Abstract
T cell:antigen presenting cell (APC) contact initiates T cell activation and is maintained by the integrin LFA-1. Talin1, a LFA-1 regulator, localizes to the immune synapse with unknown roles in T cell activation. Here, we show that talin1-deficient T cells have defects in contact-dependent T cell stopping and proliferation. While talin1-deficient T cells did not form stable interactions with APCs, transient contacts were sufficient to induce signaling. In contrast to prior models, LFA-1 polarized to T cell:APC contacts in talin1-deficient T cells but vinculin and F-actin polarization at the immune synapse was impaired. These results indicate that T cell proliferation requires sustained, talin1-mediated T cell:APC interactions and that talin1 is necessary for F-actin polarization and the stability of immune synapses.
Introduction
Naïve T cells migrate within lymph nodes and scan antigen presenting cells (APCs) for specific protein-major histocompatibility complex (MHC) combinations. Once found, triggering of the T cell Receptor (TCR) by MHC-presented cognate antigen activates intracellular signaling pathways ultimately leading to T cell proliferation and cytokine production. At a molecular level, TCR triggering contributes to the formation of the immune synapse (IS), which is comprised of TCR signaling microclusters, adhesive molecules such as the integrin LFA-1, and polarized F-actin(1). The interaction between T cells and APCs is a central event in the activation of T cells; however, the length of interactions between T cells and APCs required to induce T cell activation remains controversial. For instance, some in vitro studies suggest that long-lived interactions from 6–24 hours are required to induce full CD4+ T cell proliferation(2–5), whereas other studies show that transient interactions are sufficient to induce T cell activation(6, 7). In vivo experiments investigating T cell:APC interactions are also divided, indicating that the type of activating condition influences the stability of the interaction. Tolerizing conditions seem to promote transient interactions, whereas priming conditions seem to favor stable longer-lasting interactions with contacts maintained for hours during at least one phase of activation(8, 9).
The T cell integrin, LFA-1 (αLβ2), is required to maintain T cell adhesion to APCs expressing ICAM-1. CD4+ T cells lacking LFA-1 fail to stably conjugate with APCs(10), and CD8+ T cells fail to form stable interactions with ICAM-1-deficient dendritic cells(11). However, the relative importance of these stable interactions in terms of immune response generation differs. For instance, CD4+ T cells from LFA-1 knockout mice fail to proliferate normally in response to antigen(12) whereas CD8+ T cells are able to proliferate following ICAM-1-deficient DC stimulation but fail to develop memory responses(11).
LFA-1 is regulated both by affinity and avidity (the degree of clustering) and localizes to the immune synapse in T cell:APC conjugates(13). Following TCR stimulation, phosphorylation of the proximal scaffolding proteins LAT (linker of activated T cells) and SLP-76 (SH2 domain containing leukocyte protein of 76kD) contribute to the formation of signaling complexes that lead to Rap (a Ras-related small GTPase) activation and F-actin polarization, both of which contribute to integrin activation(14). A number of positive regulators of LFA-1 activation have been identified including talin, RapL, ADAP, SKAP55 and MST1(15). RapL and talin are thought to contribute to full T cell integrin activation through direct binding of the αL and β2 subunits, respectively. Moreover, Kindlin-III has recently been shown to modulate LFA-1 activation(16). The relative importance of these integrin-binding proteins in T cell activation remains unexplored.
While the cytoskeletal linker talin was among the first identified immune synapse components(17), its exact role in T cell biology is unclear. Talin is composed of an N terminal FERM (4.1, ezrin, radixin, moesin) domain which can regulate integrin affinity, a C terminal rod domain that contains a large number of vinculin binding sites, and a C terminal IL/WEQ domain which binds actin(18). In addition to regulating β2 integrins(15), talin can also regulate the activity of β1 and β3 integrins(19). Previous work has shown that talin is required for T cell:APC interactions through the regulation of both LFA-1 clustering and affinity(20),(21). While talin is a known component of the immune synapse and is required for T cell:APC interactions, prior studies relied on Jurkat T cell lymphoma lines and superantigen-mediated conjugation which do not allow for studies of T cell activation and proliferation. Additionally, these systems may not provide accurate models of T cell activation, because Jurkat signaling downstream of the TCR is distinctly different from primary T cells(22) and superantigen-mediated conjugation bypasses proximal signaling(23).
In addition to LFA-1, formation of T cell:APC conjugates requires the polarization of the actin cytoskeleton and its stabilization at the immune synapse(24). Actin polarization at the immune synapse is dependent on WAVE2 (WASP-family verprolin-homolgous protein 2) regulation of Arp2/3 actin nucleating complex(25) and once polarized, actin filaments are stabilized by HS-1 (hematopoetic cell specific protein 1)(26). Previous studies, using Jurkat superantigen-mediated conjugates, have shown that actin polarization precedes LFA-1 clustering(20, 21). It has been proposed that following TCR engagement, WAVE2 dependent actin polymerization contributes to vinculin- and talin- mediated integrin clustering and activation through the formation of a WAVE2-Arp2/3-vinculin complex(21). Notably, models proposing that actin polarization precedes LFA-1 polarization in T cells contrasts to findings from talin1-deficient natural killer cells, which suggest that talin1 is required for normal actin polarization at cell:cell contact sites(27).
To better understand how talin regulates LFA-1 activity, T cell:APC interactions and CD4+ T cell activation, we used conditional talin1 knockout mice to specifically delete talin1 in T cells. We found that while talin1-deficient T cells proliferated normally in response to TCR triggering by antibody, there was severely impaired contact-dependent proliferation. Using live imaging, we found that talin1-deficient cells did not form stable contacts with APCs but formed transient interactions, lasting less than 5 minutes, which were sufficient to initiate T cell signaling. Additionally, transiently interacting T cells were capable of clustering LFA-1 at the immune synapse, but despite the presence of the actin polymerizing machinery, they failed to polarize vinculin or stable F-actin to the immune synapse. Together, these findings suggest that talin1 is required for stable T cell:APC interactions, T cell proliferation, and F-actin polarization to the immune synapse.
Material and Methods
Mice
Talin1Loxp/Loxp mice were generated as previously described(28). Mice were fully backcrossed for 6 generations and crossed with mice expressing the OTII TCR transgene that recognizes OVA peptide 223–230 (Jackson Labs, Bar Harbor, ME) and CD4-Cre mice (Taconic, Hudson, NY). To obtain cells for retroviral transduction, Talin1Loxp/Loxp:OTII mice were crossed with Rosa26-CreERT2 mice(29). Genotyping was done as previously described(28). All experiments used Talin1loxp/loxp:CD4-Cre or Talin1loxp/loxp:CD4-Cre:OTII mice for talin1-deficient T cells and Talin1+/+:CD4-Cre or Talin1+/+:CD4-Cre:OTII mice for control T cells. The Institutional Animal Care and Use Committee at the University of Wisconsin approved all experimental protocols involving the use of mice.
Reagents
Antibodies for flow cytometry
PE anti-CD4, APC anti-CD4, PE anti-CD3, FITC anti-CD8, PE anti-CD62L, FITC anti-CD44, APC anti-CD69, FITC anti-CD25, PE-anti FoxP3, APC anti-CD25, PE anti-β2, PE anti-β1, FITC anti-α4, PE anti-αL, PE IFN-γ, FITC IL-2, PE IL-10, and FITC IL-4 were all from eBioscience (San Diego, CA).
Antibodies for immunoblotting/immunofluorescence
Talin (8d4), vinculin and actin were from Sigma (St. Louis, MO). β-tubulin, Zap-70, p-ZAP 70, AKT, p-AKT, WAVE2 were from Cell Signaling Technology (Danvers, MA). Nore1 was from Abcam (Cambridge, MA). p-ERK, p-JNK, and vinculin were from Santa Cruz Biotechnology (Santa Cruz, CA). p-p38, p38, ERK, JNK were from Invitrogen (Carlsbad, CA). P24/Arc (Arp2/3) was from Millpore. RIAM antibody was previously described (30) .
Cell Culture
Single cell suspensions were made from spleen of control and knockout mice between 6 and 8 weeks of age and cells were expanded for 7–10 days with OVA223–230 (Anaspec, Freemont, CA) and IL-2 (Chiron, Emeryville, CA). These OVA peptide expanded cells were used on days 7–11 for experiments. Alternatively, CD4+ T cells were isolated from cell suspension by negative selection and automacs sorting (Miltenyi, Auburn, CA). Isolated CD4+ cells were resuspended in RPMI complete supplemented with IL-2 and stimulated on plates coated with 1 µg/ml anti-CD3 (2C11) (Biolegend, San Diego, CA) and 1 µg/ml anti-CD28 (eBioscience) in RPMI supplemented with IL-2 (Chiron). These plate-activated cells were used on days 7 to 11 following isolation for in vitro assays. The LB27.4 B cell line was purchased from ATCC (Manassas, VA) and maintained in RPMI complete media.
Retroviral transduction
Phoenix viral packaging cells were transiently transfected with DNA for mCherry vector alone, GFP-PH-AKT, or UtrCH-GFP. Viral supernatants were harvested and used to infect OVA peptide expanded cells from Talin1loxp/loxp: ER-ROSA26-Cre mice on days 3–5 following isolation. On day 7, fluorescent protein expressing cells were obtained by fluorescence activated cell sorting (FACS). Cells were restimulated with irradiated splenocytes and OVA peptide weekly and treated with 250 nM (Z)-4-hydroxytamoxifen (Sigma) or ethanol control 5 days prior to use.
Characterization of leukocyte subsets and tissue distribution
Single cell suspensions were made from thymus, blood, 2 inguinal and cervical lymph nodes, and spleen from Talin1+/+:CD4-Cre and Talin1Loxp/loxp:CD4-Cre mice. Cells were counted using trypan blue exclusion, stained with antibodies as described and analyzed by flow cytometry to determine total cell and subset numbers.
Homing experiments
Naïve splenocytes from control and Talin1Loxp/Loxp:CD4-Cre mice were stained with 2.5 µM CFSE (Invitrogen) and 2.5 µM PKH-26 (Sigma), according to manufacturer’s instructions. Six million control and talin1-deficient cells were mixed together and injected intravenously into recipient mice (note: dyes were switched to control for dye affects within each experiment). One hour after injection, mice were sacrificed and cervical and inguinal lymph nodes and spleen were removed and stained with antibodies to CD4. Cells were analyzed on a FACS calibur (BD Bioscience, San Jose, CA) and the ratio of CD4+ talin1-deficient to control cells determined.
