Abstract
Intracellular signaling mechanisms translate extracellular signals, such as neuronal activity, into effects on dendrite complexity. Deciphering these mechanisms has considerable impact on understanding how the brain develops and what can go wrong in developmental disorders. How neurons regulate intracellular signaling to control their dendrite morphology remains poorly understood and is likely to be determined at the level of individual neuronal types. Calcium/Calmodulin-dependent protein kinase IV (CaMKIV) is a signaling mechanism involved in the regulation of gene expression and dendrite growth. Expression of CaMKIV is developmentally regulated in the cerebral cortex, with highest expression occurring concomitant with the period of extensive dendrite growth and elaboration. Interestingly, cortical neurons heterogeneously expressed CaMKIV in postnatal rat cortices and cortical neurons in vitro. We tested if this differential CaMKIV expression mediated distinct arborization patterns in the dendrites of pyramidal and nonpyramidal neurons. In fact, CaMKIV mediated dendrite complexity via regulation of specific morphological features of the dendrite arbor: branching and elongation, but not primary dendrite formation. We found that siRNA knockdown of CaMKIV decreased basal dendrite complexity indicating that endogenously expressed CaMKIV mediated dendrite complexity. CaMKIV was also required for activity-induced dendrite elaboration. Active CaMKIV expression in cortical neurons increased dendrite elaboration indicating that enzymatic activity was involved. These data indicated neuronal CaMKIV expression was required for basal and activity-induced dendrite complexity. Further, the data presented in this study indicates CaMKIV contributes to the diversity of dendrite arbors via restricted expression and regulation of distinct modes of dendrite elaboration.
Keywords: calcium calmodulin dependent protein kinase IV, dendrite arbor, branching, calcium signaling, siRNA
One of the most striking features of neurons is the morphological diversity of dendrites. Two major questions in dendrite development and complexity are how neurons establish specific morphological patterns and the role of neuronal activity in these events. Basic features of neuronal morphology are established prior to the arrival of afferent fibers, but as afferent fibers arrive and establish adult like distribution patterns, dendrites elaborate and dendrite spines form (Wise et al., 1979). Elevation and attenuation of neuronal activity in vivo respectively enhanced (Holloway, 1966; Volkmar and Greenough, 1972; Greenough and Volkmar, 1973) and diminished (Wiesel and Hubel, 1963; Benes et al., 1977; Rajan and Cline, 1998) dendrite complexity. Neurons in vitro respond likewise to neuronal activity (McAllister et al., 1996; Redmond et al., 2002; Vaillant et al., 2002; Yu and Malenka, 2002). Further, in vivo dendrites dramatically reorganize in response to afferent input (Steffen and Van der Loos, 1980; Wong and Ghosh, 2002). Molecular mechanisms that respond to neuronal activity are critical and when absent in humans and mice result in diminished mental capability and altered dendrite complexity (Rubinstein, 1990; Soekarman and Fryns, 1994; Bourtchuladze et al., 1994; Glazewski et al., 1999; Elgersma et al., 2004).
The mechanism by which neuronal activity conveys information to the receiving neuron resulting in structural change in dendrite complexity is, without doubt, complex (Redmond, 2008). In fact, the specific response may be dictated at the resolution of individual cortical neuronal types. For example, Juraska (1982) reported an increase in layer 3, but not layer 5, pyramidal neuron dendrite length while spine density increased in both layers 3 and 5 after eye opening in the rat. The mechanism for this differential response may involve a different reliance on glutamate receptors for mediating activity-induced morphological changes (Chen et al., 2009; Redmond et al., 2002). A difference in expression or differential regulation of intracellular signaling molecules may also contribute (Ha and Redmond, 2008; Sík et al., 1998).
In addition to regulation at the level of individual neuronal types, morphological determinants of dendrite complexity, including dendrite length, number, and branches, are independently regulated within a neuronal type. For example in layer 6 pyramidal neurons, the full-length TrkB receptor mediated branching of proximal dendrites whereas a truncated TrkB receptor mediated elongation of dendrites (Yacoubian and Lo, 2000). The difference these two TrkB receptor variants created in the dendrite arbor and complexity has significant implications for neuronal connectivity and signal processing (Häusser et al., 2000).
Although neuronal activity exerts a tremendous impact on neuronal circuits and connectivity, how a neuron regulates specific features of the dendrite arbor in response to activity is only beginning to be understood. However, the mechanisms mediating neuronal activity induced changes in neuronal complexity are well known and include calcium influx via voltage sensitive calcium channels (VSCC) and N-methyl-D-aspartate (NMDA) receptors (Redmond et al., 2002; Yu and Malenka, 2003; Wayman et al., 2006) and activation of calcium/calmodulin dependent protein kinase (CaMK) and extracellular signal related kinase (ERK) pathways to regulate cAMP Response Element Binding protein (CREB)-mediated gene transcription (Redmond et al., 2002; Vaillant et al., 2002; Wayman et al., 2006). The ERK pathway was recently found to differently modulate the morphological determinants of dendrite complexity (Ha and Redmond, 2008). This result indicates intracellular signaling mechanisms can transduce extracellular signals to regulate the mode of dendrite elaboration resulting in specific dendrite patterns. The extent to which other intracellular signaling mechanisms are selective in regulating the mode of dendrite elaboration is unclear. In the present study, we determined that CaMKIV mediated selective components of morphological complexity in basal conditions and in response to neuronal activity.
EXPERIMENTAL PROCEDURES
2.1 - Cortical Cultures
Cultures of cerebral cortical neurons were established from Long-Evans embryonic day 18 (E18) rat embryos as described in Ha and Redmond (2008). Briefly, cortices were dissected in ice-cold Hanks Balanced Salt Solution and incubated in a papain (Worthington Biochemical) solution containing L-cysteine (Sigma) in dissociation media (DM). The cortices were then transferred to an inhibitor solution containing trypsin (Sigma) and bovine serum albumin (BSA) in DM. The cortices were triturated in Basal Medium Eagle supplemented with 5% fetal bovine serum, 1mM L-glutamine, and penicillin/streptomycin (all Invitrogen). Dissociated cortical cells were plated onto poly-D-lysine and laminin (BD Biosciences) coated coverslips at a density of 0.5 × 106 cells/well in 12 well plates, 0.25 × 106 cells/well in 24 well plates, and 1.5 × 106 cells per 35mm dish. Cells were maintained in Basal Media Eagle supplemented with 1mM glutamine, 1% N-2, 5% fetal bovine serum, and penicillin/streptomycin. Media was changed at 4 days in vitro (DIV) for cultures maintained for 8 DIV.
2.2 – Neuronal transfection and stimulation
Neurons were transfected with previously described mammalian expression plasmids by a modified calcium phosphate method (Redmond et al., 2002). Transfection experiments were performed in duplicate wells and repeated multiple times. Neurons in 12 well plates were singly transfected with 0.7ug of pEGFP (Clonetech) or co-transfected with pEGFP and either CaMKIVwt, CaMKIVca, or parent vector (pv) (Sun et al., 1994) at 3 DIV at a molar ratio of 1:4 (GFP:CaMKIV). Cultures were stimulated as indicated at either 4 DIV or 7 DIV with 50mM potassium chloride (KCl) or as a control, 50mM sodium chloride (NaCl).
2.3 - siRNA
Three different double stranded siRNAs specific to rat CaMKIV were synthesized (Invitrogen) and screened for efficient CaMKIV knockdown. Effectiveness and duration of treatment necessary for siRNA to decrease CaMKIV was assayed by Western blots of HEK 293 cells transfected with siRNA and CaMKIVwt or CaMKIVres. siRNA transfections were performed using Lipofectamine 2000™ (Invitrogen). HEK 293 cells in 24 well plates were transfected with 0.7 μg of either GFP tagged wild type CaMKIV (CaMKIVwt) or siRNA resistant CaMKIV (CaMKIVres) with or without 7 pmoles (85ng) siRNA. Cells were harvested as indicated at 48hrs or 72hrs post-transfection. Cell lysates were western blotted and probed with anti-CaMKIV antibodies (1:2000; BD Transduction labs). The blots were then stripped and reprobed with anti-GFP antibodies (Invitrogen). To determine effective knockdown in neurons, 4 DIV cortical cultures plated in 24 wells were transfected with 0.2 μg plasmid DNA (pEGFP, CaMKIVwt or CaMKIVres) and 7 pmoles (85ng) of either siRNA or scrambled siRNA. At 48hrs, 72hrs, and 90 hrs post-transfection cultures were fixed with 4% paraformaldehyde (PFA) and 4% sucrose in phosphate-buffered saline (PBS), immunostained with anti-CaMKIV antibodies, imaged and intensity quantified as described below. All cultures were labeled with Hoechst to determine cell viability. No notable increase in the number of apoptotic cells was detected. Any neurons showing signs of cell death (blebbed or crenated nuclei) were excluded from analyses.
