Abstract
Background
Recent studies reported associations of the relative telomere length (RTL) and TERT variants with risk of several cancers, which has not been comprehensively investigated in squamous cell carcinoma of the head and neck (SCCHN).
Methods
We detected RTL in peripheral blood lymphocytes and genotyped six selected functional single nucleotide polymorphisms (SNPs) of the TERT gene in 888 SCCHN cases and 885 cancer-free controls of non-Hispanic whites.
Results
Overall, we did not observe significant associations between RTL and SCCHN risk (adjusted OR, 0.97; 95% CI, 0.80–1.17 for below versus above the median; Ptrend = 0.618) nor between the six TERT SNPs and SCCHN risk. We also found no associations between RTL and TERT SNPs.
Conclusions
Our results suggest that RTL and TERT functional polymorphisms may not play a major role in the etiology of SCCHN. Large prospective studies are needed to validate our findings.
Impact
Although our results suggest no association among RTL, TERT functional polymorphisms, and SCCHN risk, this study may contribute to future meta-analysis.
Keywords: genetic polymorphisms, Telomere length, TERT, head and neck cancer, molecular epidemiology
Introduction
Telomeres consist of several thousands (TTAGGG in humans)n of nucleotide repeats and a protein complex at the ends of chromosomes, maintaining genomic stability by protecting chromosomes from degradation, end-to-end fusion, and recombination (1). Human telomeres, as a marker for biological age, are approximately 10–15 kb in somatic cells and progressively shortened with each cell division. Age-dependent shortening of telomeres impairs function and viability of human cells, and both very short and very long telomeres promote carcinogenesis (2, 3), also a recognized marker carcinogenesis.
TERT encodes the reverse transcriptase component of the telomerase, necessary for the maintenance of telomere length, chromosomal stability, and cellular immortality. Normally, TERT mRNA is not expressed in most human somatic cells; however, abnormal expression of TERT mRNA and protein occurs in many cancer types, including head and neck cancer. Recently, genome-wide association studies have reported associations between TERT genotypes and risk of several cancer types (4, 5), but few studies investigated the correlation among TERT genotypes, telomere length, and cancer risk. A recent study showed that TERT-CLPTM1L variants were associated with both of the mean relative telomere length (RTL) and cancer risk (4), but another study reported no association of the TERT-CLPTM1L rs401681 SNP with RTL or cancer risk, including breast cancer, colorectal cancer and melanoma (6). One study of the FISH-measured TRL suggested a shorter RTL in head and neck cancer but the related risk was not estimated (2), and no study has investigated associations among TERT genotypes, RTL and head and neck cancer risk.
MATERIALS AND METHODS
The study subjects included 888 non-Hispanic white subjects with newly diagnosed, untreated primary tumors of squamous cell carcinoma of the head and neck (SCCHN), including the oral cavity (n = 263; 29.6%), oropharynx (n = 440; 49.6%), or larynx and hypopharynx (n = 185; 20.8%) recruited between October 1999 and October 2007, who were frequency-matched on age, sex, and ethnicity with 885 cancer-free controls identified from hospital visitors at The University of Texas M. D. Anderson Cancer Center in the same time period. The study design, selection criteria, blood collection and DNA extraction have been described elsewhere (6).
We genotyped six functional TERT single nucleotide polymorphisms (SNPs) (rs2735940 G>A; rs2736098 C>T; rs2736109 G>A; rs2853669 T>C; rs2853677 A>G and rs2853690 G>A) using the TaqMan assays with the Sequence Detection Software on an ABI-Prism7900 (Applied Biosystems, Foster City, CA). These SNPs were chosen because (1) a minor allele frequency of at least 5%, (2) location in the promoter untranslated region or cording region of the gene, and (3) previous reports of an association with risk of cancers (4, 5). Primers and probes were supplied by Applied Biosystems. For all genotypes, the assay success rate was >99% and the repeated sample’s results were 100% concordant. The mean relative telomere length (RTL) was measured by SYBR Green quantitative real time PCR as previously described (7). All methods for statistical analysis have been described elsewhere as well (6). Associations of TERT genotypes and RTL with SCCHN risk were estimated by computing the odds ratios (OR) and their 95% confidence intervals (CIs) from both univariate and multivariable logistic regression models with or without adjustment for age, sex, smoking and drinking status. To summarize published case-control association studies of TERT polymorphisms and cancer risk, we performed a meta-analysis. All statistical methods were described elsewhere for association analysis (6) and for meta-analysis (8).
