Abstract
Macrophage-derived factors, including TNFα, are known as important inducers of insulin resistance. However, the role of macrophages in insulin resistance in the liver is unclear. Hyperglycemia and insulin resistance commonly occur following acute injuries or critical illness, referred to as “critical illness diabetes”. In the present studies, the roles of macrophages in hepatic insulin resistance following surgical trauma and hemorrhage were investigated. Intravenous administration of Gadolinium chloride (GdCl3) or Clodronate-liposome (CLD-lipo) resulted in depletion of macrophages in both liver and spleen of rats. Macrophage depletion by either GdCl3 or CLD-lipo did not prevent the development of trauma and hemorrhage-induced insulin resistance in the liver of rats, as indicated by impaired hepatic insulin signaling following a 90 minute-hemorrhage period. Similarly, hepatic insulin resistance still developed in rats after removal of the spleen (splenectomy). In contrast, macrophage depletion significantly reversed the hepatic insulin resistance several hours later, following resuscitation. As a comparison, splenectomy resulted in improvement in hepatic insulin signaling following resuscitation, but to a lesser extent, suggesting both liver and spleen resident macrophages have a role in the continuation of hepatic insulin resistance following resuscitation. These studies demonstrated that the initial development of insulin resistance in liver is macrophage-independent in a rodent model of critical illness diabetes, whereas both liver and spleen macrophages have a role in the later maintenance of the insulin resistant state, following resuscitation.
Keywords: Insulin resistance, liver, spleen, trauma, hemorrhage
INTRODUCTION
Insulin regulates glucose homeostasis through suppressing glucose production in liver and promoting glucose disposal in muscle and adipose tissue. Hyperglycemia and insulin resistance commonly occur in patients after acute injuries and critical illness, which has been referred to “diabetes of injury” or “critical illness diabetes” (1–6).
Hyperglycemia after injuries or critical illness is an acute metabolic response to stress, which has been linked to increased mortality and mobility in critically ill patients (7–9). Hemorrhage is a common complication of severe injuries or complex surgeries. A rodent surgical trauma and hemorrhage model was used to investigate the mechanisms of acute insulin resistance. Previous studies indicate that trauma and hemorrhage results in rapid impairment of hepatic insulin signaling, which results in hyperglycemia and hyperinsulinemia as well as impaired glucose and insulin tolerance (10–14). The acute onset of hemorrhage-induced insulin resistance in liver has been attributed, in part, to excess production of reactive oxygen species (ROS) and activation of c-Jun N terminal kinase (JNK) and Inhibitory κB kinase (IKK) kinases (15; 16). As opposed to the initial onset of hepatic insulin resistance following trauma and hemorrhage, TNFα, plays a dominant role in continuation of the insulin resistant state later following resuscitation (12). However, the specific cellular sources of ROS or TNFα and the cell types involved in the development of hepatic insulin resistance following trauma and hemorrhage remain unclear.
Tissue macrophages play an important role in initiating an immune response and promoting inflammation and injury (17; 18). Splenic macrophages are known as an important source of inflammatory factors and splenectomy has been shown to reduce serum TNFα levels in sepsis and liver injury models in rats (19; 20). Compared with the other organs, the liver has the largest resident populations of macrophages, Kupffer cells, which are known as a major source of TNFα. Numerous studies have demonstrated the involvement of macrophages in insulin resistance as a source of proinflammatory cytokines and ROS (21–23). In the present studies, we hypothesized that macrophages, especially Kupffer cells, are a major source of ROS or TNFα after rapid blood loss, which stimulate the activation of hepatic JNK and IKK kinases and induce hepatic insulin resistance.
To directly determine the contribution of tissue macrophages in the acute development of hepatic insulin resistance following trauma and hemorrhage, two approaches of macrophage depletion were used: gadolinium chloride (GdCl3) and Clodronate-liposome (CLD-lipo). Gadolinium chloride, a rare earth metal, can form an insoluble gadolinium hydroxide colloid at neutral pH, which can be taken up by macrophages and cause their selective elimination (24). Clodronate, a non-toxic bisphosphonate, when embedded in liposomes, is taken up by macrophages, followed by intracellular release and accumulation of clodronate, which induces macrophage apoptosis (25; 26).
In the current studies, depletion of macrophages in both liver and spleen was achieved after intravenous administration of GdCl3 or CLD-lipo. Macrophage depletion did not prevent the development of insulin resistance in liver following trauma and hemorrhage, but completely reversed the hepatic insulin resistance several hours later, following resuscitation, suggesting that macrophages are not necessary for the initial onset of hemorrhage-induced hepatic insulin resistance, but have a role in the later maintenance of the insulin resistant state. As a comparison, splenectomy resulted in a lesser improvement in hepatic insulin signaling following resuscitation, suggesting both liver and spleen resident macrophages have a role in the recovery of hepatic insulin resistance following resuscitation.
RESEARCH DESIGN AND METHODS
Animal model of trauma and hemorrhage
All experimental procedures were carried out in accordance with the guidelines of the Care and Use of Laboratory Animals by the National Institutes of Health, and the experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. Male Sprague Dawley rats (220~270g, Harlan) were housed in animal facilities for at least one week before experiments. Surgical trauma and hemorrhage was performed as described previously (10–12; 14). Rats were fasted for 18~20 hrs and anesthetized by 1.5% isofluorane and a 5 cm midline laparotomy was performed. Catheters (PE-50) were placed in both femoral arteries and the right femoral vein for bleeding, monitoring of blood pressure, and resuscitation, respectively. Rats were bled rapidly to reach a Mean Arterial Pressure (MAP) of 35~40 mmHg within 10 min and maintained at 35~40 mmHg for 60min (TH60’) or 90 min (TH90’). The trauma alone (T) groups were subjected to the same surgical procedures as the trauma and hemorrhage groups, but without hemorrhage. For later time points, rats were resuscitated over 60 min after the 90 min-hemorrhage period with Ringer’s lactate (4 × the withdraw blood volume), and killed at 60 min after resuscitation (TH210’). At 1 min before harvesting, 5 U of insulin (Bovine, Sigma) or saline (500µl) was injected into the portal vein. Body temperature of the rats were not maintained during surgery or during trauma and hemorrhage.
Experimental design and administration of GdCl3 and CLD-lipo
Clodronate was a gift of Roche Diagnostics GmbH, Mannheim, Germany. The PBS containing liposome (PBS-lipo) and clodronate containing liposome (CLD-lipo) were prepared as described (27). The PBS-lipo (200 µl) or CLD-lipo (200 µl) was injected into rats via the tail vein. Another group of rats were injected with GdCl3 (Sigma, 10 mg/kg body weight) diluted in 500 µl saline. At 48 hours post-injection, surgical trauma and hemorrhage for 90 min or 210 min were performed, and tissues were harvested for analyzing insulin signaling and kinase activity.
Procedure of Splenectomy
Splenectomy was performed either during the trauma procedure (immediately after laparotomy) or 10 days prior to the trauma and hemorrhage experiments. The rats were anesthetized and a short (~2cm) left-sided laparotomy was performed. Splenic artery, vein and nerves were tightly ligated by a silk suture to prevent any blood flow and nerve signaling (“functional splenectomy”). The spleen was then removed and the abdominal incision was sutured and bathed with 1% lidocaine to reduce postoperative pain. In sham control animals, only laparotomy was performed.