In vitro proliferation
In vitro proliferation assay was performed essentially as described (31). OVA peptide expanded T cells were stained with 0.25 µM CFSE (Invitrogen) according to manufacturer’s directions and left unstimulated or stimulated with one anti-CD3/CD28 coated beads (Invitrogen) per cell or 5 ng/ml PMA and 0.5 µg/ml ionomycin (Sigma). Additionally, CD4+ T cells were stimulated with irradiated splenocytes (3000 gy) loaded with 0, 0.01, 0.1 or 1 µg/ml OVA peptide. For LFA-1 blocking experiments, IgG control antibody or 5 ug/ml LFA-1 blocking antibody clone M17/4 (eBioscience) was added. 72 hours following activation, cells were stained with anti-CD4 and CFSE dye dilution in CD4+ T cells analyzed using a FACS Caliber (BD Bioscience). The proliferative index was determined using ModFit 3.2.1 (Verity, Topsham, ME) analysis program.
In vivo proliferation was performed essentially as described(12). Briefly, CD4+ cells from wild type and knockout mice were isolated by CD4+ negative selection and stained with 2.5 µM CFSE (Invitrogen). Five million cells were injected intravenously into age/sex matched recipient mice. 18 hour later, 25 µg LPS (Sigma) or 25 µg LPS and 50 µg ovalbumin (Sigma) was injected intraperitoneally. 72 hours later, mice were sacrificed and splenocytes isolated and stained for CD4. The degree of CFSE dye dilution was determined for CD4+ T cells on a FACS Caliber (BD Bioscience).
Th1/Th2 cytokine production
Th1/Th2 cytokine profiling was done as previously described(31). Briefly, OVA peptide expanded T cells were restimulated on days 7 to 10 following isolation on 24 well plates coated with 1 µg/ml anti-CD3 (2C11) (Biolegend) along with 2 µg/ml soluble anti-CD28 (eBioscience) in the presence of Brefeldin A (eBioscience). Four hours following restimulation, cells were stained with anti-CD4 and fixed with 4% paraformaldehyde (Electron Microscopsy Services, Hatfield PA). Intracellular staining was performed. The percentage of CD4+ T cells producing cytokines was determined by flow cytometry using a FACS Calibur (BD Bioscience) and analyzed using FlowJo (Tree Star, Ashland, OR).
Immunoblotting
For immunoblotting following activation, 60×106 OVA peptide expanded control and talin1-deficient cells were resuspended in PBS (Mediatech, Manassas, VA) and coated on ice for 10 minutes with biotinylated anti-CD3 (eBioscience) prior to crosslinking with streptavidin (Jackson Labs) and incubation at 37°C for the indicated times. Alternatively, cells were left unstimulated. Cells were lysed in 50 mM Tris pH 7.6, 0.15 M NaCl, 0.1% SDS, 0.5% DOC, 1% NP-40 containing 0.2 mM PMSF, 1 µg/ml pepstatin, 2 µg/ml apoprotinin, 1 µg/ml apoprotinin, 1 µg/ml leupeptin and 1 mM sodium orthvanadate on ice and cleared by centrifugation. Protein concentration was determined by bicinchoninic acid protein assay kit (ThermoScientific, Waltham, MA), and equal concentrations of protein were added to SDS sample buffer, boiled and run on a 10% acrylamide gel. Proteins were transferred to a nitrocellulose membrane and stained. Blots were imaged with an Odyssey infrared imaging system (Licor Biotechnologies, Lincoln, NE).
Calcium Flux
Performed on OVA peptide expanded cells as previously described(31).
Adhesion assays
Adhesion assays were performed as previously described(31).
T Cell: APC conjugation assays
T cell conjugation to LB27.4 B cells was performed as described previously(31).
Live imaging
Live imaging of T cell:APC interactions was done using methods adapted from previous studies(32). LB27.4 antigen presenting cells were loaded with 2.5 µg/ml OVA peptide for 30 minutes prior to adhesion to the bottom of a poly-l-lysine (Sigma) coated glass bottom plate (in some cases LB27.4 antigen presenting cells were labeled with 2.5 µM PKH-26 (sigma)). Meanwhile, OVA peptide expanded T cells resuspended in Hanks Buffered Salt Solution (Mediatech), supplemented with 1 mM HEPES (Mediatech), 10% FBS (HyClone, Waltham, MA) and 0.25% low melt agarose (Fisher, Waltham, MA), were plated on APC and overlayed with 1 ml of mineral oil (Sigma). Cells were maintained at 37°C for the duration of acquisition. Confocal images of mCherry, GFP-PH-AKT and Lifeact-mRUBY and UtrCH-GFP localization were obtained using a laser scanning confocal microscope (Olympus, Center Valley, PA) with a 60X Plan Apo/1.45 oil immersion objective with 3 Z slice 1.8 µm apart and 1 image every 30 seconds. Conjugation of non-transduced OVA peptide expanded T cells was measured using an epifluorescent microscope (Nikon, Melville, NY) and a Coolsnap ES2 camera (Photometrics, Tuscon, AZ). One bright field image and fluorescent image was acquired every 45 seconds for 45 minutes of total imaging. Images were acquired using MetaMorph Imaging software (MDS Analytical Technologies, Downingtown, PA). Duration of conjugation was calculated as the time from initial T cell:APC contact to the T cell leaving APC.
Immunofluorescence
LB27.4 B cells were stained with 1 µM CMAC (Invitrogen) according to manufacturer’s directions and pulsed with 2.5 µg/ml OVA peptide for 30 minutes at 37°C. Equal numbers of T cells and B cells in RPMI were combined, centrifuged and incubated at 37°C for 30 minutes prior to resuspension in PBS and pulse vortexing. Cells were allowed to adhere to poly-L-lysine (Sigma) coated coverslips for 5 minutes prior to fixing with 4% paraformaldehyde (Electron Microscopsy Services) for 15 minutes. Cells were permeabilized with 0.1% Triton X-100 and blocked in goat serum. Cells were stained with primary antibodies as indicated along with FITC/TRITC conjugated anti-rat or FITC conjugated anti-rabbit secondary antibodies (Jackson Labs). Images were acquired on a laser scanning confocal microscope (Olympus) using a 60X Plan Apo/1.45 oil immersion objective with a 1X or 10x zoom factor and captured into Fluoview software (FV10-ASW version 01.07; Olympus).
Statistical Analyses
Statistical analyses were performed using Prism 4 software (GraphPad Software Inc, La Jolla, CA). Two tailed paired T-test for single comparisons or one-way analysis of variance (ANOVA) for multiple comparisons followed by Tukey post-test on continuous variable data, which was normally distributed and had equal variance.
Results
Talin1Loxp/Loxp:CD4-Cre mice develop CD4+ T cells that have impaired lymph node homing and trafficking
Talin1 is the only talin isoform expressed in T cells(33), and germline deletion of talin1 is embryonic lethal(34). To eliminate talin specifically from T cells, a Cre recombinase-mediated conditional knockout system was used to excise the talin1 gene from T cell genomic DNA. Talin1 was deleted during the double positive stage (CD4+, CD8+) of development by expression of Cre recombinase under control of the CD4 promoter (CD4-Cre). T cells from Talin1Loxp/Loxp:CD4-Cre mice have a 97% reduction in talin1 expression compared to control (Figure 1a). As controls, we used non-floxed littermates that express CD4-Cre. While fibroblasts from talin1 knockout mice showed upregulation of the closely related talin2(35), talin2 expression was not observed in control or talin1-deficient T cells (Supplemental Figure 1a).
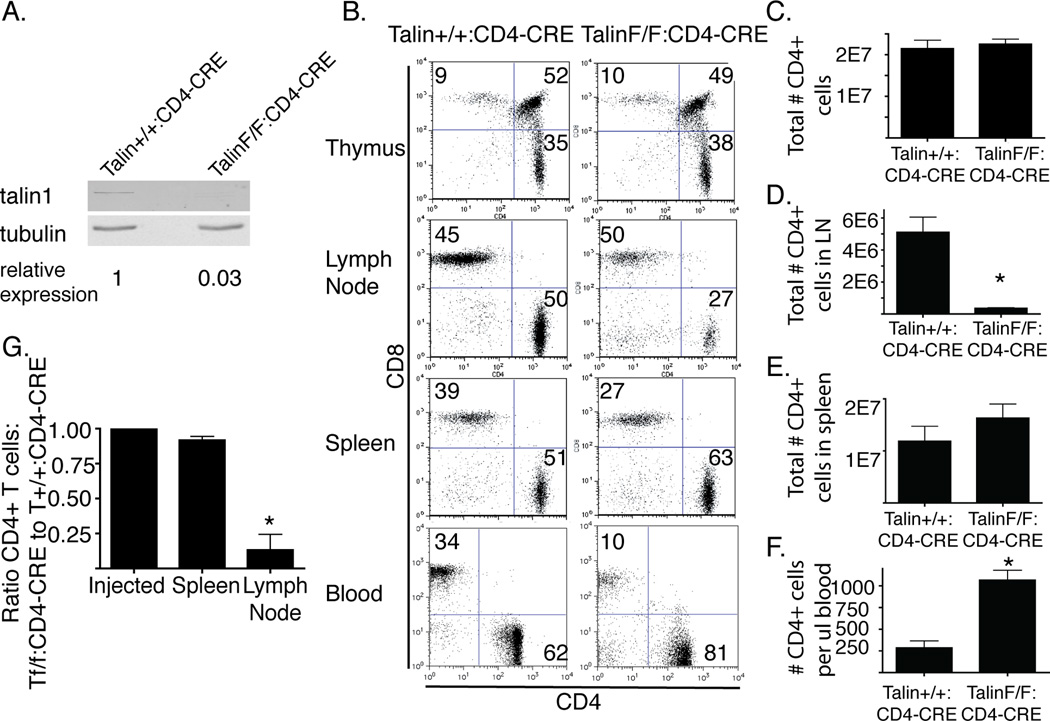
Figure 1. Talin1Loxp/Loxp:CD4-Cre mice develop CD4+ T cells that have impaired lymph node homing.
a) Representative immunoblot indicating loss of talin1 in OVA peptide expanded FACS sorted CD4+ T cells from Talin1Loxp/Loxp:CD4-Cre or control mice b) Single cell suspensions from the thymus, lymph nodes, spleen and blood were stained with CD3, CD4, and CD8 antibodies. Plots show CD3+ subset. c) The total # of CD3+/CD4+ cells in lymph node and spleen d) # CD3+/CD4+ cells in 2 cervical and inguinal lymph nodes e) # of CD3+/CD4+ cells in spleen. f) # CD3+/CD4+ cells per µl blood. Bar graph represents mean +/− standard error from 3 independent experiments using 6 mice. *=p<0.05 g) PKH26 and CFSE labeled splenocytes from Talin+/+:CD4-Cre and Talin1Loxp/Loxp:CD4-Cre mice were transferred into recipient mice in equal proportion. One hour after injection, the ratio of CD4+ talin-deficient to control cells was determined by flow cytometry. Data represent mean +/− standard error. Data are from two independent experiments using 10 mice total.