The third siRNA tested (siRNA#3) efficiently knocked down CaMKIV expression and was the siRNA used for all siRNA experiments. The sense and anti-sense sequence of siRNA#3 is CUAAGAAGCGGCUGACUAC and GUAGUCAGCCGCUUCUUAG, respectively. The sense and anti-sense sequence of scrambled siRNA is GCCAGCGUCGAAGUACUAA and UUAGUACUUCGACGCUGGC, respectively. No matches for the scrambled siRNA were identified in searches of multiple databases including the nucleotide and reseq databases. siRNA insensitive CaMKIV wild type (CaMKIVres) was generated by incorporating two base pair mismatches in the siRNA recognition region. Point mutations in CaMKIVres were generated using site directed mutagenesis kit (Stratagene). CaMKIVwt and CaMKIVres were inserted into pEGFP by PCR to generate GFP tagged versions.
2.4 - Immunocytochemistry and Immunohistochemistry
Neuronal cultures were fixed with 4% paraformaldehyde (PFA)/4% sucrose in PBS, washed with PBS and incubated in blocking buffer containing 3% BSA, 0.3% TritonX-100, 0.02% sodium azide, 3% goat serum in PBS. Coverslips were immunostained with CaMKIV (1:500; BD Transduction Labs), CaMKIV (1:500; Santa Cruz), GFP (1:1000; Invitrogen), ER81 (1:5000; Covance), pCREB (1:100; Cell Signaling), and MAP2 (1:1000; Sigma) antibodies in blocking buffer overnight at 4°C. Coverslips were then incubated with mouse or rabbit Alexa-488, -568 or -647 conjugated secondary antibodies (1:600; Invitrogen) for 2hr at room temperature. Cell nuclei were stained with Hoechst (Sigma). Any neurons showing signs of cell death (blebbed or crenated nuclei) were excluded from further analyses. Coverslips were mounted on slides using Aquamount.
Postnatal day 7 and 14 (P7 and P14) rats were perfused with 4% PFA in PBS. Brains were cryoprotected in 30% sucrose in PBS at 4°C. The brains were then embedded with Tissue TeK™ OCT compound and 40 μm coronal sections collected using a Leica cryostat. These free floating sections were immunostained as above for dissociated cells with CaMKIV (1:2000; Santa Cruz) and visualized with mouse or rabbit Alexa-488 and Alexa-568 conjugated secondary antibodies (1:600; Invitrogen). Cell nuclei were stained with Hoechst (Sigma). Sections were mounted on gelatin-coated slides and coverslipped using Aquamount. Images of stained cortical sections were captured by a Hamamatsu digital camera attached to a Zeiss Axiovert fluorescent microscope with a 4X objective.
2.5 - Western Blotting
E18 cortical neurons cultured in 35mm dishes were harvested in 2X Laemmli sample buffer by boiling lysis at 4, 8 and 10 DIV. Equal amounts of cell lysates were loaded onto 10% SDS-PAGE and transferred to nitrocellulose membranes. Membranes were incubated in blocking buffer (5% milk in TBST) for 1hr at room temperature and then probed with anti-CaMKIV (1:2000; BD Transduction Labs) antibodies in blocking buffer at 4°C overnight. Blots were incubated with HRP-conjugated secondary antibodies and visualized using chemiluminescence (Pierce). Blots were stripped and reprobed with mouse anti-GAPDH (1:20,000; Ambion) antibodies as a loading control.
2.6 – Image acquisition and analyses
Images were acquired by Hamamatsu digital camera attached to the Zeiss Axiovert fluorescent microscope with 20X and 40X objectives. To identify CaMKIV expressing (positive) and non-expressing (negative) neurons, configurations for CaMKIV images were first optimized. To standardize image acquisition, multiple transfected and non-transfected neurons were acquired without pixel saturation and capture parameters were kept the same for all the neurons imaged within the same experiment. Images of GFP and CaMKIV labeled neurons were captured for analyses using the standardized image configuration parameters. CaMKIV immunofluorescence intensity in neuronal nuclei was measured and CaMKIV mean intensity was normalized to background mean intensity. This normalized mean value was used as CaMKIV mean intensity of the neuron. Based on endogenous expression neurons were categorized as CaMKIV positive (CaMKIV expressing) and negative neurons (CaMKIV non-expressing). Intensity analyses were performed using Openlab software (Improvision).
Pyramidal and non-pyramidal neurons were analyzed. These two classes of neurons were distinguished based on distinctive features. Pyramidal neurons have a characteristic appearance that includes a pyramidal shaped cell body from which a major primary dendrite (i.e. apical dendrite) extends and gradually tapers away from the soma to terminate in a branched pattern known as an apical tuft. Several minor dendrites (i.e. basal dendrites) arise from the base of the pyramidal soma. Examples of pyramidal neurons are shown in figures 2 and 4. Nonpyramidal neurons are characterized by several primary dendrites of equivalent size that extend from the cell body. Nonpyramidal neurons are multipolar and, unlike pyramidal neurons, lack an apparent dendrite polarity. Examples of nonpyramidal neurons are shown in figure 3. The conservation in vitro of characteristic neuronal morphologies suggests that the essential mechanisms involved in the specification of neuronal morphology functions in vitro even in the absence of directional guidance cues. Dendrites greater than 10 μm in length were manually traced using Openlab software (Improvision) and quantified for total dendrite length, apical dendrite length (pyramidal), longest dendrite length (non-pyramidal), number of branch points, and number of primary dendrites. Branching index was calculated as follows: (number of branch points/average dendrite length) × 100. In general approximately 20 neurons of each cell type from 2 experiments were analyzed. Actual N values are given with each figure. In siRNA experiments, CaMKIV immunofluorescence intensity was determined as above. The experimenter was blind to all samples.
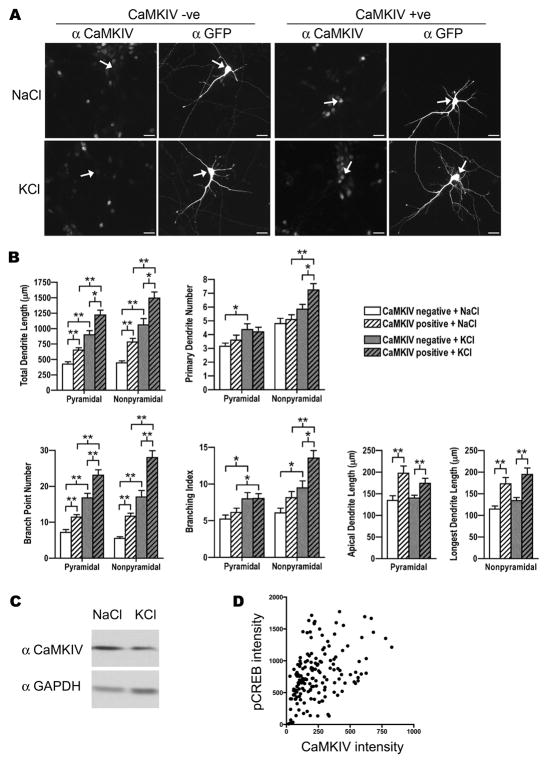
Figure 2. CaMKIV immunostained pyramidal and nonpyramidal neurons showed greater dendrite complexity in basal conditions and in response to neuronal activity.
A. Shown are examples of E18 cortical pyramidal neurons at 8 DIV that were transfected with GFP and stimulated with either 50mM KCl or 50mM NaCl as a control and immunostained with anti-CaMKIV and anti-GFP antibodies. Arrows indicate GFP transfected pyramidal neurons that are CaMKIV immunostained (CaMKIV +ve) and unstained (CaMKIV −ve) neurons. Scale bars are 20 μm.
B. CaMKIV immunostained (positive; hatched bars) and unstained (negative; solid bars) pyramidal and nonpyramidal neurons were analyzed for total dendrite length, primary dendrite number, branch point number, branching index, and either apical dendrite length (pyramidal neurons) or longest dendrite length (nonpyramidal neurons). Cultures were stimulated with either 50mM KCl (gray bars) or 50mM NaCl (white bars) as a control. Note that the neurons expressing CaMKIV endogenously had greater dendrite complexity. A minimum of 20 neurons from each class of cells from two independent experiments was analyzed. *p < 0.05, **p 0.002 (Student’s t-test, two-tailed).
C. Representative Western blot of E18 cortical neurons that were stimulated with either 50mM KCl or with 50mM NaCl (control) at 3 DIV and harvested at 4 DIV. Blots were probed with CaMKIV and GAPDH specific antibodies. GAPDH is used as a loading control. Three independent experiments did not reveal a significant difference between treatment conditions.
D. CREB was phosphorylated in CaMKIV expressing neurons after stimulation. Quantification of nuclear mean intensities of CaMKIV and pCREB in E18 cortical neurons at 8 DIV that were immunostained with CaMKIV and pCREB after treatment with 50mM KCl. Shown are 162 cells from two independent experiments.
