Abstract
We recently reported that the β1 integrin antagonist referred to as HYD1 induces necrotic cell death in myeloma cell lines as a single agent using in vitro and in vivo models. In this report we sought to delineate the determinants of sensitivity and resistance towards HYD1 induced cell death. To this end, we developed a HYD1 isogenic resistant myeloma cell line by chronically exposing H929 meyloma cells to increasing concentrations of HYD1. Our data indicate that the acquisition of resistance towards HYD1 correlates with reduced levels of the cleaved α4 integrin subunit. Consistent with reduced VLA-4 (α4β1) expression, the resistant variant showed ablated functional binding to fibronectin, VCAM-1 and the bone marrow stroma cell line HS-5. The reduction in binding of the resistant cell line to HS-5 cells translated to a compromised CAM-DR phenotype as demonstrated by increased sensitivity to melphalan and bortezomib induced cell death in the bone marrow stroma co-culture model of drug resistance. Importantly, we show that HYD1 is more potent in relapsed myeloma specimens compared to newly diagnosed patients, a finding which correlated with α4 integrin expression. Collectively, these data indicate that this novel D-amino acid peptide may represent a good candidate for pursing clinical trials in relapsed myeloma and in particular patients with high levels of α4 integrin. Moreover, our data provide further rationale for continued pre-clinical development of HYD1 and analogs of HYD1 for the treatment of multiple myeloma and potentially other tumors which home and/or metastasize to the bone.
Introduction
Multiple myeloma is a disease characterized by the homing and uncontrolled growth of malignant plasma cells within the confines of the bone marrow (1, 2). Despite the recent advances in therapy, multiple myeloma remains an incurable disease. 14,000 new cases of multiple myeloma are diagnosed each year in the United States with a five year survival rate of 37%(3). Although standard therapy will typically cause an initial response, myeloma patients ultimately develop drug resistance and become unresponsive to a variety of anti-cancer agents, a phenomenon known as multidrug resistance (MDR). Clinical observations indicate that despite divergent genetic changes typical of myeloma, current therapy is not curative in any subset of patients.
We as well as others previously reported that adhesion of myeloma and leukemia cells to components of the extracellular matrix is sufficient to cause drug resistance (4-13). We recently used a d-amino acid containing peptide (kikmviskwg) referred to as HYD1, known to block adhesion of prostate cells to extracellular matrices (14, 15) and found that in addition to blocking adhesion of multiple myeloma cells to fibronectin, that HYD1 induced caspase independent cell death in myeloma cell lines as a single agent in vitro and in vivo (16). Experimental evidence indicated that in prostate cancer cell lines that HYD1 interacts with α3 and α6 integrin (15). In order to delineate the molecular pathway of HYD1 induced caspase independent cell death, we developed an acquired isogenic HYD1 resistant H929 myeloma cell line which we refer to as H929-60 cells. In this report, we show that the acquisition of resistance towards HYD1 does not result in a phenotype that is cross-resistant to other agents used to treat myeloma, including bortezomib and melphalan. Moreover, acquisition of resistance towards HYD1 occurs at a cost in overall fitness, as the resistant variant demonstrates reduced binding to extracellular matrixes and is not resistant to melphalan or bortezomib induced cell death in the bone marrow co-culture model system. Finally, in this manuscript we show that specimens obtained from relapsed myeloma patients were significantly more sensitive to HYD1 induced cell death compared to specimens obtained from newly diagnosed patients. Collectively, our data continue to support that HYD1 is an attractive agent for treating multiple myeloma patients and may be an important strategy for the treatment of relapsed disease.
Materials and Methods
Cell Culture
NCI-H929, U226 and RPMI-8226 and HS-5 cells were obtained from the American Type Culture Collection (Rockfield, MD) Melphalan resistant 8226/LR and U266/LR6 were developed and characterized by Dr. Dalton's laboratory(7, 17, 18). Myeloma cell lines were tested for secretion of Kappa (H929) or Lambda (RPMI-8226) levels by ELISA and mycoplasm every 4 months. Resistance levels of drug selected cell lines are monitored every four months. 293FT cells were obtained from Invitrogen and grown in Iscove's DMEM (Cellgro,) supplemented with 10 % FBS. Normal bone marrow aspirate was purchased from Lonza, Inc (Allentown, NJ). Mesenchymal stroma cells (MSC) were generated by plastic adherence of the bone marrow aspirate. MSC were confirmed by CD105, Stro-1, CD29 and CD73 positivity and CD34, CD33 and CD45 negativity (data not shown). MSC were grown in MEMα/GlutaMAX™ supplemented with 10% fetal bovine serum-qualified (FBS-Q) and 1% 100x penicillin-streptomycin-glutamine (Invitrogen).
Chemical Reagents, Antibodies, and Peptides
Please refer to supplemental materials and Methods for sources of purchased materials.
Selection of a Drug-resistant Cell Line
NCI-H929 cells were exposed to increasing concentrations of HYD1 for 24 weeks. The emerging drug resistant cell line was named H929-60 and is maintained in media containing 60 ug/ml HYD1, once a week for 24 hours.
Cell Death Analysis
After treatment with HYD1, cells were washed with PBS and incubated with 2 nM TO-PRO-3 iodide for 45 minutes. The cells were analyzed for fluorescence intensity with the use of a FACSCalibur (BD Biosciences, San Jose, CA).
Measurement of Δψm
After treatment, cells were incubated for 15 minutes with 15 nM of 3,3’-dihexyloxacarbocyanine iodide (Invitrogen). Cells were washed and resuspended in PBS, and the loss of mitochondrial membrane potential was measured using FACScan.
ATP measurement
Treated and control cells were lysed in RIPA buffer (Millipore, Billerica, MD), and ATP concentrations were measured using the ENLITEN ATP bioluminescence detection kit per manufacturer's instructions (Promega, Madison, WI). ATP levels were normalized to the protein content of the lysates.