T cells in Talin1Loxp/Loxp:CD4-Cre mice developed in the thymus, and we observed similar proportions of CD4 and CD8 single and double positive cells in the knockout relative to the wild type control mice (Figure 1b). While the total number of CD4+ cells was relatively similar between wild type and Talin1Loxp/Loxp:CD4-Cre mice, we found that Talin1Loxp/Loxp:CD4-Cre mice had significantly fewer CD4+ T cells in peripheral lymph nodes and an increased number of CD4+ T cells in the blood and in the spleen (Figure 1 c–f). We also found that 1 hour after injection into recipient mice, talin1-deficient CD4+ cells failed to traffic to lymph nodes (Figure 1g), similar to findings in LFA-1 knockout mice(12, 36). Additionally, Talin1Loxp/Loxp:CD4-Cre mice had a severe impairment in regulatory T cell development, with few CD4+Foxp3+CD25+ cells found in the spleen of mice (Supplemental Figure 1b), indicating that both talin1 and β2 integrin are necessary for the normal development of regulatory T cells(37).
The few CD4+ cells that were found in the lymph nodes of Talin1Loxp/Loxp:CD4-Cre mice tended to have a more “activated” phenotype, with increased CD25 and CD69 expression and an increased percentage of memory cells with more CD44hi CD62Llow cells, although the total number was similar to control mice (Supplemental Figure 1 c, d). Talin1Loxp/Loxp:CD4-Cre mice in contrast to littermate controls developed prolapsed rectums at 8–10 weeks of age that may have been exacerbated by a helicobacter infection. For the current study, cells were isolated from Talin1Loxp/Loxp:CD4-Cre and littermate Talin1+/+:CD4-Cre controls between 6 and 8 weeks of age, prior to onset of rectal prolapse. Because of the impaired T cell trafficking and the increased percentage of activated T cells in the lymph nodes of Talin1Loxp/Loxp:CD4-Cre mice, most of the studies were done on OVA peptide expanded splenic T cells. Due to potential developmental differences between Talin1Loxp/Loxp:CD4-Cre and Talin1+/+:CD4-Cre mice, key findings were repeated in T cells from Talin1Loxp/Loxp: Rosa26-CreERT2 mice that were treated in vitro with 4-OH-tamoxifen to deplete talin1 (Supplemental Figure 2).
Talin1 is required for contact dependent CD4+ T cell proliferation
Previous work showed that the integrin, LFA-1, is required for CD4+ T cell proliferation in response to antigen-loaded APCs(12), and we hypothesized that talin would also be required. To test the effect of talin on antigen-induced T cell proliferation, we crossed Talin1Loxp/Loxp:CD4-Cre mice with mice expressing the OTII transgenic TCR, which recognizes OVA peptide 223–230. These OVA peptide expanded talin1-deficient CD4+ cells had a slight increase in αL expression compared to control cells but had no difference in α4, β1, or β2 integrin expression (Supplementary Figure 1e). We then used carboxyfluorescein succinimidyl ester (CFSE) dye dilution to assess the ability of CD4+ T cells to proliferate in response to OVA peptide loaded irradiated splenocytes. In this assay, each round of cell division resulted in a 50% dilution in CFSE, which was detected by flow cytometry and quantified as the proliferative index. While we found that control cells had a dose-dependent increase in proliferation in response to increasing concentrations of antigen, talin1-deficient T cells had impaired proliferation at all concentrations of OVA peptide (Figure 2 a, b). Additionally, we found that the LFA-1 function-blocking antibody impaired control T cell proliferation at low, but not high, concentrations of OVA peptide but did not affect proliferation of talin1-deficient cells (Supplemental Figure 1F).
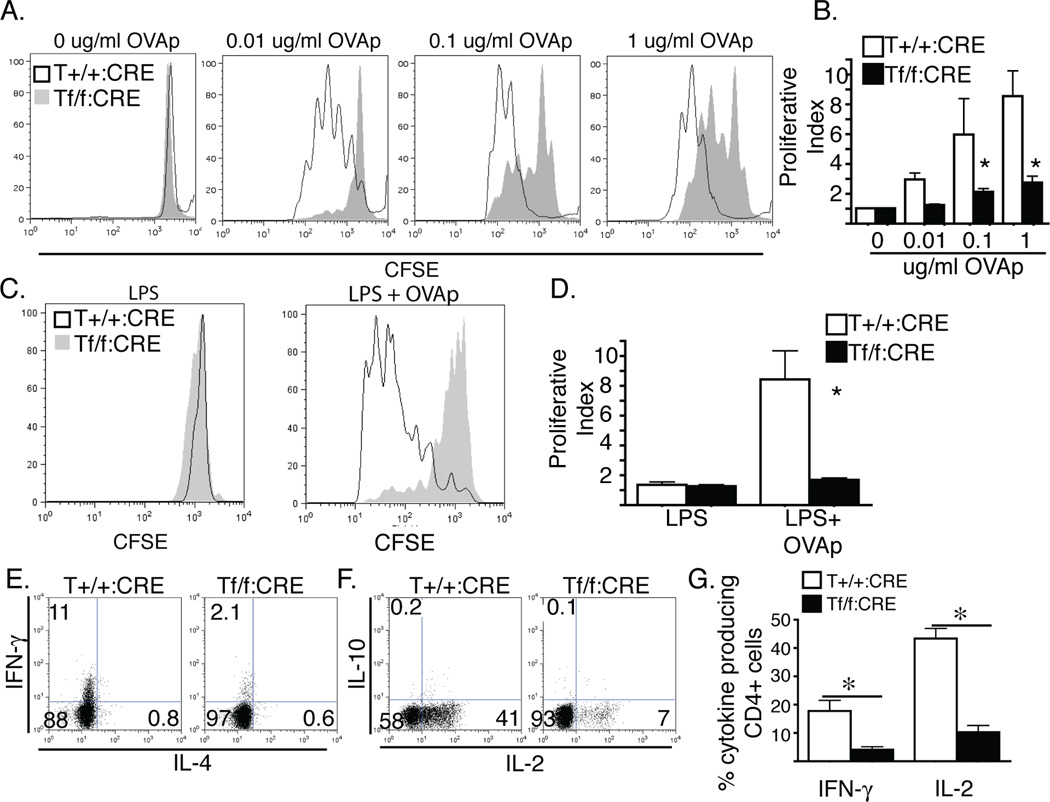
Figure 2. Talin1 is required for contact dependent CD4+ T cell proliferation.
a/b) OVA peptide expanded T cells were stained with CFSE and stimulated with irradiated splenocytes in the presence of 0, 0.01, 0.1, or 1 µg/ml OVA peptide. a) Representative histograms of CFSE dilution b) Average proliferative index +/− standard error *=p<0.05. c/d) in vivo proliferation of CFSE labeled naïve CD4+ cells injected into recipient mice 18 hours prior to stimulation with LPS or LPS and ovalbumin. d) Representative histograms of CFSE dilution e) average proliferative index +/− standard error. *=p<0.05. e/f/g) cytokine production from OVA peptide expanded T cells restimulated with anti-CD3/CD28 coated plates in the presence of brefeldin A prior to intracellular staining and flow cytometry analysis a/b) representative scatter plots of cytokine staining g) average % of CD4+ T cell producting ctyokine +/− SEM. *=P<0−.05. All plots and graphs are representative of at least three independent experiments
We next tested whether this proliferation defect also occurred in T cells in vivo. CFSE-labeled naïve CD4+ T cells were injected intravenously into wild type recipient mice 24 hours before stimulation with LPS in the presence or absence of ovalbumin. 72 hours following stimulation, we found no CFSE+ cells in the lymph nodes of mice injected with Talin1Loxp/Loxp:CD4-Cre:OTII cells. Due to the homing defect of talin1-deficient cells, we analyzed the degree of dye dilution in splenic CD4+ T cells by flow cytometry in control and talin1-deficient T cells. We found a significant decrease in the degree of dye dilution in talin1-deficient cells compared to controls, suggesting that talin1 is required for T cell proliferation in vivo (Figure 2 c, d).
Talin1 is required for contact dependent CD4+ T cell cytokine production
To determine if talin was required for cytokine production following stimulation, CD4+ T cells from Talin1+/+:CD4-Cre:OTII mice and Talin1Loxp/Loxp:CD4-Cre:OTII mice were activated with OVA peptide and seven days after isolation, cells were restimulated with plate-bound CD3 and soluble CD28 in the presence of brefeldin A. Cells were then stained with antibodies to IFN-γ, IL-4, IL-2 and IL-10. As expected, both control and talin1-deficient T cells exhibited Th1 skewing with increased production of IFN-γ and IL-2. However, there were five fold fewer talin1-deficient cells producing cytokine compared to control cells (Figure 2 e–g), indicating that talin is required for optimal Th1 cytokine production.
Talin1 is not required for contact independent CD4+ T cell proliferation
Given the observed defects in contact-dependent proliferation, we next wanted to determine if CD4+ T cells from Talin1Loxp/Loxp:CD4-Cre:OTII mice were capable of proliferating in response to contact-independent stimulation. To test this, we stimulated control and talin1-deficient T cells with anti-CD3/CD28 coated beads, which induce TCR signaling and co-stimulation, and also PMA/ionomycin, which activates PKC-θ and induces calcium signaling downstream of proximal TCR stimulation, respectively. While there were slight decreases in proliferation for talin1-deficient T cells compared to control cells following both stimulations, these differences were not statistically significant (Figure 3 a, b). These findings suggest that talin1-deficient cells have a specific defect in contact-dependent proliferation.
