Abstract
Regulatory T cells (Treg cells) play a critical role in the maintenance of airway tolerance. We report here that inhaled soluble antigen induces not only adaptive Foxp3+ Treg but also a regulatory population of CD4+ T cells in the lungs and lung-draining lymph nodes that express latency-associated peptide (LAP) on their cell surface but do not express Foxp3. Blocking the cytokines IL-10 or transforming growth factor-β (TGF-β) prevented the generation of the LAP+ Treg and Foxp3+ Treg cells in vivo, and the LAP+ Treg could also be generated concomitantly with Foxp3+ Treg in vitro by culturing naïve CD4+ T cells with antigen and exogenous TGF-β. The LAP+ Treg cells strongly suppressed naïve CD4+ T cell proliferation, and transfer of sorted OVA-specific LAP+ Treg cells in vivo inhibited allergic eosinophilia and Th2 cytokine expression in the lung, either when present at the time of Th2 sensitization or when injected after Th2 cells were formed. Furthermore, inflammatory innate stimuli from house dust mite (HDM) extract, nucleotide-binding oligomerization domain containing 2 (Nod2) ligand, and lipopolysacchride (LPS), that are sufficient for blocking airway tolerance, strongly decreased the induction of LAP+ Treg cells. Taken together, we conclude that inducible antigen-specific LAP+ Treg cells can suppress asthmatic lung inflammation and constitute a mediator of airway tolerance together with Foxp3+ Treg cells.
Introduction
The breakdown of immune tolerance in the airways leads to an abnormal response to harmless airborne antigens, characterized by T helper 2 (Th2) type inflammation and airway hyperresponsiveness. Among several possibilities for maintaining the delicate balance between airway tolerance and airway inflammation, regulatory T cells (Treg cells) have been proposed to be an essential protective mechanism (1). Several major populations of Treg cells have been studied in alternate scenarios with regard to the respiratory environment, including natural CD4+Foxp3+ Treg (2),(3), and peripheral antigen-induced adaptive CD4+ Treg that either make IL-10 but not express Foxp3 (4), or express Fox 3 with or without membrane-bound TGF-β (5),(6),(7).
TGF-β has been implicated as a key contributor to either the function or the differentiation of Treg. TGF-β is produced as part of a large complex, formed by three molecules: a latent transforming growth factor-beta binding protein (LTBP), which tethers the complex to the cell surface or the extracellular matrix (ECM); a latency associated peptide (LAP), which provides a disulfide-linked shell hindering interaction of TGF-β with its cellular receptors; and the TGF-β cytokine itself. LAP regulates TGF-β latency, likely through an RGD sequence that sequesters TGF-β (8). Mutation of LAP leads to Camurati-Engelmann disease (CED), an autosomal dominant disorder of the long bones (9), suggesting the essential role of LAP in controlling the biological activities of TGF-β. LAP has also been shown to promote chemotaxis of human monocytes and block inflammation in a murine model of delayed-type hypersensitivity (DTH) (10). This implies that LAP could function independently to modulate immune responses as well as being a marker for a TGF-β producing cell.
LAP has been found on activated CD4+Foxp3+ Treg cells in patients with activated paracoccidioidomycosis (11) and oral squamous cell carcinoma (12). These Tregs exerted their suppressive function in a TGF-β dependent fashion, suggesting LAP was a marker for the immunosuppressive cells. Hepatitis C virus (HCV) infected hepatocytes were also found to induce CD4+Foxp3+ Treg that expressed LAP, and similarly TGF-β was found to contribute to the suppressive function of these cells (13). In addition, CD8+ Treg cells generated after hematopoietic stem cell transplantation expressed high levels of LAP (14). Not all Foxp3+ Treg express LAP, however, these data have prompted some to suggest that LAP, together with IL-1 receptor type I/II (CD121a/CD121b), may be utilized to enrich for activated Foxp3+ Tregs (15). Indeed, CD4+CD25+LAP+ cells showed a potent TGF-β-dependent suppressive activity when transferred into an experimental autoimmune encephalomyelitis model (16). Most interestingly, a recent study of human T cells found a small population of LAP+CD4+ T cells that did not express Foxp3, but still exerted suppressive activity (17). Moreover, forced expression of the gene encoding TGF-β in naïve CD4+ T cells promoted membrane LAP (18) and exogenous TGF-β induced LAP expression in Foxp3 negative T cells (19). These data therefore suggest that LAP might mark Treg with suppressive potential regardless of Foxp3 expression.
We show here that LAP+ CD4+ T cells that lack Foxp3 are generated in vivo in a TGF-β and IL-10-dependent manner when mice are intranasally exposed to soluble antigen that leads to airway tolerance. Correlating with recent data (19), similar LAP+ Foxp3− CD4+ T cells could be generated in vitro in response to TGF-β, and we demonstrate that these cells are strongly suppressive and can block both the induction and effector phases of asthmatic lung inflammation. Moreover, decreased generation of LAP+ Treg cells was associated with exposure to allergens or inflammatory stimuli that are sufficient for blocking airway tolerance and that lead to Th2-driven lung inflammation. Our data therefore describe a novel population of Treg cells that may be relevant for treatment or manipulation of allergic inflammatory diseases.
Materials and Methods
Mice
C57BL/6 and B6.PLThy1a (Thy1.1) mice were from The Jackson Laboratory. OT-II TCR transgenic mice, bred in house on the BL/6 background, were used as a source of Vβ5/Vα2/Thy1.2 CD4+ T cells responsive to the peptide OVA-323–339. Foxp3/GFP reporter mice, kindly provided by Dr. Alexander Rudensky (Sloan-Kettering Institute, NY) were bred in house with OT-II TCR transgenic mice and also used as a source of Vβ5/Vα2/Thy1.2 CD4+ T cells. All experiments were conducted following the guidelines of the La Jolla Institute for Allergy and Immunology’s Institutional Animal Care and Use Committee.
Airway tolerance and lung Inflammation
Airway tolerance was induced similar to previously described protocols (7). Briefly, on day 0, mice were exposed to 100 μg soluble OVA (Worthington Biochemical Corporation) in PBS, or to PBS alone, given i.n. on 3 consecutive days. To assess the extent of tolerance, 9 days later, mice were sensitized by i.p. injection of 20 μg OVA protein (chicken egg albumin; Sigma-Aldrich), adsorbed to 4 mg aluminum hydroxide (Alum; Pierce Chemical Co.). On day 24 or later, mice were then challenged via the airways with OVA aerosol in a whole body Plexiglas box (10 mg/ml in 15 ml of PBS) for 20 min, once a day for four consecutive days, by ultrasonic nebulization. Tolerance was shown by a lack of lung inflammation, assessed as described below.
To prevent airway tolerance, soluble OVA was mixed with HDM (100 μg), the Nod2 ligand MDP (50 μg), or LPS (1 μg), given i.n. For TGFβ and IL-10R blockade experiments, one single dose of 200 μg anti-IL-10R (1B1.3a), 200 μg anti-TGF-β (1D11), or control IgG was given i.p. at the time of initial exposure to OVA.
Lung Inflammation and airway hyperresponsiveness (AHR)
Bronchoalveolar lavage (BAL) was performed 24 h after the last OVA aerosol challenge. BAL fluid was examined for cytokine content by ELISA (BD Biosciences). For cytological examination, BAL cells were spun on a slide using a Cytospin (Thermo Shandon, Pittsburgh, PA), fixed, and stained with Protocol HEMA3 (Fisher Scientific Company, L.L.C.). Differential cell count was then performed on at least 500 cells in each cytospin slide. For lung histology analysis, 5-μm sections were cut and stained with H&E (hematoxylin and eosin) for examining cell infiltration.
Airway hyperresponsiveness was assessed 24 h after the final OVA challenge in intubated and ventilated mice (FlexiVent ventilator; Scireq) anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) intraperitoneally. The dynamic airway resistance was determined using Scireq software in mice exposed to nebulized PBS or Methacholine (3, 24, 48 mg/ml).