Confocal Microscopy
To assess whether (a) HYD1 bound the cell surface and (b) whether binding of HYD1 was reduced in the resistant cell line FAM-HYD1 was used to image peptide binding in the parental and resistant cell line by confocal microscopy. A 35 mm glass bottom microwell dishes (Mattek cultureware, Ashland, MA) were plated with 10 uL Cell-tak (BD biosciences) per manufacture's instructions. Media containing 1 uM Alexa Fluor 594 wheat germ agglutinin (WGA) (Invitrogen) and 20 uM Hoechst 33342 (Invitrogen) was placed back in the plates and the samples were incubated for 30 minutes. After 30 minutes, the cells were washed and treated with media containing 6.25 μg/ml FAM conjugated HYD1 for 10 minutes. Samples were immediately viewed with a Leica DMI6000 confocal microscope (Leica Microsystems, Germany). Gain, offset, and pinhole setting were identical for all samples within the treatment group.
Reverse transcriptase polymerase chain reaction (Rt-PCR)
Rt-PCR was used to determine whether the decrease in α4 integrin protein levels in the resistant cell line was due to decreased transcription. RNA was extracted from log growth cells with RNeasy columns (Qiagen, Valencia, CA) per manufacturer's instructions. First–strand cDNA synthesis was carried out with the Quantitect Probe RT-PCR kit (Qiagen, Valencia, Ca) per manufacturer's instructions. Real time PCR primers for alpha 4 integrin were obtained from Applied Biosystems (Carlsbad, Ca). The gene-expression level was normalized using the endogenous control gene GAPDH. Real-time PCR reactions were performed using ABI 7900 Sequence Detection System (Applied Biosystems).
Cell adhesion to ECM proteins and stroma
Cell adhesion assays using extracellular matrixes were performed to as previously described (19). For stromal adhesion, 10,000 HS-5 or MSC cells were incubated overnight on immunosorb 96 well plates (Nunc, Denmark). H929 and H929-60 cells were incubated with 1uM of CMFDA (Invitrogen) for 30 minutes, washed and incubated for 45 minutes to allow unbound dye to diffuse out of the cells. Labeled cells were allowed to adhere for two hours and non-adherent cells were removed with three washes in PBS. Intensity was read on a fluorescence plate reader(9).
Transfection of shRNAs
ShRNA targeting strategies were used to determine whether α4 integrin expression was causally related to HYD1 induced cell death. α4 (TRCN0000029656) and β1 (TRCN0000029645) shRNA and non-silencing clone sets were purchased from Open Biosystems, Huntsville, AL and transfected into a lentivirus using the BLOCK-IT Lentiviral Pol II miR RNAi expression system (Invitrogen). At 72 hours of infection, 1ug/mL puromycin (Invitrogen) was added to 8226 cells to allow for the selection of a stable population of cells. For the H929 cell line transient infections were used to reduce integrin expression.
Biotin-HYD1 pull-down assay
To identify HYD1 interacting proteins biotin conjugated HYD1 was used as bait as previously described (15). Briefly, membrane pellets were solubilized in AP buffer containing 0.2% NP40 and protein was quantified using BCA reagents (Pierce, Rockford, IL). Five hundred ug of Biotin-HYD1 was bound to 30 ul of UltraLink Neutra Avidin Plus beads (Pierce) for one hour in a buffer containing 0.5 mM KCl, 0.3 mM KH2PO4, 27.6 mM NaCl and 1,6 mM Na2HPO4, pH 7.4. After one hour, the beads were washed twice in AP buffer and 50 ug of membrane extracts were added to a total volume of 500 μl and incubated with beads overnight at 4 degrees. The beads were washed five times in AP buffer containing 0.2% NP40 and samples were suspended in SDS-PAGE sample buffer and bound proteins were resolved by SDS-PAGE.
Isolation of CD138 positive and negative populations derived from Multiple Myeloma specimens
To determine whether HYD1 was active in primary patient specimens 7 newly diagnosed and 7 relapsed specimens were obtained. Myeloma patients were consented through the Total Cancer Care tissue banking protocol per IRB regulations. Mononuclear cells were separated from human blood through the use of Ficoll-Paque PLUS (GE Healthcare, UK). After separation, CD 138 positive cells were sorted using 25 MS MACS Separation Columns (Miltenyi Biotec, Germany) and CD 138 microbeads (Miltenyi) per manufacturer's instructions. For each specimen obtained α4 integrin surface expression was determined by FACS analysis and HYD1 induced cell death was determined by Topro-3 staining and FACS analysis in the CD138 positive and negative fraction.
Results
H929-60 cells are resistant to HYD1 induced cell death but do not show cross-resistance to other active myeloma agents
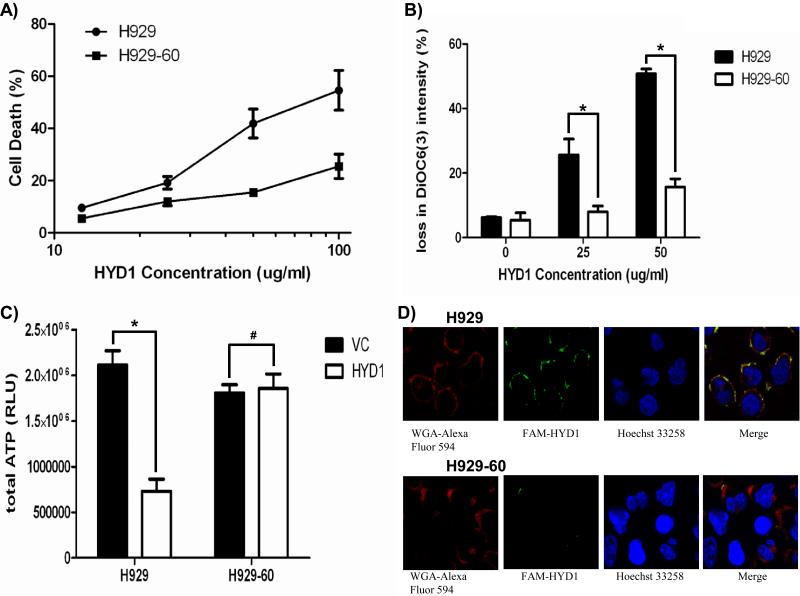
As shown in figure 1A, the H929-60 acquired resistant cell line is significantly resistant to HYD1 induced cell death (p<0.05 ANOVA) when compared to the parental H929 cells as measured by TOP-RO 3 positivity and FACS analysis. HYD1 induced cell death was previously characterized by the loss of mitochondrial membrane potential, ATP depletion, and an increase in reactive oxygen species (ROS). To determine whether the acquisition of resistance occurred upstream or downstream of mitochondria dysfunction the mitochondria membrane potential, ATP levels and ROS levels were compared following HYD1 treatment in the resistant (H929-60) and sensitive parental cell line (H929). H929-60 cells were shown to be resistant to the loss of mitochondrial membrane potential (figure 1B) and ATP levels were not depleted following HYD1 treatment (figure 1C). Finally ROS levels were reduced following HYD1 treatment in the resistant cell line compared to the parental cell line (See Supplemental figure 1). We utilized a FAM conjugated HYD1 peptide to determine whether the H929 resistant variant demonstrated a reduction in binding of FAM-HYD1 to the cell membrane As shown in figure 1D, FAM-HYD1 localizes to the plasma membrane in the parental cell line. Furthermore, the localization of FAM-HYD1 was not evenly distributed across the cell membrane, but rather FAM-HYD1 demonstrated punctuated staining in the parental cell line, suggesting potential clustering of the binding target. H929-60 cells treated with 6.25 ug/ml FAM-HYD1, demonstrated a 2.7-fold reduction in FAM-HYD1 binding relative to the parental cell line as determined by FACS analysis (see supplemental data Figure 2). Collectively, these data indicate that the mechanism causative for resistance towards HYD1 occurs upstream of mitochondrial dysfunction and generation of ROS and that the resistant mechanism is likely the result of qualitative or quantitative changes in the HYD1 binding complex located on the cell membrane.