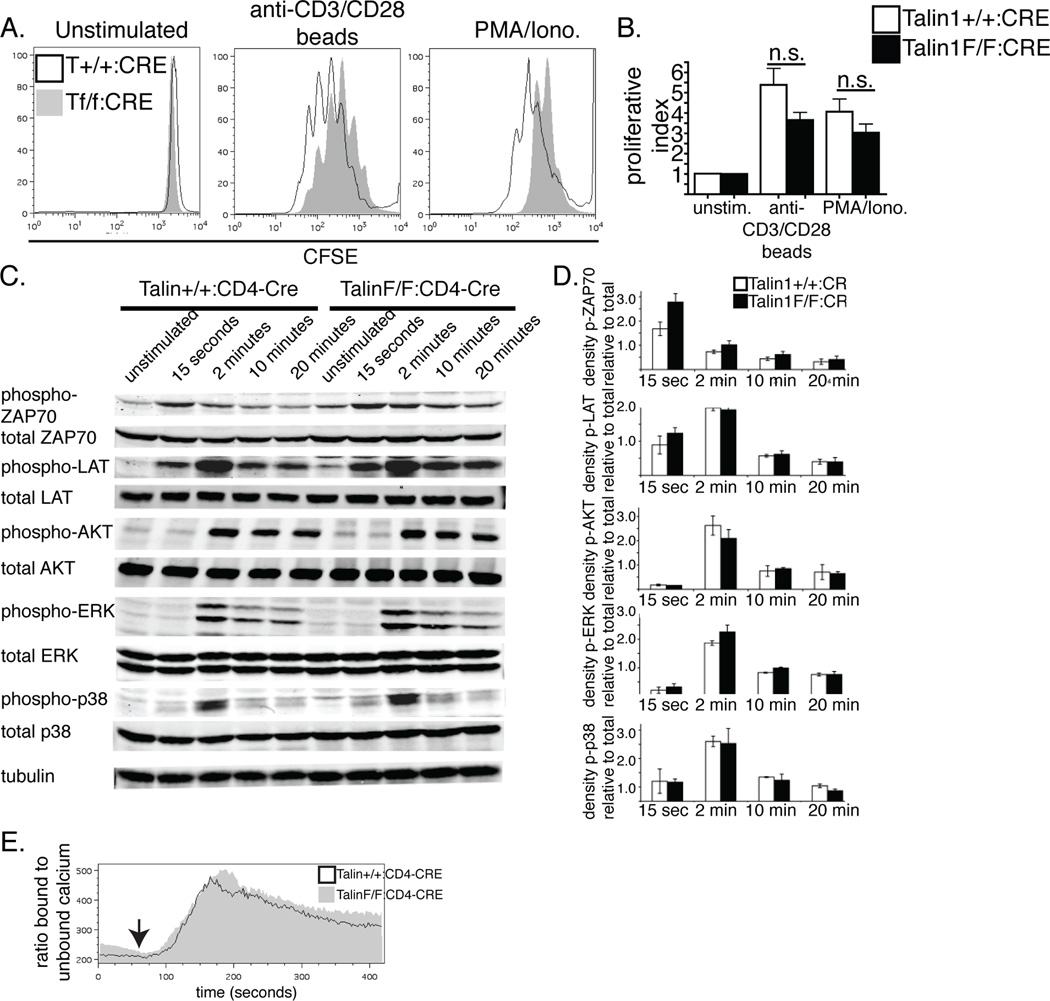
Figure 3. Talin1 is not required for contact independent CD4+ T cell proliferation and signaling.
a/b) OVA peptide expanded T cells were stained with CFSE and stimulated with anti-CD3/CD28 coated beads or PMA and ionomycin. a) Representative histograms of CFSE dilution b) Average proliferative index +/− standard error. c) Immunoblotting of control and talin1-deficient lysates following stimulation with anti-CD3 crosslinking for the indicated times. d) Band density quantification of phospho-protein relative to total. Graph represents average +/− standard error from 4 independent experiments e) OVA peptide expanded T cells loaded with Indo-1 were coated with biotinylated anti-CD3. After acquiring baseline ratio of bound:unbound calcium, streptavadin was added to induce TCR crosslinking and change in the ratio of 405 to 495 was measured. Plot is representative of 3 independent experiments
TCR induced signaling is intact in talin1-deficient cells
We were intrigued by our findings of a specific defect in contact-dependent proliferation, so we sought to better understand the differences between the contact-dependent and -independent induced proliferation. First, we looked at T cell signaling in response to TCR crosslinking. Following TCR crosslinking, control and talin1-deficient T cells showed a similar pattern of phosphorylation of ZAP-70, LAT, AKT, ERK and p38 MAPK (Figure 3 c, d). Additionally, using the calcium indicator dye, Indo-1, we found no difference in the ratio of bound to unbound calcium following TCR crosslinking, indicating that TCR signaling was similar in control and talin1-deficient cells (Figure 3 e). Together, these findings show that the impaired proliferation observed in talin1-deficient cells is not due to a global defect in TCR signal transduction.
Talin1-deficient CD4+ T cells have impaired T cell:APC contacts
Previous reports using siRNA-mediated depletion of talin have shown that talin is required for Jurkat T cell adhesion to ICAM-1 and conjugation to antigen presenting cells induced by superantigen (20, 21). To determine if talin was necessary for primary T cell adhesion and conjugation, we tested the ability of talin1-deficient cells to adhere to ICAM-1 following TCR crosslinking or PMA stimulation. Following both treatments, control T cells showed a three to four fold increase in adhesiveness, whereas no increase was observed in talin1-deficient cells (Figure 4a). These results indicate that talin is required for 2D T cell adhesion and inside-out integrin signaling induced by TCR stimulation. To determine if the defects were due to impaired integrin affinity modulation, we treated cells with MnCl2, which increases LFA-1 affinity for ligand. While control cells had a three-fold increase in adhesion to ICAM-1 following MnCl2 treatment, talin1-deficient cells did not show any statistically significant increase in adhesion (Figure 4a).
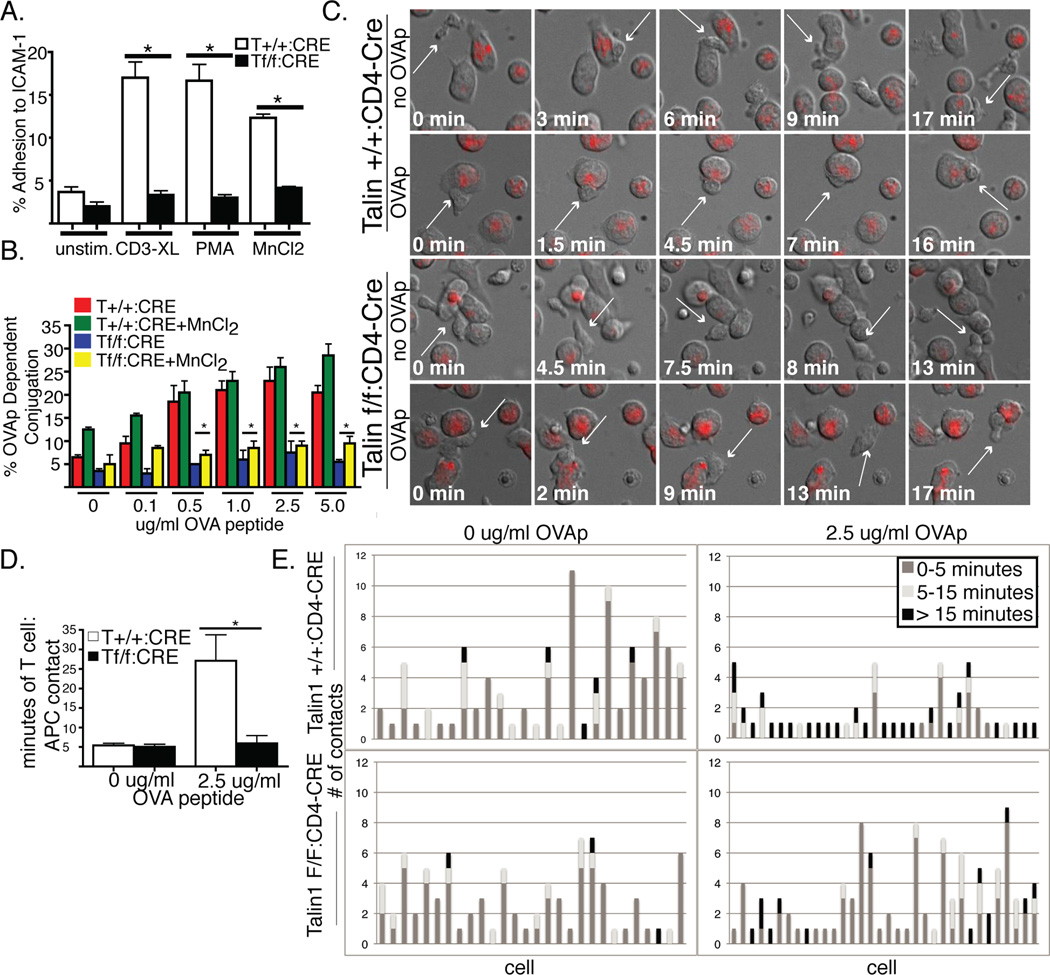
Figure 4. Talin1-deficient CD4+ T cells have impaired T cell:APC contacts.
a) OVA peptide expanded T cells were fluorescently labeled and adhered to ICAM-1 coated plates untreated or following stimulation with anti-CD3 crosslinking, PMA or MnCl2. Following washing step, the percentage of cells adherent to the plate was determined. b) OVA peptide expanded CD4+ T cells were calcein labeled and incubated with PKH-26 labeled LB27.4 cells loaded with OVA peptide +/− MnCl2. The percentage of OVA-dependent conjugation was determined by flow cytometry. c) OVA peptide expanded CD4+ T cells were imaged during interactions with red labeled LB27.4 cells +/− OVA peptide. Representative still images of T cell:APC contact are shown. White arrow follows a single T cell over time. d) Average time of T cell:APC contact e) Graphs depicting the number and duration of APC contacts made by 26 control and talin1-deficient T cells during 45 minutes of imaging. Data are representative or pooled from 3 independent experiments *=p<0.05.
We also tested the ability of talin1-deficient T cells to adhere to APCs by a flow cytometry based assay. We found that while control cells had a dose-dependent increase in antigen-dependent conjugation to the B cell lymphoma line, LB27.4, talin1-deficient cells did not adhere to APCs beyond baseline levels. Additionally, this defect in conjugation was not rescued with MnCl2, suggesting that increasing integrin affinity alone is not sufficient to rescue adhesion or conjugation (Figure 4b).
Talin1-deficient T cells did not form stable interactions with APCs
To further understand the nature of T cell:APC interactions in talin1-deficient cells, we examined T cell:APC contacts using live-3D imaging. Since talin1-deficient cells cannot adhere to a two-dimensional surface, we used a three-dimensional system comprised of low-melt agarose in the presence of serum to examine T cell:APC interactions(32). Talin1-deficient T cells displayed normal random motility in this 3D environment. In the absence of OVA peptide both control and talin1-deficient T cells showed only transient interactions with APCs and maintained a polarized morphology while migrating along APCs (Figure 4c, Supplemental movies 1–2). The average time of contact between T cells and B cells was less than 5 minutes without OVA peptide. In the presence of OVA peptide, control T cells established longer lasting interactions with APCs, with average contact times of about 30 minutes in a 45 minute movie (Figure 4 d,e, Supplemental Movie 3). Moreover, control T cells arrested migration and displayed a loss of uropod formation and cell polarization during these stable interactions with APCs. In contrast, talin1-deficient T cells in the presence of OVA peptide behaved more like T cells interacting with APCs without antigen (Figure 4c, Supplemental Movie 4): there were many contacts with an average duration of less than 5 minutes. Moreover, talin1-deficient T cells maintained a polarized morphology while contacting APCs and did not arrest migration (Figure 4 d, e). These findings indicate that stable T cell:APC interactions and antigen-induced T cell stopping require talin1.