Western Blotting analysis
Liver tissue from each animal was homogenized in lysis buffer as described previously (10; 14). The tissue lysates were centrifuged and the supernatants were stored at −80°C until use. The concentrations of the protein lysates were assayed by BCA assay as described in the manufacturer’s instruction (Pierce, Rockford, IL) and the lysates (30 µg/lane) were subjected to SDS-PAGE gel and transferred to nitrocellulose membranes. The membranes were immunoblotted with anti-PY972-IR, anti-PY612-IRS1, anti-PT183/Y185-JNK1/2, (Invitrogen, Carlsbad, CA), anti-total-IR, anti-total-IRS1 (Santa Cruz Biochnology, Santa Cruz, CA), anti-pan-Actin, anti-total-ERK, anti-P473-Akt, anti-total-Akt, anti-PS180/S181-IKKα/β (Cell Signaling, Danvers, MA) antibodies. The bands were analyzed by imaging densitometry.
Immunohistochemistry
Liver or spleen tissue was rinsed in PBS and embedded in HistoPrep Frozen Tissue Embedding Media media (Fisher Scientific Inc., MA) and snap frozen in liquid nitrogen. Frozen livers were cryosectioned (10 µm) and subjected to immunohistochemical staining with anti-CD68 antibody (AbD serotec, NC). The tissue sections were fixed in 70% ethanol overnight and blocked with 0.1% sodium azide and 0.5% H2O2 in PBS for 10 minutes. Overnight incubation with the primary antibody (Anti-CD68, 1:200 in 1% BSA) was followed by HRP-conjugated secondary antibody and DAB substrate. The sections were counterstained briefly with hematoxin and images were obtained using a Leica microscope (Leica Microsystems Inc. IL).
Plasma TNFα level measurement
Just before insulin or saline injection, blood was withdrawn from the inferior vena cava in the presence of heparin, and then centrifuged at 10000 rpm for 10min. The plasma was stored at −80 C. Plasma TNFα levels were measured by the ELISA and MSD assay (Meso Scale Discovery, Gaithersburg, MD).
Densitometry and Statistical analysis
Enhanced chemiluminescence images of immunoblots were scanned and quantified using Zero D-Scan (Scanalytics Corp., Fairfax, VA). Data is presented as the mean+/−SEM. The statistical differences were analyzed by ANOVA followed by Student-Newman-Keuls post test for comparison among multiple groups or Student’s t-test for comparison between two groups. Mann-Whitney test was used when one of the data sets was not a Gaussian distribution. P ≥ 0.05 was considered not statistically significant.
RESULTS
Depletion of macrophages in liver and spleen following intravenous administration of GdCl3
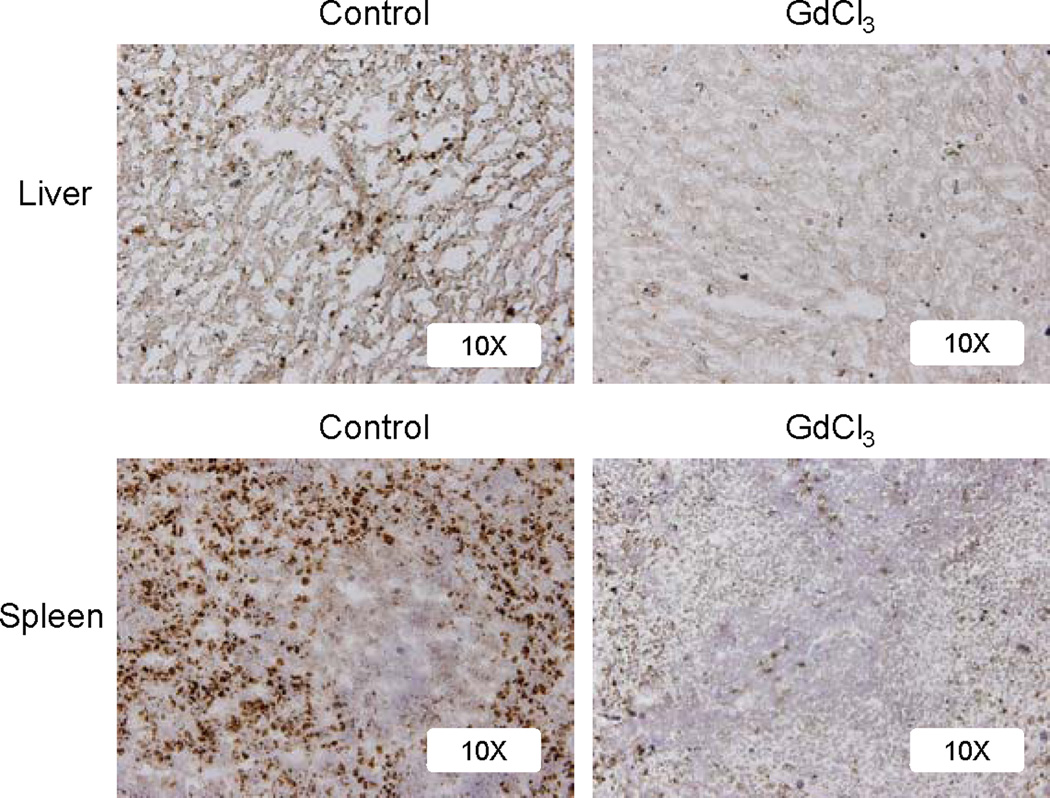
To directly determine the role of macrophages in hepatic insulin resistance following hemorrhage, GdCl3 were injected into rats via the tail vein. At 48 hours post-injection, liver and spleen tissues were frozen and sectioned and subjected to immunohisto-staining using a CD68 (a macrophage marker) antibody. Macrophages in both liver and spleen were largely ablated after administration of GdCl3 (Fig. 1).
Fig. 1. Macrophage depletion after GdCl3 administration.
Rats were injected with GdCl3 (10 mg/kg) via the tail vein. At 48 hours post-injection, surgical trauma and hemorrhage for 210 min were performed and liver and spleen tissues were harvested. Immunohistochemistry was performed on frozen liver sections with anti-CD68 antibody, followed by HRP-conjugated secondary antibody and DAB substrate. Magnification: 10×. Images are representatives of repeated experiments; the microscopic fields were randomly selected and the analysis was performed in a blinded manner.