T cell preparation and adoptive transfer
Naïve OVA-specific CD25−Foxp3− CD4+ cells were isolated from spleen and lymph nodes of OT-II/Foxp3/GFP reporter mice with naïve CD4+CD62L+ T Cell Isolation Kits (Miltenyi Biotec, CA). Cell suspensions were incubated with the biotinylated antibody cocktail, supplemented with biotinylated anti-mouse CD25, followed by magnetic anti-biotin microbeads and negative selection on LS MACS columns according to the manufacture’s instructions. The CD4+ cell purity was >90%, with >95% of resulting cells expressing the Vα2Vβ5 transgene and less than 0.1% CD4+CD25+Foxp3+ cells. These cells were either used in vitro, or were adoptively transferred (5 × 106 cells) i.v. into B6.PL Thy1.1 congenic mice to allow T cell responses to be analyzed under tolerogenic or inflammatory conditions.
To track the induction of Foxp3+ and LAP+ CD4+ T cells after adoptive transfer and induction of tolerance, lymph nodes and spleen from individual OVA-challenged mice were harvested, homogenized, and treated with red blood cell-lysing buffer (Sigma, MO) to prepare single cell suspensions. Lung tissues were minced into fragments of less than 1mm in size and digested with Collagenase D (3 mg/ml) and DNase (10 μg/ml) before homogenizing to prepare single cell suspensions. After Fc block with the 2.4G2 mAb, cells were stained with anti-Thy1.2 (53–2.1), anti-CD4 (RM4–5) (BD Biosciences) and anti-LAP (27232) (R&D systems) antibodies. Foxp3 expression was assessed by analyzing GFP expression. All samples were run on a FACS LSRII (BD Biosciences) with FlowJo (Tree Star) software.
In vitro generation and sorting of Foxp3+ and LAP+ Treg cells
Naïve CD4+ T cells from spleen and peripheral lymph nodes of OT-II/Foxp3/GFP reporter mice were isolated as above. APCs from BL/6 spleen cells were made by depleting T cells using complement fixation with Abs to Thy-1.2 (F7D5 and HO.13.4), CD4 (RL172.4), and CD8 (3.155) and were irradiated with 3,000 rad X-ray before use. Cells were cultured in RPMI 1640 medium (Invitrogen Life Technologies) with penicillin, streptomycin, glutamine, HEPES, 2-ME, and 10% FCS (Omega Scientific). For generation of Foxp3+ and LAP+ Treg cells, naïve OT-II cells were plated at 5 × 105 cells per ml with 2 × 106 cells per ml APCs, 1 μM of OVA-323-339, and 10 ng/ml of recombinant human TGF-β1 (PeproTech). At day 4, expanded OT-II cells were separated into CD4+LAP+ or CD4+Foxp3+ (GFP+) populations by sorting using a FACSAria (BD Biosciences). Expression of Treg associated markers, such as CD25, GITR, CD103, intracellular Granzyme B, intracellular CTLA-4, CD44, CD69 and TGFβRII were determined by flow cytometry by using an LSRII (BD Biosciences) with FlowJo software (Tree Star). Antibodies used were anti-LAP (BAF246) and anti-TGFβRII (R&D systems), anti-CD4 (RM4–5), anti-CD25 (PC61), anti-GITR (DTA-1), anti-CD103 (2E7), anti-Granzyme B (16G6), anti-CTLA-4 (UC10-4B9) and anti-CD44 (IM7) (BD Biosciences), anti-CD69 (H1.2F3) (eBioscience).
In vitro suppression assay
Naïve CD4+ T cells were purified from CD45.1+ OT-II TCR-Tg B6 mice and were labeled with PKH26-GL (Sigma) according to the manufacturer’s instructions. To evaluate suppressive function of Foxp3+ and LAP+ T cells, 5 × 104 PKH26-labeled CD45.1+ naïve OT-II cells were cultured with 4 × 105 irradiated T-depleted APCs and 0.5 μM OVA-323-339 for 3 days in the presence of varying numbers of CD45.2+ Treg cells. Cell division of responder T cells was assessed by dilution of PKH26 dye in the gated CD4+CD45.1+ populations. In some experiments, naïve OT-II CD4+ T cell proliferation was assessed by incorporation of [3H]thymidine (1 μCi/well), which was added for the last 8 h of culture.
In vivo studies with in vitro generated LAP+ Treg cells
In vitro generated LAP+ Treg cells (Thy1.2+) were adoptively transferred into Thy1.1 recipient mice. The recipient mice were then immunized and challenged with OVA as described. Mice were either assessed for lung inflammation, or sacrificed on day 2 or day 6 after transfer and pooled LN and spleen cells were harvested to track the transferred cells by flow cytometry as before.
Multiplex cytokine assays
Sorted T cell populations were stimulated with PMA (50 ng/ml) and Ionomycin (1 μg/ml) for 24 h. Cytokine secretion was measured using BioPlex Pro mouse cytokine assay kits (Bio-Rad Inc., Hercules, CA). All assays were carried out directly in a 96-well filtration plate (Millipore, Billerica, MA) at room temperature and protected from light. Briefly, wells were pre-wet with 100 μl assay buffer (PBS containing 1% BSA), then magnetic beads together with a standard, sample, and blank, were added in a final volume of 100 μl, and incubated together at room temperature for 30 min with continuous shaking at 500 rpm. Beads were washed three times with 100 μl wash buffer (PBS containing 1% BSA and 0.05% Tween 20). A cocktail of biotinylated detection antibodies (25 μl/well) was added to the beads for a further 30 min incubation with continuous shaking at 500 rpm. Beads were washed three times, then streptavidin-PE was added for 10 min. Beads were again washed three times and resuspended in 125 μl of assay buffer (PBS containing 1% BSA and 0.05% Tween 20). The fluorescence intensity of the beads was measured using a BioPlex array reader. BioPlex Manager 4.0 software with five-parametric-curve fitting was used for data analysis.
RT and real-time PCR
FACS sorted cells were lysed using TRIzol reagent (Invitrogen). An aliquot of total RNA (5 μg) was reverse-transcribed to cDNA using the SuperScript III (Invitrogen). The oligonucleotide primer sequences of Foxp3 were: forward primer, 5′-GGC CCT TCT CCA GGA CAG A-3′ and reverse primer, 5′-GCT GAT CAT GGC TGG GTT GT. Real-time PCR assay was carried out with LightCycler (Roche Diagnostics, Germany) using LightCycler 480 SYBR Green I master (Roche Diagnostics, Germany). Data are presented as normalized to ribosomal protein housekeeping gene L32.
Statistical analysis
Where appropriate, data were analyzed using Student’s t test. Unless otherwise indicated, data represent the mean ± SEM. P < 0.05 was considered significant and indicated by *.
Results
Inhaled antigen induces LAP+ Foxp3− CD4+ T cells
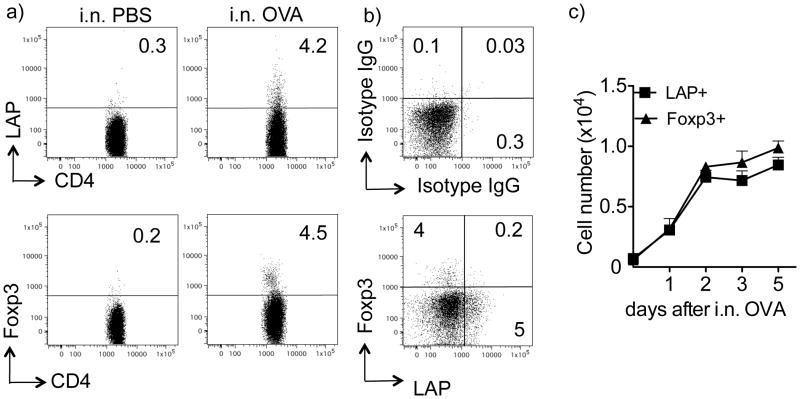
Inhalation of soluble antigen induces airway tolerance and prevents susceptibility to developing Th2-driven allergic inflammation in the lung (6, 7, 20). We previously reported the induction of antigen-specific Foxp3+ Treg during the initial 5 days of exposure to antigen in the lungs (7), but were interested in whether other types of Treg might also develop. To visualize and track the response of antigen-reactive T cells, we transferred naïve (CD25−, Foxp3−, LAP−) OVA-specific CD4 T cells from OT-II TCR transgenic Foxp3/GFP reporter mice (Thy1.2) into Thy1.1 recipients. These Thy1.1 mice were then treated with soluble OVA given i.n. once a day for 3 days, a protocol that efficiently promotes airway tolerance (7). Flow cytometry analysis of lung draining lymph nodes from individual mice, examined 2 days after the last OVA challenge, demonstrated that 3–5% of OT-II CD4+ cells were induced by antigen to express membrane LAP (Fig. 1a and 1b). In comparison, 4–6% of OT-II CD4+ cells were induced to express Foxp3, in line with our previous studies (7). These responses appeared to be systemic in that OVA-specific LAP+ and Foxp3+ T cells were also found in the spleen and lungs (Fig. S1). Most interestingly, we found that the majority of LAP+ cells did not express Foxp3, and conversely the majority of Foxp3+ cells did not express LAP (Fig. 1b). A minor population expressed both molecules. The kinetics of induction of LAP+ CD4+ T cells in the draining LN was similar to that of Foxp3+ Treg, peaking between day 2 and 5 after the initial exposure to soluble antigen (Fig. 1c).