Transient contacts are sufficient to induce signaling in talin1-deficient cells
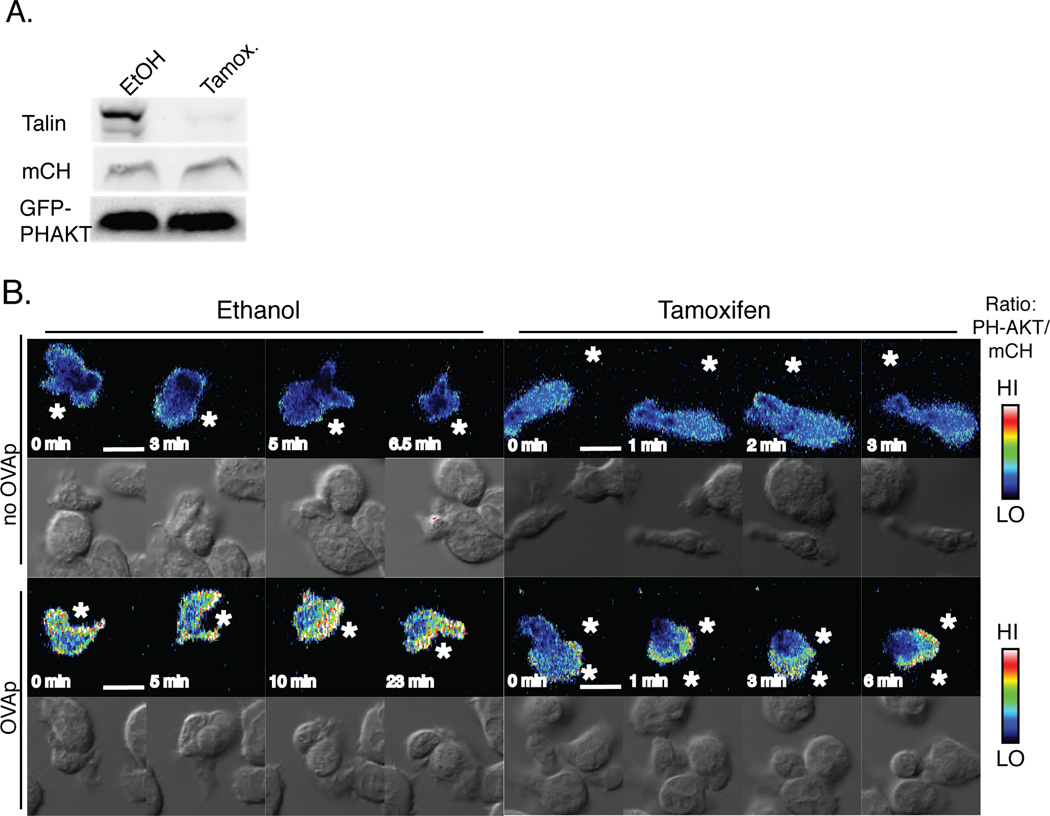
To determine if the transient T cell:APC contacts observed in talin1-deficient T cells were sufficient to induce T cell signaling, we used live imaging of a fluorescent reporter of PI3K activation in T cells contacting APCs. The PH domain of AKT, which binds to PI(3,4,5)P3 and PI(3,4)P2 and is a marker of PI3K activation, has previously been shown to polarize to the site of T cell:APC contacts (4, 38). We were unable to transfect T cells from Talin1Loxp/Loxp:CD4-Cre:OTII mice with retrovirus, consistent with the reported role of integrin activation in viral infection(39). To work around this, we retrovirally transduced T cells from Talin1Loxp/Loxp: Rosa26-CreERT2:OTII mice with constructs for mCherry and GFP-tagged PH-AKT (GFP-PH-AKT). Following an initial expansion, talin1 was depleted from T cells using treatment with 250 nM 4-hydroxytamoxifen (4-OHT). This treatment resulted in >95% reduction in talin1 protein expression in T cells (Figure 5a). T cells from Talin1Loxp/Loxp: Rosa26-CreERT2:OTII mice treated with 4-OHT had similar defects in T cell:APC contact and contact-dependent proliferation as T cells from Talin1Loxp/Loxp:CD4-Cre:OTII mice (Supplemental Figure 2). We expressed mCherry and GFP-PH-AKT to similar levels in control and 4-OHT treated cells (Figure 5a) and assessed localization of GFP-PH-AKT in control and 4-OHT treated T cells before and after stimulation with OVA peptide loaded APC. To control for volume effects at the immune synapse, the ratio of GFP-PH-AKT to mCherry was determined for T cell:APC contacts in the presence and absence of OVA peptide. While we saw no polarization of GFP-PH-AKT to the site of T cell:APC contact in the absence of OVA peptide, there was robust polarization of GFP-PH-AKT to the T cell:APC contact site in control cells in the presence of OVA peptide (Figure 5b, Supplementary Movie 5–Supplemental Movie 6). Additionally, although the 4-OHT-treated T cells from Talin1Loxp/Loxp: Rosa26-CreERT2:OTII cells did not arrest upon contact with antigen loaded APCs, GFP-PH-AKT polarized to the T cell:APC contact site in the presence, but not absence, of OVA peptide (Supplemental Movie 7–Supplemental Movie 8). This suggests that transient contacts are sufficient to induce signaling in talin1-deficient cells, although they are not able to induce T cell arrest.
Figure 5. Live imaging of signaling in Talin1-deficient cells.
OVA peptide expanded Talin1Loxp/Loxp:Rosa26-CreERT2:OTII cells were retrovirally transduced with mCherry vector and GFP-PH AKT. Prior to analysis, cells were treated with 4-OH tamoxifen or vehicle to induce genomic deletion of talin1. a) Immunoblotting shows >97% loss of talin with tamoxifen treatment. b) Ratiometric images of PH-AKT signaling relative to mCherry. White star depicts antigen presenting cell. Blots and images are representative of three independent experiments including 15 conjugation events. Scale bar represents 10 µm.
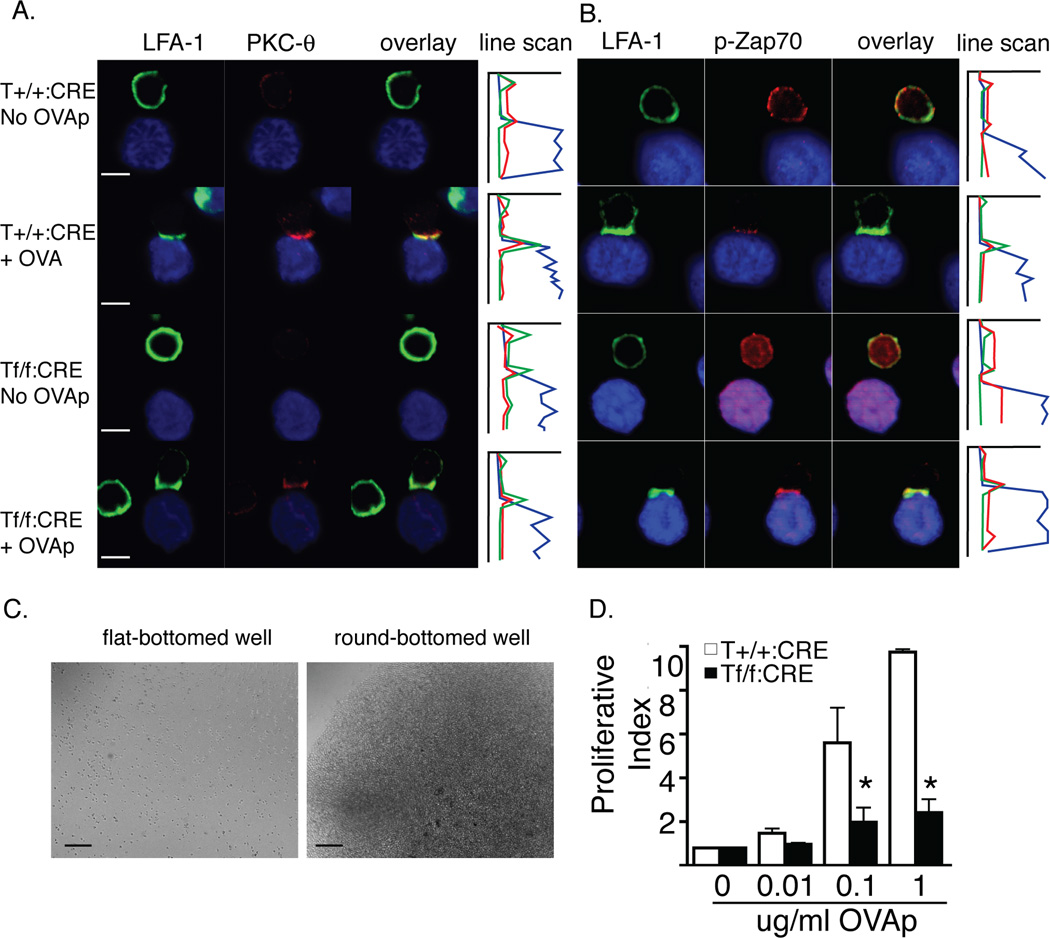
To further explore the capacity of talin1-deficient T cells to signal in response to antigen-loaded APCs, we allowed T cells to interact with APCs in the presence or absence of OVA peptide and then fixed and stained conjugates on poly-L-lysine coated coverslips. This assay only assessed the limited number of conjugates that formed; however, we found that PKC-θ, and phosphorylated Zap70 polarized to the site of T cell:APC contact in both control and talin1-deficient cells (Figure 6a, b). Surprisingly, LFA-1 also polarized to the contact site suggesting that talin is not required for LFA-1 clustering at the synapse. Additionally, we noted that RapL, which directly binds αL integrin and regulates LFA-1 clustering(40), and its regulator, RIAM, were localized to the immune synapse in talin1-deficient T cells (Supplemental Figure 3a,b). Despite the ability of transient contacts to induce signaling in talin1-deficient cells, promoting cell:cell contact using round bottomed wells was not sufficient to rescue T cell proliferation in talin1-deficient T cells (Figure 6c,d).