Figure 1. H929-60 cells are resistant to HYD1 induced cell death and demonstrated reduced binding of FAM-HYD1 to the cell surface.
A). H929 and H929-60 cells were incubated with varying concentrations of HYD1 for 6 hours and HYD1 induced cell death was determined by Topro-3 staining and FACS analysis (ANOVA test p<.05 n=9). B). Loss of mitochondrial membrane permeability was determined using DiOC6 staining following 2 hours of HYD1 treatment in H929 and H929-60 (* denotes p<.05 n=9, Student's t-test). C) ATP levels were determined in the H929 and H929-60 cell lines following 6 hours of HYD1 (50 ug/ml) treatment. (* denotes p<.05 and # denotes p>0.05, n=9, Student's t-test). D) H929 and H929-60 cells were stained with Alexa Fluor 594 wheat germ agglutinin (WGA) and Hoechst 33342 for 30 minutes. 6.25 μg/ml FAM-HYD1 was added 10 minutes prior to analysis by confocal microscopy. The experiment was repeated 3 independent times and shown is a representative experiment.
We hypothesized that since HYD1 induces necrotic cell death, selection with HYD1 would not result in a phenotype which conferred resistance to other agents commonly used to treat myeloma. To address this question, we compared the IC50 values of H929-60 and the parental H929 cells to the alkylating agent, melphalan, the topoisomerase II inhibitor, mitoxantrone, and the proteasome inhibitor, bortezomib. As predicted, H929-60 cells were not resistant to other classes of agents known to induce apoptosis commonly used to treat multiple myeloma (see Table 1). These data further support the potential advantage of targeting necrosis in combination with inducers of apoptosis for the treatment of multiple myeloma, as cross-resistance between these two agents is unlikely to occur during the course of drug treatment.
Table 1. H929-60 cells are not resistant to standard myeloma therapies.
H929 and H929-60 cells were treated with increasing concentrations of melphalan or bortezomib for 24 hours. After 24 hours, cells were stained with annexin V FITC/PI for 45 minutes and acquired on FACScan. IC50 values were determined by linear regressions. Shown is the mean and standard deviations of three independent experiments.
| Compound | H929 IC50 (n=3) | H929-60 IC50 (n=3) |
|---|---|---|
| melphalan | 11.3 +/- 2.5 μM | 13.1 +/- 3.7 μM |
| mitoxantrone | 1.42 +/- .4 μM | 1.05 +/- .7 μM |
| bortezomib | 11.3 +/- 5.1 nM | 12.2 +/- 3.9 nM |
Acquisition towards resistance to HYD1 results in reduced expression of α4, β1 integrin and ablated functional binding to fibronectin and VCAM-1
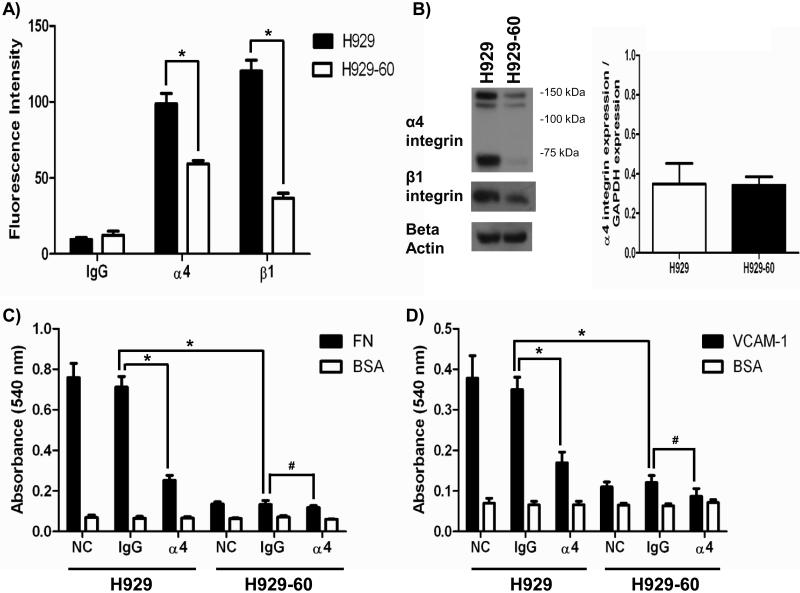
To determine whether the acquisition of resistance correlated with quantitative changes in integrin expression we screened multiple α integrin sub-units (α4, α5, αV and α6 integrin data not shown) that are commonly expressed in hematopoietic cells and determined that α4 integrin was the most abundant integrin expressed on the parental H929 cell line and expression was reduced in the resistant cell line (See Figure 2A). Using FACS analysis we determined that α4 integrin was found to be reduced by 1.8 fold. As shown in Figure 2B, the reduction at the cell surface corresponded to reduced protein expression using a whole cell whole cell lysate preparation. Interestingly, when examining the whole cell lysate the most dramatic decrease was the cleaved form of α4 integrin. In some cell types, the mature 150 kD α4 integrin is cleaved into a non-disulfide linked 80 and 70 kD fragment. For example, activation of T-cells was previously reported to correlate with increased cleavage of the mature α4 integrin(20). However, the cleavage of α4 integrin was found to not alter the adhesive properties of VLA-4 integrin to fibronectin or VCAM1(21). The attenuation of protein expression is post-transcriptionally regulated as the parental and resistant cell line demonstrated equal levels of α4 mRNA (Figure 2B). Further studies are warranted to delineate the post-transcriptional regulation of cleaved α4 integrin in the resistant cell line.