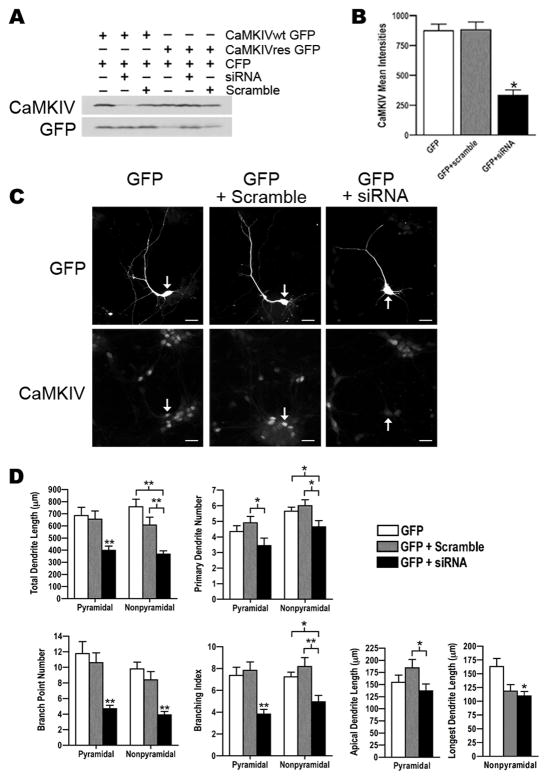
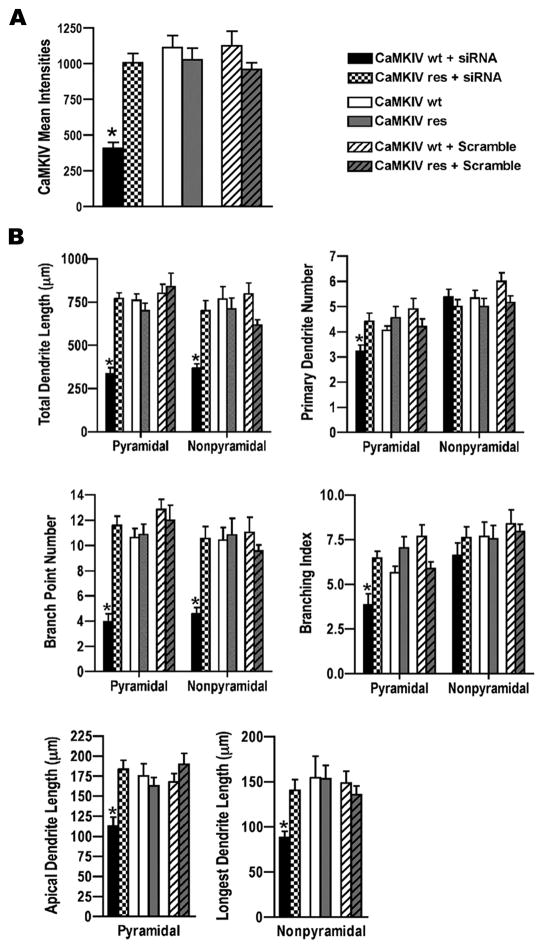
Figure 4. siRNA knockdown of CaMKIV decreased dendrite complexity.
A. Western blot analyses for CaMKIV and GFP expression in HEK 293 cells that were transfected as indicated with either GFP tagged wild type CaMKIV (CaMKIVwt) or siRNA resistant CaMKIV (CaMKIVres) plasmids and either CaMKIV siRNA or scrambled siRNA. CFP was transfected as a control and detected with GFP antibodies. Cells were harvested at 72hrs post-transfection. Note that siRNA knocked down wild type CaMKIV (lane 2) but not CaMKIVres (lane 5). Shown is a representative Western blot from two independent experiments.
B. Analyses of endogenous CaMKIV mean intensities in GFP transfected rat cortical neurons indicating siRNA knocked down endogenous CaMKIV. E18 cortical cultures were co-transfected with GFP and either CaMKIV siRNA or scrambled siRNA at 4 DIV and immunostained with anti-CaMKIV and anti-GFP antibodies at 72hrs post-transfection. A minimum of 20 cells was analyzed per group from two independent experiments. *p value < 0.05 (Student’s t-test, two-tailed).
C. Shown are images of GFP and CaMKIV immunostained neurons that were co-transfected as indicated with GFP and either scrambled siRNA or CaMKIV siRNA at 4 DIV and immunostained with anti-CaMKIV and anti-GFP antibodies 72hrs post-transfection. Arrows indicate the GFP transfected pyramidal neuron in the CaMKIV and GFP immunostained images. Scale bars are 20 μm.
D. CaMKIV siRNA transfected neurons showed a significant decrease in dendrite complexity. Pyramidal and nonpyramidal neurons transfected with GFP (white bars) or co-transfected with GFP and either scrambled siRNA (gray bars) or CaMKIV siRNA (black bars) were analyzed for total dendrite length, primary dendrite number, branch point number, branching index, and either apical dendrite length (pyramidal neurons) or longest dendrite length (nonpyramidal neurons). A minimum of 20 neurons from each class of cells from two independent experiments was analyzed. Asterisks above black bars indicate GFP+siRNA transfected neurons were significantly different from GFP and GFP+scramble. *p value < 0.05, **p value ≤ 0.002 (Student’s t-test, two-tailed).
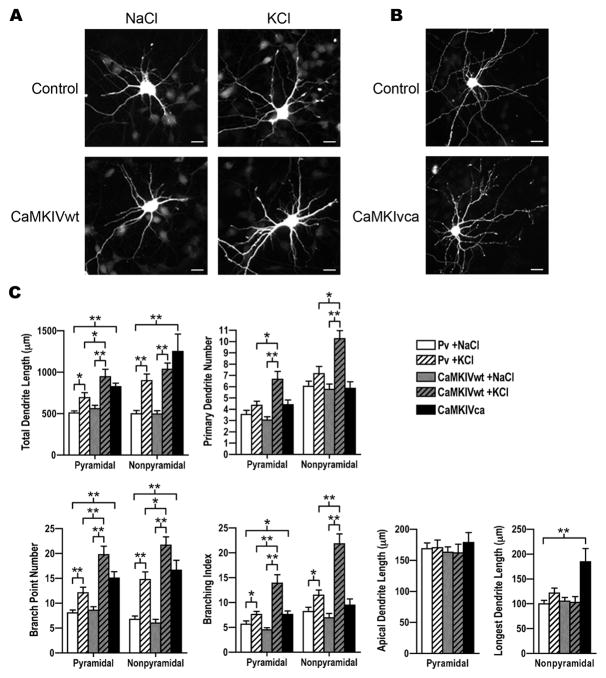
Figure 3. CaMKIV potentiated activity-induced dendrite complexity.
A. Shown are images of E18 cortical nonpyramidal neurons that were co-transfected with GFP and either empty vector (control) or wild-type CaMKIV (CaMKIVwt) plasmids at 3 DIV, stimulated with either 50mM KCl or 50mM NaCl at 4 DIV, and at 5 DIV immunostained with anti-GFP antibodies. Scale bars are 16 μm.
B. Shown are images of nonpyramidal neurons that were co-transfected with GFP and either empty vector (control) or constitutively active CaMKIV (CaMKIVca) plasmids at 3 DIV and immunostained with anti-GFP antibodies at 5 DIV. Scale bars are 16 μm.
C. Quantification of pyramidal and nonpyramidal neurons that were transfected with CaMKIVwt (gray bars) or control (pv; white bars) and either stimulated (KCl; hatched bars) or control treated (NaCl; solid bars) as indicated. CaMKIVca (black bars) transfected pyramidal and nonpyramidal neurons were untreated. Pyramidal and nonpyramidal neurons were analyzed for total dendrite length, primary dendrite number, branch point number, branching index, and either apical dendrite length (pyramidal neurons) or longest dendrite length (nonpyramidal neurons). Note that CaMKIVca transfected neurons and CaMKIVwt transfected neurons that were stimulated with KCl showed a significant increase in dendrite complexity. A minimum of 20 neurons for each class of cells from two independent experiments was analyzed. *p value < 0.05, **p value ≤ 0.002 (Student’s t-test, two-tailed).
Graphpad prism software was used for statistical analysis. Data are shown as mean ± standard error. Asterisks indicate statistically significant differences as determined by Student’s t-test (two-tailed, p values as indicated with the data). Analysis of Variance (ANOVA) with Bonferroni post-hoc analyses were also performed and yielded similar results as the Student’s t-test.
