Abstract
Background
Axonal damage and inflammatory demyelination both occur in multiple sclerosis (MS). Some studies suggest that statins, through pleiotropic effects, reduce inflammatory episodes and protect neurons. However, other studies suggest statins have disparate impacts on these pathologic processes.
Objective
To assess disability progression in MS patients receiving statin therapy.
Methods
Retrospective medical record review of an established population-based MS prevalence cohort in Olmsted County, Minnesota, comparing disability progression between patients receiving statins and controls.
Results
Duration of statin use ranged from 1.9 to 20.3 years with a mean and standard deviation of 6.8 +/− 4 years. Years between assessments ranged from 0.6 to 8.2 (75% of patients having intervals >6.4 years). The median (interquartile range) absolute change of disability among the statin group was 0 (0 to +1), compared to +0.5 (0, +1) in the no-statin group. Distributions were not significantly different (P = 0.39). The mean (standard deviation) absolute change of disability scores among the statin group was +0.69 (+1.49), not significantly different from +0.61 (+1.31) in the no-statin group. Likewise, annualized disability scores did not differ significantly (P = 0.23). Eighteen (40%) patients worsened by 1.0 or more on EDSS in the statin group and 36 (40%) in the no-statin group (P = 0.85, chi-squared test).
Conclusions
In this cohort, disability progression did not differ between those receiving statin therapy and controls. These findings support the hypothesis that statins, in doses currently prescribed for hyperlipidemia, do not affect the long-term course of multiple sclerosis.
Keywords: HMG-CoA reductase inhibitors, statins, disability, multiple sclerosis, population based
Introduction
In multiple sclerosis (MS), axonal damage occurs early and correlates with clinical disability. The mechanism of action for all disease-modifying agents currently approved for MS is through the modulation or suppression of inflammatory immune responses. Although these agents reduce inflammatory episodes and clinical exacerbations; clinically significant effects on myelin repair or disability progression are either unsupported or unknown1. Strong rationale therefore exists to more effectively suppress inflammatory episodes or to directly protect axonal integrity.
The 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are used for their cholesterol-lowering properties, but an expanding body of evidence demonstrates potentially beneficial pleiotropic effects. Relevant to neuroinflammatory disorders are increasingly better characterized immunomodulatory effects such as inhibition of expression2, 3 or binding4 of immune signaling molecules. Additionally, statins may have direct neuroprotective action by altering gene expression patterns5 or influencing apoptotic pathways in neurons6. Short-term treatment of oligodendrocyte progenitor cells with statins also enhances differentiation and induces process extension7.
Based on preclinical data, however, biologically plausible arguments can be made that statins could worsen MS. It is not clear that decreasing inflammation slows disability progression. Notably, inflammation may be necessary for myelin repair8. Also, statins inhibit cholesterol synthesis in glia9, brain cholesterol is not imported from systemic pools10, and cholesterol availability in oligodendrocytes is rate limiting for myelin elaboration.11 Statins thus interfere with oligodendroglial process dynamics7 and induce formation of abnormal myelin-like membrane sheets.12 These points raise the possibility that statin therapy may inhibit or slow myelin repair in MS lesions and thereby leave axons exposed to injury.
Animal studies and early clinical trials have fallen on both sides of this important issue. Much interest was generated in the therapeutic potential of statins in MS after the report that lovastatin improved experimental autoimmune encephalomyelitis.13 In counterbalance is the report that simvastatin inhibited central nervous system remyelination following cuprizone induced demyelination.14 Open-label trials of lovastatin15, simvastatin16, and atorvastatin alone or in combination with beta interferon17 were also promising with demonstrated reduction in the mean number of gadolinium-enhancing lesions on MRI. However, published randomized studies have been at odds with one trial reporting reduced relapse rate during combined simvastatin and beta interferon treatment18, and another trial reporting increased MRI and clinical disease activity during combined atorvastatin and beta interferon treatment.19
Several more randomized trials have been undertaken, some of which have presented preliminary results in abstract form, but final results have not been published. Primary outcome measures for these trials include MRI lesion activity and/or clinical relapses, and as such will help clarify whether statins are useful for decreasing inflammatory episodes in MS. As outlined above, however, the impact statins have on inflammatory episodes versus disability progression in MS may be disparate. Long-term follow up of patients taking statins will likely be necessary to directly address their impact on disability progression, which will be difficult to obtain in traditional double-blind clinical trials. Here we present disability outcomes in a well characterized population-based MS prevalence cohort with clinical follow up averaging seven years following initiation of statin therapy.