Figure 2. H929-60 cells have reduced α4 integrin protein levels and reduced adhesion to FN and VCAM-1.
A) Surface expression of α4 and β1 integrin on H929 and H929-60 cells was determined by FACS analysis (* denotes p<.05 n=9, Student's t-test). B) Whole cell lystates of H929 and H929-60 cells were probed for α4 integrin, β1 integrin and beta actin by Western blot analysis. α4 integrin mRNA levels were determined using real time rt-PCR. α4 integrin mRNA expression levels were normalized by dividing by GAPDH levels (p>.05 n=3). C-D) H929 and H929-60 cells were incubated with α4 blocking antibody or IgG control antibody for 30 minutes and subsequently adhered to (C) FN (40 μg/ml) or (D) VCAM-1 (10 μg/ml) coated plates for 2 hours. A representative of each experiment is shown (* denotes p<.05 or # denotes p>.05 Student's t-test).
We next sought to determine whether a reduction in the expression of VLA-4 integrin resulted in a functional reduction in adhesion to extracellular matrices. To this end, we compared the levels of adhesion of the sensitive and resistant cell line to fibronectin and the more specific ligand for α4 integrin, VCAM-1. In figure 2C-2D, H929-60 cells demonstrated a dramatic reduction in the binding to FN and VCAM-1. An α4 integrin blocking was used as a positive control for blocking the parental cell line to FN and VCAM-1.
Reducing the expression α4 and β1 integrins is causative for resistance to HYD1 induced cell death in H929 and 8226 multiple myeloma cells
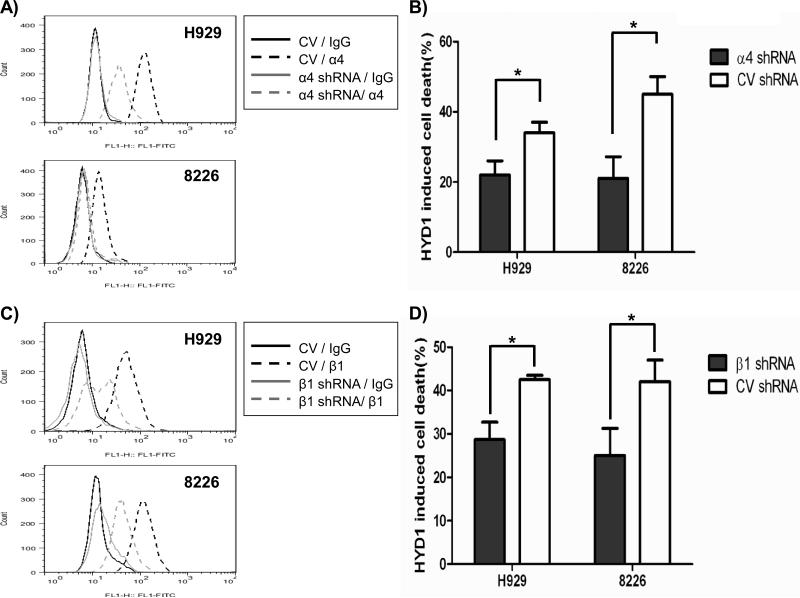
Considering H929-60 cells were resistant to HYD1 induced cell death and demonstrate reduced surface expression of the α4 integrin subunit, we next determined whether reducing the expression of α4 integrins using shRNA targeting strategies in myeloma cell lines was sufficient to induce resistance towards HYD1 induced cell death. As shown in figure 3A- and 3B, reducing the expression of α4 integrin conferred resistance to HYD1 induced cell death (p<0.05, Student's t-test) in both 8226 and H929 cells.
Figure 3. Reducing the expression of α4 and β1 integrins caused partial resistance to HYD1 induced cell death in H929 and 8226 cells.
A) and C): In H929 cells α4 and β1 integrin expression was reduced via transient infection. A: At 72 hours post infection of shRNA, α4 and β1 integrin expression was determined by FACS analysis. 8226 cells were stably infected with α4 integrin shRNA, β1 integrin shRNA or control vector shRNA. FACS analysis was used to determine expression of α4 and β1 integrin. Shown is a representative histogram from one experiment. B) and D): H929 and 8226 cells were treated with 50 μg/ml and 100ug/ml of HYD1 respectively for 6 hours. After 6 hours, cell death was analyzed by Topro-3 staining and FACS analysis. (* denotes p<.05 n=9, Student's t-test).
Since α4 integrin can heterodimerize with either β1 or β7 integrin, we tested whether reducing β1 integrin was sufficient to induce resistance to myeloma cell lines. As shown in figures 3C-D, reducing β1 integrins rendered H929 and 8226 cells resistant to HYD1 induced cell death (p<0.05, student's t-test). β1 integrin can potentially heterodimerize with 11 different α sub-units. The observation that reducing α4 or β1 integrin gave similar levels of protection indicates that the α4β1 integrin is the predominant β1 integrin partner associated with HYD1 induced cell death in myeloma cells. The observation that reducing integrin expression afforded only partial resistance may be due to (a) residual levels of α4β1 integrin remaining on the cell surface or (b) α4β1 represents only one component of the binding complex required for HYD1 induced cell death.