RESULTS
3.1 - CaMKIV is developmentally regulated and expressed by subsets of cortical neurons
We have previously shown by Western blot analysis that CaMKIV expression in the postnatal rat cortex increased dramatically in the first and second postnatal week before peaking at postnatal day 15 (Redmond et al., 2002). During this time all cortical neurons are undergoing extensive dendrite elaboration (Miller, 1981; Miller and Peters, 1981). To explore the role of CaMKIV in dendrite elaboration, the spatial expression of CaMKIV in postnatal day 7 (P7) and postnatal day 14 (P14) rat cortices was examined. Unlike many other signaling proteins, immunostaining with anti-CaMKIV specific antibodies revealed heterogeneous expression in the P7 and P14 cortex. At both ages CaMKIV expression was detected in layers 2–5 and the subplate (Figure 1A–D). A similar pattern of expression has been reported in the adult brain (Nakamura et al., 1995). This heterogeneity in CaMKIV expression was preserved in E18 cortical cultures (Figure 1E–J). Some neurons displayed intense CaMKIV immunostaining and others were not immunostained. Cell count analyses indicated the 4 DIV cultures were highly neuron rich with 97% of the cells immunostained for the neuron specific protein, MAP2. Of the MAP2 labeled neurons 40% were CaMKIV immunostained. This result indicates CaMKIV was expressed by some but not all neurons.
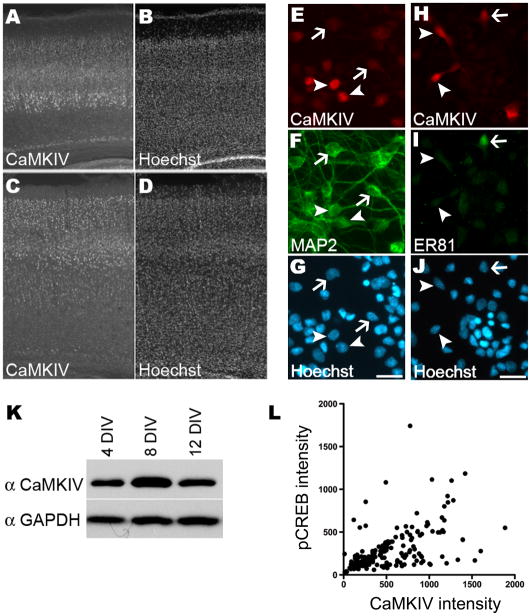
Figure 1. CaMKIV was expressed in a subset of cortical neurons and upregulated in vitro.
A–D. CaMKIV expression in the P7 and P14 rat cortex was heterogeneous. P7 and P14 rat cortices were immunostained with antibodies specific for CaMKIV (A,C) and the nuclei counterstained with Hoechst (B,D).
E–J. Representative images of E18 cortical cultures that were co-immunostained with anti-CaMKIV (E, H) and either anti-MAP2 (F) or anti-ER81 (I) antibodies are shown. Nuclei were counterstained with Hoechst (G, J). Scale bars are 20 μm. (E–G) CaMKIV immunostained (arrowheads) and unstained cells (arrows) were MAP2 labeled neurons. (H–J) CaMKIV and ER81 co-immunostained cells (arrows) as well as CaMKIV immunostained but ER81 unstained cells (arrowheads) were seen.
K. Western blot analyses of E18 cortical cultures harvested at the days in vitro (DIV) indicated and probed with antibodies specific to CaMKIV and as a loading control GAPDH.
L. Quantification of mean CaMKIV and mean pCREB intensities in E18 cortical cultures that were co-immunostained with CaMKIV and pCREB at 8 DIV. Shown are 197 neurons from two independent experiments.
At P7, CaMKIV expression was most pronounced in layer 5 cells (Figure 1A–B). To determine if CaMKIV was expressed in layer 5 cortical neurons in vitro, cultures were immunostained with antibodies to CaMKIV and ER81, a layer 5 specific marker. ER81 is an Ets transcription factor that is selectively expressed in layer 5 neurons from E15 to the adult (Hevner et al., 2003; Yoneshima et al., 2006). ER81 and CaMKIV were coexpressed in some but not all neurons in vitro. Cell count analyses determined that 20% of the ER81 immunostained neurons were also CaMKIV immunostained. Together these data suggest CaMKIV expression was heterogeneous across and within cortical layers.
The heterogeneity in CaMKIV expression in vitro and in vivo prompted us to question whether the heterogeneous expression of CaMKIV contributed to a difference between the CaMKIV expressing and nonexpressing neurons. The cerebral cortex is composed of two distinct classes of neurons, pyramidal and nonpyramidal, that display multiple differences including dendrite arborization patterns, neuronal connectivity and electrophysiological properties. We examined pyramidal neurons for CaMKIV expression and detected both CaMKIV immunostained (positive) and unstained (negative) pyramidal neurons (Figure 2A). Similarly, CaMKIV positive and negative nonpyramidal neurons were detected. This result indicated CaMKIV expression was not restricted to, nor did CaMKIV define, one of these two general morphological neuronal types.
3.2 - CaMKIV expressing pyramidal and nonpyramidal neurons displayed greater dendrite complexity
CaMKIV has been shown to be developmentally upregulated in vivo (Redmond et al., 2002). To determine whether CaMKIV expression was upregulated in vitro concurrent with dendrite elaboration as it is in vivo, Western blot analyses were performed. CaMKIV expression in vitro was concurrent with the period of dendrite elaboration and was high by 8 DIV (Figure 1K). To distinguish between an increase in the number of cells expressing CaMKIV versus an increase in amount of CaMKIV per cell, cell counts were performed at 4 and 8 DIV. At 4 and 8 DIV 40% and 44%, respectively, of MAP2 labeled neurons were colabeled with CaMKIV. This indicates very little change in the population of MAP2 neurons that expressed CaMKIV. This result and the consistency in the CaMKIV expression pattern in P7 and P14 (Figure 1) cortices suggests the increase in CaMKIV detected by Western blot analyses is due to an increase in the amount of CaMKIV expressed in each neuron rather that an increase in the number of cells that express CaMKIV. We also asked whether CaMKIV signaling was active in neurons that expressed CaMKIV in vitro by examining phosphorylation of CREB, a prominent downstream nuclear target. CaMKIV expression coincided with CREB phosphorylation (Figure 1L). This positive correlation suggests CaMKIV was active in these neurons. Together our data infers that CaMKIV contributes to neuronal differentiation.
A hallmark of neuronal differentiation is an increase in morphological complexity (Marĺn-Padilla, 1992). Although overexpression of active CaMKIV promoted dendrite growth (Redmond et al., 2002), whether endogenous CaMKIV functions in dendrite complexity has not been determined. To examine whether endogenous CaMKIV mediates a difference in dendrite complexity, the dendrite arbor of individual cortical neurons that expressed CaMKIV was quantified and compared to those that did not express CaMKIV. To visualize dendrite arbors, neurons were transfected with a mammalian expression plasmid encoding green fluorescent protein (GFP). GFP fills the entire dendrite arbor of individual neurons allowing visualization and reconstruction of the transfected neuron. The neuronal morphology and connectivity of pyramidal and nonpyramidal neurons is strikingly different (Gilbert, 1983). Further, the dendrite arbor is a significant factor in determining neuronal functionality including electrophysiological properties and connectivity (Häusser et al., 2000). These two neuronal populations also display different magnitudes of dendrite growth and remodeling in the adult (Lee et al., 2006). Therefore, the dendrites of pyramidal and nonpyramidal neurons were reconstructed and examined separately.
Pyramidal neurons that expressed CaMKIV endogenously (CaMKIV positive) displayed more complex dendrite morphologies when compared to neurons that did not express CaMKIV (CaMKIV negative) (Figure 2A,B; compare white to white hatched bars). CaMKIV positive pyramidal neurons displayed 53% greater dendrite extent (measured by total dendrite length) when compared to CaMKIV negative pyramidal neurons (652.4±36.6 versus 425.2±36.8). CaMKIV positive nonpyramidal neurons also showed 76% greater dendrite extent than CaMKIV negative nonpyramidal neurons (784±56.1 versus 445.7±31.6).
Total dendrite length is a good cumulative measure of dendrite extent. However, the dendrite pattern and specific features of the dendrite arbor are hidden in this analysis. The relative contribution of features, such as the number of primary dendrites, number of branches, and length of individual dendrites critically impacts the complexity and potential connectivity of the dendrite arbor. For example, cerebellar Purkinje neurons extend a single primary dendrite that is extensively branched whereas a typical cortical nonpyramidal neuron extends several primary dendrites that are branched, but less so than Purkinje neurons. Although the final dendrite arbor is distinctive, neurons undergo identical events in dendrite development to acquire their mature dendrite morphology. These events include dendrite initiation, dendrite elongation, and dendrite branching (Scott and Luo, 2001). We quantified dendrite initiation, branching, and elongation to determine whether CaMKIV regulates the mode by which the dendrite arbor forms. Dendrite initiation was quantified by counting the number of primary dendrites. Dendrite branching was quantified by determining the number of branch points and the branching index. Dendrite elongation was quantified by measuring the length of the apical dendrite of pyramidal neurons and the length of the longest dendrite of nonpyramidal neurons.