Figure 1. Inhalation of soluble antigen induces LAP+Foxp3− CD4+ T cells.
Naive CD4+CD25− T cells isolated from Thy1.2 OT-II TCR transgenic Foxp3/GFP reporter mice were transferred into Thy1.1 recipient mice. Recipient mice were then tolerized by exposure to soluble OVA (100 μg) in PBS given i.n. for 3 consecutive days. Control (non-tolerized) mice were exposed to PBS without OVA. (a) Representative flow dot plot of LAP and Foxp3 (GFP) expression on gated Thy1.2+ OT-II CD4+ T cells in lung-draining LN from an individual mouse at day 5 after the initial exposure to OVA. (b) Representative flow dot plot of LAP and Foxp3 (GFP) co-staining (bottom), isotype IgG (top) on gated Thy1.2+ OT-II CD4+ T cells in pooled lung-draining LN from an individual mouse at day 5 after the initial exposure to OVA. (c) Total numbers of LAP+Foxp3− and Foxp3+LAP− OT-II CD4+ T cells in lung draining LN populations on different days after the initial exposure to OVA. Data are mean numbers ± SEM from 4 individual mice per group. Data are representative of 3 independent experiments.
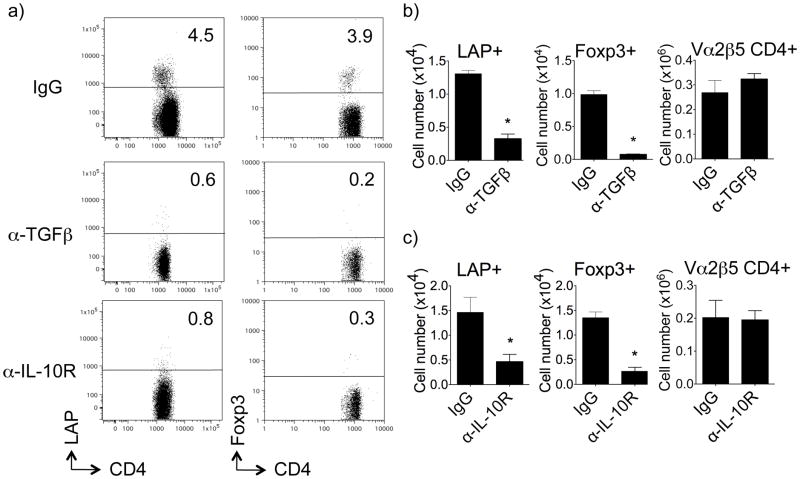
We previously showed that TGF-β plays an important role in the induction of airway tolerance. Neutralizing TGF-β at the time of inhalation of soluble antigen allowed the development of Th2-driven eosinophilia in the airway, and this corresponded with blocking the generation of antigen-specific Foxp3+ Treg cells (7). Of possible significance to our visualization of LAP+Foxp3− T cells, two recent studies found that forced expression of the TGF-β gene into CD4+CD25− T cells promoted surface LAP (18) and importantly that exogenous TGF-β induced LAP expression in Foxp3− T cells (19). Correspondingly, we found that neutralizing TGF-β in vivo when OVA was inhaled strongly reduced the generation of OVA-specific LAP+Foxp3− CD4+ cells by ~ 70%, similar to the reduction seen in OVA-specific LAP−Foxp3+ CD4+ cells (Fig. 2a, 2b and Fig. S1b). Not surprisingly, as the LAP+ and Foxp3+ population were only a fraction of the total OVA-reactive CD4 T cells, neutralizing TGF-β did not significantly alter the overall number of CD4+ OT-II cells visualized, also showing specificity for TGF-β activity (Fig. 2b). Together with our previous results (7), this implied that LAP+ cells, induced from naïve CD4+ T cells by antigen and endogenously produced TGF-β, may also be a key contributor to airway tolerance along with Foxp3+ Treg.
Figure 2. TGF-β and IL-10 are required for induction of LAP+ T cells in vivo.
Thy1.2 OT-II LAP+ and Foxp3+ T cells were tracked in vivo as described in Fig. 1. A single dose of anti-IL-10R (200 μg), anti-TGF-β (200 μg), or control IgG, was given i.p. at the time of initial exposure to i.n. OVA. (a) Representative flow cytometry dot plots of LAP and Foxp3 (GFP) expression on gated Thy1.2+ OT-II CD4+ T cells in lung-draining LN from individual mice. (b) Numbers of LAP+Foxp3− and Foxp3+LAP− OT-II CD4+ T cells, and total OT-II CD4+ cells, were calculated in lung draining lymph nodes on day 5. All results are means ± SEM from 4 individual mice per group. Data are representative of 3 independent experiments. *P < 0.05.
Another inhibitory cytokine implicated in the induction of airway tolerance is IL-10 (21). IL-10 has been reported to promote both IL-10+ Foxp3− Treg and TGF-β induced Foxp3+ Treg (22, 23). We did not visualize any IL-10-producing CD4+ T cells in the lungs or lung-draining lymph nodes (unpublished observations) in this tolerance model but we did find that blocking the IL-10R at the time of exposure to intranasal OVA strongly prevented the induction of tolerance (Fig. S2), similar to our prior observation of neutralizing TGF-β (7). Blocking IL-10R concomitantly decreased the generation of both LAP+ T cells and Foxp3+ T cells (Fig. 2a, 2c, and Fig. S1a). This did not significantly affect the total number of OVA-specific CD4+ cells visualized, also showing specificity (Fig. 2c). These data again suggested that the LAP+ T cells might represent an important regulatory population. This is in line with a study showing that LAP+ T cells generated in the gut after probiotic administration were also IL-10-dependent (24).
LAP+ T cells display regulatory activity
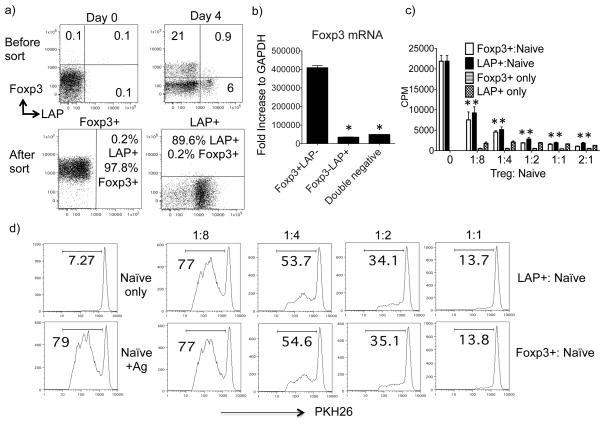
As the frequency of LAP+ T cells induced in vivo was too low to be characterized effectively, we sought to generate an equivalent population in vitro. We isolated naïve CD4+CD25− T cells from GFP−Foxp3 reporter/OT-II TCR transgenic mice and cultured them with T-depleted splenocytes and OVA peptide, in the presence of exogenous TGF-β. We found that these culture conditions that are normally used to generate Foxp3+ Treg also resulted in the generation of LAP+Foxp3− cells (LAP+ cells) over 3–5 days, similar to the population induced in vivo under tolerogenic conditions (Fig. 3a). Using cells from GFP−Foxp3 reporter mice allowed us to sort the two distinct populations. The purity of sorted LAP+Foxp3− cells was at least 89% and the purity of sorted LAP−Foxp3+ cells more than 97% (Fig. 3a). Contamination of Foxp3+ cells in the LAP+ fraction was 0.2% or less. Purity was confirmed by PCR, with sorted LAP+Foxp3− populations expressing minimal Foxp3 mRNA. As a control, Foxp3−LAP− (double negative) T cells from the same cultures (Fig. 3a) were also sorted and found to express minimal Foxp3 mRNA (Fig. 3b). This data further confirmed that LAP+Foxp3− and LAP−Foxp3+ T cells are two distinct populations.