Figure 6. Transient contacts signal normally but forcing contact cannot rescue proliferation in talin1-deficient cells.
a/b) OVA peptide expanded cells were conjugated with OVA peptide loaded LB27.4 cells and fixed on coverslips and stained with the indicated antibodies. Graph is line scan through conjugate: X axis corresponds to fluorescent intensity while Y axis corresponds to µm position through conjugate. Blue line represents APC, green line represents LFA-1, and red line represents indicated antibody. Scale bar represented 10 µm. Images are representative of three independent experiments and at least 30 conjugates. c) Brightfield images of an equal number of unstimulated cells in round- and flat-bottomed plates. Scale bar represents 100 µm. d) Bar graph depicting proliferative index of OVA peptide expanded T cells in response to OVA peptide in round-bottomed plates. Data are mean +/− standard error from three independent experiments, *=p<0.05.
Talin is required for F-actin polarization to the synapse
Since LFA-1 polarized to T cell:APC contacts in talin1-deficient T cells, we sought to better understand the organization of the immune synapse in talin1-deficient cells. T cell:APC conjugates were fixed on coverslips and stained with anti-LFA-1 and rhodamine phalloidin. LFA-1 and F-actin strongly polarized to the immune synapse in 77% and 69% of control T cells contacting antigen-loaded APC, respectively. In contrast, talin1-deficient T cells polarized LFA-1 in 72% of T cells contacting APC with antigen, whereas F-actin polarized to the immune synapse in less than 5% of conjugates formed by talin1-deficient T cells (Figure 7a). We also found that LFA-1 was able to establish a ring-like structure, corresponding to the peripheral supra-molecular activation cluster, at the immune synapse in some talin1-deficient T cell conjugates (Supplemental Figure 3b).
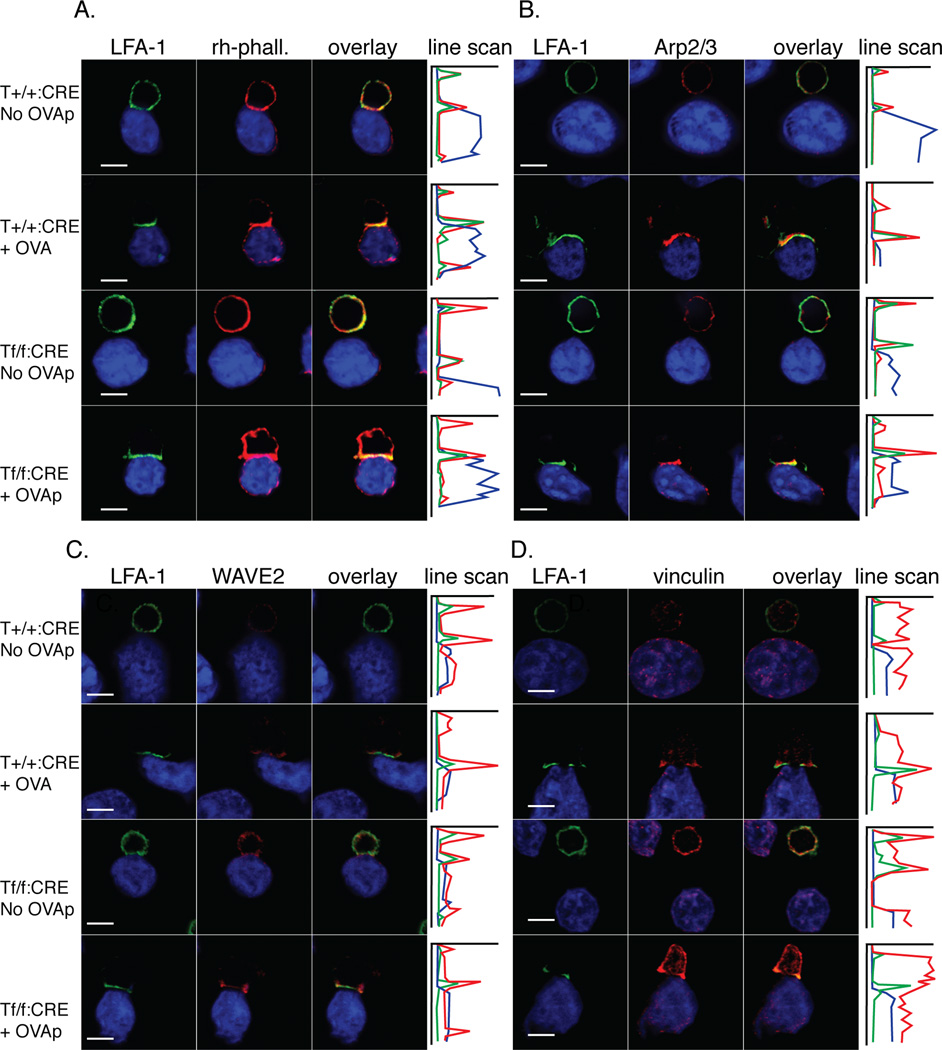
Figure 7. Talin1 is required for F-actin polarization to the synapse.
a-d) OVA peptide expanded control and talin1-deficient T cells were conjugated with LB27.4 B cells in the presence or absence of antigen, fixed on poly-L-lysine coverslips, and stained with the indicated antibodies. Graph is line scan through conjugate: X axis corresponds to fluorescent intensity while Y axis corresponds to µm position through conjugate. Blue line represents APC, green line represents LFA-1, and red line represents indicated antibody. Images are representative of 10 conjugation events from 3 independent experiments. Scale bar represents 10 µm.
To further investigate the defect in actin polarization, we used immunofluorescence to stain for components of the actin polymerizing machinery, including Arp2/3 and WAVE2. We found that both Arp2/3 and the F-actin regulator, WAVE-2, were localized at the immune synapse in both control and talin1-deficient T cells following conjugation in the presence of OVA peptide, supporting the idea that talin is not necessary for the polarization of key actin regulatory proteins to the immune synapse (Figure 7). Moreover, HS-1, which has been implicated in stabilizing F-actin at the immune synapse(26), also polarized in talin1-deficient cells (Supplemental Figure 4). In contrast, we found that vinculin failed to polarize to the immune synapse in talin1-deficient T cells compared to control T cells in the presence of antigen (Figure 7d). Taken together, our results suggest that WAVE2 and Arp2/3 are recruited to the site of T cell:APC contact in the absence of talin1 but that talin1 is necessary for the polarization of vinculin and F-actin to the immune synapse.
Talin1 is necessary for the polarization of stabilized F-actin to T cell:APC contacts
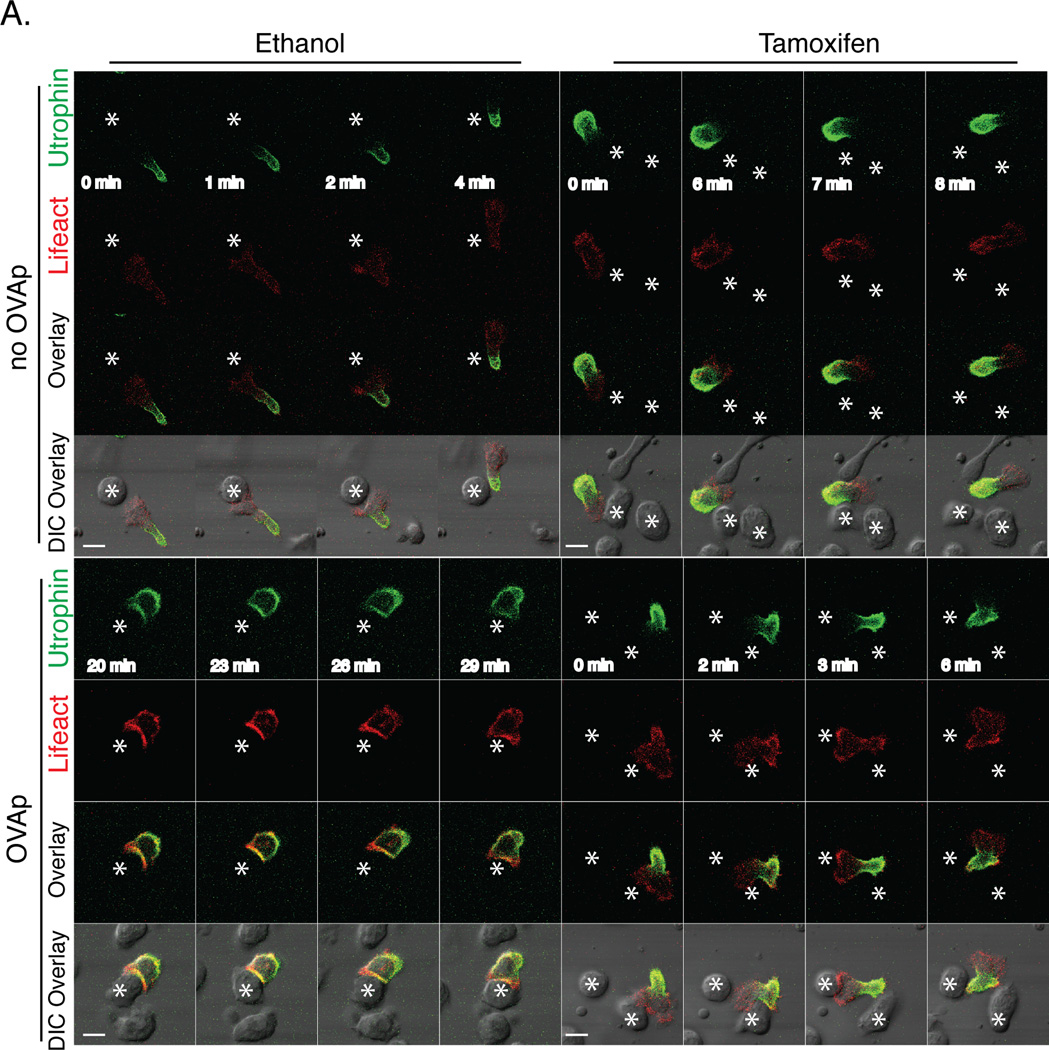
To better understand F-actin dynamics in talin1-deficient T cells contacting APC, we used live imaging to examine two different fluorescent probes that bind F-actin. m-Ruby tagged Lifeact (Lifeact-Ruby)(41) binds to all F-actin whereas GFP tagged calponin homology (CH) domain from utrophin (UtrCH-GFP) binds to stabilized F-actin(42–44). We had previously reported that UtrCH-GFP specifically localizes to the uropod of migrating neutrophils(42–44). Similarly, we found that UtrCH-GFP localizes to the uropod in motile T cells in 3D where as Lifeact-Ruby localized to the cell cortex in both the leading edge and uropod. In the absence of antigen, both control and 4-OHT treated T cells isolated from Talin1Loxp/Loxp: Rosa26-CreERT2:OTII mice retained UtrCH-GFP in the uropod during transient T cell:APC contacts (Figure 8 Supplemental Movies 9–10). In the presence of OVA peptide, control T cells lost their uropod upon contact with APCs and UtrCH-GFP established a more uniform distribution around the cell cortex with some enhancement at the T cell:APC contact site. While Lifeact-RUBY was distributed along the cell cortex, we found that occasionally Lifeact-RUBY was enriched at peripheral regions of T cell:APC contact sites, highlighting an area of active F-actin dynamics (Figure 8 Supplemental Movie 11). In contrast, talin1-deficient T cells retained UtrCH-GFP in the uropod during T cell-APC contacts in the presence of antigen, indicating that stabilized F-actin failed to polarize to the synapse without talin (Figure 8 Supplemental Movie 12). Lifeact-RUBY was enriched at the site of transient T cell:APC contacts in talin1-deficient T cells, suggesting that dynamic F-actin can polarize to the contact site between T cells and APC in the absence of talin. Taken together, our findings indicate that talin1 is necessary for the stabilization of F-actin at the immune synapse.