Pyramidal neurons displayed no significant difference between CaMKIV positive and negative neurons in the number of primary dendrites (Figure 2B; 3.6±0.3 versus 3.1±0.2). However, CaMKIV positive pyramidal neurons displayed 61% greater number of dendrite branches (11.4±0.7 versus 7.1±0.8) than CaMKIV negative neurons. In addition, the apical dendrites of CaMKIV positive pyramidal neurons were 47% longer (197.7±16.6 versus 134.4±10.9). Like pyramidal neurons, nonpyramidal neurons displayed no significant difference in the number of primary dendrites, but did display more branches and longer individual dendrite lengths (Figure 2B). The number of dendrite branches was two fold greater in CaMKIV positive nonpyramidal neurons than CaMKIV negative nonpyramidal neurons (113% greater; 11.7±0.8 versus 5.5±0.5). The longest dendrite of CaMKIV positive nonpyramidal neurons was 51% longer (172.8±14.6 versus 114.5±7.4) than CaMKIV negative neurons. Together these data indicate the greater dendrite extent in CaMKIV positive neurons is due to greater branching and elongation rather than initiation of new dendrites. Further, pyramidal and nonpyramidal neurons displayed a similar pattern of differences between CaMKIV positive and negative neurons. These data suggest that CaMKIV may participate in mediating a conserved mechanism that controls the mode of dendrite complexity in both neuronal populations.
3.3 - Endogenous expression of CaMKIV potentiated activity-induced dendrite complexity
Neuronal activity has been shown to increase dendrite growth and complexity in vivo and in vitro (reviewed in Wong and Ghosh, 2002). CaMKIV has been identified as a molecular player in activity-induced dendrite growth (Redmond et al., 2002; Kamata et al., 2007). However, the contribution of endogenous CaMKIV to activity-induced dendrite elaboration has not been shown. Therefore, we next tested whether neurons that expressed CaMKIV endogenously displayed an amplified response to neuronal activity. First, we examined the response of CaMKIV negative neurons to increased activity induced by KCl stimulation (Figure 2B; compare white to gray bars). Pyramidal and nonpyramidal neurons had a greater than two fold increase in total dendrite length when compared to control treated CaMKIV negative neurons (pyramidal, 900±67.7 versus 425.2±36.8; nonpyramidal, 1059±104.6 versus 445.7±31.6). This increase in dendrite extent was due to a dramatic increase in dendrite branching (pyramidal, 16.7±1.3 versus 7.1±0.8; nonpyramidal, 17±1.8 versus 5.5±0.5). CaMKIV negative pyramidal, but not nonpyramidal, neurons showed a significant increase in primary dendrites (4.3±0.4 versus 3.1±0.2). Interestingly, stimulation did not alter dendrite elongation in CaMKIV negative neurons.
Next we examined the response of CaMKIV positive pyramidal and nonpyramidal neurons to KCl stimulation (Figure 2B; compare white hatched to gray hatched bars). CaMKIV positive pyramidal and nonpyramidal neurons displayed nearly two-fold increase in total dendrite length when compared to control treated CaMKIV positive neurons (pyramidal, 1221±79.1 versus 652.4±36.6; nonpyramidal, 1496±97.2 versus 784±56.1). Both pyramidal and nonpyramidal CaMKIV positive neurons displayed a greater than two fold increase in branching after stimulation (pyramidal, 23.1±1.5 versus 11.4±0.7; nonpyramidal, 28±1.9 versus 11.7±0.8). CaMKIV positive nonpyramidal, but not pyramidal, neurons showed a significant increase in primary dendrites (41%; 7.2±0.4 versus 5.1±0.3). Whereas endogenous CaMKIV expression significantly increased dendrite elongation (apical dendrite length and longest dendrite length) in basal culture conditions (compare white to white hatched), stimulation did not additionally increase dendrite elongation (compare white hatched to gray hatched). This result suggests that dendrite elongation, specifically CaMKIV mediated dendrite elongation is not potentiated by neuronal activity.
Both CaMKIV negative and CaMKIV positive neurons showed significantly increased measures of dendrite elaboration after KCl stimulation. To determine whether endogenous CaMKIV expression potentiated activity induced changes in dendrite elaboration, we compared the morphological responses after stimulation of CaMKIV positive neurons to those of CaMKIV negative neurons (Figure 2A,B; compare gray to gray hatched bars). CaMKIV positive pyramidal and nonpyramidal neurons had a significantly greater total dendrite length when compared to CaMKIV negative neurons (pyramidal, 1221±79.1 versus 900±67.7; nonpyramidal, 1496±97.2 versus 1059±104.6). Both pyramidal and nonpyramidal CaMKIV positive neurons displayed a greater amount of branching than CaMKIV negative neurons after stimulation (pyramidal, 23.1±1.5 versus 16.7±1.3; nonpyramidal, 28±1.9 versus 17±1.8). Dendrite elongation (apical dendrite length and longest dendrite length) was greater in CaMKIV positive pyramidal and nonpyramidal neurons after stimulation than in CaMKIV negative neurons (pyramidal, 174.2±11.5 versus 140±6.5; nonpyramidal, 194.7±14.7 versus 134±7.3). CaMKIV positive nonpyramidal, but not pyramidal, neurons showed a significant greater number of primary dendrites (7.2±0.4 versus 5.8±0.3). Analyses of endogenous CaMKIV and pCREB levels by mean intensity measurements after stimulation at 8 DIV revealed a significant positive correlation between endogenous CaMKIV expressing neurons and pCREB after KCl stimulation (Figure 2D). Together these data suggest that endogenously expressed CaMKIV mediates a greater increase in dendrite elaboration in response to neuronal activity. Interestingly, this response was specific to dendrite branching and less so to dendrite initiation and elongation.
3.4 - Expression of wild-type CaMKIV potentiated activity-induced dendrite complexity
Previously we showed that CaMKIV expression increased concurrent with dendrite elaboration and endogenous CaMKIV expression corresponded with an extensive dendrite arbor. We next tested whether the intracellular level of CaMKIV limited dendrite elaboration. We also wanted to know if CaMKIV could promote dendrite elaboration in younger neurons. To answer these two questions, we coexpressed GFP and wild-type CaMKIV in neurons and analyzed dendrite complexity at 5 DIV instead of 8 DIV. For these analyses dendrite morphology was quantified independent of endogenous CaMKIV expression. Therefore, control transfected cultures consist of a mixed population of neurons, some expressing CaMKIV endogenously and others not. Expression of wild-type CaMKIV (CaMKIVwt) for 2 DIV did not induce a significant difference in dendrite extent, as measured by total dendrite length (Figure 3A,C; compare white and gray bars). Effectors of activity-induced signaling have been shown to restructure the dendrite arbor without significantly altering total dendrite extent (Inglis et al., 2002; Yacoubian and Lo, 2000). To determine if CaMKIVwt induced restructuring of the dendrite arbor without altering the total dendrite extent, we quantified dendrite number, branch point number, and individual dendrite length. These three measures of dendrite complexity were also unaltered in CaMKIVwt expressing neurons (Figure 3C). This indicates that CaMKIVwt did not alter dendrite complexity via changes in growth or homeostatic changes in features of complexity.
It is possible that the introduced CaMKIVwt is not fully activated in these young neurons (Chow et al., 2005). To test the role of CaMKIV activation in dendrite complexity, we first examined whether activity increased dendrite complexity at 5 DIV in control transfected cultures. As above, analyses of control transfected cultures were done independent of endogenous CaMKIV expression. Consistent with our previous results, KCl stimulation of control transfected cultures significantly increased dendrite complexity of both pyramidal and nonpyramidal neurons (Figure 3; compare white to white hatched bars). Dendrite length increased 37% in pyramidal neurons and 80% in nonpyramidal neurons (pyramidal, 695±58.8 versus 508.7±26.2; nonpyramidal, 898±81.6 versus 499.7±38.1). The activity-induced increase in dendrite length resulted from a significant increase in dendrite branching and not dendrite initiation or elongation. Pyramidal and nonpyramidal neurons displayed 50% and 123%, respectively increased number of branch points (pyramidal, 12.1±1.1 versus 8.1±0.6; nonpyramidal, 15.2±1.5 versus 6.8±0.7) and the branching index increased 35% and 40%, respectively (pyramidal, 7.6±0.6 versus 5.6±0.6; nonpyramidal, 11.5±1.1 versus 8.2±0.9). These data indicate that KCl increased complexity by increasing dendrite branching, and not by alterations in dendrite elongation or initiation. Further, these data indicates the increase in dendrite complexity was due to new growth and not restructuring of existing dendrites.
We next tested whether CaMKIVwt expression potentiated activity-induced dendrite complexity. Both pyramidal and nonpyramidal neurons expressing CaMKIVwt had greater dendrite complexity after stimulation than in basal conditions (Figure 3A,C; compare gray and gray hatched bars). In fact, multiple parameters of dendrite complexity including total dendrite length, dendrite number and dendrite branching were elevated. Pyramidal neurons expressing CaMKIVwt showed a 68% increase in total dendrite length (946±91.4 versus 562.1±39.4), a 113% increase in dendrite number (6.6±0.7 versus 3.1±0.3), and a 129% increase in dendrite branching (19.7±1.7 versus 8.6±0.7). Average dendrite length was not altered by increased activity in CaMKIVwt expressing neurons (data not shown). In addition, dendrite elongation, as measured by apical dendrite length was not altered by increasing activity in CaMKIVwt expressing neurons. When considered together, these three morphological parameters (average dendrite length, primary dendrite number, and branch point number) indicate a high branching index. In fact, the branching index increased three fold (13.9±1.7 versus 4.5±0.4).