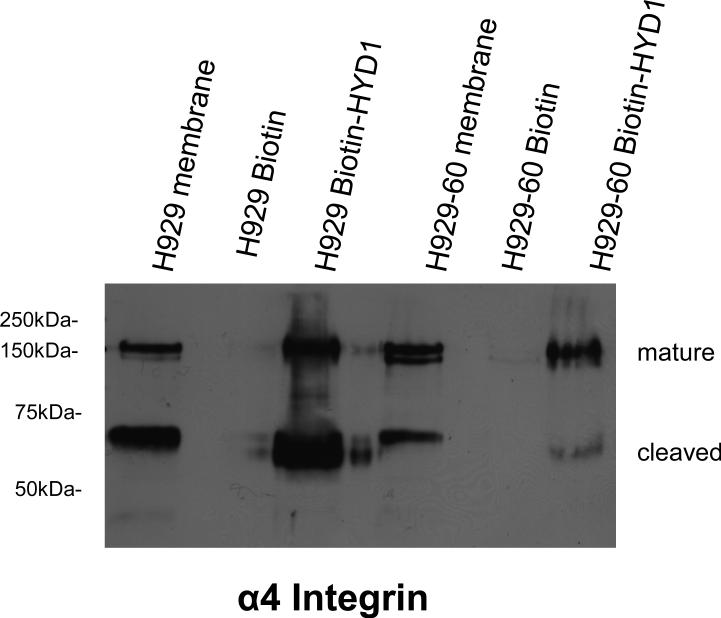
Biotin-HYD1 interacts with α4 integrin and reduced binding was observed in the acquired drug resistant cell line
Previous data indicated that biotin-HYD1 associated with α3 and α6 on prostate cancer cell lines(15). We utilized a similar strategy to determine whether biotin-HYD1 interacted with an α4 integrin containing complex and whether that interaction was attenuated in the resistant cell line. The total membrane lysate was used as a control for detection of α4 integrin. Again the reduction in the cleaved α4 integrin subunit in membrane extracts (although not as dramatic as the whole cell lysate), was most prominent in the resistant cell line compared to the mature α4 integrin subunit. Additionally, as shown in Figure 4, using biotin-HYD1 we demonstrate that the resistant cell line shows preferential decreased binding for the cleaved α4 sub-unit. Our data indicate that the cleaved form may be important with respect to functional consequences of HYD1 induced cell death. Further studies are warranted to fully understand the significance of the cleaved α4 integrin sub-unit in mediating HYD1 induced cell death and the CAM-DR phenotype.
Figure 4. Biotin-HYD1 interacts with α4 integrin and binding to cleaved α4 integrin is attenuated in the resistant H929-60 cells.
Fifty microgram of H929 and H929-60 protein obtained from membrane fractions were added to biotin or biotin-HYD1 bound NeutrAvidin beads for 18 hours as described in the methods and materials section. Biotin-HYD1 and biotin bound proteins were denatured in SDS sample buffer and α4 integrin levels were detected by western blot analysis. The experiment was repeated twice and shown is a representative experiment.
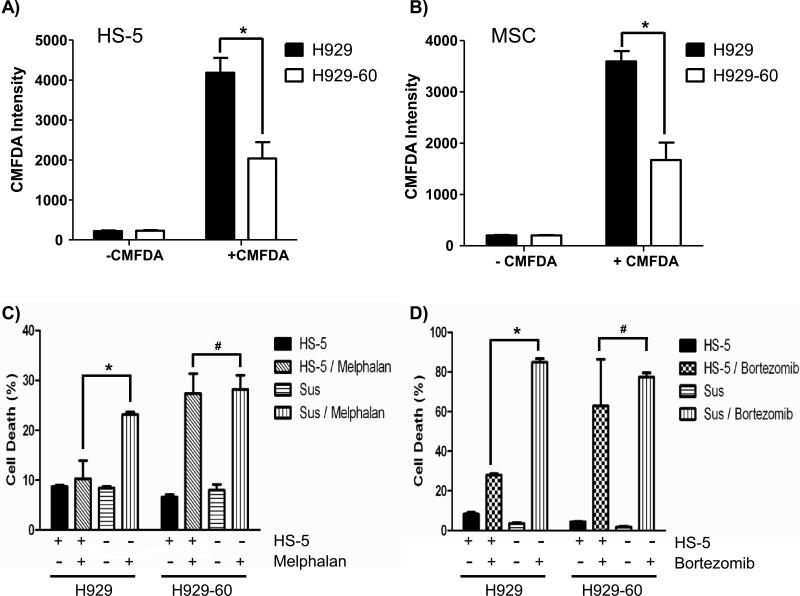
H929-60 cells displayed reduced binding to bone marrow stromal cells and demonstrate a compromised CAM-DR phenotype
In addition to attenuated adhesion to FN and VCAM-1, H929-60 cells also exhibit a reduction in adhesion to the HS-5 stromal cell line and mesenchymal stroma cells (MSC) (Figure 5A-B). We reasoned that acquisition of resistance towards HYD1, which correlated with reduced functional binding to fibronectin, VCAM-1, HS-5 stromal cells and MSC, would likely result in a compromised CAM-DR phenotype. To test this premise we utilized the HS-5 co-culture model of drug resistance. H929 and the HYD1 resistant variant H929-60 cells were treated with either melphalan or bortezomib in the presence or absence of HS-5/GFP bone marrow stromal cells. As shown in Figure 5C and 5D, the HYD1 resistant cell line was not resistant in the co-culture bone marrow stroma model to melphalan or bortezomib, respectively. There is some discrepancy in the literature whether co-culturing myeloma cells with stroma cells causes resistance to bortezomib(22). The apparent discrepancy may be the result of endpoints that measure growth compared to cell death, dose of bortezomib or the scheduling of how long myeloma cells interact with the stroma before drug exposure. Additionally, exposure to bortezomib for 24 hours was shown to downregulate α4 integrin levels and thus drug scheduling could also impact observed results (23). However, our results are consistent with recent reports showing that myeloma cells are resistant to bortezomib in MSC co-culture models. (5). Together, these data indicate that as myeloma cells are selected for resistance to HYD1 they are losing resistance to standard chemotherapy in the context of the bone marrow microenvironment due to reduced capacity to adhere to bone marrow stroma cells. Interestingly, the converse appears to be true because myeloma cell lines selected for resistance to melphalan (8226/LR5 and U266/LR6)(7, 17, 18) show increased sensitivity to HYD1 induced cell death (See Supplemental Data Table 1:). This correlated with increased α4 expression and is consistent with previous reports that selection with melphalan coincides with increased α4 integrin expression(6). The difference in sensitivity towards HYD1 or the comprised CAM-DR phenotype can not be attributed to changes in growth kinetics as the sensitive and resistant cell line demonstrate similar rates of proliferation (See Supplemental Data: Figure 3).
