Abstract
Purpose
To determine which, if any, vision variables are associated with moderate bilateral hearing loss in an elderly population.
Methods
Four hundred and forty six subjects completed a hearing screening in conjunction with measurements on a variety of vision tests including high contrast acuity, low contrast acuity measured under a variety of lighting conditions, contrast sensitivity, stereopsis, and colour vision. Logistic regression analyses were used to assess the relationship between various vision variables and hearing impairment while controlling for demographic and other co-morbid conditions.
Results
In this sample of older adults with a mean age of 79.9 years, 5.4% of individuals were moderately visually impaired (binocular high contrast VA worse than 0.54 logMAR, Snellen equivalent 6/21 or 20/70) and 12.8% were moderately bilaterally hearing impaired (hearing none of the 40 dB tones at 500, 2000 or 4000 Hz in either ear). Three measures of low contrast acuity, but not high contrast acuity or other vision measures, were significantly associated with hearing loss when controlling for age, cataract surgery history, glaucoma history and self reported stroke, all of which were significantly associated with hearing loss, although the association of glaucoma with hearing loss was negative.
Conclusions
Poorer vision for low contrast targets was associated with an increased risk of hearing impairment in older adults. Audiologists and optometrists should enquire about the other sense in cases in which a deficit is measured as individuals with dual sensory loss are at a marked disadvantage in daily life
Keywords: ageing, hearing impairment, vision impairment, acuity, dual sensory loss
Introduction
Sensory impairment is one of the most common chronic conditions of later life. The prevalence of vision impairment and hearing impairment both increase with age, as does the prevalence of dual sensory loss (vision and hearing deficits).1–11 A significant portion of the older U.S. population suffers from vision impairment, hearing impairment or both. For example, Berry et al12 reported that 21% of the older population in the U.S. has both vision and hearing impairment by the age of 70, but this is higher than typically reported. Citing survey data (i.e. self reported hearing and vision difficulties) collected by Campbell et al13 and by Crews & Campbell14, Brennan et al15 reported that visual impairment affects from 9% to 18% of older adults and that hearing loss affects from 24% to 33%. The generally higher rates of self-reported hearing loss compared to self-reported vision loss in these survey data probably reflect the everyday experience that hearing impairments sufficient to cause problems with spoken communication are more common than vision impairments sufficient to cause difficulties in daily tasks.
Of course, measured rates of vision and or hearing impairment as well as dual sensory loss also vary and depend on the criteria for impairment. Defining hearing loss as average threshold of four pure tones between 500 and 4000 Hz of >25 db in the worse ear, Cruickshanks et al4 reported that 45.9% of the Beaver Dam sample population had hearing loss. The mean age of that sample was 65.8 years. Those authors further reported that hearing loss increased with advancing age such that for every 5 years of age, the risk of hearing loss increased by almost 90%. Among those over age 80 years, hearing loss prevalence was 89.5%. The Framingham Heart Study cohort of individuals aged 60 and above had a prevalence of hearing loss of 31% when considering the better ear pure tone average threshold for frequencies between 500 and 2000 Hz and a criterion of 25 dB loss.16 In a later set of measures on an overlapping but non-identical cohort, 29% of the Framingham Study cohort had a hearing impairment when defined as >26 db pure tone average loss in the better ear for tones from 250 to 8000 Hz.7
Chia et al17 explored the association between visual impairment (defined as best corrected monocular standard high contrast acuity worse than 6/12 in the better eye) and hearing loss (average threshold > 25 db for tones of 500, 1000, 2000, and 4000Hz in the better ear). They reported that those with visual impairment had poorer hearing than those without visual impairment. Bergman & Rosenhall18 and Caban et al19 also reported an association between hearing and vision based on measured acuity and self reported hearing status. Fischer et al 20 also reported an association between hearing and vision impairments in that dual impairments were more prevalent than expected based on the independent prevalences of each sensory faculty. In the present case, the Smith-Kettlewell Institute (SKI) Study, a large elderly population was given a screening test of hearing in conjunction with testing on a broad battery of vision measures.20 These data provide an ideal opportunity to explore in greater depth the association between hearing loss and vision function among older adults. It was hypothesized that low contrast vision measures, which are affected by even sub-clinical eye disease (e.g.22), would show stronger associations with hearing impairment than the association seen for standard, high contrast acuity, since hearing loss is associated with eye disease (e.g.23–25).