Figure 8. Live Imaging of actin dynamics during T cell:APC contact.
a) OVA peptide expanded Talin1Loxp/Loxp:Rosa26-CreERT2:OTII T cells were retrovirally transduced with Lifeact-mRUBY and UtrCH-GFP and imaged during T cell contact with LB27.4 cells in the presence or absence of OVA peptide. Prior to the experiment, T cells were treated with ethanol or 4-OH tamoxifen to deplete talin1. Still images of time-lapse movie are representative of 10 contacts observed from 2 independent experiments. Scale bar represents 10 µm. * corresponds to APC
Discussion
Since the identification of talin as an immune synapse component (17), there has been considerable interest in understanding its role in LFA-1 function and T cell activation. Using mice with a specific depletion of talin1 in T cells, we now demonstrate for the first time that talin is critical for maintenance of T cell:APC contacts, contact-mediated T cell proliferation, and polarization of stable F-actin to the immune synapse. We found no evidence of defects in TCR signaling contributing to these phenotypes since protein phosphorylation following TCR crosslinking was unaltered and phospho-ZAP70 and PKC-θ localized to the immune synapse of talin1-deficient T cells. Additionally, using live imaging, we observed accumulation of GFP-PH-AKT at the site of T cell:APC contact in the absence of talin1-dependent T cell arrest. Based on our findings, we propose that the current model of LFA-1 activation in T cells should be revised to indicate that talin is dispensable for LFA-1 polarization to the synapse but is required to polarize stabilized F-actin and mediate full T cell arrest.
The defects that we observed in talin1-deficient T cell lymph node homing, APC interactions and proliferation are similar to those previously reported for CD4+ T cells isolated from LFA-1 knockout mice (12, 45). These shared phenotypes suggest that the defects observed in Talin1Loxp/Loxp:CD4-Cre T cells may primarily be due to defects in LFA-1 activation. The capacity of LFA-1 to bind its ligand, ICAM-1, is regulated by changes in affinity and clustering following TCR signaling (13). We have previously shown that talin modulates both components of LFA-1 function (20). Here, we show that talin1-deficient T cells fail to adhere to ICAM-1 and APCs but retain the ability to cluster LFA-1 at T cell:APC contacts.
Despite having this ability to cluster LFA-1, talin1-deficient T cells failed to fully polarize F-actin to the immune synapse. These findings differed from previous reports suggesting that talin is required for LFA-1 clustering, but not F-actin polarization, during Jurkat superantigen-mediated conjugation (20) (21). These differences are likely due to the types of stimulation and cell types used: Jurkat T cells treated with superantigen versus antigen-induced primary T cell conjugation. Superantigen-mediated conjugation has previously been shown to bypass proximal TCR signaling (23), and Jurkat T cell signaling is different from primary T cells (22). The use of talin knockout mice and antigen-specific primary T cells represents an advance towards better understanding the role of talin1 in T cell adhesion and activation.
While there are clear defects in LFA-1 function in the absence of talin, we found that LFA-1 is still clustered at the immune synapse in talin1-deficient T cells. In other integrin-mediated adhesions, talin1, RapL and kindlins are thought to work in concert to regulate integrin activity (19). While RapL is not required for T cell adhesion to ICAM-1 (46), both talin1 and kindlin-III have been independently shown to be important for LFA-1 dependent adhesion of T cells (16, 20). In this work, we show that RapL and the associated integrin regulatory protein RIAM are localized to the site of T cell:APC contact in talin1-deficient T cells, suggesting that RapL and/or RIAM may potentially be sufficient to cluster LFA-1. However, despite the clustering of LFA-1 at the immune synapse, LFA-1 is not functioning normally in talin1-deficient T cells since the cells have impaired adhesion to both APCs and ICAM-1-coated plates. Together, these findings indicate that although talin1 is dispensable for LFA-1 polarization, it is necessary for LFA-1-mediated adhesive function.
Based on previous studies, it was surprising to find impaired F-actin polarization at the site of T cell:APC contacts in talin1-deficient T cells. Previous work has reported that F-actin polarization to the immune synapse is required for T cell:APC interactions and full T cell proliferation since disruption of F-actin with inhibitors following conjugate formation impairs T cell activation (47), (48). To better understand the types of F-actin found at the immune synapse, we used two probes of F-actin: Lifeact, which binds to all F-actin present in the cell, and the CH domain of Utrophin, which binds specifically to stable F-actin. Since Utrophin-CH binds to stable F-actin populations, localization of Lifeact alone corresponds to areas of dynamic F-actin (44). To our knowledge, these probes have not previously been used to characterize the dynamics of F-actin at T cell:APC contact sites.
Live imaging of F-actin dynamics using Lifeact-RUBY and UtrCH-GFP showed that stable F-actin, specifically, failed to polarize to the T cell:APC contact site in talin1-deficient cells. We found that migrating talin1-deficient T cells maintained UtrCH-GFP, a marker of stabilized F-actin, at the uropod without any evidence of its re-distribution following transient T cell:APC contacts, whereas Lifeact-RUBY was enriched at T cell:APC contact sites during transient contacts. This combined with the observation that Arp2/3 and WAVE2 are at the immune synapse in talin1-deficient T cells suggests that actin polymerization at the immune synapse is intact in talin1-deficient cells but that talin is required for F-actin stabilization.
This failure to polarize F-actin in talin1-deficient cells may be due to a requirement for talin to directly bind actin at the immune synapse and provide the link to polarized integrins or it may be due to an inability to recruit vinculin to the immune synapse in the absence of talin1. Supporting the latter hypothesis, we showed that there was a defect in vinculin polarization to the immune synapse in talin1-deficient T cells even though vinculin can bind Arp2/3, which polarized normally to the synapse in the absence of talin1. Therefore, we think it is most likely that talin and vinculin work together to stabilize F-actin at the immune synapse through their interactions with LFA-1 and actin. Indeed, vinculin bound to talin has been shown to play a key role in linking F-actin to focal adhesions(49). The importance of stabilizing F-actin at the immune synapse is highlighted by the recent findings that T cells lacking leukocyte L-plastin, an actin bundling protein that mediates filamentous actin accumulation at the immune synapse, are not efficiently activated (50).
One area of controversy is the relative importance of forming stable T cell:APC interactions for T cell activation. While some in vitro studies show that T cell arrest is required for T cell proliferation and activation (2, 3), others show that transient interactions are sufficient for T cell activation (6, 7). In this paper, we demonstrate that talin1 is necessary for T cell arrest and formation of stable interactions between T cells and APCs. The failure of talin1-deficient T cells to arrest and develop stable contacts with APCs in combination with the findings of impaired contact-dependent proliferation supports the hypothesis that T cell arrest is required for T cell proliferation and that talin is a critical regulator of this process. We suspect that talin mediates a connection between F-actin and LFA-1 that is required for complete arrest of T cells on APCs since simply promoting cell-cell contact is not sufficient to restore T cell proliferation in talin1-deficient T cells.
In vivo studies investigating the importance of T cell:APC interactions indicate that T cells form stable, long-lasting interactions with APCs during priming conditions, which contribute to proliferation (10, 51). However, the requirement of these interactions for T cell proliferation have been challenged with the finding that CD8+ T cells fail to form stable interactions with ICAM-1-deficient APCs in vivo but are still able to proliferate (11). While we show that talin is required for in vivo CD4+ T cell proliferation in response to ovalbumin, the question of T cell arrest during in vivo priming is hard to address with this system since we cannot determine whether impaired proliferation in talin1-deficient T cells is due to impaired T cell:APC interactions or due to impaired T cell trafficking to lymph nodes. However, our work supports the idea that talin-mediated T cell arrest is required for full CD4+ T cell proliferation and activation.
Here, we provide evidence that talin1 is required for the formation of sustained T cell:APC interactions and T cell contact-dependent proliferation. In addition to its role in regulating LFA-1 function in T cells, it also plays a critical role in maintaining F-actin polarization to the immune synapse. While talin-dependent T cell:APC interactions seem critical in generating a number of T cell:APC responses, future challenges will be to address how talin may support the differentiation of T cells following antigenic challenge and how this differentiation may affect outcomes in response to infection and autoimmunity in vivo.
Supplementary Material
Acknowledgements
The authors would like to thank the UWCCC Flow Cytometry facility for assistance with flow cytometry and UW animal care facility for animal husbandry. We also thank Bill Bement for contribution of utrophin-GFP construct and Peter Cavnar for subcloning. Subbu Hegde provided invaluable irradiator assistance. We also thank Frank Gertler for contribution of RIAM antibody.
Footnotes
This work was supported by the National Institutes of Health Grant R01 CA085862 and NIH NIAID R01 AI068062 to A.H. Postdoctoral support was provided to A.W. by the UW Institute on Aging Training Grant (NIH#T32AG000213–17), Sanjay Asthana PI. The Wellcome Trust supported work in D.R.C’s laboratory.
Contributions
S.A.W. designed, performed and analyzed experiments and wrote the manuscript; A.J.W. performed experiments; D.A.B. provided experimental support and helped with mouse colony maintenance; S.J.M. and D.R.C. generated the Talin1flox/flox mice; T.L. provided the Rosa26-CreERT2 mice; A.H. designed and analyzed experiments and co-wrote the manuscript.