Figure 5. H929-60 cells display reduced binding to HS-5 and MSC and are not resistant to bortezomib or melphalan in the stroma co culture model.
A-B) H929 and H929-60 pre-incubated with CMFDA dye were adhered to 10,000 HS-5 or MSC cells for 2 hours. After 2 hours unadherent cells were removed and the fluorescence intensity was measured on a fluorescent plate reader. C-D): H929 and H929-60 cells were adhered to HS-5 GFP cells for 24 hours. After 24 hours samples were treated with 15 μM melphalan or 16 nM bortezomib for 24 hours. Cell death was analyzed using a FACScalibur with the GFP expressing HS-5 cells being excluded from the analysis. In A-C), a representative of three independent experiments was shown. (* =p<.05, n=9 or# =p>.05, n=9 Students t-test).
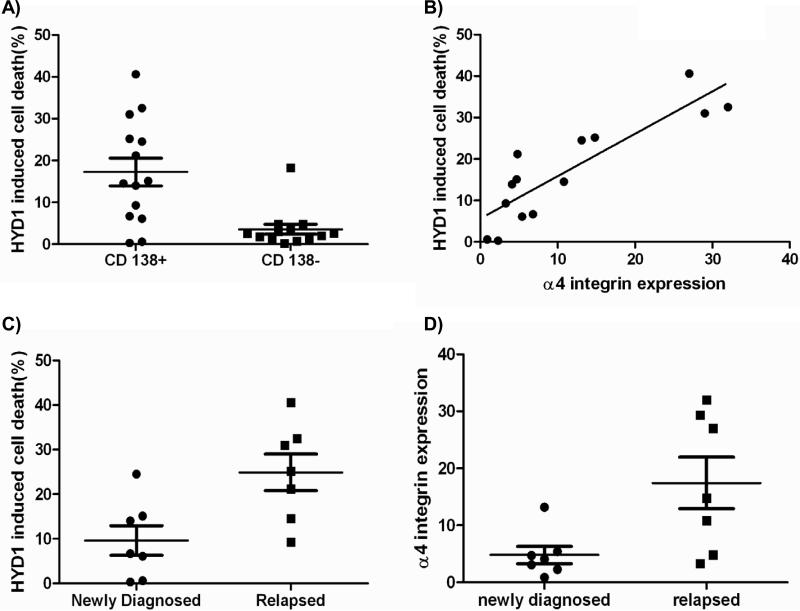
HYD1 induced cell death is increased in relapsed myeloma patient specimens compared to newly diagnosed specimens and correlates with α4 integrin expression
In order to determine whether primary myeloma specimens are sensitive to HYD1 induced cell death, we collected 7 newly diagnosed and 7 relapsed primary myeloma specimens. Immunomagnetic beads were used to enrich for CD138 positive malignant plasma cell fractions. As shown in Figure 6A, the CD138 positive tumor population was more sensitive to HYD1 induced cell death compared to the CD138 negative population. In addition to measuring cell death, we determined by FACS analysis the levels of α4 integrin expression in the CD138 positive cells. As shown in Figure 6B, HYD1 induced cell death positively correlates with α4 integrin expression. These data obtained in patient specimens correlating α4 integrin expression and sensitivity to HYD1 induced cell death is consistent with data generated in cell lines (See Table 1). Importantly, we observed that HYD1 was significantly (p<0.05, student's t-test) more active in relapsed patients compared to newly diagnosed patients (See Figure 6C). Finally as shown in Figure 6D, we show that α4 integrin levels are increased in CD138 positive cells isolated from relapsed myeloma patients compared to newly diagnosed patients (p<0.05, student's t-test). Together, these data suggest that α4 integrin expression is selected for over the course of drug treatment and may contribute to the eventual failure to therapeutically manage multiple myeloma. Additionally, patients with high levels of α4 expression may benefit from combination strategies that include targeting this specific integrin complex such as Natalizumab(24) (humanized α4 antibody) or HYD1.
Figure 6. HYD1 induced cell death in CD138+ patient samples correlates with α4 integrin expression.
A) CD138+ and CD138- cells were treated with 100 ug/ml HYD1 for 24 hours. After 24 hours cell death was measured by Topro-3 staining and FACS analysis. (p<.05, Student's t-test) B) α4 integrin expression was compared to HYD1 induced cell death using a Pearson's correlation coefficient. The CD138+ population demonstrated a significant correlation between α4 integrin expression and HYD1 induced cell death (p<.05). C-D) Specimens were separated into two groups depending on the clinical diagnosis of either; newly diagnosed or specimens obtained from patients that were considered relapsed. In C), CD138+ cells were treated with100 ug/ml HYD1 for 24 hours. After 24 hours cell death was measured by Topro-3 staining and FACS analysis (Student's t-test p<.05) In D) CD138+ cells were used to analyze for α4 integrin expression by FACS analysis. (Student's t-test p<.05)
Discussion
The development of an isogenic resistant cell line has been used by many investigators as model systems for rapidly delineating molecular determinants of response for novel as well as clinically approved agents (25-29). Selection with HYD1 resulted in a cell line with compromised adhesive interactions and failure to display a resistant phenotype in a bone marrow stroma co-culture model of drug resistance. We propose that to ensure adequate designs of trials it is essential to define biomarkers of response using patient specimens in early phases of drug development, as response markers can take time to validate and often lag behind the design of early clinical trials(30) To this end in this report, we demonstrate that α4 integrin expression positively correlated with HYD1 induced cell death. Additionally, HYD1 was more potent in specimens obtained from relapsed myeloma patients compared to newly diagnosed myeloma patients. These data suggest that HYD1 will likely be most effective in relapsed patients demonstrating high levels of α4 expression. It is attractive to speculate that agents which disrupt cell adhesion may indeed be a good strategy for comprising the overall fitness of cells in the context of the bone marrow microenvironment. Agents that disrupt cell adhesion may be critical to test the development of evolutionary based double bind strategies, where resistance to a drug is predicted to occur at the cost of fitness within the niche. The utilization of double bind strategies is a unique concept recently proposed by Gatenby and colleagues for delaying the emergence of resistant variants in the treatment of cancer and indeed HYD1 may fit the criteria for an agent to test this unique therapeutic strategy (31).