Methods
Patients
The indices of the Rochester Epidemiology Project were used to identify all known cases of multiple sclerosis among patients residing in Olmsted County, MN in 2000.20 The study authors reviewed medical records of each case to extract medical information and confirm diagnosis. Definite (clinical or laboratory supported) multiple sclerosis cases were defined using the Poser criteria.21 Extracted information included demographic data, age at onset and diagnosis, use of immunomodulatory therapy, and disease course. This prevalence cohort was then characterized in regard to disability. Expanded Disability Status Scale (EDSS) scores were established through personal interview and a full neurologic examination. In order to limit inter-rater variability, all scores were assigned by a single investigator (SJP). Some patients with severe disability were visited in their homes or in nursing home facilities. For patients unwilling to be seen, a telephone interview was performed.
In the retrospective study presented here, this MS prevalence cohort was further characterized. All patients received their care at Mayo Clinic or Olmsted Medical Center and thus ascertainment bias and statin exposure misclassification can be expected to be minimal. This study was approved by the institutional review boards of both institutions. We reviewed the medical records of each patient to extract information in regard to statin use, cardiovascular comorbidities, and current disability. For every patient in the prevalence cohort, review of the most recent clinical assessment in the medical record established grades for each of the Kurtzke Functional Systems and subsequent assignment of an EDSS score. Here again, in order to limit inter-rater variability, all scores were assigned by a single investigator (MMP). Data was collected and scores were assigned without knowledge of previous scores.
Matching
A logistic regression model was fit with statin treatment considered the event and the following background covariates included as predictors: gender, clinical course, year of birth, age of onset, and age at diagnosis. The estimated probability of being in the treatment group (propensity score) was calculated for each patient given his/her background covariates. Propensity scores were used to match each patient treated with statins to two non-treated patients, which is an efficient way to obtain covariate balance between groups.22 Matching was also undertaken with cardiovascular comorbidity, in addition to all covariates noted above, included as a predictor. A number of non-treated patients had propensity scores near zero in this model, which allowed balanced matching of treated patients to only one non-treated patient.
Analysis
EDSS assessments from 2000 were used as baseline values and more recent EDSS assessments as follow-up values. Data were compared as either absolute change or annualized change. Group-wise differences between treated versus untreated patients utilized a two-sided Wilcoxon rank-sum/Mann-Whitney U test. The area under the receiver operating characteristic curve (AUROC) can be interpreted as the estimated probability that a randomly selected no-statin patient has a greater increase in EDSS than a randomly selected statin patient. Data were also compared as the proportion of patients worsened by 1.0 or more on the EDSS. Group-wise comparisons utilized a chi-squared test.
Results
The 2000 Olmsted County prevalence cohort includes 201 patients with clinically or laboratory-supported definite MS. Years between EDSS assessments ranged from 0.6 to 8.2 with 75% of patients having intervals >6.4 years. At the time of chart review, 156 (78%) patients were not known to have been treated with statins, whereas 45 (22%) patients were identified as receiving these medications. Statin and no-statin patients were comparable in baseline characteristics except for age (Table 1; median year of birth 1943 vs. 1951). This and more subtle differences motivated matching methods to obtain a more comparable no-statin group in order to reduce confounding and bias.
Table 1.
Demographics of Olmsted County MS prevalence cohort
| Variable | Statin (n = 45) Median (range) or No. (%) |
No statin (n = 156) Median (range) or No. (%) |
Matched no statin (n = 90) Median (range) or No. (%) |
CV matched no statin (n = 45) Median (range) or No. (%) |
|---|---|---|---|---|
| Female | 26 (58) | 114 (73) | 56 (62) | 27 (60) |
| Year of birth | 1943 (1921–1958) | 1951 (1911–1983) | 1946 (1911–1973) | 1944 (1913–1966) |
| Age at onset, y. | 33 (15–56) | 31 (12–58) | 33 (14 – 58) | 33 (17–54) |
| Age at diagnosis, y. | 38 (23–65) | 35 (15–61) | 39 (18 – 59) | 40 (20–56) |
| Relapsing-remitting MS | 27 (60) | 103 (66) | 54 (60) | 25 (56) |
| Primary progressive MS | 2 (4) | 9 (6) | 5 (6) | 3 (7) |
| Secondary progressive MS | 16 (36) | 44 (28) | 31 (34) | 17 (38) |
| Immunomodulatory therapya | 7 (26) | 43 (42) | 15 (28) | 4 (16) |
| Any vascular comorbidityb | 39 (87) | 64 (41) | 45 (50) | 37 (82) |
Number (%) of relapsing-remitting MS patients.
