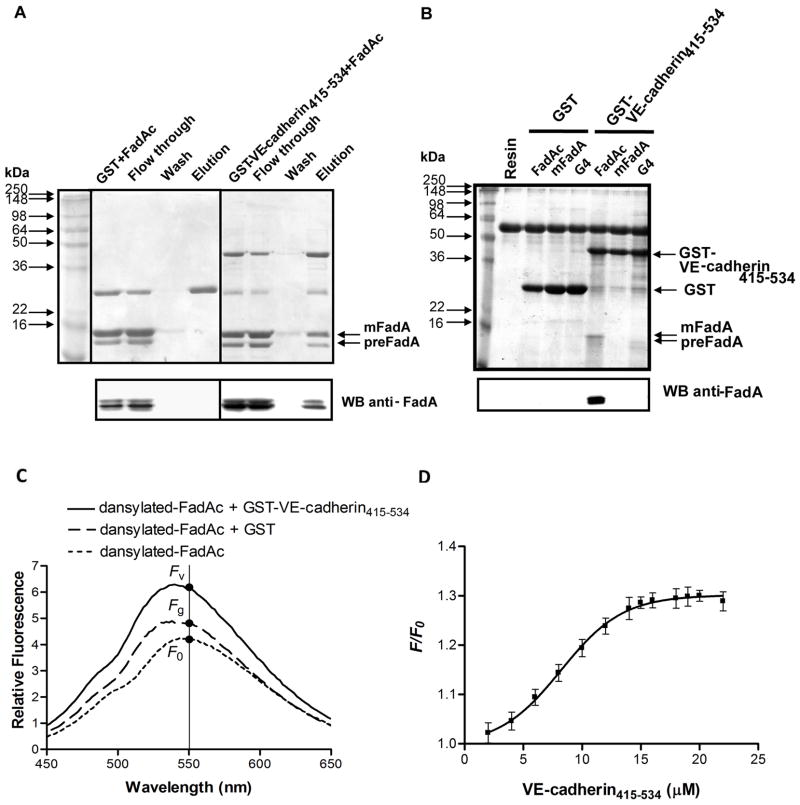
Binding of FadAc to VE-cadherin415–534 was examined by affinity column chromatography and co-precipitation assay using purified FadAc and E. coli lysates expressing FadAc, respectively. The VE-cadherin415–534 region was conjugated to the C-terminus of the Glutathione-S-Transferase (GST). The GST-VE-cadherin415–534 fusion protein or GST alone was mixed with purified FadAc and then passed through a glutathione column. FadAc was eluted from the column only when mixed with GST-VE-cadherin415–534 but not when mixed with GST (Figure 2A).
Figure 2. Binding of FadAc to VE-cadherin415–534.
(A) Purified FadAc mixed with GST or GST-VE-cadherin415–534 proteins were loaded onto the glutathione column. Following elution, the samples (2 μg/lane) were loaded onto 12% SDS-PAGE, followed by Coomassie blue staining (top panel) or Western blot using anti-FadA antibodies (bottom panel). (B) Co-precipitation assay was performed by mixing GST or GST-VE-cadherin415–534 with E. coli lysate expressing FadAc, mFadA, or the FadA mutant G4, respectively. The GST complexes were captured with equilibrated GST-bind resin, and eluted with 4x Laemmli buffer. One-fifth of the elution was subjected to 12% SDS-PAGE followed by Coomassie blue staining (top panel) and Western blot using anti-FadA antibodies (bottom panel). (C) Representative fluorescence emission spectra of dansylated-FadAc (4 μM) alone, or in the presence of 20 μM GST or 20 μM GST-VE-cadherin415–534. (D) Increased fluorescence intensity due to the presence of VE-cadherin415–534 (F) relative to that of dansylated-FadAc alone (F0) was plotted against the concentration of VE-cadherin415–534 added, where F = Fv − Fg, with Fv being the fluorescent intensity emitted in the presence of GST-VE-cadherin415–534 and Fg being that emitted in the presence of GST. The data were fit to the Boltzmann sigmoidal equation and the dissociation constant was calculated as described in the experimental procedures.
In the co-precipitation assay, GST-VE-cadherin415–534 was incubated with E. coli BL21(DE3) lysates expressing FadAc, mFadA, or the G4 mutant (replacing each of the four residues in the non-alpha-helical loop region with glycine). This experiment differs from Figure 2A in that the E. coli proteins serve as non-specific competitors. The G4 mutant was stably expressed, producing both pre-FadA and mFadA (data not shown). Whereas FadAc-GST-VE-cadherin415–534 complex was precipitated by glutathione resins, neither mFadA nor G4 co-precipitated with GST-VE-cadherin415–534 (Figure 2B). The GST protein alone did not co-precipitate with FadAc from the E. coli lysate indicating that co-precipitation of FadAc by GST-VE-cadherin415–534 was unlikely due to GST-FadAc or resin-FadAc interactions. Together, these results indicate that FadAc binds directly to VE-cadherin415–534 and that both pre-FadA and the non-alpha-helical loop region are required for binding.
Affinity of FadAc for VE-cadherin415–534 was determined using dansylated-FadAc. Dansyl chloride is non-fluorescent until it reacts with amines, producing a stable fluorogenic protein derivative, i.e. dansylated FadAc. Upon binding to the hydrophobic side chains of the partner protein, i.e. VE-cadherin415–534, fluorescence emission will increase. This method has been used to measure dissociation constants (Kd) (Margueron et al., 2009, Wu et al., 2006). As shown in Figure 2C, the fluorescence emission was significantly increased when dansylated-FadAc was incubated with GST-VE-cadherin415–534, compared to with GST or alone. The Kd between FadAc and VE-cadherin415–534 was calculated to be 15.9 ± 0.6 μM (Figure 2D).
FadAc colocalizes with VE-cadherin on HUVEC and modifies distribution of VE-cadherin
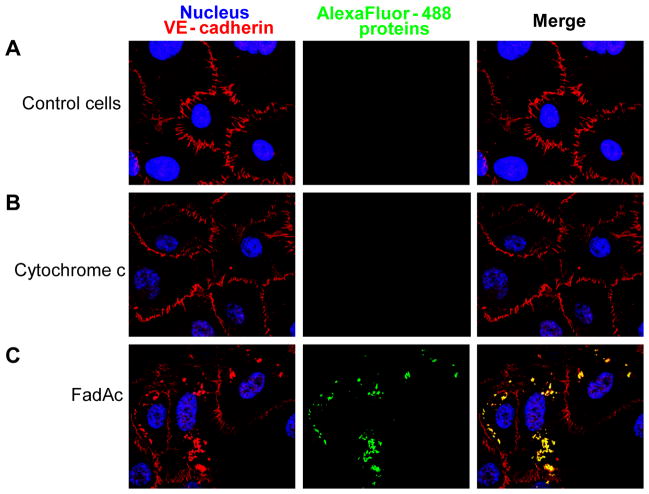
Alexa Fluor 488-conjugated FadAc, or Alexa Fluor 488-conjugated cytochrome c, which has a similar molecular weight as that of the FadA monomer and serves as a control, was incubated with HUVEC, followed by immuno-labeling of VE-cadherin. Confocal microscopy revealed binding of HUVEC by FadAc, but not by cytochrome c. FadAc co-localized with VE-cadherin (Figure 3). Moreover, the distribution pattern of VE-cadherin was altered in the presence of FadAc. In the untreated or cytochrome c-treated cells, VE-cadherin localized exclusively at the cell-cell junctions as expected (Figure 3). In cells bound by FadAc, however, VE-cadherin was detected both at the cell-cell junctions and in the cytoplasm compartments (Figure 3), suggesting that FadAc caused translocation of VE-cadherin.