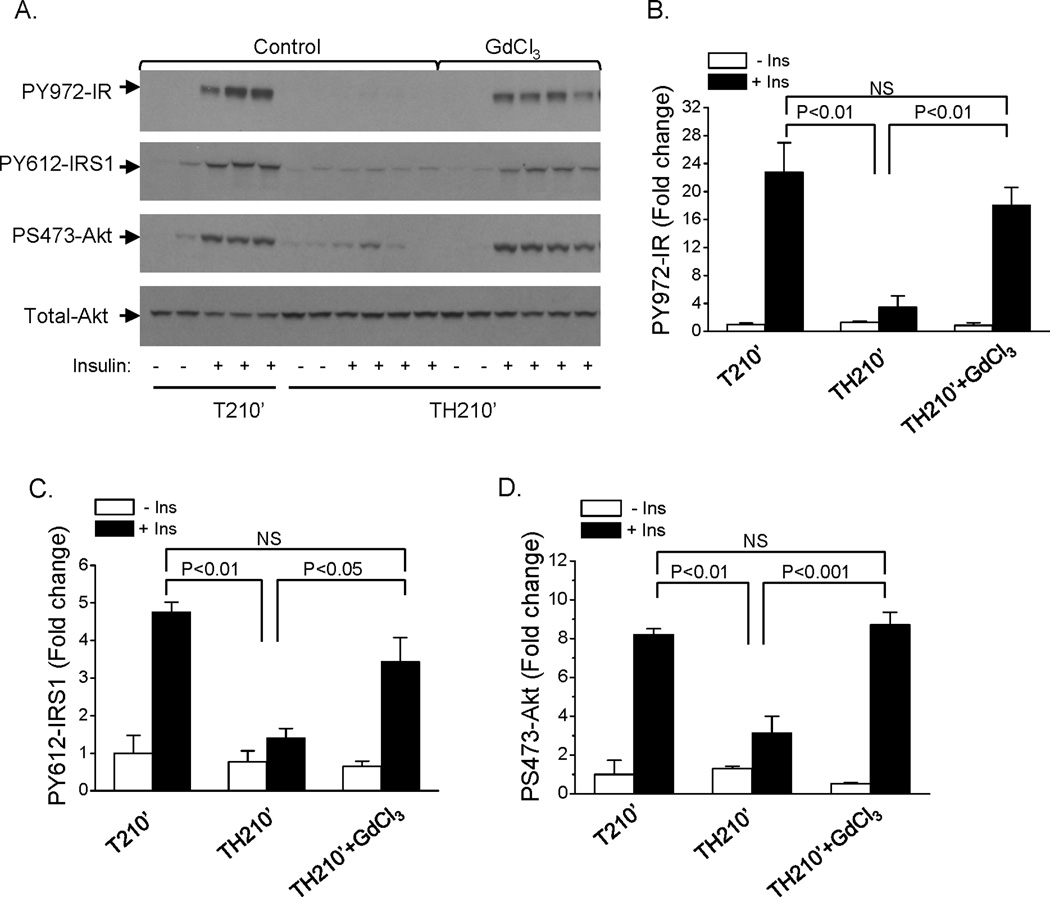
Hemorrhage-induced hepatic insulin resistance still developed after macrophage depletion by GdCl3
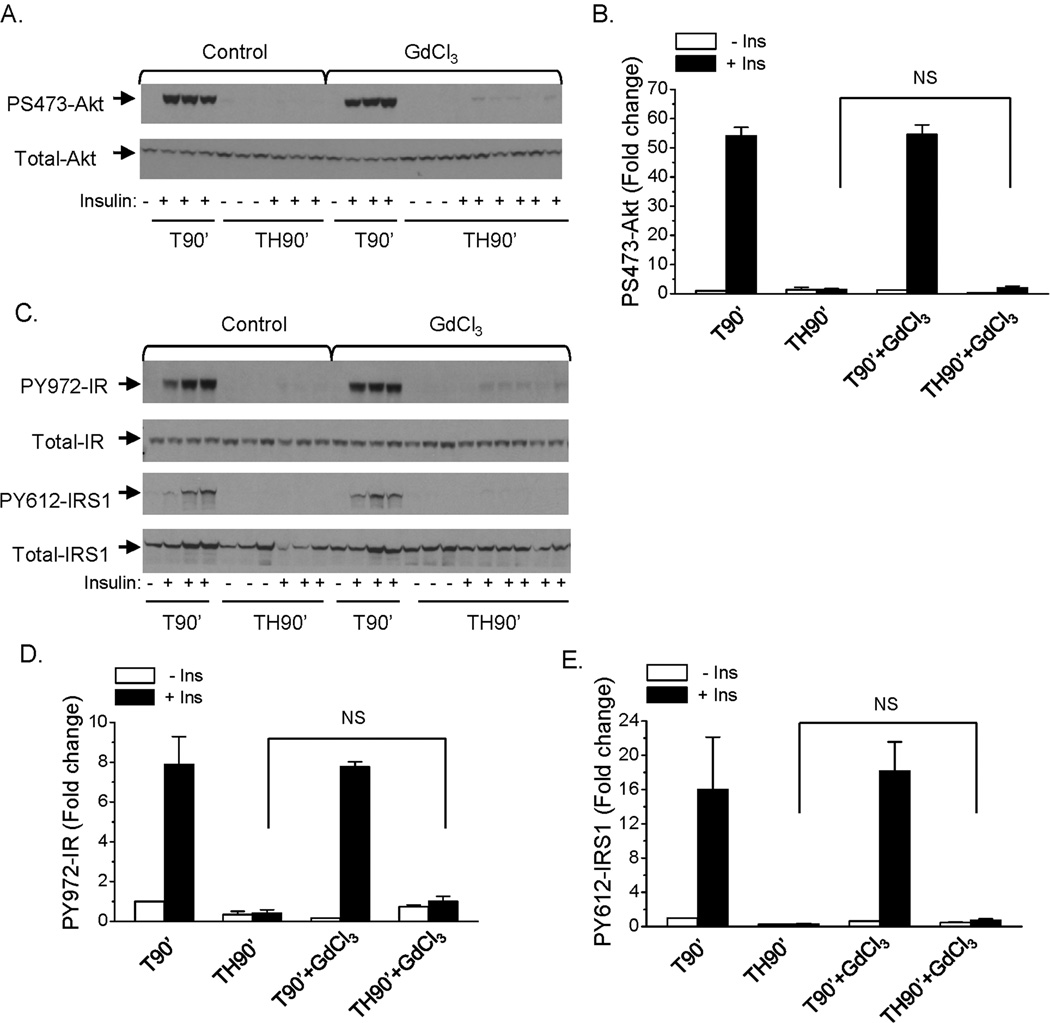
We previously reported that hepatic insulin resistance occurs in rats 90 min following trauma and hemorrhage (10–12; 14; 28). The rats were injected with GdCl3 and 48 hours post-injection, surgical trauma and hemorrhage for 90 min was performed. At the end of the 90 min hemorrhage period, prior to resuscitation, insulin was injected into the rats via the hepatic portal vein to determine the hepatic insulin signaling. Consistent with previous findings in our laboratory, insulin-induced S473-Akt phosphorylation was completely abolished after trauma and hemorrhage for 90 min in the non-injected control groups, indicating development of hemorrhage-induced hepatic insulin resistance (Fig. 2A, B). The ability of insulin to induce Akt phosphorylation was still impaired following the 90 min hemorrhage period (TH90’) after macrophage depletion by GdCl3 pretreatment (Fig. 2A, B). The expression of total Akt protein was not changed, whether the animal was treated with GdCl3 or not (Fig. 2A).
Fig. 2. Insulin signaling in liver following trauma and hemorrhage for 90 min with macrophage depletion by GdCl3.
Rats were injected with GdCl3 (10 mg/kg) via the tail vein, at 48 hours post-injection, surgical trauma and hemorrhage for 90 min was performed and liver tissues were harvested. (A). Liver protein extracts were subjected to Western Blot analysis with anti-PS473-Akt and total-Akt antibodies. (B) The level of PS473-Akt was presented as the mean +/− SEM in the graph (n=3 for each group); (C) Liver protein extracts were subjected to Western Blot analysis with anti-PY972-IR, PY612-IRS1, total-IRS1 and total-IR antibodies. (TH90’: trauma and hemorrhage for 90 min; T90’: trauma alone for 90 min). The levels of PY972-IR (D) and PY612-IRS1 (E) was presented as the mean +/− SEM in the graphs (n=3–5 for each group).
Insulin signaling upstream of Akt was examined next. The expression of total IR and IRS1 proteins were not changed by GdCl3 treatment (Fig. 2C). Similar to what occurred with P-Akt, insulin induced tyrosine phosphorylation of the insulin receptor and IRS1 (Y972-IR and Y612-IRS1, respectively) after trauma and hemorrhage for 90 min were not improved with GdCl3 pretreatment compared to control group (Fig. 2C, D, E). These data suggest that liver and spleen macrophages are not important in mediating the initial development of hemorrhage-induced hepatic insulin resistance, since the insulin resistance developed whether or not macrophages had been depleted.