Methods
Subjects
Four hundred and forty six subjects completed a hearing screening at the second follow-up (third visit) of the SKI longitudinal study of vision and function in the elderly. The mean age of this sample was 79.9 years (range 67 to 107 years) and 43% were male. The SKI study sample is a random sample of older adults living in Marin County in northern California and has been described in detail elsewhere.20
Tests and Procedures
The vision measures and procedures for testing have been described in detail elsewhere.20 The test battery considered here was comprised of eight measures. Standard distance high contrast acuity and low contrast acuity (20% contrast) were measured with Bailey-Lovie Charts.26,27 Low contrast (10% contrast) acuity at near (40 cm) was measured first in the absence and then in the presence of a 3300 cd/m2 surrounding glare source (Berkeley Glare Test28). Low contrast acuity at low luminance was measured at near using the SKILL Card Dark Chart.29 This chart presents low contrast (15% contrast) letters on a dark gray background that reduces the effective luminance by 1 log unit (in this case from 150 to 15 cd/m2). Contrast sensitivity was assessed using the Pelli-Robson Chart30 at a non-standard test distance of 3 meters. Data from Mäntyjärvi and Laitinen31 show that there was no difference between contrast sensitivity measured with the Pelli-Robson chart at 1 vs. 3 metres. Stereoacuity was measured using the Frisby Stereo Test32 presented at 40 cm. At this test distance, best measurable performance was better than or equal to 85 arc seconds (pass all three test plates) and worst performance was worse than 340 arc sec (fail all three test plates). Colour vision was assessed using the Farnsworth-Munsell D-15 test under MacBeth Lamp illumination.33,34 All testing was done binocularly with habitual correction for distance or near as appropriate.
As noted above, some studies define hearing loss severity on the basis of performance in the better ear, others in the worse ear, and yet others in terms of both ears. A screening test of hearing was performed on each ear of participants. Hearing aids, if used, were not worn during testing. Pure tones at 500, 2000 and 4000 Hz were presented at 40 db twice to each ear using a Welch Allyn AudioScope® screening audiometer in a quiet room. Subjects were to indicate that they heard a tone by raising a hand. Participants were considered to have failed the screening (be hearing impaired) if no tone was heard on either trial for all frequencies in either ear (i.e. both ears had thresholds exceeding 40 dB for all tones). This criterion is equivalent to the usual definition of moderate bilateral hearing loss.
In addition to vision and hearing measures, self-reported information on education level, medical conditions (hypertension, heart disease, diabetes, stroke, cancer, and arthritis) and eye conditions (cataract surgery, glaucoma, age-related macular degeneration [AMD]) were obtained. Records from participants’ eye care provider were requested for all participants who granted permission (N=431, 97%), and of these complete concurrent information* was available for 72.4% of participants (N=323). For the remaining participants, we had to rely on a combination of self-report and eye record data for 24.2% (N=108) and only self-report data for 3.4% (N=15).
Depressive symptoms were assessed using the 10-item version of the Center for Epidemiologic Studies-Depression Scale (CES-D), which has been used in previous survey studies of older adults35 with a cut-off depression symptom score of 10.
The research followed the tenets of the Declaration of Helsinki. Informed consent was obtained from the subjects after explanation of the nature and possible consequences of the study. The research was approved by the institutional review board of the Smith-Kettlewell Eye Research Institute.
Data Analysis
Data were analyzed using SPSS 6.1 for the Macintosh. Descriptive statistics (means, medians, and standard deviations) were computed for continuous measures, and frequency distributions were obtained for the categorical measures. Initial univariate analyses to assess the difference between hearing impaired and non-impaired participants were carried out for each of the potential covariates using Student’s t-tests (continuous) and chi-square (categorical).
Logistic regression analyses were used to assess the relationship between various vision variables and hearing impairment while controlling for demographic and other co-morbid conditions. Odds Ratios (ORs) along with 95% confidence intervals are presented for each measure. Measures for which the 95% confidence intervals do not include 1.0 are significantly associated with hearing impairment.
Results
Overall Prevalence of Vision and Hearing Losses
Figure 1 illustrates the association between prevalence of hearing impairment (solid bars), vision impairment (white bars) and combined hearing and vision impairment (gray bars) across age in this aged sample. Not surprisingly, hearing impairment was least frequent (1.6% of the age group) among the youngest participants (aged 67 – 74), increased to approximately 9%, between ages 75–79 and ages 80–84 and then increased dramatically to 32.7% from 85 years of age onward. The overall rate of hearing impairment, defined as failure to hear any 40 dB tone in either ear, for this rather aged group (aged 79.9 ± 7.5 yrs) was 12.8% (57/446 individuals).
Figure 1.