Figure 3. Co-localization of FadAc and VE-cadherin by double immunofluorescent staining and confocal microscopy.
HUVEC monolayers were blocked in growth medium supplemented with 1% BSA and incubated for 1 hr at 37°C, followed by 2 hr incubation with BSA (A), Alexa-Fluor 488-conjugated cytochrome c (B), or Alexa-Fluor 488-conjugated FadAc (green) (C). Cells were washed and permeabilized, followed by incubation with mouse monoclonal antibody anti-VE-cadherin (red) and Alexa-Fluor-594-conjugated chicken-anti-rabbit polyclonal antibodies and counterstained with DAPI (blue). The cells were observed using a laser scanning confocal microscope. The yellow color results from the merge of red and green, indicating co-localization of VE-cadherin and FadAc.
VE-cadherin is required for efficient cell attachment by F. nucleatum
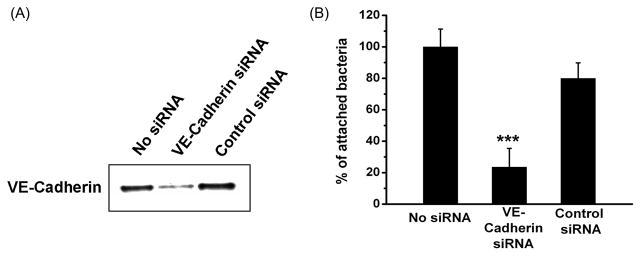
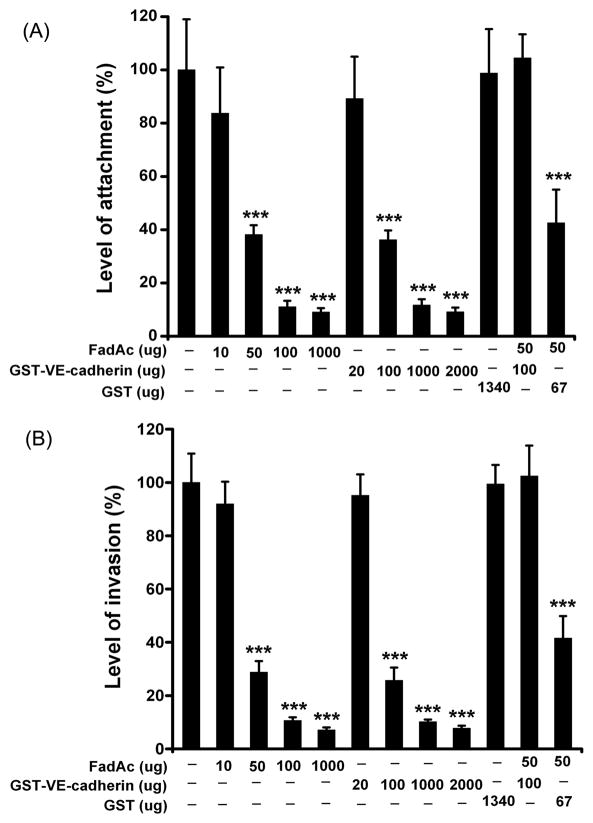
Expression of VE-cadherin in HUVEC was knocked down by siRNA, as confirmed by Western blot using anti-VE-cadherin antibodies (Figure 4A). F. nucleatum 12230 binding to the siRNA-transfected cells was compared to that of untreated cells or cells treated with non relevant siRNA. Inhibition of VE-cadherin expression by siRNA resulted in approximately 3-fold reduction of attachment by F. nucleatum 12230 (Figure 4B), indicating that VE-cadherin is required for F. nucleatum to bind to HUVEC. To confirm, an inhibitory attachment assay was performed. F. nucleatum binding to HUVEC was inhibited in the presence of FadAc or GST-VE-cadherin415–534 in a dose-dependent manner, but not by GST alone. FadAc and GST-VE- cadherin415–534, but not GST alone, also inhibited F. nucleatum invasion of HUVEC cells in a dose-dependent manner and to similar extents as inhibition of attachment (Figure 5B), indicating that FadAc-VE-cadherin-mediated cell attachment is a pre-requisite of F. nucleatum invasion. The inhibition rate augmented with increasing concentrations of FadAc or GST-VE- cadherin415–534 to a maximum of >10-fold (Figure 5A & 5B). Since FadAc forms polymers, it is difficult to estimate its molar concentrations. FadAc and VE-cadherin415–534 started to exhibit inhibitory effects at 50 μg and 100 μg, respectively, indicating that they were likely close to equimolar at these concentrations. This is supported by the observation that when 50 μg FadAc and 100 μg VE-cadherin were mixed together they nullified each other’s inhibitory effect (Figure 5A & 5B).
Figure 4. VE-cadherin is required for F. nucleatum to attach to HUVEC.
(A) Western blot of HUVEC cells transfected with 100 nM anti-CDH5 siRNA pool or non-targeting siRNA Pool#2 from Dharmacon using rabbit polyclonal anti-VE-cadherin antibodies. Approximately 5×103 cells were loaded onto each lane. The blot shown is a representative of three independent experiments. (B) Attachment of F. nucleatum 12230 to HUVEC cells treated in (A). Expressed are relative attachment levels with that exhibited by F. nucleatum 12230 to untreated cells designated as 100%. Data are the means ± SD from three independent experiments performed at least in triplicate. *** p<0.001 based on t tests.
Figure 5. Dose-dependent inhibition of F. nucleatum attachment and invasion by FadAc and VE-cadherin.
The proteins pre-incubated with HUVEC were indicated below the x axis. The level of F. nucleatum 12230 attachment (A) or invasion (B) was expressed as the percentage of bacteria recovered following cell lysis relative to the total number of bacteria initially added. Data are the means ± SD from two independent experiments each performed in triplicates. *** p<0.001 based on t tests.
FadAc alters endothelial cell integrity
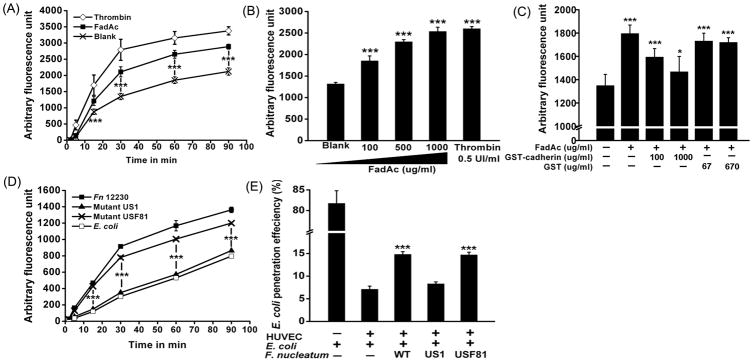
HUVEC monolayers grown in trasnwell inserts were incubated with FadAc and Texas Red-conjugated dextran. The permeability of HUVEC was determined by measuring the fluorescence “leaked” into the lower chamber. Thrombin, known to increase the endothelial permeability (Kumar et al., 2009), was used as a positive control. At 50 μg/ml, the lowest concentration required for effective binding (Xu et al., 2007), FadAc induced a significant increase of permeability at as early as 15 min following treatment, as compared to the basal penetration of the non-treated cells (Figure 6A). Penetration of Texas Red-conjugated dextran increased with the FadAc concentrations, reaching maximum at 1 mg/ml, similar to that caused by thrombin (Figure 6B). The FadAc-induced permeability was inhibited with increasing concentrations of GST-VE-cadherin415–53. Permeability was reduced close to basal level at 1 mg/ml GST-VE-cadherin415–53, while GST alone had no inhibitory effect (Figure 6C). FadAc treatment of HUVEC did not change cell morphology or induce cell death as shown by Trypan Blue staining (Figure 7), indicating that FadAc-induced permeability was not due to cell toxicity.