No effects of macrophage depletion on activation of stress signaling pathways in liver following trauma and hemorrhage
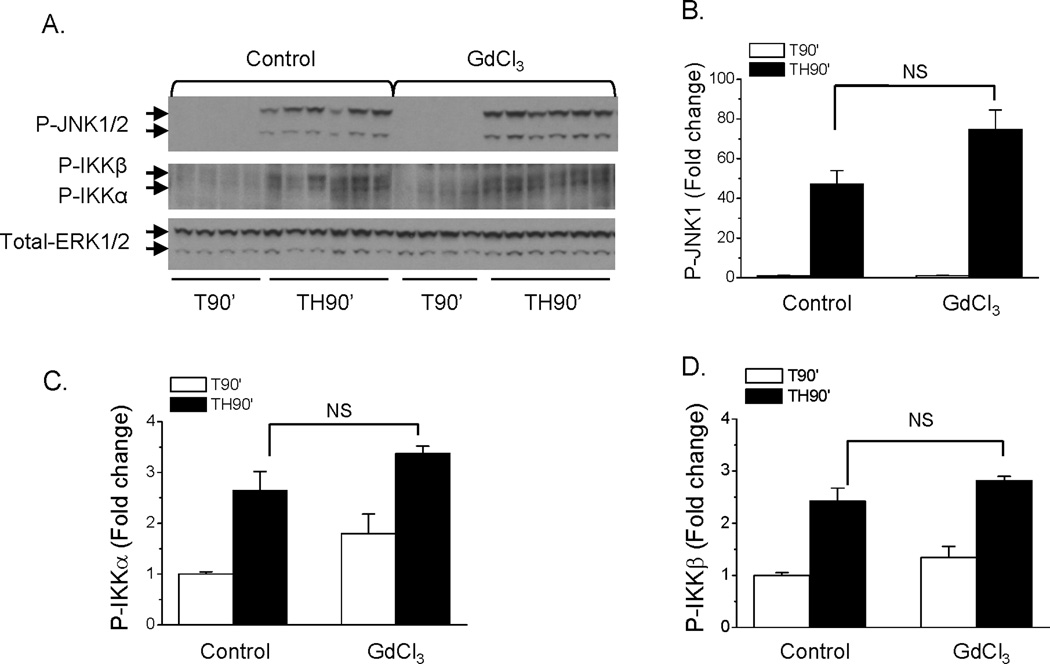
Our previous studies demonstrated activation of stress signaling pathways in liver, including the JNK and IKK pathways, which contribute to the onset of hepatic insulin resistance after trauma and hemorrhage (16). The effects of macrophage depletion on hemorrhage-induced activation of hepatic JNK and IKK kinases were examined. Consistent with our previous studies, in the control groups without GdCl3, hemorrhage resulted in increased activation/phosphorylation of JNK1/2 (Fig. 3A, B), IKKα (Fig. 3A, C) and IKKβ (Fig. 3A, D). After pretreatment with GdCl3, activation of JNK1/2, IKKα, and IKKβ were not altered after trauma and hemorrhage for 90 min compared to non-treated control groups (Fig. 3A, B, C, D). This suggests that activation of JNK1 and IKK kinases following trauma and hemorrhage are macrophage-independent.
Fig. 3. Effect of macrophage depletion by GdCl3 on the activation of JNK and IKK kinases in liver following trauma and hemorrhage.
Rats were injected with GdCl3 (10 mg/kg) via the tail vein, at 48 hours post-injection, surgical trauma and hemorrhage for 90 min was performed and liver tissues were harvested. (A). Liver protein extracts were subjected to Western Blot analysis with anti-PT181/Y183-JNK1/2, PS181-IKKβ/PS180-IKKα, and total-ERK antibodies. (B, C, D). The levels of P-JNK1 (B), P-IKKα (C) and P-IKKβ (D) in liver was presented as the mean +/− SEM in the graph (n=4–9 for each group; TH90’: trauma and hemorrhage for 90 min; T90’: trauma alone for 90 min).
No effects of macrophage depletion by CLD-lipo on the onset of hemorrhage-induced hepatic insulin resistance
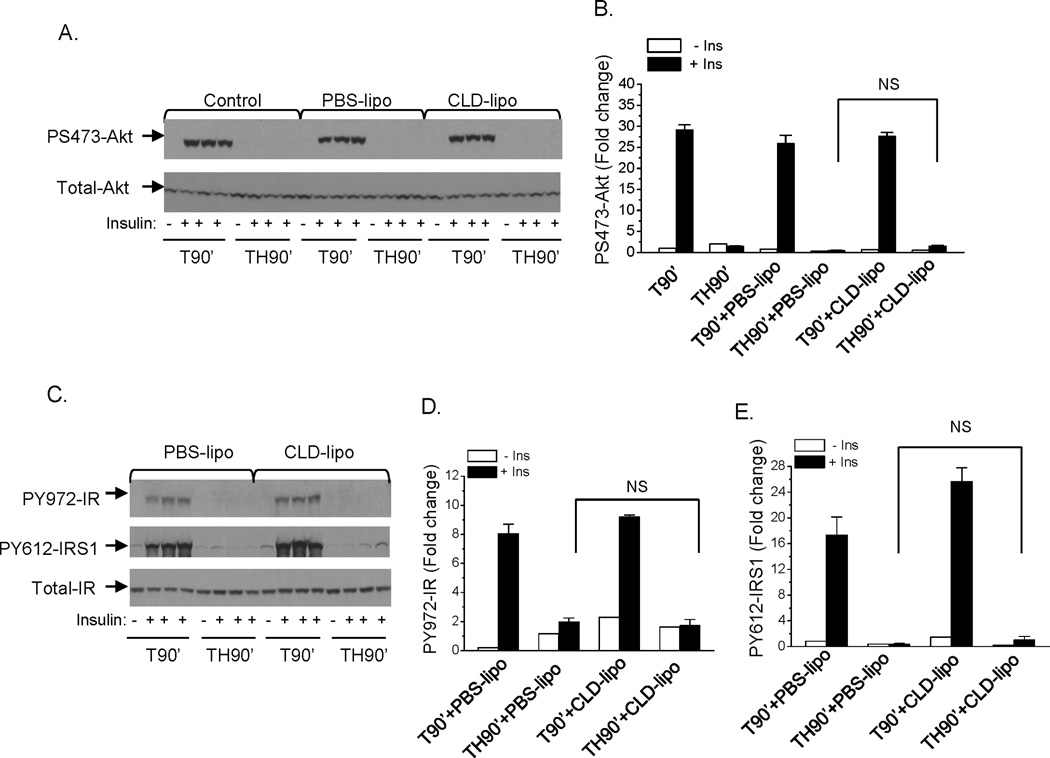
To confirm the findings with GdCl3, another approach of macrophage depletion was used, where CLD-lipo was injected into the rats via tail vein at 48 hours before surgical procedures, and either the trauma alone or the trauma and hemorrhage protocols were performed.. Similar to what was found with GdCl3, CLD-lipo pretreatment did not improve insulin signaling in liver after trauma and hemorrhage for 90 min, as determined by the levels of insulin-induced S473-Akt, Y972-IR, Y612-IRS1 phosphorylation (Fig. 4A, B, C, D, E). This further confirmed that the development of hepatic insulin resistance following trauma and hemorrhage is macrophage independent. Similar to what was found with GdCl3, activation of JNK, IKKα, and IKKβ were not affected by CLD-lipo (data not shown).