Figure 3. LAP+Foxp3− T cells inhibit naïve CD4+ T cell proliferation in vitro.
Naïve CD4+ T cells from OT-II TCR transgenic GFP/Foxp3 reporter mice were cultured with T-depleted splenocytes and OVA peptide (1 μM) in the presence of exogenous TGF-β (10 ng/ml). Cells were analyzed and sorted on day 4. (a) Top: Foxp3 versus LAP staining on CD4 T cells before and after culture. Bottom: Purity of the sorted LAP+Foxp3− and LAP−Foxp3+ CD4+ T cell populations. (b) Foxp3 mRNA levels in sorted LAP+Foxp3− and LAP−Foxp3+ populations, analyzed using real-time PCR. Data are normalized to the housekeeping gene GAPDH. (c–d) FACS sorted LAP+Foxp3− and LAP−Foxp3+ OT-II T cells (1 × 105) were cultured, alone or at varying ratios with naïve CD4+CD25− OT-II T cells, plus irradiated (3000 rads) syngeneic splenocytes in the presence of 0.5 μM OVA peptide. (c) Proliferation was assessed after a pulse with [3H] thymidine for the last 16 h of a 72-h incubation period. Data are presented as means ± SEM. (d) Responder naïve T cells were labeled with PKH26 dye and division was assessed by loss of the dye after 3 days. Data represent one out of three independent experiments. *P < 0.05.
To test whether in vitro generated OVA-specific LAP+ T cells could act as Treg, we cocultured the sorted LAP+ or Foxp3+ T cells with naïve OT-II T cells, T-depleted APC, and OVA peptide, and measured proliferation either by tritiated thymidine incorporation or after PKH26 labeling of the responder T cells (Fig. 3c and d). LAP+ T cells were hypoproliferative to antigen in vitro, similar to Foxp3+ Treg, when compared to naïve CD4+ T cells (Fig. 3c). However, LAP+ T cells strongly inhibited the division of naïve T cells in a dose-dependent manner (Fig. 3c and d). Importantly, the potency of suppression was similar to that of Foxp3+ Treg showing the activity could not derive from contaminating Foxp3+ cells. These data confirmed that TGF-β-induced LAP+(Foxp3−) T cells could display regulatory activity, in line with recently published results (17) (19).
Phenotype of in vitro generated LAP+ Treg cells
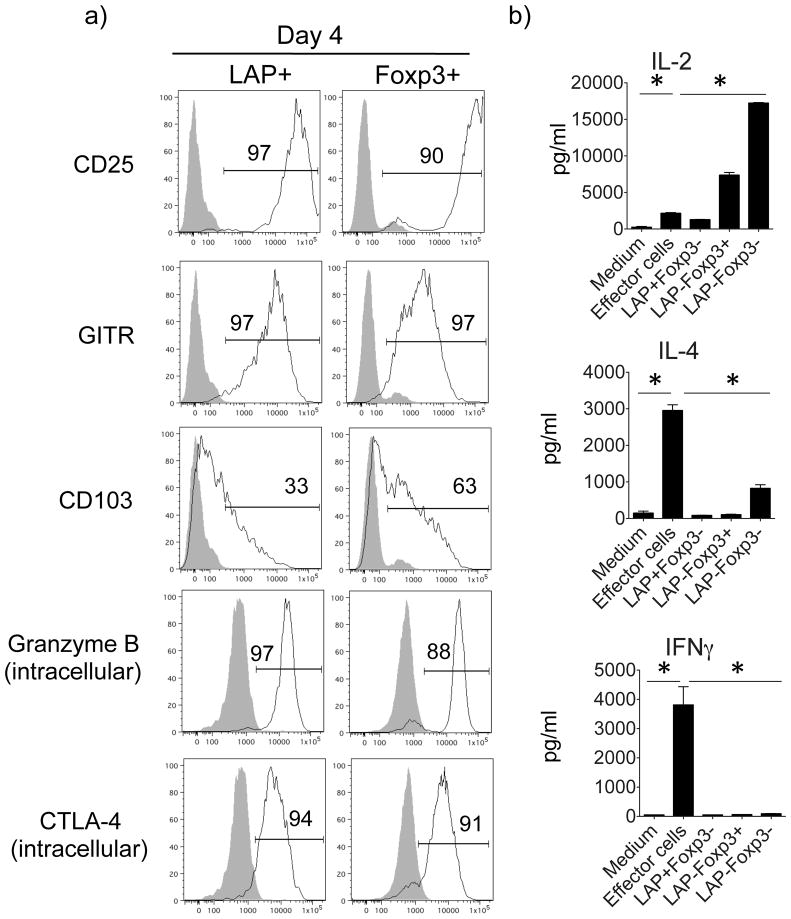
To further pursue similarities or differences between LAP+ Treg and Foxp3+ Treg cells, we assessed a number of molecules reported to be associated with Treg (Fig. 4a and Fig. S3). When LAP+ Treg cells were first visualized on day 2 of the TGF-β-induced cultures, CD25 was quickly upregulated on a small portion (18%) of Foxp3+ cells, but not on LAP+ cells (not shown). By day 4, the majority of the LAP+ and Foxp3+ cells expressed high levels of CD25 (Fig. 4a), which was maintained on day 6, albeit at a lower level on LAP+ cells (Fig. S3a). GITR (TNFRSF18), a receptor belonging to the TNFR superfamily that has also been associated with Foxp3+ Treg, was also rapidly expressed and maintained at high levels on both LAP+ and Foxp3+ cells. CD103, the integrin αEβ7, has additionally been reported to be expressed on Foxp3+ Treg cells (25),(26), and again was markedly upregulated on both LAP+ and Foxp3+ cells to a similar extent. We also examined the Treg-associated suppressive molecules Granzyme B and CTLA-4. Granzyme B, a serine protease, which is responsible for the induction of apoptosis in target cells, was shown to be highly upregulated in CD4+CD25+(Foxp3+) Treg and served as one mechanism for cell contact-mediated suppression (27, 28). Granzyme B was upregulated to high levels on the majority of LAP+ and Foxp3+ cells on day 4 (Fig. 4a). Expression was maintained over 6 days (Fig. S3a), but at lower levels in LAP+ cells, suggesting that the expression of intracellular Granzyme B might be transient. CTLA-4, another suppressive molecule expressed by Foxp3+ Treg cells, can attenuate T cell activation either directly or indirectly through interaction with CD80 and CD86 (B7) molecules on antigen-presenting cells. Again, intracellular CTLA-4 was strongly expressed by both LAP+ and Foxp3+ T cells on day 4 (Fig. 4a) and largely maintained at day 6 (Fig. S3a). We also assessed expression of CD44, CD69, and TGFβRII (Fig. S3b). In addition to its important role in T cell activation, migration, and apoptosis (29, 30), CD44 has been associated with Foxp3 expression and Treg function (31, 32) and promoting surface expression of TGFβ (33). We found that CD44 was upregulated on 46% of LAP+ and 65% of Foxp3+ cells (Fig. S3b). Signaling from the T cell early activation marker CD69 can induce the synthesis of TGFβ (34) and CD69+Foxp3−CD4+ Tregs have been shown to suppress T cell proliferation through membrane-bound TGFβ (35). We found that more LAP+ cells expressed higher levels of CD69 (Fig. S3b). TGFβRII expression has also been reported to be important for Treg maintenance (36, 37), and the induced LAP+ T cells expressed higher levels compared to Foxp3+ cells (Fig. S3b). This data is in line with reports showing that murine CD4+CD25−LAP+ and human CD4+LAP+ cells express CD69 and TGFβRII (17, 38).
Figure 4. Phenotype of TGF-β-induced LAP+Foxp3− T cells.
LAP+Foxp3− and LAP−Foxp3+ OT-II T cells were generated as described in Fig. 3. (a) Representative expression of CD25, GITR, CD103, Granzyme B and CTLA-4 (black line), with isotype controls (gray shade), at day 4 on gated LAP+Foxp3− and LAP−Foxp3+ CD4+ T cells. (b) Cytokine secretion profiles of TGF-β-induced sorted LAP+Foxp3−, LAP−Foxp3+, and LAP−Foxp3− T cells. Positive control populations (Effector cells) were from cultures of OT-II splenocytes stimulated with OVA peptide in non-skewing conditions for 4 days. T cells were restimulated with PMA (50 ng/ml) and Ionomycin (1 μg/ml) for 24h. IL-2, IL-4 and IFNγ levels were measured using mouse cytokine multiplex kits. Similar data were obtained in 3 independent experiments. *P < 0.05.