Similarly, nonpyramidal neurons showed a 108% increase in total dendrite length (1035±76.4 versus 497.3±40.1), a 79% increase in dendrite number (10.2±0.7 versus 5.7±0.4), a 271% increase in dendrite branch points (21.5±1.7 versus 5.8±0.8), and a 216% increase in the branching index (21.8±1.9 versus 6.9±0.8). However, there was no detectable change in dendrite elongation as measured by the longest dendrite length. Average dendrite length was also unaltered by increased activity in CaMKIVwt expressing neurons (data not shown). These results show CaMKIVwt potentiated the ability of both pyramidal and nonpyramidal neurons to elaborate dendrites in response to stimulation. Interestingly, CaMKIVwt signaling was biased in that it preferentially potentiated an effect on dendrite branching and initiation rather than elongation in these younger cortical neurons. These results indicate CaMKIVwt promoted a highly branched dendrite arbor in response to stimulation.
3.5 - Active CaMKIV mediated dendrite elaboration independent of stimulation
To test whether an enzymatically active CaMKIV is sufficient to mediate select components of dendrite complexity we expressed constitutively active CaMKIV (CaMKIVca) along with GFP in E18 cortical neurons and analyzed dendrite complexity (Figure 3B,C; black bars). Pyramidal neurons that expressed CaMKIVca showed 63% greater total dendrite length when compared to control transfected neurons (826.1±43.6 versus 508.7±26.2). CaMKIVca expressing nonpyramidal neurons showed an even more dramatic (2.5 fold) difference in total dendrite length (1251±211.4 versus 499.7±38.1). CaMKIVca expression nearly doubled (15.0±1.3 versus 8.1±0.6) the number of branch points of pyramidal neurons and nearly tripled (15.9±1.9 versus 6.8±0.7) the number of branch points of nonpyramidal neurons. However, CaMKIVca expression had no significant effect on the number of primary dendrites. This result, when compared to the increase in primary dendrites detected in stimulated CaMKIVwt transfected neurons (gray hatched bars) suggests additional activity-induced mechanisms might also have contributed to dendrite initiation after stimulation. Although CaMKIVca expression did not alter the length of apical dendrites of pyramidal neurons, the length of the longest dendrite increased 85% (184.8±26.3 versus 99.9±6.8) in nonpyramidal neurons. These results show active CaMKIV, independent of external stimulation, was sufficient to replicate augmentation induced by neuronal activity of dendrite branching in pyramidal and nonpyramidal neurons and dendrite elongation in nonpyramidal neurons.
3.6 - CaMKIV was critical for dendrite complexity
To test whether CaMKIV was essential for dendrite complexity we screened several siRNAs matching CaMKIV for effective reduction of CaMKIV expression. Of the siRNAs targeting CaMKIV tested the most effective CaMKIV siRNA is shown in figure 4A. This siRNA to CaMKIV knocked down overexpressed wild type CaMKIV in HEK cells. Two silent point mutations were created in the siRNA-targeted region of CaMKIV to generate CaMKIVres. The siRNA did not reduce expression of CaMKIVres, indicating CaMKIVres was resistant to siRNA knockdown. In addition, a scrambled siRNA had no effect on wild type and CaMKIVres expression. Together these data indicate the siRNA effectively knocked down CaMKIV expression. We next tested if CaMKIV siRNA effectively knocked down endogenous CaMKIV in E18 cortical neurons. CaMKIV siRNA was cotransfected with GFP into cultured neurons and CaMKIV expression assayed 72 hours post transfection by quantitative immunofluorescence. This analysis indicated the siRNA dramatically knocked down endogenous CaMKIV expression [333.2±45.3 versus 880.9±66.5 (scramble RNA) or 874.1±54.2 (no RNA) mean intensity] in cortical neurons (Figure 4B).
Knockdown of endogenous CaMKIV with this siRNA resulted in less complex neurons than control and scramble siRNA transfected neurons (Figure 4C,D; compare black to white or gray bars). Total dendrite length was reduced 39–52% in CaMKIV siRNA transfected neurons compared to controls [pyramidal, 399.2±34.5 versus 655.3±67.1 (scramble RNA) or 685.4±67.5 (no RNA); nonpyramidal, 367.4±27.4 versus 606.8±65.4 (scramble RNA) or 758.2±61.9 (no RNA)]. The reduction of dendrite branching was most dramatic. Both pyramidal and nonpyramidal CaMKIV siRNA transfected neurons had a 60% decrease in the number of dendrite branches [pyramidal, 4.70±0.4 versus 10.60±1.3 (scramble RNA) or 11.75±1.6 (no RNA); nonpyramidal, 3.90±0.4 versus 8.40±1.0 (scramble RNA) or 9.80±0.9 (no RNA)]. Further, the branching index decreased 48% and 32% in pyramidal and nonpyramidal neurons, respectively [pyramidal, 3.8±0.4 versus 7.8±0.7 (scramble RNA) or 7.3±0.7 (no RNA); nonpyramidal, 4.9±0.6 versus 8.2±0.8 (scramble RNA) or 7.2±0.4 (no RNA)].
An effect on primary dendrites and elongation was also detected in CaMKIV siRNA transfected neurons. The number of primary dendrites was decreased with CaMKIV siRNA [pyramidal, 3.45±0.5 versus 4.90±0.4 (scramble RNA) or 4.35±0.4 (no RNA); nonpyramidal, 4.65±0.4 versus 6.0±0.4 (scramble RNA) or 5.65±0.3 (no RNA)]. Apical dendrite length in CaMKIV siRNA transfected pyramidal neurons when compared to scramble RNA transfected was shorter (137.0±14.0 versus 184.6±17.3). Nonpyramidal neurons transfected with CaMKIV siRNA showed a decrease in longest dendrite length when compared to control (no RNA transfected) (109.7±8.3 versus 163.1±14.9). Although scramble RNA transfected nonpyramidal neurons compared to the no RNA transfected control showed a decrease in longest dendrite length (118.7±12.0 versus 163.1±14.9), this result was not replicated in later experiments (Figures 5B and 6). These results indicate CaMKIV was essential for basal dendrite complexity.
Figure 5. CaMKIVres rescued CaMKIV siRNA decrease in dendrite complexity.
A. Analyses of CaMKIV mean intensities in GFP transfected cortical neurons indicated CaMKIV siRNA knocked down coexpressed CaMKIVwt but not coexpressed CaMKIVres. E18 cortical cultures were co-transfected with GFP and either CaMKIV siRNA (black and checker board bars) or scrambled siRNA (white hatched and gray hatched bars) and either wild type CaMKIV (CaMKIVwt; black, white, and white hatched bars) or siRNA resistant CaMKIV (CaMKIVres; checker board, gray, and gray hatched bars) at 4 DIV. Cultures were immunostained with anti-CaMKIV and anti-GFP antibodies at 72hrs post-transfection. A minimum of 20 cells was analyzed per group from two independent experiments. *p value < 0.05 (Student’s t-test, two-tailed).
B. CaMKIV siRNA decreased CaMKIVwt, but not CaMKIVres, mediated dendrite complexity. Cortical neurons were co-transfected with GFP and either CaMKIVwt or CaMKIVres and either scrambled siRNA or CaMKIV siRNA as indicated above in A. Transfected neurons were analyzed for total dendrite length, primary dendrite number, branch point number, branching index, and either apical dendrite length (pyramidal neurons) or longest dendrite length (nonpyramidal neurons). A minimum of 18 neurons from each class of cells from two independent experiments was analyzed. Asterisks above solid black bars indicate CaMKIVwt+siRNA transfected neurons were significantly different from CaMKIVres+siRNA transfected neurons. *p value < 0.01 (Student’s t-test, two-tailed).
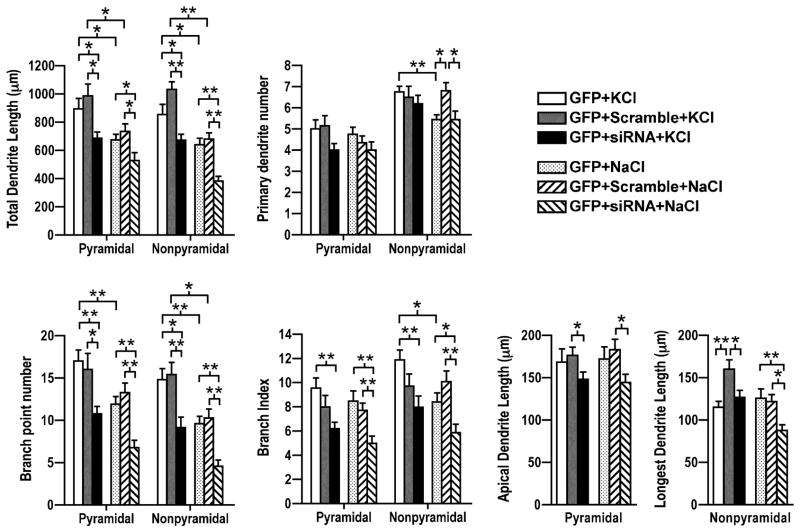
Figure 6. CaMKIV mediated activity-induced dendrite complexity.