Excluding hypercholesterolemia.
MS = multiple sclerosis.
CV = cardiovascular disease.
In addition, a recent study suggested that cardiovascular comorbidity is associated with an increased risk of disability progression in MS.23 In the cohort presented here, statins were prescribed based on the presence of hypercholesterolemia. Expectedly, cardiovascular comorbidities were more common in the statin group (Table 2). We therefore undertook matching both including and excluding cardiovascular disease as a covariate, thereby enabling comparisons to address this issue. Given that between group comparisons utilizing either matched group were markedly similar, we primarily report findings with cardiovascular disease excluded as a covariate. This yielded larger group size and, therefore, increased statistical power.
Table 2.
Vascular Comorbidities
| Comorbidity | Statin (n=45) No. of patients (%) |
No statin (n=156) No. of patients (%) |
Matched no statin (n=90) No. of patients (%) |
|---|---|---|---|
| Diabetes | 17 (38) | 11 (7) | 8 (9) |
| Heart Disease | 12 (27) | 15 (10) | 14 (16) |
| Hypertension | 38 (84) | 58 (37) | 39 (43) |
| Hypercholesterolemia | 45 (100) | 36 (23) | 23 (26) |
| Peripheral vascular disease | 2 (4) | 0 (0) | 0 (0) |
| Any vascular comorbiditya | 39 (87) | 64 (41) | 45 (50) |
Excluding hypercholesterolemia.
Duration of statin use at most recent clinical assessment ranged from 1.9 to 20.3 years with a mean and standard deviation of 6.8 +/− 4 years. During the observation period, 28 (62%) patients were continued on a single statin, whereas 11 (24%) and 6 (13%) were transitioned between two or three, respectively. Simvastatin was most common, followed by atorvastatin, lovastatin, pravastatin and fluvastatin (Table 3).
Table 3.
Distribution of statin use
| Medication | Ever used No. of patients (%) |
Exclusively used No. of patients (%) |
|---|---|---|
| Simvastatin | 32 (71) | 17 (38) |
| Atorvastatin | 20 (44) | 9 (20) |
| Lovastatin | 9 (20) | 2 (4) |
| Pravastatin | 4 (9) | 0 (0) |
| Fluvastatin | 3 (7) | 0 (0) |
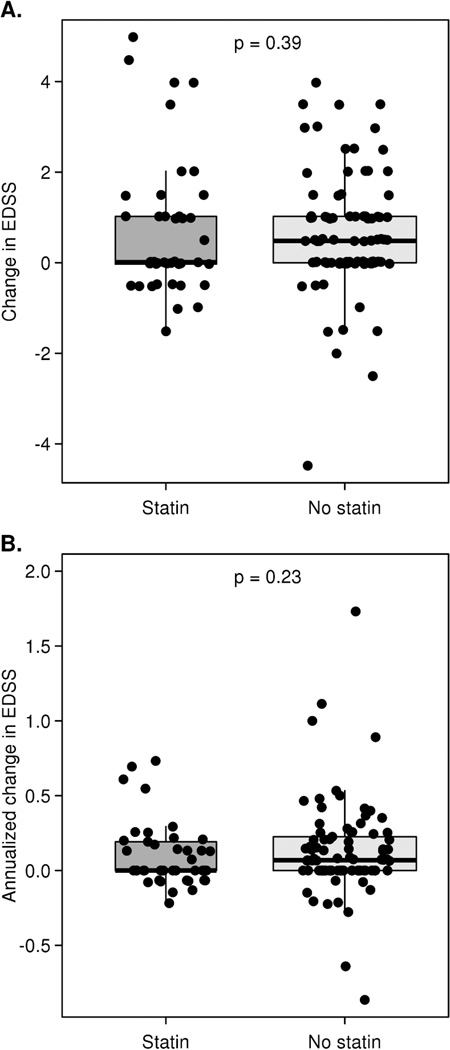
Change in EDSS score was compared between the statin group, the entire no-statin group, and both matched subsets (Table 4). Comparisons were markedly similar in either case. The median (interquartile range [IQR]) absolute change among the statin group was 0 (0 to +1) with a range from −1.5 to +5. The median (IQR) in the matched no-statin group was +0.5 (0, +1) with a range of −4.5 to +4.0. These distributions are summarized in panel A of figure 1 and were quite similar with an AUROC of 0.56 (95% CI, 0.44 to 0.64), a difference that was not significant (P = 0.39, Wilcoxon rank-sum test). Between group comparisons of the mean (standard deviation [SD]) absolute change in EDSS scores demonstrated no significant difference; the statin group was +0.69 (+1.49) and the matched no-statin group was +0.61 (+1.31).