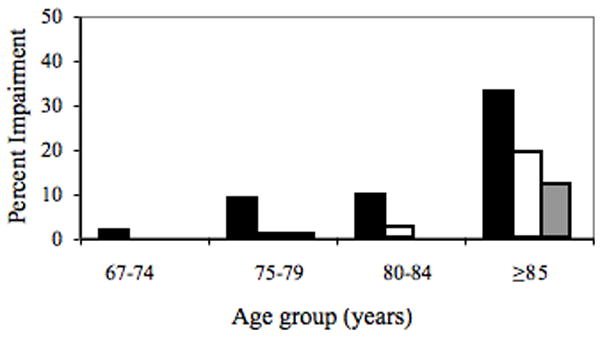
Rates of hearing impairment (black bars), vision impairment (white bars) and dual sensory impairment (gray bars) across age groups.
The frequency of vision impairment (binocular visual acuity worse than 0.54 logMAR, Snellen equivalent 6/21 or 20/70) across age is indicated by the white bars in Figure 1. Using this definition, there was no vision impairment among the group less than 75 years of age. Only 0.8% of individuals aged 75 to 79 years and 2.3 % of those aged 80–84 had a vision impairment. The rate rose to 19.1% among those aged 85 and older. Overall, 5.4% of this sample had a visual impairment as defined as acuity of worse than 0.54 logMAR (Snellen 6/21 or 20/70). The median acuity of the whole sample was 0.10 logMAR (Snellen 6/7.5 or 20/25).
Among those aged 85+ years, dual sensory loss was present in 11.8% of the subjects. At younger ages dual sensory impairment was seen only among those aged 75–75.99 years, at a rate <1.0%.
Demographics of hearing impaired and non-hearing impaired groups
Table 1 presents a comparison of the hearing impaired and non-hearing impaired groups on demographic measures and co-morbid conditions. Not surprisingly, those with hearing loss were older (by about 9 years) than those without hearing loss. Consistent with this is the fact that those with hearing loss were more likely to have self-reported history of stroke, a diagnosis of age-related maculopathy, and to have had cataract surgery. The groups did not differ on any of the other factors evaluated (sex, years of education, depression, glaucoma, or self–reported diagnoses of hypertension, diabetes, cancer, or arthritis).
Table 1.
Comparison of demographic characteristics of hearing impaired and non hearing impaired participants.
| Characteristics | Not Hearing Impaired (n=389) | Hearing Impaired (n=57) | |
|---|---|---|---|
| Mean age in years (SD) | 78.8 (6.6) | 87.7 (8.4)* | |
| Sex (% male) | 42.4% | 47.4% | |
| Mean years of education (SD) | 15.1 (2.6) | 14.9 (3.3) | |
| % Depression (Score ≥ 10) | 8.2% | 9.3% {n=54} | |
| % Cataract Surgery | 29.4% {n=385} | 66.1%* | |
| % ARM History | 17.2% {n=383} | 33.3%† {n=54} | |
| % Glaucoma History | 19.6% {n=382} | 17.0% {n=53} | |
| % Self-Reported Medical Diagnoses | Hypertension | 51.9% | 50.9% |
| Heart Disease | 37.8% | 49.1% | |
| Diabetes | 8.7% | 12.3% | |
| Stroke | 7.2% | 22.8%* | |
| Cancer | 36.0% | 45.6% | |
| Arthritis | 58.1% | 63.2% | |
Bold text indicates a significant difference between the groups. Key: {N} Data from hearing impaired and non-hearing impaired participants when not N=57 and N=389 respectively.
p<0.001;
p<0.01; Non-bold text = p>0.10.
To take into account the effect of age on the relationship between hearing and demographic status, logistic regression controlling for age was performed on each covariate in Table 1 individually. Table 2 presents the results and illustrates that three factors were significantly associated with hearing impairment when age was taken into account: cataract surgery, glaucoma and self-reported stroke. Having a history of cataract surgery approximately doubled the likelihood of hearing loss. Glaucoma was also associated with hearing loss, but the association was negative; for this sample having glaucoma was associated with a decreased likelihood of hearing loss. Self-reported stroke history increased the odds of hearing impairment by a factor of 2.69. Once age was taken into account, history of age-related maculopathy was no longer related to hearing status.
Table 2.