Figure 6. FadA increases the permeability of the endothelial barrier.
A–C. Confluent HUVEC monolayers in transwells were incubated with 50 μg/ml FadAc (■), 0.5 UI/mL Thrombin (◇), or untreated (×) (A); or with increasing concentrations of FadAc (B); or with 50 μg/ml FadAc in combination with varying concentrations of GST or GST-VE-cadherin415–534, or untreated (C). Cell permeability was assessed by adding Texas Red-conjugated dextran to the upper chamber (final concentration = 1 mg/ml). At indicated times (A), or after 30 min (B and C), aliquots from the lower chamber were taken and the fluorescence was measured. D. Cells were incubated with F. nucleatum 12230 (■), fadA-deletion mutant US1 (▲), fadA-complementing strain USF81(×), or E coli DH5α (□), each at an MOI=100, followed by the addition of fluorescent dextran. The permeability assay was performed as described above. E. E. coli was added to the transwell inserts with or without HUVEC, or in combination with F. nucleatum 12230, US1, or USF81. At the indicated times, aliquots were taken from the lower chamber and plated on LB agar plates followed by incubation in air. The rate of E. coli penetration was calculated as percent of bacteria recovered from the lower chamber over the total bacteria added to the upper chamber. Data are the means ± SD from at least three independent experiments performed in duplicates or triplicates. * p<0.05 and *** p<0.001 based on t test (A to D) and ANOVA test followed by Bonferroni’s post test (E).
Figure 7. HUVEC viability assay.
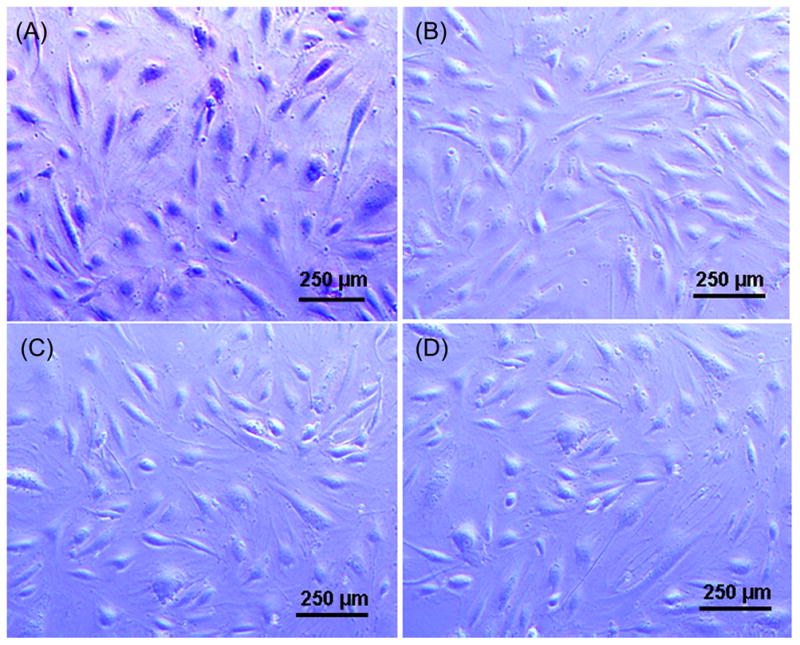
HUVEC were incubated with 4% formaldehyde (A), untreated (B), incubated with 1 mg/ml of FadAc (C) or F. nucleatum 12230 at an MOI of 1000:1 (D) for 2.5 hours, and stained with Trypan Blue. Cells were observed immediately under a light microscope immediately. A representative field for each condition is shown. The dead cells were stained blue (A) while the viable cells remained unstained (B–D). FadA adhesin (green) from Fusobacteirum nucleatum co-localizes with VE-cadherin (red) on the endothelial cells, causing VE-cadherin to relocate from cell-cell junctions (top two rows) to intracellular compartments (bottom row).
The effect of cell permeability was also tested using the whole bacteria (Figure 6D). HUVEC monolayers were incubated with F. nucleatum 12230, the fadA-deletion mutant US1, the fadA-complementing clone USF81, and E. coli DH5α, respectively. Those incubated with F. nucleatum 12230 and USF81 demonstrated significantly increased permeability to dextran compared to those treated with US1 or E. coli, indicating the increase of cell permeability was caused specifically by FadA.
Using the transwell assay, the ability of F. nucleatum to facilitate other microorganisms, such as E. coli, to pass across the HUVEC monolayers was tested. When E. coli was added to the fibronectin-coated transwell without HUVEC, >80% of the initial inoculum ended up in the lower chamber, indicating the membrane was permissible to penetration. In the presence of HUVEC, E. coli penetration was reduced by >10 fold, to <7%, indicating the monolayer formed an efficient barrier. E. coli penetration of HUVEC increased by 2-fold when co-incubated with F. nucleatum 12230 or USF81, but not with US1 (Figure 6E). These results demonstrate that F. nucleatum facilitates penetration of the HUVEC monolayers by E. coli in a FadA-dependent manner.
DISCUSSION
We have shown previously that FadA was required for F. nucleatum to adhere to and invade host epithelial and endothelial cells in vitro and to colonize the murine placenta in vivo (Ikegami et al., 2009, Xu et al., 2007). This surface exposed protein likely interacts with an endothelial receptor to disseminate through circulation and to colonize in the placenta. We used the yeast-two-hybrid technology to perform a high throughput screening of a cDNA library of the human placenta. Two members of the cadherin family were identified, PCDH1 and the endothelial cell-specific cell-junction VE-cadherin. Unlike VE-cadherin which belongs to the classical cadherin family, PCDH1 is a member of the non-clustered group of protocadherins with unique features compared to the classical cadherins. These proteins possess various numbers of extracellular subdomains similar to cadherins but display intracellular domains without significant homologies (Sano et al., 1993). Protocadherins appear to be involved mainly in neuronal development and function even though their expression has been detected throughout the body in mice (Redies et al., 2008). PCDH1 is expressed in the cerebral cortex in the adult mouse brain (Krishna et al., 2011). Although its precise function remains poorly described, as a cell-cell junction protein, PCDH1 may be a target for FadA (and F. nucleatum) to interact with brain cells. Sequence comparison of the FadA-binding region of VE-cadherin and the corresponding regions of E-, P-, N-cadherin reveals 29–33% identity and 44–48% similarity, respectively (data not shown). It may be possible that FadA binds to different types of cells by binding to the corresponding regions of different cadherins. For this study, our focus was on F. nucleatum interaction with endothelial cells, thus we concentrated our efforts on VE-cadherin, a cell-junction molecule crucial for the integrity of the endothelial barrier and vascular morphogenesis (Gory-Faure et al., 1999, Vestweber, 2008, Vittet et al., 1997).
Through affinity column chromatography and co-precipitation assays, binding between FadAc and VE-cadherin was confirmed. Such interaction required both pre-FadA and mFadA, and involved the non-alpha helical loop of the protein. Using dansylated FadAc, the Kd for FadAc and VE-cadherin415–534 is calculated to be 15.9 ± 0.6 μM, similar to that reported for the Listeria monocytogenes internalin A (InlA) and its receptor E-cadherin (kd = 8 ± 4 μM) (Wollert et al., 2007). Silencing of VE-cadherin as well as inhibitory attachment and invasion assays using purified FadAc or VE-cadherin415–534 demonstrated that FadA binding to VE-cadherin is required for F. nucleatum to adhere to and invade endothelial cells.