References
- 1.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol. 2003;4:749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 3.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 4.Costello PS, Gallagher M, Cantrell DA. Sustained and dynamic inositol lipid metabolism inside and outside the immunological synapse. Nat Immunol. 2002;3:1082–1089. doi: 10.1038/ni848. [DOI] [PubMed] [Google Scholar]
- 5.Benvenuti F, Lagaudrière-Gesbert C, Grandjean I, Jancic C, Hivroz C, Trautmann A, Lantz O, Amigorena S. Dendritic cell maturation controls adhesion, synapse formation, and the duration of the interactions with naive T lymphocytes. J Immunol. 2004;172:292–301. doi: 10.4049/jimmunol.172.1.292. [DOI] [PubMed] [Google Scholar]
- 6.Faroudi M, Zaru R, Paulet P, Muller S, Valitutti S. Cutting edge: T lymphocyte activation by repeated immunological synapse formation and intermittent signaling. J Immunol. 2003;171:1128–1132. doi: 10.4049/jimmunol.171.3.1128. [DOI] [PubMed] [Google Scholar]
- 7.Gunzer M, Schafer A, Borgmann S, Grabbe S, Zanker KS, Brocker EB, Kampgen E, Friedl P. Antigen presentation in extracellular matrix: interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity. 2000;13:323–332. doi: 10.1016/s1074-7613(00)00032-7. [DOI] [PubMed] [Google Scholar]
- 8.Bousso P. T-cell activation by dendritic cells in the lymph node: lessons from the movies. Nat Rev Immunol. 2008;8:675–684. doi: 10.1038/nri2379. [DOI] [PubMed] [Google Scholar]
- 9.Hugues S, Boissonnas A, Amigorena S, Fetler L. The dynamics of dendritic cell-T cell interactions in priming and tolerance. Curr Opin Immunol. 2006;18:491–495. doi: 10.1016/j.coi.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med. 2004;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scholer A, Hugues S, Boissonnas A, Fetler L, Amigorena S. Intercellular adhesion molecule-1-dependent stable interactions between T cells and dendritic cells determine CD8+ T cell memory. Immunity. 2008;28:258–270. doi: 10.1016/j.immuni.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Kandula S, Abraham C. LFA-1 on CD4+ T cells is required for optimal antigen-dependent activation in vivo. J Immunol. 2004;173:4443–4451. doi: 10.4049/jimmunol.173.7.4443. [DOI] [PubMed] [Google Scholar]
- 13.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5:546–559. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 14.Abram CL, Lowell CA. The ins and outs of leukocyte integrin signaling. Annu Rev Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burbach BJ, Medeiros RB, Mueller KL, Shimizu Y. T-cell receptor signaling to integrins. Immunol Rev. 2007;218:65–81. doi: 10.1111/j.1600-065X.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- 16.Manevich-Mendelson E, Feigelson SW, Pasvolsky R, Aker M, Grabovsky V, Shulman Z, Kilic SS, Rosenthal-Allieri MA, Ben-Dor S, Mory A, Bernard A, Moser M, Etzioni A, Alon R. Loss of Kindlin-3 in LAD-III eliminates LFA-1 but not VLA-4 adhesiveness developed under shear flow conditions. Blood. 2009;114:2344–2353. doi: 10.1182/blood-2009-04-218636. [DOI] [PubMed] [Google Scholar]
- 17.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 18.Critchley DR. Biochemical and structural properties of the integrin-associated cytoskeletal protein talin. Annu Rev Biophys. 2009;38:235–254. doi: 10.1146/annurev.biophys.050708.133744. [DOI] [PubMed] [Google Scholar]
- 19.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simonson WT, Franco SJ, Huttenlocher A. Talin1 regulates TCR-mediated LFA-1 function. J Immunol. 2006;177:7707–7714. doi: 10.4049/jimmunol.177.11.7707. [DOI] [PubMed] [Google Scholar]
- 21.Nolz JC, Medeiros RB, Mitchell JS, Zhu P, Freedman BD, Shimizu Y, Billadeau DD. WAVE2 regulates high-affinity integrin binding by recruiting vinculin and talin to the immunological synapse. Mol Cell Biol. 2007;27:5986–6000. doi: 10.1128/MCB.00136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartelt RR, Cruz-Orcutt N, Collins M, Houtman JCD. Comparison of T cell receptor-induced proximal signaling and downstream functions in immortalized and primary T cells. PLoS ONE. 2009;4:e5430. doi: 10.1371/journal.pone.0005430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bueno C, Lemke CD, Criado G, Baroja ML, Ferguson SS, Rahman AK, Tsoukas CD, McCormick JK, Madrenas J. Bacterial superantigens bypass Lck-dependent T cell receptor signaling by activating a Galpha11-dependent, PLC-beta-mediated pathway. Immunity. 2006;25:67–78. doi: 10.1016/j.immuni.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–259. doi: 10.1146/annurev.immunol.26.021607.090347. [DOI] [PubMed] [Google Scholar]
- 25.Nolz JC, Gomez TS, Zhu P, Li S, Medeiros RB, Shimizu Y, Burkhardt JK, Freedman BD, Billadeau DD. The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation. Curr Biol. 2006;16:24–34. doi: 10.1016/j.cub.2005.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez TS, McCarney SD, Carrizosa E, Labno CM, Comiskey EO, Nolz JC, Zhu P, Freedman BD, Clark MR, Rawlings DJ, Billadeau DD, Burkhardt JK. HS1 functions as an essential actin-regulatory adaptor protein at the immune synapse. Immunity. 2006;24:741–752. doi: 10.1016/j.immuni.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mace EM, Zhang J, Siminovitch KA, Takei F. Elucidation of the integrin LFA-1-mediated signaling pathway of actin polarization in natural killer cells. Blood. 2010;116:1272–1279. doi: 10.1182/blood-2009-12-261487. [DOI] [PubMed] [Google Scholar]
- 28.Nieswandt B, Moser M, Pleines I, Varga-Szabo D, Monkley S, Critchley D, Fassler R. Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo. J Exp Med. 2007;204:3113–3118. doi: 10.1084/jem.20071827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo K, McMinn JE, Ludwig T, Yu YH, Yang G, Chen L, Loh D, Li C, Chua S, Jr, Zhang Y. Disruption of peripheral leptin signaling in mice results in hyperleptinemia without associated metabolic abnormalities. Endocrinology. 2007;148:3987–3997. doi: 10.1210/en.2007-0261. [DOI] [PubMed] [Google Scholar]
- 30.Lafuente EM, van Puijenbroek AAFL, Krause M, Carman CV, Freeman GJ, Berezovskaya A, Constantine E, Springer TA, Gertler FB, Boussiotis VA. RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev Cell. 2004;7:585–595. doi: 10.1016/j.devcel.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 31.Wernimont SA, Legate KR, Simonson WTN, Fassler R, Huttenlocher A. PIPKI gamma 90 negatively regulates LFA-1-mediated adhesion and activation in antigen-induced CD4+ T cells. J Immunol. 2010;185:4714–4723. doi: 10.4049/jimmunol.1001445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedman RS, Jacobelli J, Krummel MF. Surface-bound chemokines capture and prime T cells for synapse formation. Nat Immunol. 2006;7:1101–1108. doi: 10.1038/ni1384. [DOI] [PubMed] [Google Scholar]
- 33.Debrand E, El Jai Y, Spence L, Bate N, Praekelt U, Pritchard CA, Monkley SJ, Critchley DR. Talin2 is a large and complex gene encoding multiple transcripts and protein isoforms. FEBS Journal. 2009;276:1610–1628. doi: 10.1111/j.1742-4658.2009.06893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monkley SJ, Pritchard CA, Critchley DR. Analysis of the mammalian talin2 gene TLN2. Biochem Biophys Res Commun. 2001;286:880–885. doi: 10.1006/bbrc.2001.5497. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Jiang G, Cai Y, Monkley SJ, Critchley DR, Sheetz MP. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nature Cell Biology. 2008 doi: 10.1038/ncb1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berlin-Rufenach C, Otto F, Mathies M, Westermann J, Owen MJ, Hamann A, Hogg N. Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1-deficient mice. J Exp Med. 1999;189:1467–1478. doi: 10.1084/jem.189.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marski M, Kandula S, Turner JR, Abraham C. CD18 is required for optimal development and function of CD4+CD25+ T regulatory cells. J Immunol. 2005;175:7889–7897. doi: 10.4049/jimmunol.175.12.7889. [DOI] [PubMed] [Google Scholar]
- 38.Harriague J, Bismuth G. Imaging antigen-induced PI3K activation in T cells. Nat Immunol. 2002;3:1090–1096. doi: 10.1038/ni847. [DOI] [PubMed] [Google Scholar]
- 39.Hioe CE, Chien PC, Lu C, Springer TA, Wang XH, Bandres J, Tuen M. LFA-1 expression on target cells promotes human immunodeficiency virus type 1 infection and transmission. J Virol. 2001;75:1077–1082. doi: 10.1128/JVI.75.2.1077-1082.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katagiri K, Maeda A, Shimonaka M, Kinashi T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat Immunol. 2003;4:741–748. doi: 10.1038/ni950. [DOI] [PubMed] [Google Scholar]
- 41.Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, Sixt M, Wedlich-Soldner R. Lifeact: a versatile marker to visualize F-actin. Nat Methods. 2008;5:605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burkel BM, von Dassow G, Bement WM. Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophin. Cell Motil Cytoskeleton. 2007;64:822–832. doi: 10.1002/cm.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cooper KM, Bennin DA, Huttenlocher A. The PCH family member proline-serine-threonine phosphatase-interacting protein 1 targets to the leukocyte uropod and regulates directed cell migration. Mol Biol Cell. 2008;19:3180–3191. doi: 10.1091/mbc.E08-02-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoo SK, Deng Q, Cavnar PJ, Wu YI, Hahn KM, Huttenlocher A. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev Cell. 2010;18:226–236. doi: 10.1016/j.devcel.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graf B, Bushnell T, Miller J. LFA-1-mediated T cell costimulation through increased localization of TCR/class II complexes to the central supramolecular activation cluster and exclusion of CD45 from the immunological synapse. J Immunol. 2007;179:1616–1624. doi: 10.4049/jimmunol.179.3.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miertzschke M, Stanley P, Bunney TD, Rodrigues-Lima F, Hogg N, Katan M. Characterization of interactions of adapter protein RAPL/Nore1B with RAP GTPases and their role in T cell migration. J Biol Chem. 2007;282:30629–30642. doi: 10.1074/jbc.M704361200. [DOI] [PubMed] [Google Scholar]
- 47.Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J Exp Med. 1995;181:577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 49.Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol. 2007;179:1043–1057. doi: 10.1083/jcb.200703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang C, Morley SC, Donermeyer D, Peng I, Lee WP, Devoss J, Danilenko DM, Lin Z, Zhang J, Zhou J, Allen PM, Brown EJ. Actin-Bundling Protein L-Plastin Regulates T Cell Activation. J Immunol. 2010 doi: 10.4049/jimmunol.1001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.