Odds Ratios and lower and upper 95% confidence intervals for associations between demographic variables and hearing impairment, controlling for age.
| Variable | Unit | Odds Ratio | Lower confidence interval | Upper confidence interval |
|---|---|---|---|---|
| Sex | Male | 1.62 | 0.86 | 3.03 |
| Years of education | Per year | 1.06 | 0.95 | 1.18 |
| Depression score (CESD) | score ≥10 | 0.84 | 0.28 | 2.51 |
| Cataract surgery history | Yes | 1.97* | 1.01 | 3.85 |
| ARMD history | Yes | 0.71 | 0.33 | 1.54 |
| Glaucoma history | Yes | 0.37* | 0.15 | 0.88 |
| Self reported hypertension | Yes | 0.82 | 0.44 | 1.53 |
| Self-reported Heart disease | Yes | 1.34 | 0.72 | 2.49 |
| Self-reported diabetes | Yes | 1.46 | 0.55 | 3.91 |
| Self-reported cancer | Yes | 1.26 | 0.68 | 2.34 |
| Self-reported stroke | Yes | 2.69* | 1.18 | 6.15 |
| Self-reported arthritis | Yes | 0.87 | 0.46 | 1.67 |
Bold text* indicates a significant association (p<0.05).
Association Between Individual Vision Measures and Hearing Loss
Overall, in this study group, 5.4% of individuals were moderately visually impaired (binocular high contrast VA worse than 0.54 logMAR, Snellen 6/21 or 20/70) and 12.8% were moderately bilaterally hearing impaired (hearing none of the 40 dB tones in either ear). If vision and hearing impairments were independent, the probability of having both would be the product of the separate impairment probabilities. In this case, we would expect dual sensory loss in 0.7% of people. In fact, the prevalence of dual sensory loss was over four times higher (3.1%), indicating that the two kinds of impairment are associated. The rate of dual sensory loss across age is illustrated by the gray bars in Figure 1. Only one person below age 85 had both vision and hearing impairments. However, 11.8% of those aged 85 or older had both impaired hearing and vision.
In univariate analysis, which does not take age and other covariates into account, all vision measures were significantly associated with hearing impairment. Table 3 presents the results of logistic regression examining the association between individual vision measures and hearing loss, after controlling for age, cataract surgery history, glaucoma history and self reported stroke. Three vision measures showed significant associations with hearing loss: low contrast acuity, low contrast acuity at low luminance, and low contrast, and acuity in glare. For any of these three measures, those who perform poorly were 40% – 50% more likely to have moderate bilateral hearing loss than those who scored well on that measure. This was true for each of the three measures.
Table 3.
Odds ratios and lower and upper 95% confidence intervals for the association of vision measures with hearing impairment controlling for age, cataract surgery history, glaucoma history and self reported stroke.
| Vision variable | Unit | Odds Ratio | Lower confidence interval | Upper confidence interval |
|---|---|---|---|---|
| High contrast visual acuity | 0.3 log unit | 1.32 | 0.92 | 1.90 |
| Low contrast (20%) visual acuity | 0.3 log unit | 1.21 | 0.88 | 1.67 |
| Low contrast (10%) acuity | 0.3 log unit | 1.50* | 1.02 | 2.22 |
| Contrast Sensitivity | 0.3 log unit | 1.23 | 0.89 | 1.70 |
| Low contrast/low luminance acuity | 0.3 log unit | 1.46* | 1.07 | 1.98 |
| Low contrast acuity in glare | 0.3 log unit | 1.40* | 1.02 | 1.91 |
| Stereo acuity | pass/fail | 1.15 | 0.87 | 1.51 |
| Color vision | pass/fail | 1.64 | 0.78 | 3.46 |
Bold text* indicates a significant association (p<0.05).
Table 4 presents the odds ratios of those health factors associated with hearing loss, as well as age and sex and the SKILL Dark Chart performance, which was included because it had the highest Wald statistic of the three vision measures that were associated with hearing loss. In this final model, only age, glaucoma history, self-reported stroke and SKILL Dark Chart acuity were significantly associated with hearing impairment. Each five year age increment increased the likelihood of hearing impairment by 81%. Those with glaucoma history were significantly less likely to have had hearing impairment than those with no history of glaucoma. Those who reported having had at least one stroke were over 3.6 times as likely to have had a hearing impairment than those who reported not ever having had a stroke.
Table 4.
Odds ratios and upper and lower 95% confidence limits for a model including only factors significantly associated with hearing impairment.
| Variable | Unit | Odds Ratio | Lower confidence interval | Upper confidence interval |
|---|---|---|---|---|
| Age | (5 year increase) | 1.81** | 1.35 | 2.43 |
| Sex | Male | 1.89 | 0.96 | 3.73 |
| Cataract surgery | Yes | 2.13 | 0.89 | 5.10 |
| Glaucoma history | No | 0.28* | 0.11 | 0.71 |
| Self-reported stroke | Yes | 3.64* | 1.42 | 9.33 |
| SKILL Dark Chart Acuity | (0.3 log unit) | 1.46* | 1.07 | 1.98 |
Bold text indicates a significant association (*p<0.05; **≤0.001).