In our pregnant murine model, F. nucleatum crosses the endothelium to colonize the placenta causing localized inflammation and fetal demise (Han et al., 2004, Liu et al., 2007). VE-cadherin is naturally expressed in placental tissues, as part of the adherent junctions of the endothelial cells (Dye et al., 2001, Leach et al., 2000). It is present at the contact sites between trophoblast cells and decidual endothelial cells (Bulla et al., 2005). Binding to VE-cadherin could be the first step for F. nucleatum to colonize the placenta. F. nucleatum can subsequently reach the amniotic fluid and the fetus or other compartments of the feto-placental unit by interacting with other host-cell partners. F. nucleatum is also capable of overcoming the blood-brain barrier causing brain abscesses (Heckmann et al., 2003, Kai et al., 2008). VE-cadherin may be a key component in enabling F. nucleatum to cross the brain endothelium.
VE-cadherin is normally expressed at the cell junction for the integrity of the endothelium. Co-localization of FadAc and VE-cadherin altered the distribution of the latter. We speculate that upon binding, FadA triggers VE-cadherin-mediated internalization, resulting in loss of contact at cell-cell junction, which may be the cause of the increased permeability. The FadA-mediated permeability was incompletely inhibited by VE-cadherin415–534, indicating other host factors may also be involved. Weakening of the endothelial monolayer integrity by FadA is a property shared by no other bacterial component thus far.
Alteration of the endothelial integrity allows not only F. nucleatum but also other bacteria in the close proximity, such as E. coli, to cross the endothelium. Thus, F. nucleatum may serve as an “enabler”, facilitating systemic dissemination of other microorganisms. In the oral cavity, a wide variety of microbial species bind to F. nucleatum, a phenomenon termed “co-aggregation” (Rickard et al., 2003, Saito et al., 2009, Saito et al., 2008, Shaniztki et al., 1997). The coaggregation and increased endothelium permeability may explain why extra-oral infections involving F. nucleatum are often polymicrobial (Ghafghaichi et al., 2010, Martin et al., 2007, Ohyama et al., 2009). A wide variety of oral species in saliva and dental plaque have been demonstrated to translocate to the murine placenta in a pooled population (Fardini et al., 2010). In human, F. nucleatum has been found in the intra-amniotic cavity both as a sole infectious organism and in mixed infections (Han et al., 2009). It is possible other species can cross the maternofetal barrier once the endothelial integrity is impaired in the presence of F. nucleatum.
VE-cadherin has been linked to virulence mechanisms of pathogens (Coureuil et al., 2009, Sheets et al., 2005, MacIntyre et al., 2002). However, its involvement has always been indirect. The Type-IV pili of Neisseria meningitidis has been shown to bind to brain endothelial cells. Upon binding, VE-cadherin is recruited to the site of the N. meningitidis colonies (Coureuil et al., 2009). Cysteine proteases from the periodontal pathogen Porphyromonas gingivalis W83, designated gingipains, cause the cleavage of VE-cadherin (Sheets et al., 2005). Another study showed that infection with Chlamydia pneumoniae causes an increased expression of VE-cadherin in human brain microvascular endothelial cells (MacIntyre et al., 2002). To the best of our knowledge, this is the first time VE-cadherin has been identified to directly mediate bacterial adherence. InlA binds to EC1 of E-cadherin (Lecuit et al., 1999, Schubert et al., 2002), which FadA does not bind (unpublished results). Instead, FadA targets a region encompassing the second half of EC4 and the first half of EC5 on VE-cadherin. This region is not involved in homophilic interactions (Ahrens et al., 2003), nor has it been shown to be bound by other components. Thus, F. nucleatum may have found a unique niche for binding to the endothelial cells with little competition from anything else.
Utilizing a cadherin to promote cell attachment and potentially invasion raises the question of accessibility. Adherent junction cadherins are not usually accessible at the apical surface of the cells. Then how can the bacteria reach such molecules? In the case of L. monocytogenes, it has been shown that E-cadherin is transiently exposed at the apical surface of enterocytes during the apoptotic cell extrusion. Attachment and invasion of L. monocytogenes is preferentially observed at these sites (Pentecost et al., 2006). Similar exposition of VE-cadherin may occur in the endothelium. It has been reported that VE-cadherin harbors a basal-to-apical flow at cell junctions showing that it is not a static but a dynamic system since in tissues, cells are able to relocate (Kametani & Takeichi, 2007). One could postulate that VE-cadherin may be transiently exposed at the apical site of endothelial cells which may allow binding by FadA. In addition, the increase of cell permeability modified by FadA may also help to enhance accessibility by the bacteria at the destabilized cell-cell junctions. In the case of entering in the blood vessel, F. nucleatum would target endothelial cell from the basal site, where VE-cadherin would likely be more accessible. This would constitute a port of entry in the systemic dissemination from the oral cavity.
We have previously shown that F. nucleatum, which does not possess any known Type III secretion system (Kapatral et al., 2002), adheres to and then invades the host cells through a zipper-like mechanism which requires cytoskeleton rearrangement (Han et al., 2000). It would be interesting to evaluate the role of VE-cadherin in this process. The co-localization experiment has provided the first indication that VE-cadherin may mediate the invasion of F. nucleatum. Internalization of VE-cadherin occurs through a clathrin-dependent mechanism (Xiao et al., 2003, Xiao et al., 2005). Certain invasive pathogens used clathrin-dependent internalization of the cellular receptor they bind to, such as Candida albicans with N-cadherin (Phan et al., 2005) and L. monocytogenes with hepatocyte growth-factor receptor Met (Veiga & Cossart, 2005). Recently, L. monocytogenes InlA interaction with E-cadherin has been shown to cause post-translational modification of E-cadherin and trigger endocytosis in relation with clathrin recruitment (Bonazzi et al., 2008). Studies are currently under way to further characterize the interactions between FadA and VE-cadherin and their subsequent events.
Overall, our results demonstrate that F. nucleatum, a natural inhabitant of the oral cavity, possesses in its molecular repertoire an efficient “tool”, the adhesin FadA, to interact specifically with the endothelium through the VE-cadherin receptor. This process may be important for F. nucleatum to cross the blood barriers, which are important virulence mechanisms for systemic dissemination and for reaching deeper tissues. In addition, we demonstrate in vitro that F. nucleatum is a potential “enabler” microorganism which can facilitate other bacteria to cross the endothelium, a possible reason why F. nucleatum is often found in mixed infections. This investigation sheds new light on possible mechanisms of microbial dissemination, and especially in mixed-species infections.
EXPERIMENTAL PROCEDURES
Bacterial strains, cell culture and plasmids
Wild type F. nucleatum 12230 (Han et al., 2000), the fadA-deletion mutant US1 (Han et al., 2005) and the fadA-complementing strain USF81 (Ikegami et al., 2009) were cultivated using Columbia blood agar supplemented with 5 μg/ml hemin (Sigma), 1 μg/ml menadione (Sigma), and 5% defibrinated sheep blood (Cleveland Scientific, OH) and incubated at 37°C with 5% CO2, 10% H2, and 85% N2. E coli DH5α and BL21(DE3) (Invitrogen) were cultured in LB broth aerobically at 37°C.
Human Umbilical Vein Endothelial Cells (HUVEC) were purchased from Lonza (Waskerville, MD) and cultured in F12-K supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA), 30 μg/ml of Endothelial Cell Growth Supplements (BD Biosciences, San Jose, CA), 0.1 mg/ml heparin (Sigma, Saint Louis, MO) and 100 UI/ml penicillin and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA).