RESULTS
Of the subjects, 37.9% and 49.9% of the 888 cases (aged 56.8 ± 11.3 with 74.8% male) with SCCHN were current smokers and drinkers, respectively, which were higher than that (15.1% and 40.8%) for the 885 cancer-free controls (aged 55.4 ± 11.0 with 74.4% male) (P <0.001 for both). The genotype frequencies of the rs2735940 G>A; rs2736098 C>T; rs2736109 G>A; rs2853669 T>C; and rs2853677 A>G SNPs were in agreement with the Hardy-Weinberg equilibrium [P = 0.663, 0.546, 0.530, 0.863, and 0.358, respectively, except for rs2853690 G>A (P = 0.003)]. Overall, no significant associations between these six TERT SNPs and SCCHN risk were observed after adjustment for age, sex, and smoking and alcohol status (Table 1).
Table 1.
Genotype frequencies of the TERT polymorphisms among SCCHN cases and control subjects and their associations with SCCHN risk
| Genotype | Cases n = 888 (%) |
Controls n = 885 (%) |
Adjusted OR(95%CI)a |
|---|---|---|---|
| rs2735940 G>A | |||
| GG | 224 (25.2) | 221 (25.0) | 1.00 |
| GA | 440 (49.6) | 436 (49.3) | 0.99 (0.78–1.26) |
| AA | 224 (25.2) | 228 (25.7) | 1.03 (0.79–1.36) |
| rs2736098 C>T | |||
| CC | 481 (54.2) | 468 (52.9) | 1.00 |
| CT | 351 (39.5) | 356 (40.2) | 0.97 (0.79–1.19) |
| TT | 56 (6.3) | 61 (6.9) | 0.86 (0.58–1.29) |
| rs2736109 G>A | |||
| GG | 319 (35.9) | 313 (35.4) | 1.00 |
| GA | 427 (48.1) | 419 (47.3) | 1.00 (0.81–1.24) |
| AA | 142 (16.0) | 153 (17.3) | 0.92 (0.69–1.22) |
| rs2853669 T>C | |||
| TT | 428 (48.2) | 425 (48.0) | 1.00 |
| TC | 381 (42.9) | 375 (42.4) | 1.02 (0.84–1.26) |
| CC | 79 (8.9) | 85 (9.6) | 0.91 (0.64–1.29) |
| rs2853677 A>G | |||
| AA | 294 (33.1) | 311 (35.1) | 1.00 |
| AG | 448 (50.5) | 416 (47.0) | 1.09 (0.88–1.36) |
| GG | 146 (16.4) | 158 (17.9) | 1.00 (0.75–1.33) |
| rs2853690 G/A | |||
| GG | 596 (67.1) | 618 (69.8) | 1.00 |
| GA | 265 (29.8) | 228 (25.8) | 1.17 (0.94–1.45) |
| AA | 27 (3.0) | 39 (4.4) | 0.69 (0.41–1.16) |
Adjusted by age, sex, smoking and drinking status.