Fig. 4. Insulin signaling in liver following trauma and hemorrhage for 90 min with macrophage depletion by CLD-lipo.
Rats were injected with PBS-lipo (200 µl) or CLD-lipo (200 µl) via the tail vein, at 48 hours post-injection, surgical trauma and hemorrhage for 90 min was performed and liver tissues were harvested. (A). Liver protein extracts were subjected to Western Blot analysis with anti-PS473-Akt and total-Akt antibodies; (B) The level of PS473-Akt was presented as the mean +/− SEM in the graph (n=3 for each group); (C). Liver protein extracts were subjected to Western Blot analysis with anti-PY972-IR, PY612-IRS1 and total-IR antibodies. The levels of PY972-IR (D) and PY612-IRS1 (E) was presented as the mean +/− SEM in the graphs. (n=3–5 for each group. TH90’: trauma and hemorrhage for 90 min; T90’: trauma alone for 90 min).
No effects of splenectomy on the onset of hemorrhage-induced hepatic insulin resistance
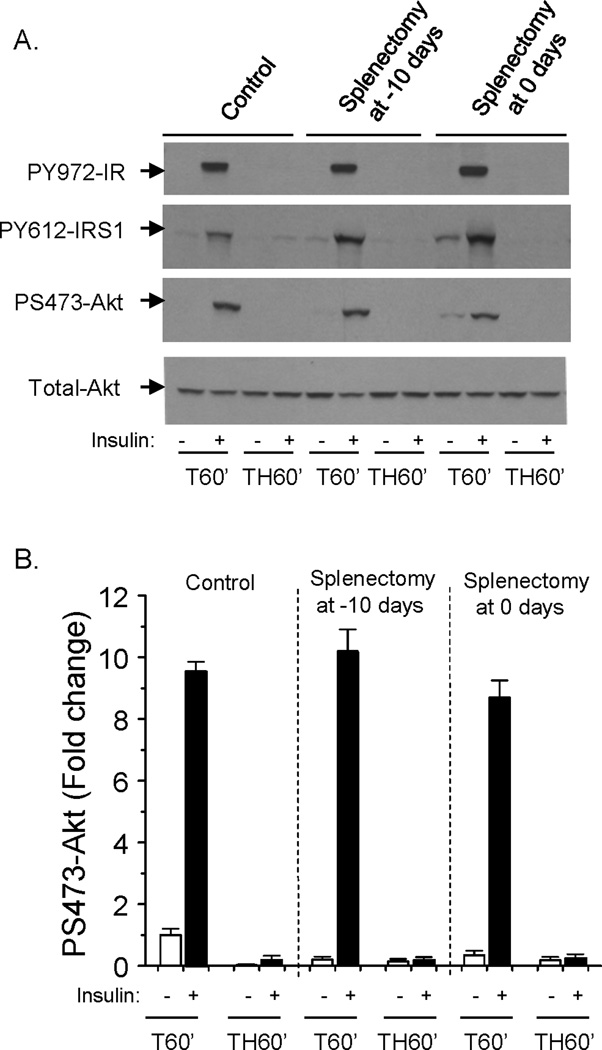
To determine a potential role of the spleen in the acute development of hepatic insulin resistance following trauma and hemorrhage, splenectomy was performed at either 10 days before or immediately before hemorrhage. Hepatic insulin signaling following trauma and hemorrhage for 60 min (TH60’) was not improved after splenectomy, as insulin signaling determined by insulin-induced phosphorylation of Y972-IR, Y612-IRS1 and S473-Akt (Fig. 5A, B). This suggests that the spleen is not necessary for the acute development of hemorrhage-induced hepatic insulin resistance.
Fig. 5. Hepatic insulin signaling following trauma and hemorrhage for 60 min after splenectomy.
Splenectomy was performed on rats at 10 days before or immediately prior to the surgical trauma and hemorrhage procedure. (A). Liver protein extracts were subjected to Western Blot analysis with anti-PS473-Akt, PY612-IRS1, PY972-IR, and total-Akt antibodies. (B). The level of PS473-Akt is presented as the mean +/− SEM in the graph. (ins: insulin; n=3 rats for each group; TH60’: trauma and hemorrhage for 60 min; T60’: trauma alone for 60 min).
Recovery of hepatic insulin signaling following macrophage depletion
As a dominant role of TNFα in the later maintenance of insulin resistance has been indicated in our previous studies, the effect of macrophage depletion on hepatic insulin signaling later, following resuscitation, was next determined. Unlike what was found at the earlier time point (TH90’), the ability of insulin to induce phosphorylation of Akt, IR and IRS1 in the liver of rats several hours following hemorrhage and resuscitation (TH210’) was significantly increased after macrophage depletion by GdCl3 (Fig. 6 A, B, C, D). This suggests that macrophages have an important role in maintaining the insulin resistant state later, despite not being necessary for the onset of insulin resistance.
Fig. 6. Insulin signaling in liver following trauma and hemorrhage for 210 min after resuscitation with macrophage depletion by GdCl3.
Rats were injected with GdCl3 (10 mg/kg) via the tail vein, at 48 hours post-injection, surgical trauma and hemorrhage for 90 min were performed and after resuscitation over 60 min and recovery in the cage for 60 min, liver tissues were harvested. (A). Liver protein extracts were subjected to Western Blot analysis with anti-PS473-Akt, PY972-IR, PY612-IRS1, and total-Akt antibodies. The levels of PY972-IR (B), PY612-IRS1 (C), and PS473-Akt (D) are presented as the mean +/− SEM in the graph (n=2–4 for each group; TH210’: trauma and hemorrhage for 90 min + resuscitation for 60 min + recovery for 60 min; T210’: trauma alone for 210 min).
Expression of IGFBP-1 is normally inhibited by insulin via activation of the PI3K/Akt pathway (10). Similar to our previous studies measuring IGFBP-1 mRNA by Northern blots (10; 12), there was an increase in IGFBP-1 by trauma alone, and a further 1.5-fold induction by the combination of trauma and hemorrhage. Treatment of rats with GdCl3 completely abolished the hemorrhage-induced increase in IGFBP-1, significantly (p<0.05) reducing the level to that of the trauma-only control animal. This suggests that macrophage depletion could also restore insulin’s action downstream of Akt activation.