Next, we assessed the ability of Treg cells to secrete cytokines (Fig. 4b and Fig. S4). We sorted LAP+Foxp3− and LAP−Foxp3+ OT-II T cells, and as a control LAP−Foxp3− T cells, from 4-day TGF-β-induced cultures as described in Fig. 3a. These cells were restimulated with PMA and Ionomycin for 24h. Splenocytes from OT-II mice were cultured with OVA peptide under neutral (non-skewing) conditions for 4 days, and then also restimulated with PMA and Ionomycin as an additional positive control (labeled Effector cells). LAP+Foxp3− Treg cells displayed a largely anergic-type phenotype when compared to the unskewed effector cell population, producing little/no inflammatory cytokines or chemokines, including IL-2, IL-4, and IFNγ (Fig. 4b and Fig. S4). This profile was again similar to Foxp3+ Treg, although Foxp3+ cells and not LAP+ cells did produce significant levels of MCP-1α and β (Fig. S4). In contrast, the LAP−Foxp3− T cells isolated from the same TGF-β-induced cultures produced a substantial amount of IL-2, some IL-4 and IL-17, and MCP-1α and β, suggesting they were largely undifferentiated (Fig. 4b and S4). Neither LAP+ nor Foxp3+ Treg cells produced substantial levels of IL-10 (Fig. S4). Taken together, these results showed that antigen-induced LAP+ Treg cells were phenotypically very similar to antigen-induced Foxp3+ Treg cells.
LAP+ Treg cells suppress the onset of asthmatic lung inflammation in vivo
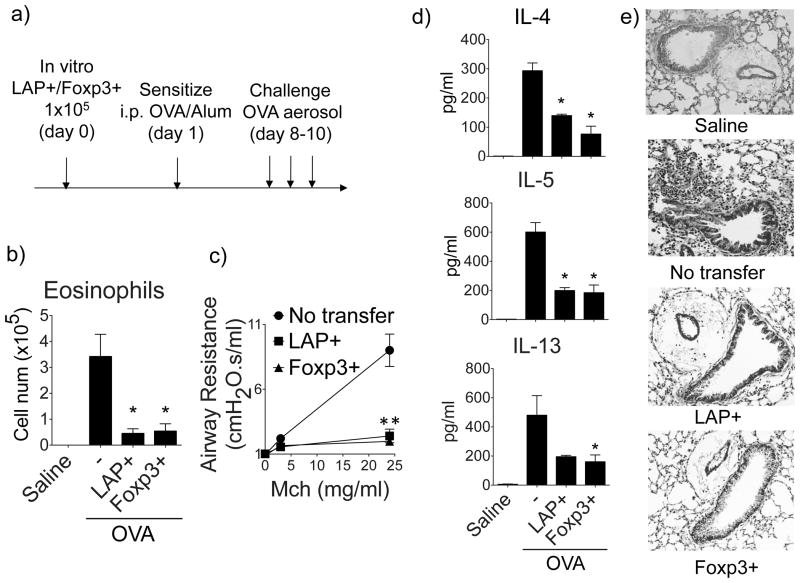
To investigate the in vivo suppressive function of the induced LAP+ Treg cells, we sorted LAP+ and Foxp3+ OT-II T cells as in Fig. 3 and adoptively transferred them into syngeneic BL/6 host mice. The recipients were then sensitized with OVA in Alum and subsequently challenged with OVA via the airways to induce lung inflammation (Fig. 5a). 1 × 105 LAP+ Treg cells substantially inhibited the induction of eosinophilia in the airways (Fig. 5b), airway hyperresponsiveness (AHR) to methacholine (Fig. 5c), Th2 cytokines in BAL (Fig. 5d), and peribronchial cell infiltration in lung parenchyma (Fig. 5e). Adoptively transferred Foxp3+ Treg cells (1 × 105) also strongly prevented the development of the Th2 inflammatory response, suggesting that LAP+ Treg and Foxp3+ Treg are equally potent as regulatory populations that can promote airway tolerance. To eliminate the possibility that the observed suppression by sorted LAP+ Treg cells was due to the minor contamination of Foxp3+ Treg cells (0.2%, Fig. 3a), we repeated the experiment using 1 × 103 sorted Foxp3+ Treg, which is 1% of the 105 Treg cells that were able to strongly inhibit the asthmatic response. 103 Foxp3+ Treg failed to suppress the OVA-induced eosinophilia and lung inflammation (Fig. S3c, S3d, and not shown), confirming that the observed suppression by sorted LAP+ Treg cells was not due to contamination of Foxp3+ Treg cells.
Figure 5. LAP+ T cells prevent induction of asthmatic lung inflammation in vivo.
LAP+Foxp3− or Foxp3+LAP− OT-II T cells, generated in vitro as described in Fig. 3, were sorted and transferred into naïve BL/6 recipient mice. One day later, the mice were then sensitized with OVA (20 μg) in Alum (4 mg) and subsequently challenged with OVA aerosol to induce lung inflammation. (a) Protocol timeline. (b) Eosinophils in BAL. (c) Airway hyperresponsiveness to Methacholine, assessed using a FlexiVent. (d) Cytokines in BAL by ELISA. (e) Representative H&E staining of lung sections. Data are means ± SEM from 3 mice per group. Similar data were generated from 3 independent experiments. *P < 0.05.
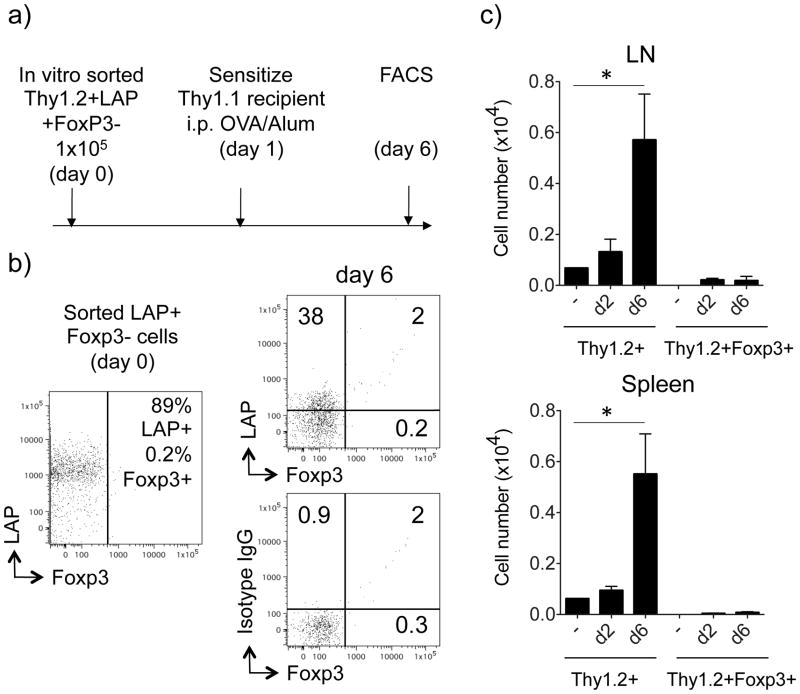
To investigate whether the LAP+ Treg cells converted to Foxp3+ Treg cells upon antigen encounter in vivo, we sorted Thy1.2+ LAP+ T cells as described in Fig. 3a and transferred them into Thy1.1 recipient mice. The mice were then immunized with OVA in Alum as above and the transferred Thy1.2+ cells were visualized after immunization (Fig. 6). The expression of LAP was downregulated on the majority of cells (6±1 % positive) on day 2 (data not shown), whereas 38±8% were positive on day 6 (Fig. 6b). Importantly, there was no significant expression of Foxp3 detected in the majority of T cells, indicating that the transferred LAP+ Treg cells did not convert to Foxp3+ Treg cells. Enumerating the total number of Thy1.2+ T cells in pooled LN and spleen showed strong expansion of the transferred population between 2 and 6 days, but only a fraction were Foxp3+ cells throughout this time period, confirming minimal conversion and accumulation of Foxp3+ cells (Fig. 6c).
Figure 6. LAP+ T cells do not convert to Foxp3+ T cells in vivo.