Discussion
In this study we found dual sensory loss in 3.1% of participants. This is much higher (by a factor of 4.5) than expected from the rates of hearing impairment (12.8%) and vision impairment defined by high contrast acuity (5.4%) assuming the two types of impairment were independent (0.69%). This is consistent with the finding of an association between visual impairment (defined by standard acuity) and hearing impairment reported by Chia et al17 as well as the findings of a study by Caban et al19 which was based on self-reported vision and hearing problems. Hearing status and vision impairment are not independent in older adults.
In the present study, however, standard high contrast acuity was not significantly associated with hearing impairment once age and other co-morbid conditions were taken into account. The vision variable that was most strongly associated with a co-existing hearing loss was low contrast (15%) acuity under low luminance conditions (SKILL Dark Chart). However, low contrast (10%) acuity with and without glare present were also significant risk factors for hearing loss.
The finding of an association between standard high contrast visual acuity and hearing loss in previous studies, even when age and other covariates were taken into account, may be due to the criteria for vision and hearing loss used. Chia et al17 used a milder definition of hearing loss (an average threshold of 4 tones (500, 1000, 2000, 4000 Hz) of > 25 dB in the better ear), compared to our requirement that the threshold for all three of the tested tones (500, 2000, 4000 Hz) exceed 40 dB in both ears. Chia et al.’s study sample was also younger (average age 69.8 years versus 79.9 years in the present study). Interestingly, those authors reported a tendency toward a stronger association between hearing and impaired visual acuity in younger participants (< age 70). Bergman & Rosenhall18 also reported an association among men younger than 70 years old, but not older individuals. The mean age of this sample was 79.9 years, with the youngest participants 67 years old. The older age of this sample may thus also account, in part, for the lack of a significant association between hearing loss and visual acuity when covariates are included in the model. The lack of association between standard high contrast acuity and hearing loss may also reflect that the vast majority of this sample (94.6%) maintained acuity better than 0.54 logMAR (6/21 or 20/70), reducing the likelihood of finding associations of visual acuity with hearing. Finally, the much larger sample size of the Chia et al17 study (2334 individuals) may have also contributed to their finding of a significant association between standard high contrast acuity and hearing loss.
In the final model, the vision test with the highest association with hearing impairment was the SKILL Dark Chart, a test of low contrast vision at reduced luminance. We have previously found that the SKILL Dark Chart to often be abnormal in the presence of normal standard high contrast acuity and shows greater variation among individuals, even among those with good acuity (6/12 or 20/40 or better).21 We attribute the current findings to the sensitivity of the SKILL Card to preclinical as well as frank disease. Low contrast, low luminance testing is highly sensitive to eye disease (e.g.36–38) and associations between hearing loss and age-related eye disease have been documented.17,23–25
Others have reported significant associations between cataract surgery and hearing loss (e.g.17,25). In this study, history of cataract surgery was a significant predictor of hearing loss in univariate analysis and remained significant when age was taken into account. However, in the final model, which included a vision function measure, having had cataract surgery was no longer associated with hearing status. This suggests that the vision measure - low contrast acuity measured at low luminance - was a stronger predictor of hearing status than cataract surgery.
Though the frequency of age-related maculopathy was twice as high in the hearing impaired than among those with normal hearing (Table 1; p<0.01), age-related maculopathy was not related to hearing loss once age and other covariates were taken into account. Klein et al24 reported an association between late (but not early) AMD and hearing loss. In this study, we did not explore the association between hearing loss and late AMD in particular. Chia et al17 found weak associations between any ARM and hearing loss in their somewhat younger sample. Further, the association between age-related maculopathy and hearing loss was stronger among their younger (< age 70 years) subjects. Given that the mean age of our subjects was nearly ten years older than this, the lack of association seen here is consistent with their report.
The observed association between hearing loss and stroke history is in agreement with earlier reports (e.g.39). The most perplexing finding of this study is that having a history of glaucoma was associated with a decreased likelihood of hearing impairment when age was included in the logistic regression model. No obvious underlying cause for this negative association presents itself. There have been several studies examining whether glaucoma and hearing loss are (positively) associated, with mixed results favouring a lack of association, (e.g.40–42), but none have reported the negative association seen here. Further, beta blockers, often prescribed for glaucoma, have been shown to be associated with poorer hearing, at least in women.43 Thus, the negative association between glaucoma and hearing loss remains unexplained. The possibility exists that the association is due to chance, particularly in light of the very wide confidence intervals around the odds ratio (Table 4). Because, for a portion of the study sample, information about the presence or absence of glaucoma was based on self-report, it is possible that misreporting of glaucoma status contributed to this surprising finding. For example, Douglas et al44 found that more than 10% of people with visual impairment could not identify their own underlying eye disease, even when prompted. Therefore additional analyses were performed omitting the 123 individuals for whom self-report was used in part or solely to determine eye health status. However, the negative association between glaucoma and hearing loss remained (OR = 0.27, 95% CI = 0.09 – 0.79).