Partial recovery of hepatic insulin signaling following splenectomy
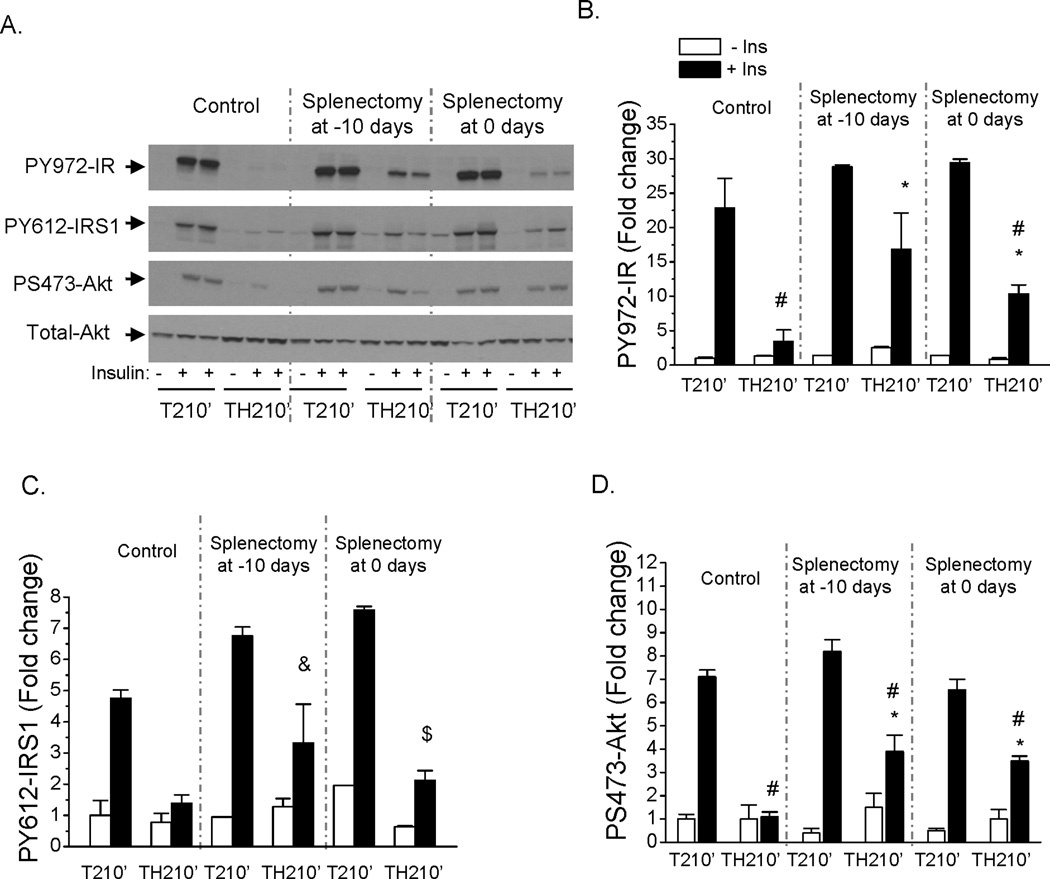
To better understand whether the later maintenance of hepatic insulin resistance is mainly attributed to local liver macrophages or systemic factors derived from the spleen, insulin signaling in the liver of rats after spleen removal was examined. As opposed to almost complete recovery in insulin signaling with macrophage depletion of both liver and spleen, splenectomy at either 10 days before or right before hemorrhage resulted in a partial improvement in insulin induced PY972-IR, PY612-IRS1 and PS473-Akt at 210 min following trauma and hemorrhage after resuscitation (Fig. 7A, B, C, D). This suggests that both liver and spleen have a role in maintaining the hepatic insulin resistant state later, following resuscitation.
Fig. 7. Partial recovery of hepatic insulin signaling following trauma and hemorrhage for 210 min after splenectomy.
Splenectomy was performed on rats at 10 days before or immediately prior to the surgical trauma and hemorrhage procedure. After a hemorrhage period of 90 min, resuscitation over 60 min and recovery in the cage for 60 min, liver tissues were harvested. (A). Liver protein extracts were subjected to Western Blot analysis with anti-PS473-Akt, PY972-IR, PY612-IRS1, and total-Akt antibodies. The levels of PY972-IR (B), PY612-IRS1 (C), and PS473-Akt (D) are presented as the mean +/− SEM in the graph (n=2–4 for each group; TH210’: trauma and hemorrhage for 90 min + resuscitation for 60 min + recovery for 60 min; T210’: trauma alone for 210 min; #: P<0.05 vs T210’+ins in control or splenectomized groups respectively; * : P<0.05 vs TH210’ +ins in non-splenectomized control group; &: P=0.064 vs TH210’ +ins in non-splenectomized control group; $: P=0.055 vs TH210’ +ins in non-splenectomized control group).
Macrophage depletion or splenectomy resulted in decreased circulating TNFα level following resuscitation
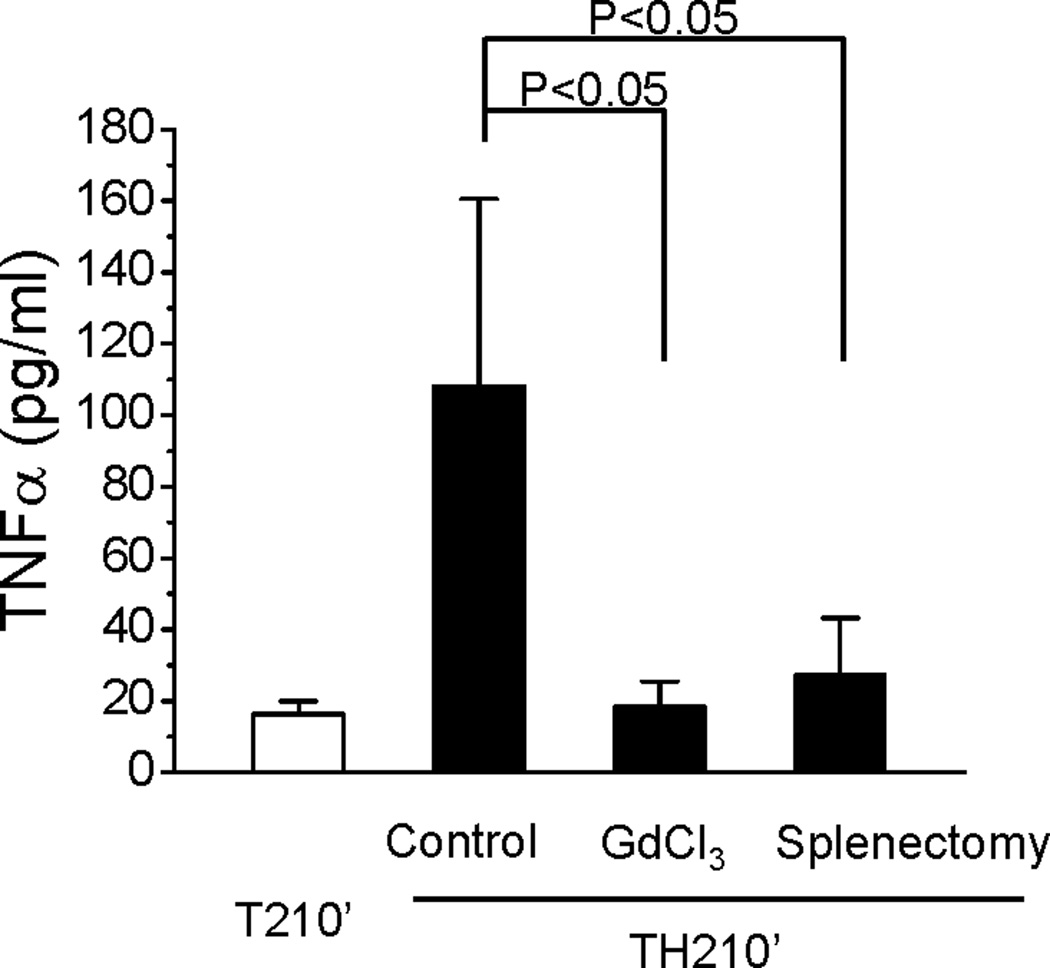
We next determined the effects of macrophage depletion or splenectomy on the levels of circulating TNFα in rats following trauma and hemorrhage and resuscitation. Consistent with previous data (11), at 210 min following trauma, hemorrhage and resuscitation, circulating TNFα increased to an average of 108 pg/ml compared to 16 pg/ml following trauma alone. After depletion of Kupffer cells and splenic macrophages by GdCl3, the level of circulating TNFα decreased significantly to an average of 18 pg/ml (Fig. 8) at 210 min following trauma and hemorrhage, similar to the value in the absence of hemorrhage. Circulating TNFα also decreased in rats after removal of spleen at 10 days before surgery and 210 min after trauma and hemorrhage (27.3pg/ml, Fig. 8), approaching the values in the absence of hemorrhage or following GdCl3, and significantly less than trauma and hemorrhage without splenectomy. This data confirms that macrophages in general, and the spleen in particular, are important sources of circulating TNFα following trauma, hemorrhage and resuscitation, and this TNFα has important role in the maintenance of the insulin resistant state in the liver of rats later following resuscitation (12).