Table 4.
Change in EDSS score over observation period
| Variable | Statin (n = 45) | No statin (n = 156) | Matched no statin (n = 90) | CV matched no statin (n = 45) |
|---|---|---|---|---|
| EDSS change | ||||
| Median (IQR) | 0.0 (0.0, +1.0) | +0.5 (0.0, +1.0) | +0.5 (0.0, +1.0) | +0.5 (0.0, +1.0) |
| Mean (SD) | +0.69 (+1.49) | +0.59 (+1.40) | +0.61 (+1.31) | +0.57 (+1.35) |
| Range | −1.5 to +5.00 | −4.5 to +7.5 | −4.5 to +4.0 | −4.5 to +3.5 |
| Annualized EDSS change | ||||
| Median (IQR) | 0.0 (0.0, +0.2) | +0.1 (0.0, +0.2) | +0.1 (0.0, +0.2) | +0.1 (0.0, +0.2) |
| Mean (SD) | +0.18 (+0.62) | +0.14 (+0.37) | +0.15 (+0.40) | +0.12 (+0.27) |
| Range | −0.22 to +4.0 | −0.86 to +2.5 | −0.86 to +2.5 | −0.64 to +1.11 |
EDSS = Expanded Disability Status Scale.
IQR = interquartile range.
SD = standard deviation.
CV = cardiovascular disease.
Figure 1.
Box plots with superimposed data points showing absolute (panel A) and annualized (panel B) change in EDSS between the 2000 assessment and most recent assessment among subjects matched for gender, course, year of birth, age at onset, and age at diagnosis. Data points are randomly shifted along the horizontal axis to reduce overlap. The thick line in each box represents the median value. For the statin group, the median coincides with the bottom of the box representing the lower quartile. The P-values at the top of each plot are based on a Wilcoxon rank-sum/Mann-Whitney U test. One treated patient with an annualized change of +4 and one untreated patient with an annualized change of +2.5 are not shown in panel B.
To account for subject variability in duration of follow-up from baseline to the most recent clinical assessment, we annualized the EDSS change. Median (IQR) annual change in EDSS for the statin group was 0 (0, +0.2) compared to +0.1 (0, +0.2) for the matched no-statin group. As shown in panel B of figure 1, these annualized distributions were also quite similar (AUROC 0.56, 95% CI 0.46 to 0.66; P = 0.23, Wilcoxon rank-sum test). Here again, between group comparisons of the mean (SD) annual change in EDSS scores were not significantly different; the statin group was +0.18 (+0.62) and the matched no-statin group was +0.15 (+0.40).
Given that not all patients had worsened disability scores, we report the fraction who worsened by 1.0 or more on the EDSS; a meaningful alternate way of viewing the data. Since all but three patients had at least one year of follow-up, and two of those were 0.9 years, all are included in this analysis. Among the statin group, 18 (40%) patients worsened compared to 36 (40%) patients in the matched no-statin group (Table 5). These proportions are equal and not statistically different (P = 0.85, chi-squared test).
Table 5.