Visual impairment in itself impacts both physical and social functioning, whereas hearing impairment largely affects social functioning45. Dual sensory impairment, hearing and vision loss, compromises the two primary modes of communication, listening and speech reading, potentially leading to social isolation. It also limits the information that the individual can glean from the environment, rendering tasks of daily living more difficult and thereby imposes limits on an individual’s ability to function in daily life thereby broadly impacting quality of life (e.g. 20,46). Not surprisingly, dual sensory loss impacts depression, cognitive and general function, activities of daily living and quality of life more than a deficit in vision or hearing alone (e.g.14,15,17,47,48). Dual sensory loss is significantly more common than expected based on prevalence of hearing loss and eye/vision disorders.
As hearing declines, reliance on vision increases for tasks such as understanding speech. The current study indicates that vision impairment for low contrast targets (~10% contrast), particularly when presented under poor illumination, is more prevalent among the hearing impaired. This suggests that individuals with hearing impairment should carry out activities such as conversing with others under bright illumination without glare. Further, those speaking with hearing impaired individuals should do what they can to increase the contrast of facial features by, for example, wearing lipstick.
The findings have significance for clinicians, both audiologists and eye care practitioners, in that finding a deficit in one domain (e.g. vision) indicates an increased likelihood of deficits in the other domain (e.g. hearing). It is suggested that audiologists consider including a brief test of low contrast vision, such as low contrast acuity. Likewise, eye care practitioners should consider performing a screening test of hearing on their patients. Depending on the severity of the dual sensory loss, referral for rehabilitation may be called for.
Acknowledgments
Supported by a National Eye Institute grant (EY09855 to JAB), an RERC grant from the National Institute on Disability and Rehabilitation Research (NIDRR) to JAB, and by the Smith-Kettlewell Eye Research Institute.
Footnotes
Complete, concurrent information” indicates that the time period between the eye examination and vision test was either 1) less than 2 months, or 2) we were able to confirm the same eye status from subsequent eye examination records.
Conflict of interest: All authors: None
References
- 1.Kahn HA, Leibowitz HM, Ganley JP, et al. The Framingham Eye Study. I. Outline and major prevalence findings. Am J Epidemiol. 1977;106(1):17–32. doi: 10.1093/oxfordjournals.aje.a112428. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Klein BEK, Linton KLP, De Mets DL. The Beaver Dam Eye Study: Visual acuity. Ophthalmology. 1991;98:1310–1315. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 3.Attebo K, Mitchell P, Smith W. Visual acuity and causes of visual loss in Australia: The Blue Mountains Eye Study. Ophthalmology. 1996;103:357–64. doi: 10.1016/s0161-6420(96)30684-2. [DOI] [PubMed] [Google Scholar]
- 4.Cruikshanks KJ, Wiley TL, Tweed TS, et al. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin: The epidemiology of hearing loss study. Am J Epidemiol. 1998;148:79–86. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- 5.Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 6.Gopinath B, Rochtchina E, Wang JJ, Schnecider J, Leeder SR, Mitchell P. Prevalence of age-related hearing loss in older adults: Blue Mountains Study. Arch Intern Med. 2009;169(4):415–416. doi: 10.1001/archinternmed.2008.597. [DOI] [PubMed] [Google Scholar]
- 7.Gates GA, Cooper JC, Kannel WB, Miller NJ. Hearing in the elderly: The Framingham Cohort, 1983–1985. Part I. Basic audiometric test results. Ear Hear. 1990;11:247–252. [PubMed] [Google Scholar]
- 8.Smith SL, Bennett LW, Wilson RH. Prevalence and characteristics of dual sensory impairment (hearing and vision) in a veteran population. J Rehabil Res Dev. 2008;45:597–610. doi: 10.1682/jrrd.2007.02.0023. [DOI] [PubMed] [Google Scholar]