Sorted Thy1.2+LAP+Foxp3− T cells, generated in vitro as described in Figs. 3 and 5, were transferred into Thy1.1 recipient mice. One day later, the recipient mice were immunized with OVA (20 μg) and alum (4 mg). (a) Protocol timeline. (b) The expression of surface LAP and intracellular Foxp3 on gated Thy1.2+ T cells was analyzed in pooled LN and spleen on day 6 after the immunization. Representative dot plot of T cells before and 6 days after the transfer. Isotype control staining for LAP shown in bottom plot. (c) Total numbers of recovered Thy1.2+ T cells in pooled lymph nodes and spleens on day 2 and day 6, compared to recovered Thy1.2+ Foxp3+ T cells. All results are means ± SEM from 4 individual mice per group. Data are representative of 3 independent experiments. *P < 0.05.
LAP+ Treg cells suppress asthmatic lung inflammation induced by primed T cells
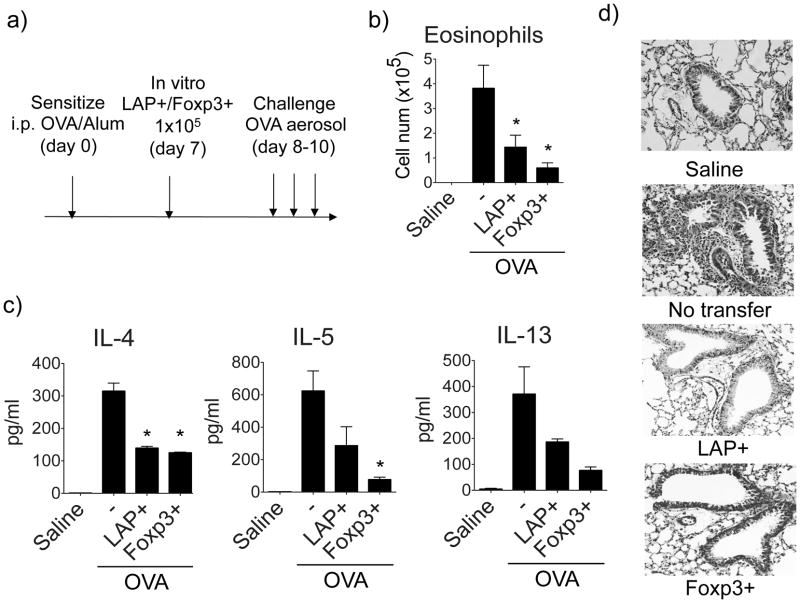
We further tested the in vivo suppressive ability of LAP+ Treg cells injected after the initial generation of pathogenic T cells. Sorted OVA-specific CD4+LAP+ T cells, as described in Fig. 3a, were transferred into recipient mice previously sensitized one week earlier with OVA/Alum. The recipients were subsequently challenged with OVA aerosol to induce lung inflammation (Fig. 7a). 1 × 105 LAP+ T cells again markedly inhibited OVA-induced eosinophilia in the airways (Fig. 7b), Th2 cytokines in BAL (Fig. 7c), and peribronchial cell infiltration in lung parenchyma (Fig. 7d). This indicates that LAP+ Treg cells are able to suppress effector T cell activation during the peak of inflammatory responses. In comparison, Foxp3+ Treg cells displayed a slightly stronger inhibitory activity in suppressing effector T cell driven lung inflammation (Fig. 7b, 7c, and 7d). Collectively, these data show that LAP+Foxp3− T cells induced with antigen are a bona fide regulatory population that can suppress asthmatic lung inflammation with activity similar to conventional Foxp3+ Treg cells.
Figure 7. LAP+ T cells suppress asthmatic lung inflammation in sensitized mice.
FACS sorted LAP+Foxp3− or Foxp3+LAP− OT-II T cells, generated as described in Fig. 3, were transferred into BL/6 mice that had been sensitized with OVA and Alum for 7 days. The recipients were then challenged with OVA aerosol to induce lung inflammation starting one day later. (a) Protocol timeline. (b) Eosinophils in BAL. (c) Cytokines in BAL by ELISA. (d) Representative H&E staining of lung sections. Data are means ± SEM from 3 mice per group. Data are representative of 3 independent experiments. *P < 0.05.
Allergens and pro-inflammatory microbial-associated molecular patterns suppress the generation of LAP+ Treg cells
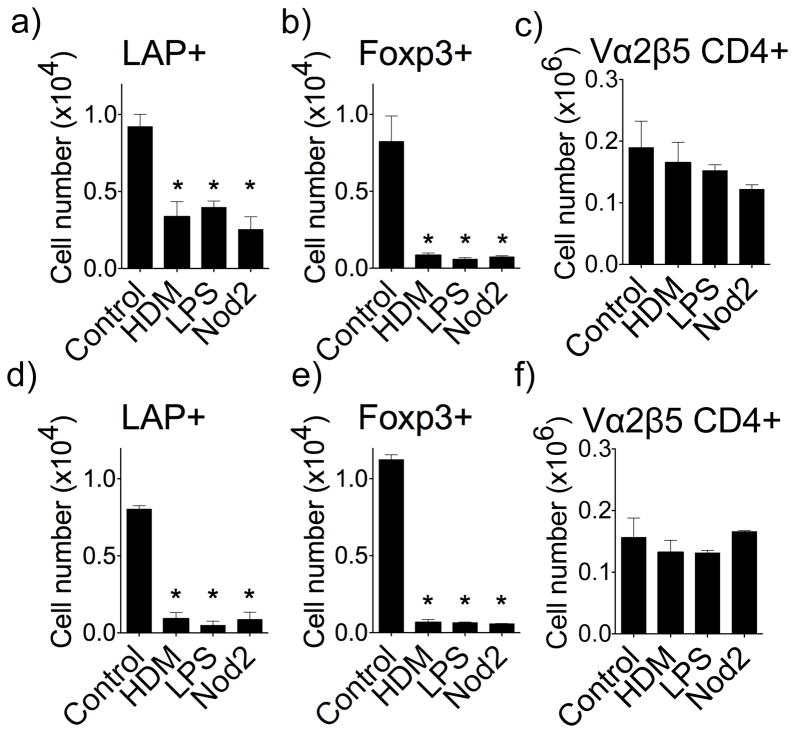
We, and others, have previously shown that allergens, or isolated microbial-associated molecular patterns (MAMPs), such as LPS that targets TLR4, and peptidoglycan derivatives that target the intracellular Nod2 receptor, can prevent the induction of airway tolerance and lead to susceptibility to development of Th2-driven lung inflammation (7, 39),(40). Although this activity is at least in part through antagonizing the generation of Foxp3+ Treg, our data suggested that LAP+ Treg may also be controlled by these stimuli. House Dust Mite (HDM) extract, the Nod2 ligand MDP (muramyl dipeptide), and LPS, were then given concurrently with soluble OVA intranasally, and the induction of LAP+ Treg cells was tracked as described in Fig. 1. HDM, LPS, and Nod2 ligand strongly inhibited the generation of antigen-specific LAP+ T cells in the lung draining lymph nodes (Fig. 8a), lung tissue (Fig. 8d), and spleen (Fig. S7a), in addition to blocking the induction of Foxp3+ T cells (Figs. 8b, 8e, and Fig. S1c). HDM, LPS, and Nod2 ligand did not significantly affect the total number of OVA-specific CD4+ T cells visualized, again showing specificity (Fig. 8c and 8f). Therefore, allergens and associated MAMPs likely antagonize airway tolerance by preventing the generation of both LAP+ Treg and Foxp3+ Treg cells.
Figure 8. Allergens and pro-inflammatory microbial-associated molecular patterns suppress the generation of LAP+ Treg cells.
The generation of LAP+Foxp3− and Foxp3+LAP− OT-II T cells was tracked in vivo as described in Fig. 1. HDM extract, MDP, or LPS, were given i.n. concurrently with soluble OVA. (a–f) Numbers of LAP+Foxp3−, LAP−Foxp3+, and total OT-II CD4+ T cells in lung draining lymph nodes (a–c) and lung tissue (d–f). Data are means ± SEM from 3 mice per group. Data are representative of 3 independent experiments. *P < 0.05.