CaMKIV siRNA decreased activity-induced dendrite complexity. Quantification of pyramidal and nonpyramidal neurons that were co-transfected with GFP and either scrambled siRNA (gray, R-L hatched bars) or CaMKIV siRNA (black, L-R hatched bars) at 4 DIV, stimulated (KCl; white, gray, black bars) or control treated (NaCl; dotted, R-L hatched, L-R hatched bars) as indicated at 7 DIV, and analyzed at 8 DIV. Transfected neurons were analyzed for total dendrite length, primary dendrite number, branch point number, branching index, and either apical dendrite length (pyramidal neurons) or longest dendrite length (nonpyramidal neurons). A minimum of 20 neurons from each class of cells from two independent experiments was analyzed. *p value < 0.05; **p value < 0.002 (Student’s t-test, two-tailed).
3.7 - Rescue of CaMKIV siRNA knockdown restored dendrite complexity
To test whether the deficits in dendrite complexity in the siRNA transfected neurons were specifically due to the loss of CaMKIV we performed rescue experiments. Neurons were cotransfected with GFP and either CaMKIVwt or CaMKIVres and either CaMKIV siRNA or scrambled siRNA. At 72 hours post transfection CaMKIV siRNA effectively knocked down coexpressed CaMKIVwt but not CaMKIVres in HEK cells (Figure 4A) and in cortical neurons (Figure 5A; 403±42.6 versus 1008±63.7 mean intensity).
Consistent with knockdown of endogenous CaMKIV, both pyramidal and nonpyramidal neurons cotransfected with CaMKIVwt and CaMKIV siRNA had reduced total dendrite length, number of branch points, and measures of dendrite elongation (apical dendrite length and longest dendrite length) (Figure 5B). Pyramidal neurons cotransfected with CaMKIVwt and CaMKIV siRNA showed a 58% decrease in total dendrite length (335.6±34.5 versus 801.4±52.5), a 35% decrease in dendrite number (3.2±0.3 versus 4.9±0.4), a 70% decrease in dendrite branch point number (3.9±0.6 versus 12.8±0.8), and a 33% reduction in apical dendrite length (113±10.8 versus 168.2±10.0) when compared those transfected with scramble siRNA (Figure 5B; compare black to white hatched bars). CaMKIVwt and CaMKIV siRNA cotransfected nonpyramidal neurons showed a similar diminished dendrite complexity with a 54% decrease in total dendrite length (367.4±25.2 versus 797.8±63.4), a 59% decrease in dendrite branch point number (4.5±0.5 versus 11±1.2), and a 40% reduction in longest dendrite length (88.8±6.3 versus 148.8±13.1) when compared to CaMKIVwt and scramble siRNA cotransfected neurons.
Whereas CaMKIV siRNA decreased dendrite complexity in CaMKIVwt transfected neurons, it did not alter dendrite complexity in CaMKIVres transfected neurons (Figure 5B; compare checkered bars with gray, gray hatched, white, and white hatched bars). Total dendrite length, primary dendrite number, branch point number, branching index, apical dendrite length, and longest dendrite length were not significantly different between scramble and no RNA transfected neurons. Coexpression of CaMKIVres with CaMKIV siRNA completely restored to normal all parameters of dendrite complexity decreased by CaMKIV siRNA (Figure 5B; compare checkered to black bars). The ability of CaMKIVres to rescue CaMKIV siRNA inhibition of dendrite complexity indicates the diminished dendrite complexity was due to the specific loss of CaMKIV.
3.8 - CaMKIV was critical for activity-induced dendrite elaboration
Previously we showed that constitutively active CaMKIV was sufficient to mediate increased dendrite complexity (Figure 3). Further, endogenously expressed CaMKIV and CaMKIVwt potentiated activity-induced dendrite complexity (Figures 2 and 3). We next asked whether CaMKIV was necessary for activity-induced dendrite complexity. Neurons were cotransfected with GFP and either CaMKIV siRNA or scramble RNA and either stimulated with KCl or as a control, NaCl. Knockdown of CaMKIV blocked activity-induced dendrite complexity. CaMKIV siRNA transfected neurons were less complex than control and scramble RNA transfected neurons (Figure 6; compare black to white and gray bars). Total dendrite length was not significantly different in KCl stimulated CaMKIV siRNA transfected neurons compared to unstimulated controls [pyramidal, 686.4±45.0(CaMKIV siRNA+KCl) versus 733.6±53.8 (scramble RNA+NaCl) or 674.9±38.9 (no RNA+NaCl); nonpyramidal, 672.2±41.6 (CaMKIV siRNA+KCl) versus 679±44.1 (scramble RNA+NaCl) or 640.9±45.8 (no RNA+NaCl)]. CaMKIV siRNA also significantly blocked activity-induced increase in the number of dendrite branches [pyramidal, 10.8±0.9 (CaMKIV siRNA+KCl) versus 16.0±1.9 (scramble RNA+KCl) or 17.0±1.3 (no RNA+KCl); nonpyramidal, 9.2±1.2 (CaMKIV siRNA+KCl) versus 15.4±1.4 (scramble RNA+KCl) or 14.8±1.3 (no RNA+KCl)]. An increased branching index was also blocked in CaMKIV siRNA knocked down neurons after stimulation [pyramidal, 6.2±0.5 (CaMKIV siRNA+KCl) versus 8.0±1.0 (scramble RNA+KCl) or 9.6±0.9 (no RNA+KCl); nonpyramidal, 8.0±0.9 (CaMKIV siRNA+KCl) versus 10.2±1.3 (scramble RNA+KCl) or 11.9±0.9 (no RNA+KCl)]. Consistent with earlier results (Figure 2), measurements of elongation (apical dendrite length and longest dendrite length) were not increased with KCl stimulation of control or CaMKIV siRNA transfected neurons. Scramble RNA transfected nonpyramidal neurons showed an increase in longest dendrite length. An increase in elongation was not detected in any other experiments suggesting this result is likely a nonspecific effect. Consistent with our previous results (Figure 4) CaMKIV siRNA in unstimulated (basal) conditions attenuated apical dendrite length, longest dendrite length, branch point number, branching index, and total dendrite length. Together these results indicate that CaMKIV is necessary to mediate basal and activity-induced dendrite elaboration.
DISCUSSION
We report in the present study that knockdown of CaMKIV expression with RNAi decreased dendrite extent and complexity in basal conditions. However, previous studies reported differing results when CaMKIV activity was decreased. When kinase dead and dominant negative CaMKIV were expressed in neurons total dendrite growth decreased (Redmond et al, 2002; Wayman et al., 2004; Tai et al., 2008). Kamata et al. (2007) added a nuclear localization sequence (NLS) to kinase dead CaMKIV and reported similar results; basal total dendrite growth was attenuated. However, Wayman et al. (2004; 2006) expressed a NLS tagged dominant negative CaMKIV and failed to detect a significant effect on total dendrite growth. It is not clear why the two NLS tagged CaMKIVs yielded different results. Dissimilar efficacies and mechanisms of action as inhibitors of endogenous CaMKIV may have contributed to the differing results. We used RNAi to specifically knockdown endogenous CaMKIV expression and rescued expression with a RNAi resistant CaMKIV. The attenuation and rescue of total dendrite growth we report in the current CaMKIV RNAi studies more stringently demonstrate that dendrite complexity is CaMKIV-dependent and support the conclusion that endogenous CaMKIV is crucial for mediating dendrite growth.
The features of a dendrite arbor are independently regulated so that a dendrite arbor acquires a distinct three dimensional architecture characteristic of a particular neuronal type (e.g. cerebellar Purkinje neurons and cortical layer 5 pyramidal neurons). We report in the current study that CaMKIV modulated the dendrite arbor through increased formation of dendrite branches and elongation of primary dendrites. We found that both pyramidal and nonpyramidal CaMKIV endogenously expressing neurons had greater branching that was diminished when CaMKIV was knocked down but was rescued by expression of siRNA resistant CaMKIV. Further, expression of a constitutive active CaMKIV increased branching in pyramidal and nonpyramidal neurons. Data from our study also support a role for CaMKIV in dendrite elongation. Pyramidal and nonpyramidal neurons that expressed endogenous CaMKIV had longer apical dendrites and longer individual dendrite lengths, respectively. The longest dendrite length of nonpyramidal neurons was decreased when endogenous and expressed wild type CaMKIV were knocked down and was rescued by expression of siRNA resistant CaMKIV. Knockdown of endogenous CaMKIV in pyramidal neurons trended to decreased apical dendrite length. However, apical dendrite length was decreased with siRNA knockdown of expressed wild type CaMKIV that was rescued by siRNA resistant CaMKIV. A constitutive active CaMKIV increased longest dendrite length of nonpyramidal neurons but not the apical dendrite length of pyramidal neurons. This difference in response of pyramidal and nonpyramidal neurons hints that mechanisms downstream of CaMKIV mediating elongation may be differently regulated in these two morphological cell types. Together these results show that CaMKIV not only regulated total dendrite growth (e.q. total amount of dendrites), but did so in a manner that specifically regulated the pattern of the dendrite arbor.