- 9.Jee J, Wang JJ, Rose KA, Lindley R, Landau P, Mitchell P. Vision and hearing impairment in aged care clients. Ophthalmic Epidemiol. 2005;12:199–205. doi: 10.1080/09286580590969707. [DOI] [PubMed] [Google Scholar]
- 10.Brabyn JA, Schneck ME, Haegerstrom-Portnoy G, Lott LA. Dual sensory loss: Overview of problems, visual assessment, and rehabilitation. Trends Amplif. 2007;11:219–226. doi: 10.1177/1084713807307410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults. Arch Int Med. 2008;168:1522–1530. doi: 10.1001/archinte.168.14.1522. [DOI] [PubMed] [Google Scholar]
- 12.Berry P, Mascia J, Steinman BA. Vision and hearing loss in older adults: Double trouble. Care Manag J. 2004;5(1):35–40. doi: 10.1891/cmaj.5.1.35.61260. [DOI] [PubMed] [Google Scholar]
- 13.Campbell VA, Crews JE, Moriarty DG, Zack MM, Blackman DK. Surveillance for sensory impairment, activity limitation, and health-related quality of life among older adults—United States, 1993–1997. MMWR CDC Surveill Summ. 1999;48:131–156. [PubMed] [Google Scholar]
- 14.Crews JE, Campbell VA. Vision impairment and hearing loss among community-dwelling Americans: Implications for health and functioning. Am J Public Health. 2004;94(5):823–829. doi: 10.2105/ajph.94.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brennan M, Horowitz A, Su YP. Dual sensory loss and its impact on everyday competence. Gerontologist. 2005;45(3):337–346. doi: 10.1093/geront/45.3.337. [DOI] [PubMed] [Google Scholar]
- 16.Moscicki EK, Elkins EF, Baum HM, McNamara PM. Hearing loss in the elderly: An epidemiologic study of the Framingham Heart Study cohort. Ear Hear. 1985;6(4):184–190. [PubMed] [Google Scholar]
- 17.Chia EM, Mitchell P, Rochtchina E, Foran S, Golding M, Wang JJ. Association between vision and hearing impairment and their combined effects on quality of life. Arch Ophthalmol. 2006;124:1465–1470. doi: 10.1001/archopht.124.10.1465. [DOI] [PubMed] [Google Scholar]
- 18.Bergman B, Rosenhall U. Vision and hearing in old age. Scand Audiol. 2001;30:255–263. doi: 10.1080/01050390152704779. [DOI] [PubMed] [Google Scholar]
- 19.Caban AJ, Lee DJ, Gomez-Marin O, Lam BL, Zheng DD. Prevalence of concurrent hearing and visual impairment in US Adults: The National Health Interview Survey, 1997–2002. Am J Public Health. 2005;95:1940–1942. doi: 10.2105/AJPH.2004.056671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer ME, Cruickshanks KJ, Klein BEK, Klein R, Schubert CR, Wiley TL. Multiple sensory impairment and quality of life. Ophthalmic Epidemiology. 2009;16:346–53. doi: 10.3109/09286580903312236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haegerstrom-Portnoy G, Schneck ME, Brabyn J. Seeing into old age: Vision function beyond acuity. Optom Vis Sci. 1999;76:141–158. doi: 10.1097/00006324-199903000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Lovie-Kitchin JE, Feigl B. Assessment of age-related maculopathy using subjective vision tests. Clin Exp Optom. 2005;88:292–303. doi: 10.1111/j.1444-0938.2005.tb06713.x. [DOI] [PubMed] [Google Scholar]
- 23.Liew G, Wong TY, Mitchell P, Newall P, Smith W, Wang JJ. Retinal microvascular abnormalities and age-related hearing loss: The Blue Mountains Hearing Study. Ear Hear. 2007;28:394–401. doi: 10.1097/AUD.0b013e3180479388. [DOI] [PubMed] [Google Scholar]
- 24.Klein R, Cruikshanks KJ, Klein BEK, Nondahl DM, Wiley T. Is age-related maculopathy related to hearing loss? Arch Ophthalmol. 1998;116:360–365. doi: 10.1001/archopht.116.3.360. [DOI] [PubMed] [Google Scholar]
- 25.Klein BEK, Cruickshanks KJ, Nondahl DM, Klein R, Dalton DS. Cataract and hearing loss in a population-based study: The Beaver Dam Studies. Am J Ophthalmol. 2001;132:537–543. doi: 10.1016/s0002-9394(01)01126-6. [DOI] [PubMed] [Google Scholar]
- 26.Bailey IL, Lovie J. New design principles for visual acuity letter charts. Am J Optom Physiol Opt. 1976;53:740–745. doi: 10.1097/00006324-197611000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Ferris FL, Kassof A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–96. [PubMed] [Google Scholar]