Plasmid pYWH417-6 carrying the entire fadA gene from F. nucleatum 12230 was used to express FadAc (Nithianantham et al., 2009). Plasmid pYH1490 is a derivative of pYWH417-6 with the FadA signal peptide deleted, expressing mFadA alone. Plasmid pYH1471 carrying the FadA mutant G4 with each of the four residues in the non-alpha helical region, T66, R67, F68, and Y69, replaced with glycine was constructed with primers Gly4 forward (5′-CAAGCAGAAGCTAACGGAGGAGGAGGAAAATCTCAATATCAATTAGC3′) and Gly4 reverse ‘5’-GCTAATTGATATTGAGATTTCCTCCTCCTCCTGTTAGCTTCTGCTTG-3′) using the QuickChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA).
For the yeast two-hybrid experiments, the coding sequence for full-length FadA from pYWH417-6 was amplified by PCR and cloned into pB29 as an N-terminal fusion to LexA (N-FadA-LexA-C). The construct was verified by sequencing analysis of the entire insert before being used as a bait to screen a random-primed human placenta cDNA library constructed in pP6. Plasmids pB29 and pP6 were derived from plasmids pBTM116 (Vojtek & Hollenberg, 1995) and pGADGH {Bartel, 1993 #485}, respectively.
To construct the GST-VE cadherin fusion, VE-cadherin region 415–534 was amplified by PCR from the identified Y2H prey vector clone with the following primers (Fwd- 5′-GGCCGGATCCAAGGGCCAGTTCTTCCGAGT-3′/Rev-5′-GGCCGAATTCGGCCGTGTTATCGTGATTAT-3′) containing a BamHI and EcoRI site, respectively. The amplicon was digested and directionally cloned into pGEX-6-P1 (GE Healthcare, Piscataway, NJ) to obtain a fusion protein with the GST tag at the N-terminal of the VE-cadherin region415–534. The construct was verified by sequencing analysis using the pGEX primers (Fwd-5′-GGGCTGGCAAGCCACGTTTGGTG-3′/Rev-5′-CCGGGAGCTGCATGTGTCAGAGG-3′) by the Genomics Core Facility at Case Western Reserve University (Cleveland, OH) and designated as plasmid pYWH1621. A correct clone was transformed into E. coli BL21 (DE3) to produce strain YH1621.
Yeast Two-hybrid screening
Yeast two-hybrid screening was performed by Hybrigenics, S.A., Paris, France (http://www.hybrigenics.com). A total of 76 million clones (8-fold the complexity of the library) were screened using a mating approach with Y187 (matα) and L40ΔGal4 (matα) yeast strains as previously described (Fromont-Racine et al., 1997). A group of 37 His+ colonies were selected on a medium lacking tryptophan, leucine and histidine. The prey fragments of the positive clones were amplified by PCR and sequenced at their 5′ and 3′ junctions. The resulting sequences were used to identify the corresponding interacting proteins in the GenBank database (NCBI).
Preparation of protein samples and protein purification
Pre-FadA-mFadA complex (FadAc), mFadA, and the FadA mutant G4 were purified as previously described (Xu et al., 2007). E. coli cultures grown to O.D.600 = 0.5 were induced by 0.1 mM IPTG for two and half hours followed by lysis in 8M Urea, 0.3M NaCl, 50 mM NaH2PO4 pH 8.0. The lysate supernatant was collected by centrifugation and dialyzed against 10 mM Tris-HCl pH 7.5. Total proteins concentration was measured by BCA.
GST or the recombinant GST-VE-cadherin415–534 proteins were purified using the GST-Bind Resin (EMD Chemicals, Gibbstown, NJ) according the manufacturer’s instructions. Briefly, the bacteria were cultured in LB medium till OD600 ~0.8 before IPTG was added to a final concentration of 0.1 mM. After incubation for two and half hours at 37°C, the bacteria were harvested and the pellet was resuspended in GST bind/wash buffer (43 mM Na2HPO4, 14.7 mM KH2PO4, 1.37 M NaCl, 27 mM KCl, pH 7.3) containing 0.1 mg/ml lysozyme (Sigma, Saint Louis, CO), 0.1% CA-630 (USB, Cleveland, OH), 1mM PMSF (Thermo Scientific, Rockford, IL) and 1x Halt anti-protease cocktail (Pierce, Rockford, IL), followed by sonication. After centrifugation, the clear supernatant was mixed with GST-Bind resin equilibrated in GST bind/wash buffer for 1 hour at 4°C with gentle shaking. After three washes, GST or GST-VE-cadherin415–534 protein was eluted from the resin with the GST elution buffer (10 mM glutathione 50 mM Tris-HCl, pH 8.0).
Affinity column chromatography
Purified FadAc (3 mg) and GST or GST-VE-cadherin415–534 proteins (3 mg) (in 50 mM Na2HPO4 pH 7.5) were incubated with 5 ml of pre-equilibrated GST-bind resin for 10 min with gentle shaking and then loaded onto the column. After washing with binding buffer (4.3 mM Na2HPO4, 1.5 mM KH2PO4 and pH 7.3), the proteins were eluted with 10 mM reduced glutathione in 0.05 M Tris-HCl pH 8.0. The presence of FadAc in the eluted fraction was detected by Western blot.
GST co-precipitation assay
An aliquot of 1.2 nmoles of GST or GST-VE-cadherin415–534 was incubated overnight at 4°C with 100 μg of total protein of E. coli lysate expressing FadAc. The mixtures were then incubated with equilibrated GST-bind resin (EMD Chemicals, Gibbstown, NJ) for 1 hour at 4°C. After several washes with GST bind wash buffer containing 0.1% BSA, 10% glycerol and 0.5% CA-630, the GST complexes were eluted in 4x Laemmli buffer by boiling in dry bath at 90°C for 5 min. The presence of FadAc in the co-precipitated complexes was detected by Western blot.
Determination of protein dissociation constant
A total of 500 μg FadAc was incubated with 5 μl of 10 mg/ml dansyl chloride (Invitrogen, Carlsbad, CA) at 4 °C for 5 hr in 50 mM Na2HPO4, 10 mM NaHCO3 and pH 7.5. Free dansyl chloride was removed by extensive dialysis against binding buffer (10mM Tris and pH 8.0). The extent of labeling was 0.72 mol of dye per mol of FadAc, as determined by absorbance at 340 nm according to manufacturer’s instruction. The concentration of FadAc was measured by the Bradford assay using BSA as a standard. The molar concentration of GST or GST-VE-cadherin415–534 was measured by using a molar extinction coefficient at 280 nm. Dansylated-FadAc (4 μM) was incubated with increasing concentrations of GST or its fusion protein at 4°C overnight. The solution was excited using an SLM 8000C spectrofluorimeter (SLM Instruments, Rochester, NY) at 340 nm, and the fluorescence emission were measured in the wavelength range of 450–650 nm. The difference between fluorescence intensity emitted at 550 nm in the presence of GST-VE-cadherin415–534 (Fv) and that in the presence of GST (Fg) (i.e. F = Fv − Fg) relative to the fluorescence emitted by FadAc alone (F0) was plotted against the protein concentrations, and the data were fitted to a Boltzmann sigmoidal equation with the Prism 4 software (GraphPad Software Inc., San Diego, CA). The dissociation constant was calculated as previously described (Bertrand et al., 1994). In brief, the fractional degree of saturation of dansylated-FadA was determined by a = (F−F0)/(F∞ − F0), where F∞ is the fluorescence signal at saturation level. 1/(1 − a) was plotted against the protein concentrations divided by a. The data of this plot were fitted to a straight line by linear regression. Kd was determined from the reciprocal of the slope.