Fig. 8. Levels of circulating TNFα following trauma and hemorrhage for 210 min after resuscitation with macrophage depletion or splenectomy.
Rats were injected with GdCl3 (2 days before surgical procedures) or subjected to splenectomy (10 days before surgical procedures) as previously described. Surgical trauma and hemorrhage for 90 min were performed and after resuscitation over 60 min and recovery in the cage for 60 min, liver tissues were harvested. The level of plasma TNFα are presented as the mean +/− SEM in the graph (n=4 –10 for each group; TH210’: trauma and hemorrhage for 90 min + resuscitation for 60 min + recovery for 60 min; T210’: trauma alone for 210 min).
DISCUSSION
With a surgical trauma and hemorrhage model of critical illness diabetes, we have previously demonstrated a causative role of ROS in the rapid development of hemorrhage-induced hepatic insulin resistance and a dominant role of TNFα in the later maintenance of the insulin resistant state (11; 12; 14). The aim of the present work is to understand the specific cellular sources of ROS or TNFα and the type of cells involved in the pathogenesis of acute insulin resistance. Our current data demonstrated that macrophages, in both liver and spleen, are not necessary for the acute onset of insulin resistance following trauma and hemorrhage, but contribute to the maintenance of the insulin resistant state later after resuscitation.
Following hemorrhage or experimental liver ischemia, most studies have focused on the activity and function of macrophages after resuscitation or reperfusion, in which macrophages are classically activated and produce a number of pro-inflammatory factors and ROS (29; 30). However, not much is known about the activity and role of macrophages during the hemorrhage or ischemia period, prior to resuscitation or reperfusion. We previously found that increased production of ROS and activation of the cell stress (JNK) and inflammatory (IKK/NFκB) pathways occur within minutes following hemorrhage, which play a key role in the rapid development of hepatic insulin resistance, partly through inducing inhibitory IRS-1 serine phosphorylation (15; 16).. CLD-lipo and GdCl3 have been widely used experimentally in elucidating the role of macrophages and are able to deplete macrophages in both the liver and spleen (25; 26; 31–34). In the present studies, both approaches, GdCl3, and CLD-lipo, were used, and demonstrated that after depletion of macrophages, hepatic insulin resistance still developed after the combination of trauma and hemorrhage. In addition, the development of hepatic insulin resistance cannot be prevented by splenectomy. Therefore the initial development of hepatic insulin resistance, during the hemorrhage period, was macrophage-independent. In the absence of macrophages in the liver and spleen, the observed activation of JNK and IKK kinases in liver after trauma and hemorrhage likely occurs within hepatocytes, in which they directly interact with IR or IRS proteins and inhibit insulin signal transduction.
Our previous studies demonstrated that inhibition of ROS by NAC resulted in decreased activation of JNK and IKK kinases in liver (15), suggesting ROS is the upstream inducer of JNK and IKK activation in liver following trauma and hemorrhage. The unaltered activation of JNK and IKK following macrophage depletion suggests that macrophages are not a major source of ROS during the hemorrhage period. Thus, other cells, such as hepatocytes or endothelial cells, may be the source of ROS in response to trauma and hemorrhage. When macrophages are eliminated prior to trauma and hemorrhage, other cells are capable of providing sufficient ROS which results in activation of JNK and IKK, and, therefore, insulin resistance can still develop in the absence of Kupffer cells and splenic macrophages.
The recovery of hepatic insulin signaling, later, following resuscitation, by depletion of macrophages suggests that macrophages are important for maintenance of the insulin resistant state following its initial development. Our previous findings indicate that increased circulating TNFα has a role in hepatic insulin resistance following resuscitation (12), but TNFα does not increase rapidly enough to explain the initial development of insulin resistance (manuscript submitted). In the present studies, the circulating level of TNFα following resuscitation, several hours after the initial trauma and hemorrhage, was largely decreased after macrophage depletion, that is depletion of Kupffer cells, splenic macrophages, and other macrophage populations. In addition, the circulating level of TNFα was also significantly decreased by removal of the spleen, suggesting that much of the increased TNFα following trauma and hemorrhage was from the spleen. Thus, tissue macrophages in the liver or spleen, are likely activated in response to blood loss and resuscitation to release sufficient inflammatory factors, such as TNFα, which acts systemically or locally on hepatocytes and inhibits hepatic insulin signaling.
The spleen is reported as one of the main sites producing inflammatory mediators, including TNFα, in response to ischemia reperfusion injury (19; 20). Compared to GdCl3 treatment, which deplete macrophages in both liver and spleen with nearly complete reversal of insulin resistance following resuscitation, removal of the spleen also resulted in improvement in hepatic insulin signaling, but to a lesser extent. This suggests that the spleen has a role in maintaining the insulin resistant state following resuscitation, at least in part by inhibiting hepatic insulin signaling, and is an important source of circulating TNFα.
In summary, the present studies demonstrated differential roles of macrophages between the initial development and later maintenance of hemorrhage-induced hepatic insulin resistance. Our findings suggest that the initial onset of insulin resistance is macrophage independent, whereas following resuscitation macrophages in both liver and spleen are activated and release factors, such as TNFα locally and into the circulation, which result in the maintenance of insulin resistance.
ACKNOWLEDGEMENTS
We would like to thank Drs. Li Li,, and John L. Franklin for their help with techniques. We would like to acknowledge the UAB Comparative Pathology Laboratory for frozen tissue cryosection. We would also like to acknowledge Dr. Nico van Rooijen and Roche Diagnostics GmbH, Mannheim, Germany for providing the CLD-liposome reagent. We also thank the Metabolism Core lab of the UAB Nutrition and Obesity Research Center (P30 DK56336) for help in measuring TNFα levels.
The research is supported by grants from the National Institutes of Health (DK 62071), the Department of Defense (W81XWH-0510387) and the Veterans Administration Merit Review to J.L.M.