- 28.Bailey IL, Bullimore MA. A new test of disability glare. Optom Vis Sci. 1991;68:911–917. doi: 10.1097/00006324-199112000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Haegerstrom-Portnoy G, Brabyn J, Schneck ME, Jampolsky A. The SKILL card: An acuity test of reduced luminance and contrast. Invest Ophthalmol Vis Sci. 1997;38:207–218. [PubMed] [Google Scholar]
- 30.Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clin Vis Sci. 1988;2:187–199. [Google Scholar]
- 31.Mäntyjärvi M, Laitinen T. Normal values for the Pelli-Robson contrast sensitivity test. J Cataract Refract Surg. 2001;27(2):261–6. doi: 10.1016/s0886-3350(00)00562-9. [DOI] [PubMed] [Google Scholar]
- 32.Frisby JP. The Frisby stereotest: Amended instructions. Br Orthopt J. 1980;37:108. [Google Scholar]
- 33.Farnsworth D. Panel D-15 Manual. New York: The Psychological Corp; 1947. The Farnsworth Dichotomous Test for Color Blindness. [Google Scholar]
- 34.Adams AJ, Haegerstrom-Portnoy G. Color deficiency. In: Amos JF, editor. Diagnosis and Management in Vision Care. Boston: Butterworths; 1987. pp. 671–713. [Google Scholar]
- 35.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 36.Luckiesh M. Test charts representing a variety of visual tasks. Am J Ophthalmol. 1944;27:270–75. [Google Scholar]
- 37.Sloan LL. Variation of acuity with luminance in ocular disease and anomalies. Doc Ophthalmol. 1969;26:384–93. doi: 10.1007/BF00943999. [DOI] [PubMed] [Google Scholar]
- 38.Sunness JA, Rubin GS, Broman A, Applegate CA, Bressler NM, Hawkins BS. Low luminance visual dysfunction as a predictor of subsequent visual acuity loss from geographic atrophy in age-related macula degeneration. Ophthalmol. 2008;115(9):1480–8. doi: 10.1016/j.ophtha.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gopinath G, Schneider J, Rochtchina E, Leeder SR, Mitchell P. Association between age-related hearing loss and stroke in an older population. Stroke. 2011;40:1496–1498. doi: 10.1161/STROKEAHA.108.535682. [DOI] [PubMed] [Google Scholar]
- 40.Hayreh SS, Podhajsky P, Zimmerman MB. Ocular and optic nerve head ischemic disorders and hearing loss. Am J Ophthalmol. 1999;128:606–611. doi: 10.1016/s0002-9394(99)00246-9. [DOI] [PubMed] [Google Scholar]
- 41.Shapiro A, Siglock TJ, Ritch R, Malinoff R. Lack of association between hearing loss and glaucoma. Am J Otol. 1997;18(2):172–174. [PubMed] [Google Scholar]
- 42.Yazdani S, Tousi A, Pakravan M, Faghihi AR. Sensorineural hearing loss in pseudoexfoliation syndrome. Ophthalmology. 2008;115(3):425–429. doi: 10.1016/j.ophtha.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 43.Lee FS, Matthews LJ, Mills JH, Dubno JR, Adkins WY. Gender-specific effects of medicinal drugs on hearing levels of older persons. Otolaryngol Head Neck Surg. 1998;118(2):221–227. doi: 10.1016/S0194-5998(98)80020-X. [DOI] [PubMed] [Google Scholar]
- 44.Douglas G, Pavel S, Corcoran C, Uperjesi F. Individual’s recollections of their experiences in eye clinics and understanding of their eye condition: results from a survey of visually impaired people in Britain. Ophthalmic and Physiological Optics. 2010;30:748–57. doi: 10.1111/j.1475-1313.2010.00784.x. [DOI] [PubMed] [Google Scholar]
- 45.Wallhagen MI, Strawbridge WJ, Shema SJ, Kurata J, Kaplan GA. Comparative impact of hearing and vision impairment on subsequent functioning. JAGS. 2001;49:1086–92. doi: 10.1046/j.1532-5415.2001.49213.x. [DOI] [PubMed] [Google Scholar]
- 46.Brennan M, Bally SJ. Psychosocial adaptations to dual sensory loss in middle and late adulthood. Trends Amplif. 2007;11(4):281–300. doi: 10.1177/1084713807308210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Capella-McDonnall ME. The effects of single and dual sensory loss on symptoms of depression in the elderly. Int J Geriatr Psychiatry. 2005;20:855–861. doi: 10.1002/gps.1368. [DOI] [PubMed] [Google Scholar]
- 48.Lin MY, Gutierrez PR, Stone KL, et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc. 2004;52:1996–2002. doi: 10.1111/j.1532-5415.2004.52554.x. [DOI] [PubMed] [Google Scholar]