Subjects with worsened disability
| Worsening of EDSS ≥ 1.0a | Statin (n=45) No. of patients (%) |
No statin (n=156) No. of patients (%) |
Matched no statin (n=90) No. of patients (%) |
|---|---|---|---|
| Yes | 18 (40) | 51 (33) | 36 (40) |
| No | 27 (60) | 105 (67) | 54 (60) |
Subjects with at least 1-point worsening of EDSS by last follow up.
Discussion
This retrospective study demonstrates neither benefit nor harm of statin therapy in regard to disability progression in a population-based prevalence cohort of MS patients. Treatment randomization was not possible, but much longer clinical follow up enables disability progression to be more directly assessed. Although EDSS is not a linear scale, neither in level of disability from one step to the next nor time spent at each stage, inclusion of a broad range of scores through a population-based approach limits potential bias. This cohort is diverse in terms of important characteristics such as disease course and immunomodulatory therapy use, but employed matching techniques efficiently balance covariates while limiting selection bias that would be introduced through non population-based inclusion criteria. Between group comparisons utilizing either matched group were markedly similar.
One limitation of this study is that sample sizes are relatively small. However, the key finding of this report is that differences in EDSS change were found to be quite small over a longer duration of follow-up than can be done in a standard clinical trial. Another limitation of this study is the fraction of patients without significant disability progression, which differs from the usual entry criteria for a clinical trial. This possibly led to an underestimation of statins’ effect in patients with worsening disability. However, the range of clinical severity observed in this study is reflective of the population-based nature of the cohort and informs generalizability in clinical practice. Our data provide some evidence against there being a clinically important effect of statins on MS disability and that a false negative/Type II error is possible but unlikely.
Statins have numerous pleiotropic effects and their impact on immunomodulation may differ from their impact on disability progression in MS. Preclinical and early clinical studies have demonstrated potential benefit as well as potential harm. Several clinical trials underway or recently completed will help clarify the impact on inflammatory episodes, but will not likely directly address disease progression as our study does. The study presented here contains the longest clinical follow up of MS patients taking statins reported thus far, and as such directly addresses disability and disease progression.
Whether or not statins are shown to be useful in MS in the short term (1 to 2 years) remains an open question. However, they will certainly continue to be used for cardiovascular indications, and understanding their safety in MS patients is important. Even though the doses used in cardiovascular disease and clinical trials in MS for immune modulation may be different, it is unknown what doses may have an impact on MS. Our study, with some limitations, supports the hypothesis that statins used in doses currently prescribed for managing hyperlipidemia do not affect the long term course of MS.
Acknowledgement
This study was supported in part by Mayo Clinic Department of Neurology, grants from the NIH (NS24180 and NS32129), a pilot grant from the National MS Society (PP1391), and from grants by the Hilton and Applebaum Foundations. Population-based assessment was made possible by the Rochester Epidemiology Project (AG034676).
References
- 1.Compston A. The basis for treatment in multiple sclerosis. Acta Neurol Scand Suppl. 2006;183:41–47. doi: 10.1111/j.1600-0404.2006.00614.x. [DOI] [PubMed] [Google Scholar]
- 2.Sadeghi MM, Tiglio A, Sadigh K, O'Donnell L, Collinge M, Pardi R, et al. Inhibition of interferon-gamma-mediated microvascular endothelial cell major histocompatibility complex class II gene activation by HMG-CoA reductase inhibitors. Transplantation. 2001;71:1262–1268. doi: 10.1097/00007890-200105150-00014. [DOI] [PubMed] [Google Scholar]