ABBREVIATION
- CLD-lipo
Clodronate-liposome
- GdCl3
Gadolinium chloride
- IR
insulin receptor
- IRS
insulin receptor substrate
- PI3K
phosphoinositide 3-kinase
- IKK
Inhibitory κB kinase
- JNK
c-Jun N-teminal kinase
- TH
trauma and hemorrhage
- T
trauma alone
- ERK
extracellular signal-regulated kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ikezu T, Okamoto T, Yonezawa K, Tompkins RG, Martyn JA. Analysis of thermal injury-induced insulin resistance in rodents. Implication of postreceptor mechanisms. J Biol Chem. 1997;272:25289–25295. doi: 10.1074/jbc.272.40.25289. [DOI] [PubMed] [Google Scholar]
- 2.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 3.Thorell A, Nygren J, Ljungqvist O. Insulin resistance: a marker of surgical stress. Curr Opin Clin Nutr Metab Care. 1999;2:69–78. doi: 10.1097/00075197-199901000-00012. [DOI] [PubMed] [Google Scholar]
- 4.McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. 2001;17:107–124. doi: 10.1016/s0749-0704(05)70154-8. [DOI] [PubMed] [Google Scholar]
- 5.Agwunobi AO, Reid C, Maycock P, Little RA, Carlson GL. Insulin resistance and substrate utilization in human endotoxemia. J Clin Endocrinol Metab. 2000;85:3770–3778. doi: 10.1210/jcem.85.10.6914. [DOI] [PubMed] [Google Scholar]
- 6.Cree MG, Wolfe RR. Postburn trauma insulin resistance and fat metabolism. Am J Physiol Endocrinol Metab. 2008;294:E1–E9. doi: 10.1152/ajpendo.00562.2007. [DOI] [PubMed] [Google Scholar]
- 7.Bochicchio GV, Sung J, Joshi M, Bochicchio K, Johnson SB, Meyer W, Scalea TM. Persistent hyperglycemia is predictive of outcome in critically ill trauma patients. J Trauma. 2005;58:921–924. doi: 10.1097/01.ta.0000162141.26392.07. [DOI] [PubMed] [Google Scholar]
- 8.Gore DC, Chinkes D, Heggers J, Herndon DN, Wolf SE, Desai M. Association of hyperglycemia with increased mortality after severe burn injury. J Trauma. 2001;51:540–544. doi: 10.1097/00005373-200109000-00021. [DOI] [PubMed] [Google Scholar]
- 9.Laird AM, Miller PR, Kilgo PD, Meredith JW, Chang MC. Relationship of early hyperglycemia to mortality in trauma patients. J Trauma. 2004;56:1058–1062. doi: 10.1097/01.ta.0000123267.39011.9f. [DOI] [PubMed] [Google Scholar]
- 10.Ma Y, Wang P, Kuebler JF, Chaudry IH, Messina JL. Hemorrhage induces the rapid development of hepatic insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2003;284:G107–G115. doi: 10.1152/ajpgi.00217.2002. [DOI] [PubMed] [Google Scholar]
- 11.Ma Y, Toth B, Keeton AB, Holland LT, Chaudry IH, Messina JL. Mechanisms of hemorrhage-induced hepatic insulin resistance: role of tumor necrosis factor-alpha. Endocrinology. 2004;145:5168–5176. doi: 10.1210/en.2004-0524. [DOI] [PubMed] [Google Scholar]
- 12.Xu J, Kim HT, Ma Y, Zhao L, Zhai L, Kokorina N, Wang P, Messina JL. Trauma and Hemorrhage-Induced Acute Hepatic Insulin Resistance: Dominant Role of Tumor Necrosis Factor (TNF)-alpha. Endocrinology. 2008;149:2369–2382. doi: 10.1210/en.2007-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Messina JL. Acute insulin resistance following injury. Trends Endocrinol Metab. 2009;20:429–435. doi: 10.1016/j.tem.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Thompson LH, Zhao L, Messina JL. Tissue Specific Difference in the Molecular Mechanisms for the Development of Acute Insulin Resistance Following Injury. Endocrinology. 2009;150:24–32. doi: 10.1210/en.2008-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhai L, Ballinger SW, Messina JL. Role of Reactive Oxygen Species in Injury-induced Insulin Resistance. Mol Endocrinol. doi: 10.1210/me.2010-0224. 10 A.D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang S, Messina JL. Role of Inhibitory {kappa}B Kinase and c-Jun N-Terminal Kinase in the Development of Hepatic Insulin Resistance in Critical Illness Diabetes. Am J Physiol Gastrointest Liver Physiol. 2011 doi: 10.1152/ajpgi.00148.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G256–G265. doi: 10.1152/ajpgi.00550.2001. [DOI] [PubMed] [Google Scholar]
- 18.Zhu XL, Zellweger R, Zhu XH, Ayala A, Chaudry IH. Cytokine gene expression in splenic macrophages and Kupffer cells following haemorrhage. Cytokine. 1995;7:8–14. doi: 10.1006/cyto.1995.1002. [DOI] [PubMed] [Google Scholar]
- 19.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang H, Meng F, Li W, Tong L, Qiao H, Sun X. Splenectomy ameliorates acute multiple organ damage induced by liver warm ischemia reperfusion in rats. Surgery. 2007;141:32–40. doi: 10.1016/j.surg.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 21.Permana PA, Menge C, Reaven PD. Macrophage-secreted factors induce adipocyte inflammation and insulin resistance. Biochem Biophys Res Commun. 2006;341:507–514. doi: 10.1016/j.bbrc.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 22.de LC, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582:97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adding LC, Bannenberg GL, Gustafsson LE. Basic experimental studies and clinical aspects of gadolinium salts and chelates. Cardiovasc Drug Rev. 2001;19:41–56. doi: 10.1111/j.1527-3466.2001.tb00182.x. [DOI] [PubMed] [Google Scholar]
- 25.Abshagen K, Eipel C, Kalff JC, Menger MD, Vollmar B. Loss of NF-kappaB activation in Kupffer cell-depleted mice impairs liver regeneration after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1570–G1577. doi: 10.1152/ajpgi.00399.2006. [DOI] [PubMed] [Google Scholar]
- 26.van RN, Sanders A. Elimination, blocking, and activation of macrophages: three of a kind? J Leukoc Biol. 1997;62:702–709. doi: 10.1002/jlb.62.6.702. [DOI] [PubMed] [Google Scholar]
- 27.van RN, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 28.Thompson LH, Kim HT, Ma Y, Kokorina N, Messina JL. Acute muscle-type specific insulin resistance following injury. Mol Med. 2008;11–12:715–723. doi: 10.2119/2008-00081.Thompson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwabe RF, Brenner DA. Mechanisms of Liver Injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583–G589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 30.Jarrar D, Wang P, Cioffi WG, Bland KI, Chaudry IH. Critical role of oxygen radicals in the initiation of hepatic depression after trauma hemorrhage. J Trauma. 2000;49:879–885. doi: 10.1097/00005373-200011000-00015. [DOI] [PubMed] [Google Scholar]
- 31.O'Neill PJ, Ayala A, Wang P, Ba ZF, Morrison MH, Schultze AE, Reich SS, Chaudry IH. Role of Kupffer cells in interleukin-6 release following trauma-hemorrhage and resuscitation. Shock JID - 9421564. 1994;1:43–47. doi: 10.1097/00024382-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Meijer C, Wiezer MJ, Diehl AM, Schouten HJ, Schouten HJ, Meijer S, van RN, van Lambalgen AA, Dijkstra CD, van Leeuwen PA. Kupffer cell depletion by CI2MDP-liposomes alters hepatic cytokine expression and delays liver regeneration after partial hepatectomy. Liver. 2000;20:66–77. doi: 10.1034/j.1600-0676.2000.020001066.x. [DOI] [PubMed] [Google Scholar]
- 33.Sturm E, Havinga R, Baller JF, Wolters H, van RN, Kamps JA, Verkade HJ, Karpen SJ, Kuipers F. Kupffer cell depletion with liposomal clodronate prevents suppression of Ntcp expression in endotoxin-treated rats. J Hepatol. 2005;42:102–109. doi: 10.1016/j.jhep.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Jiang S, Gavrikova TA, Pereboev A, Messina JL. Adenovirus infection results in alterations of insulin signaling and glucose homeostasis. Am J Physiol Endocrinol Metab. 2010;298:E1295–E1304. doi: 10.1152/ajpendo.00723.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]