Discussion
Treg cells have been demonstrated to maintain airway tolerance in mouse models of asthma, and increased numbers of Treg or Treg cell activity have been associated with current treatments that reduce allergic and asthmatic symptoms. CD4+CD25+Foxp3+ Treg numbers were observed to increase in patients undergoing allergen immunotherapy, for example after exposure to escalating doses of HDM (41), grass pollen (42) and venom (43). Enhanced numbers of Foxp3+ Treg have also been found in Glucocorticoid-treated asthmatic patients (44), and histamine receptor (H4R) agonists were shown to enrich the Foxp3+ Treg population in a mouse model of asthma (45). These data strongly support the idea that methods to induce regulatory T cells, or the adoptive transfer of regulatory T cells, would be efficacious in the prevention and treatment of lung inflammatory disease. Here we report a novel Treg population bearing surface LAP that does not express Foxp3, but which expresses Treg-associated suppressive molecules, including Granzyme B and CTLA-4. These cells were equally potent at suppressing asthmatic lung inflammation in the mouse suggesting they are another population of regulatory T cells that should be considered as a target of analysis or therapy for allergic disease.
Interestingly, a recent report found that a small percentage (1.32%) of CD4+ cells in human peripheral blood expressed LAP (17). These LAP+ cells did not express Foxp3, were hypo-proliferative, and exhibited suppressive activity in vitro. Therefore, it is highly likely that the LAP+ Treg that we describe are similar to these human peripheral LAP+ Treg cells, although there was an apparent discrepancy between the cytokine secretion profiles in that our cells made little/no inflammatory cytokines whereas the human cells made IL-8, IL-9, IL-10, and IFN-γ. However, collectively the data suggest that CD4+LAP+ Treg cells may have potential in cell-based immunotherapy for treating asthma, as well as being another Treg population that can be used to track the activity of alternate forms of therapy for asthma.
The antigen-induced CD4+LAP+ Treg cells that we visualized in vivo and in vitro lacked Foxp3, even after in vivo re-activation with antigen, whereas the majority of Foxp3+ Treg generated concomitantly did not express LAP. This is in contrast to some reports that have found activated Foxp3+ Treg can also express surface LAP. Our in vivo and in vitro analyses did show that there was a small proportion of Foxp3+LAP+ cells generated in response to TGF-β, but they were strongly out numbered by single positive cells. The discrepancy between our observations and other investigators that have visualized LAP on Foxp3+ T cells might due to either different environments to which the T cells were exposed, variations in stimulation conditions, and/or differences in the T cells that were analyzed. We used naïve T cells from a TCR transgenic mouse stimulated with antigen to become adaptive iTreg, whereas for example, one study observed LAP expression on mouse CD4+CD25+ nTreg that were restimulated with anti-CD3 and IL-2 for 3 days (46). Another report observed 50–70 % human Foxp3+ cells expressing LAP (15), which were likely largely nTreg, obtained from peripheral blood and expanded for 14–21 days before restimulation with anti-CD3/CD28. Oida et al. used conditions most similar to our systems, stimulating BALB/c CD4+CD25− T cells that would have been a mixture of naïve and memory T cells, with plate-bound anti-CD3/anti-CD28 and 10 ng/ml TGFβ. They found LAP expression in Foxp3− cells as well as the majority of Foxp3+ cells (47). Whether they would have seen the same result with naïve T cells stimulated with antigen is not clear.
Oral anti-CD3 administration has been shown to induce a population of CD4+LAP+ regulatory cells (48), which were associated with suppressive activity in a variety of autoimmune disease models, including autoimmune encephalomyelitis (16, 38), diabetes (49, 50), colitis (51), and arthritis (52). At least in one case when analyzed, these CD4+LAP+ cells co-expressed Foxp3 (16). However, given our data and others mentioned above, it is likely that some Treg were LAP+Foxp3− as oral anti-CD3 is most similar to mucosal immunization with soluble antigen as we used here. It has been proposed that LAP could be utilized for isolating Foxp3+ Treg cells (15). We suggest that isolation of Treg exclusively based on LAP expression might also include the Foxp3− cells, which would be advantageous as these LAP+Foxp3− T cells are potent suppressors and likely co-operate with Foxp3+ Treg to limit autoreactivity and inadvertent inflammatory responses such as those induced by allergens.
Supplementary Material
Acknowledgments
This work was supported by NIH grants AI070535 and CA91837 to M.C.
We thank YanFei Adams for technical assistance. This is manuscript # 1382 from the La Jolla Institute for Allergy and Immunology.
References
- 1.Lloyd CM, Hawrylowicz CM. Regulatory T cells in asthma. Immunity. 2009;31:438–449. doi: 10.1016/j.immuni.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005;202:1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joetham A, Takeda K, Taube C, Miyahara N, Matsubara S, Koya T, Rha YH, Dakhama A, Gelfand EW. Naturally occurring lung CD4(+)CD25(+) T cell regulation of airway allergic responses depends on IL-10 induction of TGF-beta. J Immunol. 2007;178:1433–1442. doi: 10.4049/jimmunol.178.3.1433. [DOI] [PubMed] [Google Scholar]
- 4.Hawrylowicz CM, O’Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5:271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 5.Ostroukhova M, Qi Z, Oriss TB, Dixon-McCarthy B, Ray P, Ray A. Treg-mediated immunosuppression involves activation of the Notch-HES1 axis by membrane-bound TGF-beta. J Clin Invest. 2006;116:996–1004. doi: 10.1172/JCI26490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostroukhova M, Seguin-Devaux C, Oriss TB, Dixon-McCarthy B, Yang L, Ameredes BT, Corcoran TE, Ray A. Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and FOXP3. J Clin Invest. 2004;114:28–38. doi: 10.1172/JCI20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duan W, So T, Croft M. Antagonism of airway tolerance by endotoxin/lipopolysaccharide through promoting OX40L and suppressing antigen-specific Foxp3+ T regulatory cells. J Immunol. 2008;181:8650–8659. doi: 10.4049/jimmunol.181.12.8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, Xiong X, Munger JS. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol. 2007;176:787–793. doi: 10.1083/jcb.200611044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saito T, Kinoshita A, Yoshiura K, Makita Y, Wakui K, Honke K, Niikawa N, Taniguchi N. Domain-specific mutations of a transforming growth factor (TGF)-beta 1 latency-associated peptide cause Camurati-Engelmann disease because of the formation of a constitutively active form of TGF-beta 1. J Biol Chem. 2001;276:11469–11472. doi: 10.1074/jbc.C000859200. [DOI] [PubMed] [Google Scholar]
- 10.Ali NA, Gaughan AA, Orosz CG, Baran CP, McMaken S, Wang Y, Eubank TD, Hunter M, Lichtenberger FJ, Flavahan NA, Lawler J, Marsh CB. Latency associated peptide has in vitro and in vivo immune effects independent of TGF-beta1. PLoS ONE. 2008;3:e1914. doi: 10.1371/journal.pone.0001914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira MC, de Oliveira RT, da Silva RM, Blotta MH, Mamoni RL. Involvement of regulatory T cells in the immunosuppression characteristic of patients with paracoccidioidomycosis. Infect Immun. 2010;78:4392–4401. doi: 10.1128/IAI.00487-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasparoto TH, de Souza Malaspina TS, Benevides L, de Melo EJ, Jr, Costa MR, Damante JH, Ikoma MR, Garlet GP, Cavassani KA, da Silva JS, Campanelli AP. Patients with oral squamous cell carcinoma are characterized by increased frequency of suppressive regulatory T cells in the blood and tumor microenvironment. Cancer Immunol Immunother. 2010;59:819–828. doi: 10.1007/s00262-009-0803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall CH, Kassel R, Tacke RS, Hahn YS. HCV+ hepatocytes induce human regulatory CD4+ T cells through the production of TGF-beta. PLoS ONE. 2010;5:e12154. doi: 10.1371/journal.pone.0012154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Bertucci AM, Ramsey-Goldman R, Burt RK, Datta SK. Regulatory T cell (Treg) subsets return in patients with refractory lupus following stem cell transplantation, and TGF-beta-producing CD8+ Treg cells are associated with immunological remission of lupus. J Immunol. 