Western blot analysis
To detect FadA, samples were separated by 12% SDS-PAGE followed by Coommassie blue staining and immunodetected with the mouse monoclonal anti-FadA clone 5G11-3G8 antibody (Xu et al., 2007) and horse radish peroxidase (HRP)-conjugated goat anti-mouse IgG (Pierce, Rockford, IL). To examine the expression of the VE-cadherin in HUVEC after siRNA treatment (see below), cells were harvested with enzyme free cell dissociation buffer (Invitrogen, Carlsbad, CA), counted, and resuspended in 4x Laemmli buffer. The equivalent of 5×103 cells was loaded onto each lane and subjected to 10% SDS-PAGE. VE-cadherin was detected using rabbit polyclonal antibody (Abcam, Cambridge, MA) and the HRP-conjugated goat anti-rabbit IgG.
Protein co-localization by double immunofluorescent labeling and confocal microscopy
FadAc and cytochrome c (Sigma, St Louis, MO) were each conjugated to Alexa Fluor 488 (Invitrogen, Carlsbad, CA) as previously described (Xu et al., 2007). HUVEC cells were seeded in 35mm glass bottom microwell dishes (Matek Corp, Ashland, MA) and grown to confluence. Cells were placed in complete growth medium supplemented with 1% BSA and incubated for 1 hour at 37°C. Alexa Fluor 488-conjugated FadAc or cytochrome c was added to the cells at a final concentration of 50 μg/ml and incubated for 2 hours. After washes, the cells were fixed with 4% formaldehyde for 15 min at room temperature and washed in D-PBS Ca2+ Mg2+ (Invitrogen, Carlsbad, CA) containing 0.1% glycine for 10 min. The ceells were then blocked in 1% BSA in D-PBS Ca2+ Mg2+ for 30 min, followed by overnight incubation with mouse anti-VE-cadherin monoclonal antibodies (BD Biosciences, Bedford, MA) diluted 1:50 in 0.2% BSA in D-PBS Ca2+ Mg2+ at 4°C. After three washes, cells were incubated with Alexa Fluor 594-conjugated chicken polyclonal anti-mouse antibodies for 1 hour, washed, and counter-stained with DAPI before being mounted in Fluoromout G (Southern Biotech, Birmingham, AL) containing 2.5% DABCO (Acros Organics, Morris Plains, NJ). The samples were visualized with a Leica TCS SP2 AOBS filter-free UV/spectral confocal laser scanner on an inverted DM IRE2 microscope. The experiment was repeated at least three times.
Modulation of VE-cadherin expression by siRNA
HUVEC cells were seeded at 2.5×104 cells per well in 96-well plates and grown overnight. The siGENOME SMARTpool siRNA anti-CDH5 was purchased from Dharmacon (Lafayette, CO) and was used at 100 nM with Dharmafect I transfection reagent (Dharmacon) according the manufacturer’s instructions. As a negative control, siGENOME Non-targeting siRNA Pool#2 from Dharmacon (Lafayette, CO) was used. At 24 hours following transfection, the cells were placed in complete growth medium and incubated for an additional 24 hours before being used for the attachment assay (see below).
Tissue culture attachment and invasion assays
For HUVEC cells treated with or without siRNA, F. nucleatum 12230 was added to the cells at a multiplicity of infection (MOI) of 5. Following one-hour incubation at 37°C, the monolayers were washed 3 times with D-PBS pH 7.1 supplemented with Ca2+ and Mg2+. Cells were lyzed with water for 20 min at 37°C. Serial dilutions of the lysates were plated onto blood agar plates to enumerate the total cell-associated bacteria. For invasion assays, the bacteria were incubated with the monolayers at 37°C for 4 hrs, followed by washes with PBS. Fresh media containing 300 μg/ml gentamicin and 200 μg/ml metronidazole were added to the monolayers and incubated for an additional hour to kill extracellular bacteria. The cells were then washed and lyzed with water as described above. The levels of attachment and invasion were expressed as the percentage of bacteria recovered following cell lysis relative to the total number of bacteria initially added. The experiment was performed in triplicates and repeated at least three times. For the inhibition assays, HUVEC cells were seeded at 8×104 cells per well in 24-well plates and grown to confluence. Proteins were added to the cells as indicated and incubated for 45 min prior to the addition of F. nucleatum. The attachment and invasion assays were performed as described above. Each experiment was performed in triplicates and repeated twice.
Fluorescence permeability assay
Costar Transwell inserts (6.5 mm diameter and 0.4 μm pore size; Corning, NY) was coated with 50 μL of 7 μg/ml fibronectin (Millipore, Billerica, MA) before HUVECs were inoculated at a density of 3×104 cells per well and allowed to grow to confluence. Cells were incubated with FadAc of indicated concentrations or 0.5 UI/ml Thrombin. A total of 5 μl of 20 mg/ml Texas Red-conjugated dextran (Invitrogen, Carlsbad, CA) was added to the upper chamber (final concentration = 1mg/ml). At the indicated times, an aliquot of 25 μl of media was removed from the lower chamber and diluted 1:20. The fluorescence in the diluted aliquots was measured in triplicates in a microplate reader (BioTek, Winooski, VT). Each experiment was performed in duplicates and repeated at least three times. For the inhibitory experiments, a total of 50 μg of FadAc, 100 or 1000 μg of GST-VE-cadherin415–534, or 67 or 670 μg of GST was added either alone or in combination to the cells and incubated for 45 minutes, followed by the permeability assay as described above. When using the whole bacteria, log-phase cultures of F. nucleatum 12230, US1, USF81 and E coli DH5α were washed twice in D-PBS Ca2+ Mg2+, followed by centrifugation and resuspension to a final concentration of 6×108 CFU/ml in serum free cell medium. The bacteria were added to HUVEC at an MOI of 100:1. For controls, equal volumes of D-PBS Ca2+ Mg2+ without bacteria were added to the monolayers. After one hour, the cells were washed and incubated with fluorescent dextran, followed by fluorescent measurement of the lower chamber as described above. At least three independent experiments were performed, with each in duplicates.
E. coli transwell penetration assay
HUVEC cells were inoculated into fibronectin–coated Costar Transwell inserts as described above. Log-phase cultures of F. nucleatum 12230, US1, USF81 and E coli DH5α were washed twice in D-PBS Ca2+ Mg2+, followed by centrifugation and resuspension in serum free cell medium to a final concentration of 6×109 CFU/ml (for F. nucleatum), or 6×108 CFU/ml (for E coli). F. nucleatum was added to HUVEC at an MOI of 1000:1 and E. coli at an MOI of 100:1, respectively. After one-hour incubation at 37°C, aliquots were taken from the lower chamber and plated on LB agar plates followed by overnight incubation at 37°C in air to enumerate the number of E. coli recovered from the lower chamber. Each experiment was performed in duplicates and repeated three times.
Cell viability assay
FadAc (at a final concentration of 1 mg/ml) or F. nucleatum 12230 at an MOI of 1000:1 was added to the HUVEC monolayers and incubated for 2.5 hours. HUVECs treated with 4% formaldehyde or untreated were used as positive and negative controls, respectively. Trypan Blue (Invitrogen) was added to the monolayers at 1:10 dilution and incubated for 5 minutes. The cells were observed immediately under the microscope. Non-viable cells were stained blue while viable cells remained unstained.
Acknowledgments
The authors thank Carl Venezia and Mary Barkley for help with measurements using the spectrofluorimeter, and Zhenghe Wang for discussion and reading the manuscript. This work was supported in part by NIH grants RO1 DE14924, KO2 DE16102, and R21 DE17165 to Y.W.H.