Our data indicate that reducing α4β1 integrin expression was sufficient to confer partial drug resistance. However, since only partial resistance was observed, we predict that additional components contained within the HYD1 binding complex may contribute to HYD1 induced cell death. Further studies are warranted for delineating the entire HYD1 interacting complex using unbiased approaches which is currently a focus of our laboratory. In the drug resistant variant cell line, we observed a predominant reduction in the cleavage of α4 integrin compared to the mature form. It is intriguing to speculate whether the cleaved fragment of α4 integrin is required for HYD1 induced cell death and whether the cleaved form has any significance with respect to disease progression in multiple myeloma.
Recent data demonstrate that targeting α4 integrin in a syngeneic mouse 5TGM1 model via monoclonal antibody treatment reduced the tumor burden in the bone marrow, spleen and liver(32). Moreover, the VCAM-1/VLA-4 axis increases MIP-1 alpha and beta levels and increases the ability of myeloma cells to support osteoclastogenesis(33). Based on these findings, it will be important to determine whether HYD1 inhibits the ability of myeloma cells to disrupt bone homeostasis by either inhibiting the activation of osteoclasts or disrupting the ability of myeloma cells to inhibit the differentiation of osteoblasts(34-36). Another strategy for targeting integrins is to inhibit pathways required for inside-out activation of VLA-4 integrins. Thus VLA-4 can be modulated by regulating the affinity for ligand as well as clustering or avidity of the integrin heterodimer(37, 38). A potential strategy could include targeting Rap1 which is known to be required for inside out activation of VLA-4(39). Another approach is inhibition of CXCR4, where recently, Azab et al. showed that AMD3100 inhibits adhesion of myeloma cells to stroma and sensitized myeloma cells to chemotherapy in co-culture models of drug resistance(5). However, HYD1 is unique to our knowledge, as in addition to blocking cell adhesion, HYD1 induces necrotic cell death directly on the myeloma cell, a finding that was not observed with α4 blocking antibody or RGD containing peptides (data not shown) Historically, drug development has focused on aberrations in signaling intrinsic to the tumor cells using unicellular models. However, we and others have argued that tumors evolve in the context of the microenvironment, and thus it is feasible that some phenotypes observed in tumors such as drug resistance and metastasis will only be expressed in the context of cues derived from the microenvironment (22, 40-42).
Supplementary Material
Acknowledgements
This work was supported by National Cancer Institute R01CA122065 (LAH) and by the Multiple Myeloma Research Foundation (LAH)
References
- 1.Anderson KC, Kyle RA, Dalton WS, Landowski T, Shain K, Jove R, et al. Multiple Myeloma: New Insights and Therapeutic Approaches. Hematology (Am Soc Hematol Educ Program) 2000:147–65. doi: 10.1182/asheducation-2000.1.147. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–73. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics. CA Cancer J Clin. 2010 doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Aoudjit F, Vuori K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene. 2001;20:4995–5004. doi: 10.1038/sj.onc.1204554. [DOI] [PubMed] [Google Scholar]
- 5.Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X, et al. The CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009 doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93:1658–67. [PMC free article] [PubMed] [Google Scholar]
- 7.Hazlehurst LA, Enkemann SA, Beam CA, Argilagos RF, Painter J, Shain KH, et al. Genotypic and phenotypic comparisons of de novo and acquired melphalan resistance in an isogenic multiple myeloma cell line model. Cancer Res. 2003;63:7900–6. [PubMed] [Google Scholar]
- 8.Mori Y, Shimizu N, Dallas M, Niewolna M, Story B, Williams PJ, et al. Anti-alpha4 integrin antibody suppresses the development of multiple myeloma and associated osteoclastic osteolysis. Blood. 2004;104:2149. doi: 10.1182/blood-2004-01-0236. [DOI] [PubMed] [Google Scholar]
- 9.Nefedova Y, Landowski TH, Dalton WS. Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia. 2003;17:1175–82. doi: 10.1038/sj.leu.2402924. [DOI] [PubMed] [Google Scholar]
- 10.Nefedova Y, Sullivan DM, Bolick SC, Dalton WS, Gabrilovich DI. Inhibition of Notch signaling induces apoptosis of myeloma cells and enhances sensitivity to chemotherapy. Blood. 2008;111:2220–9. doi: 10.1182/blood-2007-07-102632. [DOI] [PubMed] [Google Scholar]
- 11.Sethi T, Rintoul RC, Moore SM, MacKinnon AC, Salter D, Choo C, et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med. 1999;5:662–8. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 12.Morin PJ. Drug resistance and the microenvironment: nature and nurture. Drug Resist Updat. 2003;6:169–72. doi: 10.1016/s1368-7646(03)00059-1. [DOI] [PubMed] [Google Scholar]
- 13.Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, Fujimi A, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med. 2003;9:1158–65. doi: 10.1038/nm909. [DOI] [PubMed] [Google Scholar]
- 14.DeRoock IB, Pennington ME, Sroka TC, Lam KS, Bowden GT, Bair EL, et al. Synthetic peptides inhibit adhesion of human tumor cells to extracellular matrix proteins. Cancer Res. 2001;61:3308–13. [PubMed] [Google Scholar]
- 15.Sroka TC, Pennington ME, Cress AE. Synthetic D-amino acid peptide inhibits tumor cell motility on laminin-5. Carcinogenesis. 2006 doi: 10.1093/carcin/bgl005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nair RR, Emmons MF, Cress AE, Argilagos RF, Lam K, Kerr WT, et al. HYD1-induced increase in reactive oxygen species leads to autophagy and necrotic cell death in multiple myeloma cells. Mol Cancer Ther. 2009;8:2441–51. doi: 10.1158/1535-7163.MCT-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellamy WT, Dalton WS, Gleason MC, Grogan TM, Trent JM. Development and characterization of a melphalan-resistant human multiple myeloma cell line. Cancer Res. 1991;51:995–1002. [PubMed] [Google Scholar]