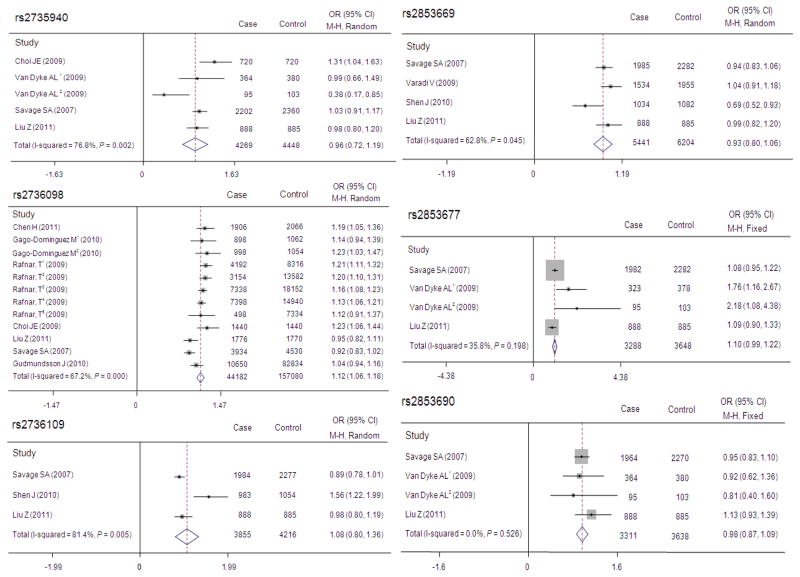
We then performed a mini meta-analysis of available from published studies on the association between TERT polymorphisms and cancer risk (Figure 1), and found that, overall, the pooled data showed that TERT functional polymorphisms were not significantly associated with cancer risk, except for the rs2736098 (OR, 1.12; 95% CI, 1.06–1.18) for 22,091 cancer cases and 78,540 controls. For RTL, carriers of shorter or longer RTL did not have altered SCCHN risk (adjusted OR = 0.97, 95% CI = 0.80–1.17), nor was any trend of associations, when RTL was categorized into quartiles (Ptrend = 0.618) (Table 2). Finally, we did not find any evidence of associations among the SNPs analyzed, RTL and SCCHN risk before or after adjustment for age, sex, smoking and drinking status (data not shown).
Fig. 1.
Meta-analysis of associations between 6 SNPs and risk of cancers. OR and 95%CI were calculated using a dominant model for rs2735940, 2736109, rs2853669, rs2853677 and rs2853690 and an allelic model for rs2736098. Van Dyke AL1 and Van Dyke AL2 represented for lung cancer studies in Caucasian and African Americans, respectively; Gago-Dominguez M1 and Gago-Dominguez M2 represented bladder cancer studies in Caucasian and Chinese, respectively, and Rafnar, T1–5 represented studies for basal cell, lung, bladder, prostate and cervical cancers, respectively.
Table 2.
Comparison and association of the relative telomere lengths between patients with SCCHN and cancer-free controls
| RTL (T/S ratio) | Cases
|
Controls
|
P | Crude OR (95% CI) | Adjusted OR (95% CI)b | ||
|---|---|---|---|---|---|---|---|
| n (%) | Mean ± SD | n (%) | Mean ± SD | ||||
| Overall | 888 (100) | 2.07 ± 5.05 | 885 (100) | 1.90 ± 4.53 | 0.460a | ||
| By median | |||||||
| ≥1.09 | 467 (52.6) | 3.59 ± 6.59 | 445 (50.3) | 3.40 ± 6.01 | 0.651b | 1.00 | 1.00 |
| <1.09 | 421 (47.4) | 0.38 ± 0.33 | 440 (49.7) | 0.38 ± 0.34 | 0.987b | 0.91 (0.76–1.10) | 0.97 (0.80–1.17) |
| By quartile | |||||||
| 4th (≥2.28)c | 230 (25.9) | 5.64 ± 8.95 | 219 (24.8) | 4.16 ± 8.18 | 0.631b | 1.00 | 1.00 |
| 3rd (1.09–2.28) | 237 (26.7) | 1.61 ± 0.34 | 226 (25.5) | 1.62 ± 0.35 | 0.824b | 1.00 (0.77–1.30) | 1.00 (0.76–1.31) |
| 2nd (0.25–1.09) | 219 (24.7) | 0.63 ± 0.26 | 217 (24.5) | 0.67 ± 0.25 | 0.134b | 0.96 (0.74–1.25) | 1.01 (0.77–1.33) |
| 1st (<0.25) d | 202 (22.8) | 0.09 ± 0.08 | 223 (25.2) | 0.09 ± 0.06 | 0.308b | 0.86 (0.66–1.13) | 0.92 (0.70–1.22) |
Two-sided Student’s t tests for differences between cases and controls.
Adjusted by age, sex, smoking and drinking status.
Fourth quartile represents longest quartile of relative telomere length.
First quartile represents shortest quartile of relative telomere length.
DISCUSSION
In this study of associations among RTL, TERT SNPs and SCCHN risk, the largest of all published case-control studies, only next to a recent breast cancer study (8), we found no evidence of associations in 888 SCCHN patients and 885 cancer-free controls in a non-Hispanic white population. This finding is consistent with our mini meta-analysis results. Our study sample size had a statistical power of 80% to detect an OR of 0.611 or 1.508 with an average TERT risk genotype of 10% or to detect a difference in RTL as small as 0.257, compared with the reported 0.9 from the only one published study of 92 head and neck cancer cases and 92 controls (2).