- 3.Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 4.Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 5.Johnson-Anuna LN, Eckert GP, Keller JH, Igbavboa U, Franke C, Fechner T, et al. Chronic administration of statins alters multiple gene expression patterns in mouse cerebral cortex. J Pharmacol Exp Ther. 2005;312:786–793. doi: 10.1124/jpet.104.075028. [DOI] [PubMed] [Google Scholar]
- 6.Johnson-Anuna LN, Eckert GP, Franke C, Igbavboa U, Muller WE, Wood WG. Simvastatin protects neurons from cytotoxicity by up-regulating Bcl-2 mRNA and protein. J Neurochem. 2007;101:77–86. doi: 10.1111/j.1471-4159.2006.04375.x. [DOI] [PubMed] [Google Scholar]
- 7.Miron VE, Rajasekharan S, Jarjour AA, Zamvil SS, Kennedy TE, Antel JP. Simvastatin regulates oligodendroglial process dynamics and survival. Glia. 2007;55:130–143. doi: 10.1002/glia.20441. [DOI] [PubMed] [Google Scholar]
- 8.Foote AK, Blakemore WF. Inflammation stimulates remyelination in areas of chronic demyelination. Brain. 2005;128:528–539. doi: 10.1093/brain/awh417. [DOI] [PubMed] [Google Scholar]
- 9.Bjorkhem I, Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol. 2004;24:806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- 10.Jurevics H, Morell P. Cholesterol for synthesis of myelin is made locally, not imported into brain. J Neurochem. 1995;64:895–901. doi: 10.1046/j.1471-4159.1995.64020895.x. [DOI] [PubMed] [Google Scholar]
- 11.Saher G, Brugger B, Lappe-Siefke C, Mobius W, Tozawa R, Wehr MC, et al. High cholesterol level is essential for myelin membrane growth. Nat Neurosci. 2005;8:468–475. doi: 10.1038/nn1426. [DOI] [PubMed] [Google Scholar]
- 12.Maier O, De Jonge J, Nomden A, Hoekstra D, Baron W. Lovastatin induces the formation of abnormal myelin-like membrane sheets in primary oligodendrocytes. Glia. 2009;57:402–413. doi: 10.1002/glia.20769. [DOI] [PubMed] [Google Scholar]
- 13.Stanislaus R, Pahan K, Singh AK, Singh I. Amelioration of experimental allergic encephalomyelitis in Lewis rats by lovastatin. Neurosci Lett. 1999;269:71–74. doi: 10.1016/s0304-3940(99)00414-0. [DOI] [PubMed] [Google Scholar]
- 14.Miron VE, Zehntner SP, Kuhlmann T, Ludwin SK, Owens T, Kennedy TE, et al. Statin therapy inhibits remyelination in the central nervous system. Am J Pathol. 2009;174:1880–1890. doi: 10.2353/ajpath.2009.080947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sena A, Pedrosa R, Graca Morais M. Therapeutic potential of lovastatin in multiple sclerosis. J Neurol. 2003;250:754–755. doi: 10.1007/s00415-003-1070-8. [DOI] [PubMed] [Google Scholar]
- 16.Vollmer T, Key L, Durkalski V, Tyor W, Corboy J, Markovic-Plese S, et al. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet. 2004;363:1607–1608. doi: 10.1016/S0140-6736(04)16205-3. [DOI] [PubMed] [Google Scholar]
- 17.Paul F, Waiczies S, Wuerfel J, Bellmann-Strobl J, Dorr J, Waiczies H, et al. Oral high-dose atorvastatin treatment in relapsing-remitting multiple sclerosis. PLoS One. 2008;3:e1928. doi: 10.1371/journal.pone.0001928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Togha M, Karvigh SA, Nabavi M, Moghadam NB, Harirchian MH, Sahraian MA, et al. Simvastatin treatment in patients with relapsing-remitting multiple sclerosis receiving interferon beta 1a: a double-blind randomized controlled trial. Mult Scler. 2010;16:848–854. doi: 10.1177/1352458510369147. [DOI] [PubMed] [Google Scholar]
- 19.Birnbaum G, Cree B, Altafullah I, Zinser M, Reder AT. Combining beta interferon and atorvastatin may increase disease activity in multiple sclerosis. Neurology. 2008;71:1390–1395. doi: 10.1212/01.wnl.0000319698.40024.1c. [DOI] [PubMed] [Google Scholar]
- 20.Pittock SJ, Mayr WT, McClelland RL, Jorgensen NW, Weigand SD, Noseworthy JH, et al. Disability profile of MS did not change over 10 years in a population-based prevalence cohort. Neurology. 2004;62:601–606. doi: 10.1212/wnl.62.4.601. [DOI] [PubMed] [Google Scholar]
- 21.Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 22.Stuart EA. Matching methods for causal inference: A review and a look forward. Stat Sci. 2010;25:1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marrie RA, Rudick R, Horwitz R, Cutter G, Tyry T, Campagnolo D, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010;74:1041–1047. doi: 10.1212/WNL.0b013e3181d6b125. [DOI] [PMC free article] [PubMed] [Google Scholar]