- 18.Chen Q, Van der Sluis PC, Boulware D, Hazlehurst LA, Dalton WS. The FA/BRCA pathway is involved in melphalan-induced DNA interstrand cross-link repair and accounts for melphalan resistance in multiple myeloma cells. Blood. 2005;106:698–705. doi: 10.1182/blood-2004-11-4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hazlehurst LA, Valkov N, Wisner L, Storey JA, Boulware D, Sullivan DM, et al. Reduction in drug-induced DNA double-strand breaks associated with beta1 integrin-mediated adhesion correlates with drug resistance in U937 cells. Blood. 2001;98:1897–903. doi: 10.1182/blood.v98.6.1897. [DOI] [PubMed] [Google Scholar]
- 20.Bednarczyk JL, Szabo MC, McIntyre BW. Post-translational processing of the leukocyte integrin alpha 4 beta 1. J Biol Chem. 1992;267:25274–81. [PubMed] [Google Scholar]
- 21.Teixido J, Parker CM, Kassner PD, Hemler ME. Functional and structural analysis of VLA-4 integrin alpha 4 subunit cleavage. J Biol Chem. 1992;267:1786–91. [PubMed] [Google Scholar]
- 22.McMillin DW, Delmore J, Weisberg E, Negri JM, Geer DC, Klippel S, et al. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat Med. 2009;16:483–9. doi: 10.1038/nm.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noborio-Hatano K, Kikuchi J, Takatoku M, Shimizu R, Wada T, Ueda M, et al. Bortezomib overcomes cell-adhesion-mediated drug resistance through downregulation of VLA-4 expression in multiple myeloma. Oncogene. 2009;28:231–42. doi: 10.1038/onc.2008.385. [DOI] [PubMed] [Google Scholar]
- 24.Baker DE. Natalizumab: overview of its pharmacology and safety. Rev Gastroenterol Disord. 2007;7:38–46. [PubMed] [Google Scholar]
- 25.Gutman D, Morales AA, Boise LH. Acquisition of a multidrug-resistant phenotype with a proteasome inhibitor in multiple myeloma. Leukemia. 2009;23:2181–3. doi: 10.1038/leu.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banerjee D, Mayer-Kuckuk P, Capiaux G, Budak-Alpdogan T, Gorlick R, Bertino JR. Novel aspects of resistance to drugs targeted to dihydrofolate reductase and thymidylate synthase. Biochim Biophys Acta. 2002;1587:164–73. doi: 10.1016/s0925-4439(02)00079-0. [DOI] [PubMed] [Google Scholar]
- 27.Mahon FX, Deininger MW, Schultheis B, Chabrol J, Reiffers J, Goldman JM, et al. Selection and characterization of BCR-ABL positive cell lines with differential sensitivity to the tyrosine kinase inhibitor STI571: diverse mechanisms of resistance. Blood. 2000;96:1070–9. [PubMed] [Google Scholar]
- 28.Morgan SE, Kim R, Wang PC, Bhat UG, Kusumoto H, Lu T, et al. Differences in mutant p53 protein stability and functional activity in teniposide-sensitive and -resistant human leukemic CEM cells. Oncogene. 2000;19:5010–9. doi: 10.1038/sj.onc.1203865. [DOI] [PubMed] [Google Scholar]
- 29.Robey RW, Polgar O, Deeken J, To KW, Bates SE. ABCG2: determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007;26:39–57. doi: 10.1007/s10555-007-9042-6. [DOI] [PubMed] [Google Scholar]
- 30.Khleif SN, Doroshow JH, Hait WN. AACR-FDA-NCI Cancer Biomarkers Collaborative consensus report: advancing the use of biomarkers in cancer drug development. Clin Cancer Res. 16:3299–318. doi: 10.1158/1078-0432.CCR-10-0880. [DOI] [PubMed] [Google Scholar]
- 31.Gatenby RA, Silva AS, Gillies RJ, Frieden BR. Adaptive therapy. Cancer Res. 2009;69:4894–903. doi: 10.1158/0008-5472.CAN-08-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olson DL, Burkly LC, Leone DR, Dolinski BM, Lobb RR. Anti-alpha4 integrin monoclonal antibody inhibits multiple myeloma growth in a murine model. Mol Cancer Ther. 2005;4:91–9. [PubMed] [Google Scholar]
- 33.Abe M, Hiura K, Ozaki S, Kido S, Matsumoto T. Vicious cycle between myeloma cell binding to bone marrow stromal cells via VLA-4-VCAM-1 adhesion and macrophage inflammatory protein-1alpha and MIP-1beta production. J Bone Miner Metab. 2009;27:16–23. doi: 10.1007/s00774-008-0012-z. [DOI] [PubMed] [Google Scholar]
- 34.Epstein J, Yaccoby S. Consequences of interactions between the bone marrow stroma and myeloma. Hematol J. 2003;4:310–4. doi: 10.1038/sj.thj.6200313. [DOI] [PubMed] [Google Scholar]
- 35.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–94. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 36.Yaccoby S, Wezeman MJ, Henderson A, Cottler-Fox M, Yi Q, Barlogie B, et al. Cancer and the microenvironment: myeloma-osteoclast interactions as a model. Cancer Res. 2004;64:2016–23. doi: 10.1158/0008-5472.can-03-1131. [DOI] [PubMed] [Google Scholar]
- 37.Abram CL, Lowell CA. The ins and outs of leukocyte integrin signaling. Annu Rev Immunol. 2009;27:339–62. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–47. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Bruyn KM, Rangarajan S, Reedquist KA, Figdor CG, Bos JL. The small GTPase Rap1 is required for Mn(2+)- and antibody-induced LFA-1- and VLA-4-mediated cell adhesion. J Biol Chem. 2002;277:29468–76. doi: 10.1074/jbc.M204990200. [DOI] [PubMed] [Google Scholar]
- 40.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hazlehurst LA, Landowski TH, Dalton WS. Role of the tumor microenvironment in mediating de novo resistance to drugs and physiological mediators of cell death. Oncogene. 2003;22:7396–402. doi: 10.1038/sj.onc.1206943. [DOI] [PubMed] [Google Scholar]
- 42.Nair RR, Tolentino J, Hazlehurst LA. The bone marrow microenvironment as a sanctuary for minimal residual disease in CML. Biochem Pharmacol. 80:602–12. doi: 10.1016/j.bcp.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.