References
- Ahrens T, Lambert M, Pertz O, Sasaki T, Schulthess T, Mege RM, Timpl R, Engel J. Homoassociation of VE-cadherin follows a mechanism common to “classical” cadherins. J Mol Biol. 2003;325:733–742. doi: 10.1016/s0022-2836(02)01286-x. [DOI] [PubMed] [Google Scholar]
- Bertrand B, Wakabayashi S, Ikeda T, Pouyssegur J, Shigekawa M. The Na+/H+ exchanger isoform 1 (NHE1) is a novel member of the calmodulin-binding proteins. Identification and characterization of calmodulin-binding sites. J Biol Chem. 1994;269:13703–13709. [PubMed] [Google Scholar]
- Bonazzi M, Veiga E, Pizarro-Cerda J, Cossart P. Successive post-translational modifications of E-cadherin are required for InlA-mediated internalization of Listeria monocytogenes. Cell Microbiol. 2008;10:2208–2222. doi: 10.1111/j.1462-5822.2008.01200.x. [DOI] [PubMed] [Google Scholar]
- Bulla R, Villa A, Bossi F, Cassetti A, Radillo O, Spessotto P, De Seta F, Guaschino S, Tedesco F. VE-cadherin is a critical molecule for trophoblast-endothelial cell interaction in decidual spiral arteries. Exp Cell Res. 2005;303:101–113. doi: 10.1016/j.yexcr.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Cheung WY, Bellas J. Case report: Lemierre syndrome presenting with fever and pharyngitis. Am Fam Physician. 2007a;75:979–980. [PubMed] [Google Scholar]
- Cheung WY, Bellas J. Fusobacterium: elusive cause of life-threatening septic thromboembolism. Can Fam Physician. 2007b;53:1451–1453. [PMC free article] [PubMed] [Google Scholar]
- Cigarran S, Neches C, Lamas JM, Garcia-Trio G, Alonso M, Saavedra J. A case report of a pyogenic liver abscess caused by Fusobacterium nucleatum in a patient with autosomal dominant polycystic kidney disease undergoing hemodialysis. Ther Apher Dial. 2008;12:91–95. doi: 10.1111/j.1744-9987.2007.00548.x. [DOI] [PubMed] [Google Scholar]
- Coureuil M, Mikaty G, Miller F, Lecuyer H, Bernard C, Bourdoulous S, Dumenil G, Mege RM, Weksler BB, Romero IA, Couraud PO, Nassif X. Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science. 2009;325:83–87. doi: 10.1126/science.1173196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye JF, Jablenska R, Donnelly JL, Lawrence L, Leach L, Clark P, Firth JA. Phenotype of the endothelium in the human term placenta. Placenta. 2001;22:32–43. doi: 10.1053/plac.2000.0579. [DOI] [PubMed] [Google Scholar]
- Fardini Y, Chung P, Dumm R, Joshi N, Han YW. Transmission of diverse oral bacteria to murine placenta: evidence for the oral microbiome as a potential source of intrauterine infection. Infect Immun. 2010;78:1789–1796. doi: 10.1128/IAI.01395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromont-Racine M, Rain JC, Legrain P. Toward a functional analysis of the yeast genome through exhaustive two-hybrid screens. Nat Genet. 1997;16:277–282. doi: 10.1038/ng0797-277. [DOI] [PubMed] [Google Scholar]
- Ghafghaichi L, Troy S, Budvytiene I, Banaei N, Baron EJ. Mixed infection involving Actinomyces, Aggregatibacter, and Fusobacterium species presenting as perispinal tumor. Anaerobe. 2010;16:174–178. doi: 10.1016/j.anaerobe.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Gory-Faure S, Prandini MH, Pointu H, Roullot V, Pignot-Paintrand I, Vernet M, Huber P. Role of vascular endothelial-cadherin in vascular morphogenesis. Development. 1999;126:2093–2102. doi: 10.1242/dev.126.10.2093. [DOI] [PubMed] [Google Scholar]
- Han YW, Fardini Y, Chen C, Iacampo KG, Peraino VA, Shamonki JM, Redline RW. Term stillbirth caused by oral Fusobacterium nucleatum. Obstet Gynecol. 2010;115:442–445. doi: 10.1097/AOG.0b013e3181cb9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YW, Ikegami A, Rajanna C, Kawsar HI, Zhou Y, Li M, Sojar HT, Genco RJ, Kuramitsu HK, Deng CX. Identification and characterization of a novel adhesin unique to oral fusobacteria. J Bacteriol. 2005;187:5330–5340. doi: 10.1128/JB.187.15.5330-5340.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YW, Redline RW, Li M, Yin L, Hill GB, McCormick TS. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun. 2004;72:2272–2279. doi: 10.1128/IAI.72.4.2272-2279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol. 2009;47:38–47. doi: 10.1128/JCM.01206-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YW, Shi W, Huang GT, Kinder Haake S, Park NH, Kuramitsu H, Genco RJ. Interactions between periodontal bacteria and human oral epithelial cells: Fusobacterium nucleatum adheres to and invades epithelial cells. Infect Immun. 2000;68:3140–3146. doi: 10.1128/iai.68.6.3140-3146.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann JG, Lang CJ, Hartl H, Tomandl B. Multiple brain abscesses caused by Fusobacterium nucleatum treated conservatively. Can J Neurol Sci. 2003;30:266–268. doi: 10.1017/s0317167100002717. [DOI] [PubMed] [Google Scholar]
- Ikegami A, Chung P, Han YW. Complementation of the fadA mutation in Fusobacterium nucleatum demonstrates that the surface-exposed adhesin promotes cellular invasion and placental colonization. Infect Immun. 2009;77:3075–3079. doi: 10.1128/IAI.00209-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai A, Cooke F, Antoun N, Siddharthan C, Sule O. A rare presentation of ventriculitis and brain abscess caused by Fusobacterium nucleatum. J Med Microbiol. 2008;57:668–671. doi: 10.1099/jmm.0.47710-0. [DOI] [PubMed] [Google Scholar]
- Kametani Y, Takeichi M. Basal-to-apical cadherin flow at cell junctions. Nat Cell Biol. 2007;9:92–98. doi: 10.1038/ncb1520. [DOI] [PubMed] [Google Scholar]
- Kapatral V, Anderson I, Ivanova N, Reznik G, Los T, Lykidis A, Bhattacharyya A, Bartman A, Gardner W, Grechkin G, Zhu L, Vasieva O, Chu L, Kogan Y, Chaga O, Goltsman E, Bernal A, Larsen N, D’Souza M, Walunas T, Pusch G, Haselkorn R, Fonstein M, Kyrpides N, Overbeek R. Genome sequence and analysis of the oral bacterium Fusobacterium nucleatum strain ATCC 25586. J Bacteriol. 2002;184:2005–2018. doi: 10.1128/JB.184.7.2005-2018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornstra JJ, Veenendaal D, Bruyn GA, de Graaf H. Septic arthritis due to Fusobacterium nucleatum. Br J Rheumatol. 1998;37:1249. doi: 10.1093/rheumatology/37.11.1249. [DOI] [PubMed] [Google Scholar]
- Krishna KK, Hertel N, Redies C. Cadherin expression in the somatosensory cortex: evidence for a combinatorial molecular code at the single-cell level. Neuroscience. 2011;175:37–48. doi: 10.1016/j.neuroscience.2010.11.056. [DOI] [PubMed] [Google Scholar]
- Kumar P, Shen Q, Pivetti CD, Lee ES, Wu MH, Yuan SY. Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev Mol Med. 2009;11:e19. doi: 10.1017/S1462399409001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach L, Lammiman MJ, Babawale MO, Hobson SA, Bromilou B, Lovat S, Simmonds MJ. Molecular organization of tight and adherens junctions in the human placental vascular tree. Placenta. 2000;21:547–557. doi: 10.1053/plac.2000.0541. [DOI] [PubMed] [Google Scholar]