2009;183:6346–6358. doi: 10.4049/jimmunol.0901773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran DQ, Andersson J, Hardwick D, Bebris L, Illei GG, Shevach EM. Selective expression of latency-associated peptide (LAP) and IL-1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood. 2009;113:5125–5133. doi: 10.1182/blood-2009-01-199950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen ML, Yan BS, Bando Y, Kuchroo VK, Weiner HL. Latency-associated peptide identifies a novel CD4+CD25+ regulatory T cell subset with TGFbeta-mediated function and enhanced suppression of experimental autoimmune encephalomyelitis. J Immunol. 2008;180:7327–7337. doi: 10.4049/jimmunol.180.11.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandhi R, Farez MF, Wang Y, Kozoriz D, Quintana FJ, Weiner HL. Cutting edge: human latency-associated peptide+ T cells: a novel regulatory T cell subset. J Immunol. 2010;184:4620–4624. doi: 10.4049/jimmunol.0903329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oida T, Weiner HL. Overexpression of TGF-ss 1 gene induces cell surface localized glucose-regulated protein 78-associated latency-associated peptide/TGF-ss. J Immunol. 2010;185:3529–3535. doi: 10.4049/jimmunol.0904121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oida T, Weiner HL. TGF-beta induces surface LAP expression on murine CD4 T cells independent of Foxp3 induction. PLoS ONE. 5:e15523. doi: 10.1371/journal.pone.0015523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsitoura DC, DeKruyff RH, Lamb JR, Umetsu DT. Intranasal exposure to protein antigen induces immunological tolerance mediated by functionally disabled CD4+ T cells. J Immunol. 1999;163:2592–2600. [PubMed] [Google Scholar]
- 21.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 22.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 23.Stock P, Akbari O, Berry G, Freeman GJ, Dekruyff RH, Umetsu DT. Induction of T helper type 1-like regulatory cells that express Foxp3 and protect against airway hyper-reactivity. Nat Immunol. 2004;5:1149–1156. doi: 10.1038/ni1122. [DOI] [PubMed] [Google Scholar]
- 24.Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J Immunol. 2005;174:3237–3246. doi: 10.4049/jimmunol.174.6.3237. [DOI] [PubMed] [Google Scholar]
- 25.Banz A, Peixoto A, Pontoux C, Cordier C, Rocha B, Papiernik M. A unique subpopulation of CD4+ regulatory T cells controls wasting disease, IL-10 secretion and T cell homeostasis. Eur J Immunol. 2003;33:2419–2428. doi: 10.1002/eji.200324205. [DOI] [PubMed] [Google Scholar]
- 26.Zelenika D, Adams E, Humm S, Graca L, Thompson S, Cobbold SP, Waldmann H. Regulatory T cells overexpress a subset of Th2 gene transcripts. J Immunol. 2002;168:1069–1079. doi: 10.4049/jimmunol.168.3.1069. [DOI] [PubMed] [Google Scholar]
- 27.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 29.Sherman L, Sleeman J, Herrlich P, Ponta H. Hyaluronate receptors: key players in growth, differentiation, migration and tumor progression. Current opinion in cell biology. 1994;6:726–733. doi: 10.1016/0955-0674(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 30.Ayroldi E, Cannarile L, Migliorati G, Bartoli A, Nicoletti I, Riccardi C. CD44 (Pgp-1) inhibits CD3 and dexamethasone-induced apoptosis. Blood. 1995;86:2672–2678. [PubMed] [Google Scholar]
- 31.Firan M, Dhillon S, Estess P, Siegelman MH. Suppressor activity and potency among regulatory T cells is discriminated by functionally active CD44. Blood. 2006;107:619–627. doi: 10.1182/blood-2005-06-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bollyky PL, Lord JD, Masewicz SA, Evanko SP, Buckner JH, Wight TN, Nepom GT. Cutting edge: high molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:744–747. doi: 10.4049/jimmunol.179.2.744. [DOI] [PubMed] [Google Scholar]
- 33.Bollyky PL, Falk BA, Long SA, Preisinger A, Braun KR, Wu RP, Evanko SP, Buckner JH, Wight TN, Nepom GT. CD44 costimulation promotes FoxP3+ regulatory T cell persistence and function via production of IL-2, IL-10, and TGF-beta. J Immunol. 2009;183:2232–2241. doi: 10.4049/jimmunol.0900191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sancho D, Gomez M, Sanchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136–140. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Han Y, Guo Q, Zhang M, Chen Z, Cao X. CD69+ CD4+ CD25− T cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-beta 1. J Immunol. 2009;182:111–120. doi: 10.4049/jimmunol.182.1.111. [DOI] [PubMed] [Google Scholar]
- 36.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Ochi H, Abraham M, Ishikawa H, Frenkel D, Yang K, Basso AS, Wu H, Chen ML, Gandhi R, Miller A, Maron R, Weiner HL. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25− LAP+ T cells. Nat Med. 2006;12:627–635. doi: 10.1038/nm1408. [DOI] [PubMed] [Google Scholar]
- 39.Cates EC, Fattouh R, Wattie J, Inman MD, Goncharova S, Coyle AJ, Gutierrez-Ramos JC, Jordana M. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J Immunol. 2004;173:6384–6392. doi: 10.4049/jimmunol.173.10.6384. [DOI] [PubMed] [Google Scholar]
- 40.Duan W, Mehta AK, Magalhaes JG, Ziegler SF, Dong C, Philpott DJ, Croft M. Innate signals from Nod2 block respiratory tolerance and program T(H)2-driven allergic inflammation. J Allergy Clin Immunol. 2010;126:1284–1293. e1210. doi: 10.1016/j.jaci.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Hehir RE, Gardner LM, de Leon MP, Hales BJ, Biondo M, Douglass JA, Rolland JM, Sandrini A. House dust mite sublingual immunotherapy: the role for transforming growth factor-beta and functional regulatory T cells. Am J Respir Crit Care Med. 2009;180:936–947. doi: 10.1164/rccm.200905-0686OC. [DOI] [PubMed] [Google Scholar]
- 42.Radulovic S, Jacobson MR, Durham SR, Nouri-Aria KT. Grass pollen immunotherapy induces Foxp3-expressing CD4+ CD25+ cells in the nasal mucosa. J Allergy Clin Immunol. 2008;121:1467–1472. e1461. doi: 10.1016/j.jaci.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Pereira-Santos MC, Baptista AP, Melo A, Alves RR, Soares RS, Pedro E, Pereira-Barbosa M, Victorino RM, Sousa AE. Expansion of circulating Foxp3+)D25bright CD4+ T cells during specific venom immunotherapy. Clin Exp Allergy. 2008;38:291–297. doi: 10.1111/j.1365-2222.2007.02887.x. [DOI] [PubMed] [Google Scholar]
- 44.Karagiannidis C, Akdis M, Holopainen P, Woolley NJ, Hense G, Ruckert B, Mantel PY, Menz G, Akdis CA, Blaser K, Schmidt-Weber CB. Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J Allergy Clin Immunol. 2004;114:1425–1433. doi: 10.1016/j.jaci.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 45.Morgan RK, McAllister B, Cross L, Green DS, Kornfeld H, Center DM, Cruikshank WW. Histamine 4 receptor activation induces recruitment of FoxP3+ T cells and inhibits allergic asthma in a murine model. J Immunol. 2007;178:8081–8089. doi: 10.4049/jimmunol.178.12.8081. [DOI] [PubMed] [Google Scholar]
- 46.Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O’Shea JJ, Shevach EM. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J Exp Med. 2008;205:1975–1981. doi: 10.1084/jem.20080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oida T, Weiner HL. TGF-beta induces surface LAP expression on murine CD4 T cells independent of Foxp3 induction. PLoS ONE. 2010;5:e15523. doi: 10.1371/journal.pone.0015523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ochi H, Abraham M, Ishikawa H, Frenkel D, Yang K, Basso A, Wu H, Chen ML, Gandhi R, Miller A, Maron R, Weiner HL. New immunosuppressive approaches: oral administration of CD3-specific antibody to treat autoimmunity. J Neurol Sci. 2008;274:9–12. doi: 10.1016/j.jns.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishikawa H, Ochi H, Chen ML, Frenkel D, Maron R, Weiner HL. Inhibition of autoimmune diabetes by oral administration of anti-CD3 monoclonal antibody. Diabetes. 2007;56:2103–2109. doi: 10.2337/db06-1632. [DOI] [PubMed] [Google Scholar]
- 50.Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K, Wu HY, Weiner HL. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci U S A. 2010;107:9765–9770. doi: 10.1073/pnas.0908771107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, Strober W. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172:834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 52.Wu HY, Maron R, Tukpah AM, Weiner HL. Mucosal anti-CD3 monoclonal antibody attenuates collagen-induced arthritis that is associated with induction of LAP+ regulatory T cells and is enhanced by administration of an emulsome-based Th2-skewing adjuvant. J Immunol. 2010;185:3401–3407. doi: 10.4049/jimmunol.1000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.