The TERT gene has been identified as a catalytic subunit and a key regulator of telomerase activity, and over-expression of TERT is thought to be involved in the tumorigenesis of various cancers, including SCCHN. Although several association studies evaluated the role of TERT polymorphisms in cancer risk, the results are inconclusive, mostly because of small samples included in the published studies. One recent large case-control study did not find an association of TERT SNP (rs401681) with risk of cancers of the breasts, colorectum and skin, nor with telomere length (7). Telomere length in blood lymphocytes is considered a tumor marker (2, 3), but the results from association studies are also inconclusive in some cancers. One recent prospective study of breast cancer failed to validate the findings from previous large case-control study performed by the same group (9), similarly as we found in our recent meta-analysis of 11,255 cases and 13,101 controls from 21 publications (8), namely, the case-control findings were not confirmed by prospective studies, suggesting that single larger, well-design prospective studies are warranted to confirm the reported findings.
Supplementary Material
Acknowledgments
Grant support: National Institutes of Health grants R01 ES 11740-07 and CA 131274-01 (to Q. W.) and CA 16672 (to M. D. Anderson Cancer Center).
We thank Margaret Lung and Kathryn L. Tipton for their assistance in recruiting the subjects, Min Zhao, Hongping Yu, Jianzhong He, and Kejing Xu for their laboratory assistance.
Abbreviations
- TERT
Telomerase reverse transcriptase
- SNP
single nucleotide polymorphism
- SCCHN
squamous cell carcinoma of the head and neck
- OR
odds ratio
- CI
confidence interval
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Conflict of Interest Statement: None declared.
References
- 1.de Lange T. Shelterin the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–10. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 2.Wu X, Amos CI, Zhu Y, Zhao H, Grossman BH, Shay JW, et al. Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst. 2003;95:1211–8. doi: 10.1093/jnci/djg011. [DOI] [PubMed] [Google Scholar]
- 3.Gramatges MM, Telli ML, Balise R, Ford JM. Longer relative telomere length in blood from women with sporadic and familial breast cancer compared with healthy controls. Cancer Epidemiol Biomarkers Prev. 2010;19:605–13. doi: 10.1158/1055-9965.EPI-09-0896. [DOI] [PubMed] [Google Scholar]
- 4.Rafnar T, Sulem P, Stacey SN, Geller F, Gudmundsson J, Sigurdsson A, et al. Sequence variants at the TERT-CLPTM1L locus associate with many cancer types. Nat Genet. 2009;41:221–7. doi: 10.1038/ng.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen J, Gammon MD, Wu HC, Terry MB, Wang Q, Bradshaw PT, et al. Multiple genetic variants in telomere pathway genes and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2010;19:219–28. doi: 10.1158/1055-9965.EPI-09-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu H, Huang YJ, Liu Z, Wang LE, Li G, Sturgis EM, Johnson DG, Wei Q. Effects of MDM2 promoter polymorphisms and p53 codon 72 polymorphism on risk and age at onset of squamous cell carcinoma of the head and neck. Mol Carcinog. 2011;50:697–706. doi: 10.1002/mc.20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pooley KA, Tyrer J, Shah M, Driver KE, Leyland J, Brown J, et al. No association between TERT-CLPTM1L single nucleotide polymorphism rs401681 and mean telomere length or cancer risk. Cancer Epidemiol Biomarkers Prev. 2010;19:1862–5. doi: 10.1158/1055-9965.EPI-10-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma H, Zhou Z, Wei S, Liu Z, Pooley KA, Dunning AM, et al. Shortened telomere length is associated with increased risk of cancer: a meta-analysis. PLoS One. 2011;6:e20466. doi: 10.1371/journal.pone.0020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pooley KA, Sandhu MS, Tyrer J, Shah M, Driver KE, Luben RN, Bingham SA, Ponder BA, Pharoah PD, Khaw KT, Easton DF, Dunning AM. Telomere length in prospective and retrospective cancer case-control studies. Cancer Res. 2010;70:3170–6. doi: 10.1158/0008-5472.CAN-09-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.