- Lecuit M, Dramsi S, Gottardi C, Fedor-Chaiken M, Gumbiner B, Cossart P. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 1999;18:3956–3963. doi: 10.1093/emboj/18.14.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Redline RW, Han YW. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J Immunol. 2007;179:2501–2508. doi: 10.4049/jimmunol.179.4.2501. [DOI] [PubMed] [Google Scholar]
- MacIntyre A, Hammond CJ, Little CS, Appelt DM, Balin BJ. Chlamydia pneumoniae infection alters the junctional complex proteins of human brain microvascular endothelial cells. FEMS Microbiol Lett. 2002;217:167–172. doi: 10.1111/j.1574-6968.2002.tb11470.x. [DOI] [PubMed] [Google Scholar]
- Margueron R, Justin N, Ohno K, Sharpe ML, Son J, Drury WJ, 3rd, Voigt P, Martin SR, Taylor WR, De Marco V, Pirrotta V, Reinberg D, Gamblin SJ. Role of the polycomb protein EED in the propagation of repressive histone marks. Nature. 2009;461:762–767. doi: 10.1038/nature08398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CA, Wijesurendra RS, Borland CD, Karas JA. Femoral vein thrombophlebitis and septic pulmonary embolism due to a mixed anaerobic infection including Solobacterium moorei: a case report. J Med Case Reports. 2007;1:40. doi: 10.1186/1752-1947-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore WE, Moore LV. The bacteria of periodontal diseases. Periodontol. 1994;2000(5):66–77. doi: 10.1111/j.1600-0757.1994.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Nithianantham S, Xu M, Yamada M, Ikegami A, Shoham M, Han YW. Crystal structure of FadA adhesin from Fusobacterium nucleatum reveals a novel oligomerization motif, the leucine chain. J Biol Chem. 2009;284:3865–3872. doi: 10.1074/jbc.M805503200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama H, Nakasho K, Yamanegi K, Noiri Y, Kuhara A, Kato-Kogoe N, Yamada N, Hata M, Nishimura F, Ebisu S, Terada N. An unusual autopsy case of pyogenic liver abscess caused by periodontal bacteria. Jpn J Infect Dis. 2009;62:381–383. [PubMed] [Google Scholar]
- Pentecost M, Otto G, Theriot JA, Amieva MR. Listeria monocytogenes invades the epithelial junctions at sites of cell extrusion. PLoS Pathog. 2006;2:e3. doi: 10.1371/journal.ppat.0020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan QT, Fratti RA, Prasadarao NV, Edwards JE, Jr, Filler SG. N-cadherin mediates endocytosis of Candida albicans by endothelial cells. J Biol Chem. 2005;280:10455–10461. doi: 10.1074/jbc.M412592200. [DOI] [PubMed] [Google Scholar]
- Redies C, Heyder J, Kohoutek T, Staes K, Van Roy F. Expression of protocadherin-1 (Pcdh1) during mouse development. Dev Dyn. 2008;237:2496–2505. doi: 10.1002/dvdy.21650. [DOI] [PubMed] [Google Scholar]
- Rickard AH, Gilbert P, High NJ, Kolenbrander PE, Handley PS. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 2003;11:94–100. doi: 10.1016/s0966-842x(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Saini S, Gupta N, Batra G, Arora DR. Role of anaerobes in acute pelvic inflammatory disease. Indian J Med Microbiol. 2003;21:189–192. [PubMed] [Google Scholar]
- Saito A, Inagaki S, Ishihara K. Differential ability of periodontopathic bacteria to modulate invasion of human gingival epithelial cells by Porphyromonas gingivalis. Microb Pathog. 2009;47:329–333. doi: 10.1016/j.micpath.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Saito A, Inagaki S, Kimizuka R, Okuda K, Hosaka Y, Nakagawa T, Ishihara K. Fusobacterium nucleatum enhances invasion of human gingival epithelial and aortic endothelial cells by Porphyromonas gingivalis. FEMS Immunol Med Microbiol. 2008;54:349–355. doi: 10.1111/j.1574-695X.2008.00481.x. [DOI] [PubMed] [Google Scholar]
- Sano K, Tanihara H, Heimark RL, Obata S, Davidson M, St John T, Taketani S, Suzuki S. Protocadherins: a large family of cadherin-related molecules in central nervous system. EMBO J. 1993;12:2249–2256. doi: 10.1002/j.1460-2075.1993.tb05878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert WD, Urbanke C, Ziehm T, Beier V, Machner MP, Domann E, Wehland J, Chakraborty T, Heinz DW. Structure of internalin, a major invasion protein of Listeria monocytogenes, in complex with its human receptor E-cadherin. Cell. 2002;111:825–836. doi: 10.1016/s0092-8674(02)01136-4. [DOI] [PubMed] [Google Scholar]
- Shaniztki B, Hurwitz D, Smorodinsky N, Ganeshkumar N, Weiss EI. Identification of a Fusobacterium nucleatum PK1594 galactose-binding adhesin which mediates coaggregation with periopathogenic bacteria and hemagglutination. Infect Immun. 1997;65:5231–5237. doi: 10.1128/iai.65.12.5231-5237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets SM, Potempa J, Travis J, Casiano CA, Fletcher HM. Gingipains from Porphyromonas gingivalis W83 induce cell adhesion molecule cleavage and apoptosis in endothelial cells. Infect Immun. 2005;73:1543–1552. doi: 10.1128/IAI.73.3.1543-1552.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga E, Cossart P. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat Cell Biol. 2005;7:894–900. doi: 10.1038/ncb1292. [DOI] [PubMed] [Google Scholar]
- Vestweber D. VE-cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler Thromb Vasc Biol. 2008;28:223–232. doi: 10.1161/ATVBAHA.107.158014. [DOI] [PubMed] [Google Scholar]
- Vittet D, Buchou T, Schweitzer A, Dejana E, Huber P. Targeted null-mutation in the vascular endothelial-cadherin gene impairs the organization of vascular-like structures in embryoid bodies. Proc Natl Acad Sci U S A. 1997;94:6273–6278. doi: 10.1073/pnas.94.12.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtek AB, Hollenberg SM. Ras-Raf interaction: two-hybrid analysis. Methods Enzymol. 1995;255:331–342. doi: 10.1016/s0076-6879(95)55036-4. [DOI] [PubMed] [Google Scholar]
- Wollert T, Heinz DW, Schubert WD. Thermodynamically reengineering the listerial invasion complex InlA/E-cadherin. Proc Natl Acad Sci U S A. 2007;104:13960–13965. doi: 10.1073/pnas.0702199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Hanson SM, Francis DJ, Vishnivetskiy SA, Thibonnier M, Klug CS, Shoham M, Gurevich VV. Arrestin binding to calmodulin: a direct interaction between two ubiquitous signaling proteins. J Mol Biol. 2006;364:955–963. doi: 10.1016/j.jmb.2006.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K, Allison DF, Kottke MD, Summers S, Sorescu GP, Faundez V, Kowalczyk AP. Mechanisms of VE-cadherin processing and degradation in microvascular endothelial cells. J Biol Chem. 2003;278:19199–19208. doi: 10.1074/jbc.M211746200. [DOI] [PubMed] [Google Scholar]
- Xiao K, Garner J, Buckley KM, Vincent PA, Chiasson CM, Dejana E, Faundez V, Kowalczyk AP. p120-Catenin regulates clathrin-dependent endocytosis of VE-cadherin. Mol Biol Cell. 2005;16:5141–5151. doi: 10.1091/mbc.E05-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Yamada M, Li M, Liu H, Chen SG, Han YW. FadA from Fusobacterium nucleatum utilizes both secreted and nonsecreted forms for functional oligomerization for attachment and invasion of host cells. J Biol Chem. 2007;282:25000–25009. doi: 10.1074/jbc.M611567200. [DOI] [PubMed] [Google Scholar]