CaMKIV mediation of increased branching would enhance local connectivity of the neuron. Increased elongation of dendrites, however, could result in invasion of adjacent cortical columns and layers, like that reported when activity was altered in the somatosensory cortex (Hickmott and Steen, 2005). Activity can also significantly alter a neuron’s dendrite arbor in the adult animal (Hickmott and Steen, 2005; Kozorovitskiy et al., 2005; Lee et al., 2006). CaMKIV null, dnCaMKIV, and CaMKIV overexpressing transgenic mice display behaviors consistent with CaMKIV involvement in specific forms of learning (Ho et al., 2000; Kang et al., 2001; Wei et al., 2002; Fukushima et al., 2008; Takao et al., 2010). CaMKIV’s role in regulating dendrite arbors in the adult cerebral cortex awaits examination. Although it is uncertain if this effect is primary or a secondary effect due to altered granule cells, cerebellar Purkinje neurons show attenuated dendrite arbors in CaMKIV null mice (Kokubo et al., 2009).
Neurons of the cerebral cortex show distinctive responses to neuronal activity (Juraska, 1982; Häusser et al., 2000). Although the factors determining these distinctive responses are poorly understood, neuronal type (pyramidal or nonpyramidal), complement of neurotransmitters (e.g. glutamate and GABA), location (laminar position and cortical region), and developmental age contribute (Juraska, 1982; Häusser et al., 2000; Maravall et al., 2004). In this study we show CaMKIV modulates specific features of the dendrite arbor in response to activity. Further, we show that activity-induced dendrite complexity is dependent on CaMKIV. Together our data suggests CaMKIV contributes to the diversity of dendrite complexity via restricted expression and mediation of the mode of dendrite elaboration. Like CaMKIV, the ERK pathway also selectively modulated features of the dendrite arbor. Whereas CaMKIV increased dendrite branching and elongation, ERK signaling increased the formation of primary dendrites and dendrite branching but did not alter dendrite elongation (Ha and Redmond, 2008). Quantification of dendrite growth, a measure of the cumulative dendrite arbor, indicated ERK and CaMKIV were required for dendrite elaboration, but did not reveal the mode-specific changes in dendrite complexity mediated by ERK and CaMKIV (Redmond et al., 2002). The results from the current study suggest that distinct signaling pathways exert selective morphological outcomes even though the stimulus and route of calcium entry are identical.
Different responses to neuronal activity are also elicited by the site of calcium entry. Calcium that enters via VSCCs is different in its ability to activate signaling mechanisms that induce dendrite growth and gene transcription than calcium that enters via NMDA receptors (Bading et al., 1993; Redmond et al., 2002). However, calcium entry via Transient receptor potential channel 6 (TRPC6) increased dendrite complexity similar to that of VSCC (Tai et al., 2008). As reported with VSCC activation, CaMKIV and CREB mediated signaling were essential for conveying TRPC6 induced dendrite complexity. Interestingly, TRPC6 expression increased hippocampal neuron dendrite branching and total dendrite length but did not increase primary dendrites (Tai et al., 2008). Dendrite elongation was not examined. This result, along with the current study indicate multiple routes of calcium entry at the plasma membrane can activate CaMKIV resulting in comparable morphological changes. Further, these results suggest the mechanism and dynamics of CaMKIV activation are shared. Together these studies suggest that the pathway activated has a significant role in determining the biological outcome independent of the route of activation.
CaMKIV is primarily nuclearly localized and phosphorylates nuclearly localized proteins such as CREB and CBP to regulate gene transcription (Jensen et al., 1991; Sun et al., 1994; Mayr and Montminy, 2001; Impey et al., 2002). Tai et al. (2008) and Redmond et al. (2002) showed CaMKIV mediated dendrite growth was via CREB and CBP-regulated transcription. We found that CREB phosphorylation was elevated in CaMKIV expressing neurons. Pyramidal and nonpyramidal neurons displayed similar CaMKIV mediated changes in dendrite complexity suggesting a conserved CaMKIV downstream signaling mechanism. Together these data suggests the CaMKIV mediated dendrite branching and elongation reported in this study are also via CREB-mediated signaling.
In the quest to understand how neurons translate activity into neuron specific changes in the dendrite arbor we have found that cortical neurons differentially express CaMKIV in vivo and in vitro. As a result, the outcome on basal and activity-induced dendrite complexity is distinguishable based upon CaMKIV expression. Neurons that expressed CaMKIV displayed more complex dendrite arbors. Neurons are also able to temporally regulate the activation of ubiquitously expressed signaling proteins, such as ERK, to elicit different morphological responses to neuronal activity (Ha and Redmond, 2008). Together these data indicate neurons spatially and temporally control activity-induced mechanisms to regulate biological outcomes such as dendrite complexity.
Highlights.
Developing cortical neurons heterogeneously expressed CaMKIV in vivo and in vitro.
siRNA knockdown of CaMKIV decreased basal and activity-dependent dendrite complexity.
CaMKIV is required for activity-dependent dendrite elaboration.
CaMKIV mediated specific modes of dendrite elaboration: branching and elongation.
Acknowledgments
We thank Kathleen Wallis and Xiaolan Yi for technical assistance and Seungshin Ha for helpful advice and discussions during the course of the study. This work was supported by a National Institutes of Health R01 grant to LRH.
ABBREVIATIONS
- ANOVA
Analysis of Variance
- BSA
Bovine Serum Albumin
- CaMKIV
Calcium/Calmodulin-dependent protein kinase IV
- CaMKIVca
constitutively active CaMKIV
- CaMKIVres
siRNA resistant CaMKIV
- CaMKIVwt
wild type CaMKIV
- CBP
CREB Binding Protein
- CREB
cAMP Response Element Binding protein
- DIV
days in vitro
- E15
Embryonic day 15
- E18
Embryonic day 18
- ER81
Ets transcription factor
- ERK
Extracellular signal related kinase
- GABA
Gamma-Aminobutyric Acid
- GAPDH
Glyceraldehyde 3-phosphate Dehydrogenase
- GFP
Green Fluorescent Protein
- HEK 293
Human Embryonic Kidney 293 cells
- MAP2
Microtubule Associated Protein 2
- NLS
Nuclear Localization Sequence
- NMDA
N-methyl-D-aspartate
- P7
Postnatal day 7
- P14
Postnatal day 14
- PCR
Polymerase Chain Reaction
- pCREB
CREB phosphorylated on Serine 133
- pEGFP
plasmid encoding Enhanced Green Fluorescent Protein
- PFA
Paraformaldehyde
- RNA
Ribonucleic Acid
- RNAi
RNA interference
- siRNA
small interfering RNA
- TrkB
TrkB tyrosine kinase receptor
- TRPC6
Transient receptor potential channel 6
- VSCC
voltage sensitive calcium channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- Benes FM, Parks TN, Rubel EW. Rapid dendritic atrophy following deafferentation: an EM morphometric analysis. Brain Res. 1977;122:1–13. doi: 10.1016/0006-8993(77)90658-8. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Chen W, Prithviraj R, Mahnke AH, McGloin KE, Tan JW, Gooch AK, Inglis FM. AMPA glutamate receptor subunits 1 and 2 regulate dendrite complexity and spine motility in neurons of the developing neocortex. Neuroscience. 2009;159:172–182. doi: 10.1016/j.neuroscience.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow FA, Anderson KA, Noeldner PK, Means AR. Autonomous activity of Calcium/Calmodulin-dependent protein kinase IV is required for its role in transcription. J Biol Chem. 2005;280:20530–20538. doi: 10.1074/jbc.M500067200. [DOI] [PubMed] [Google Scholar]
- Elgersma Y, Sweatt JD, Giese KP. Mouse genetic approaches to investigating calcium/calmodulin-dependent protein kinase II function in plasticity and cognition. J Neurosci. 2004;24(39):8410–8415. doi: 10.1523/JNEUROSCI.3622-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima H, Maeda R, Suzuki R, Suzuki A, Nomoto M, Toyoda H, Wu LJ, Xu H, Zhao MG, Ueda K, Kitamoto A, Mamiya N, Yoshida T, Homma S, Masushige S, Zhuo M, Kida S. Upregulation of Calcium/Calmodulin-Dependent Protein Kinase IV improves memory formation and rescues memory loss with aging. J Neurosci. 2008;28(40):9910–9919. doi: 10.1523/JNEUROSCI.2625-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD. Microcircuitry of the visual cortex. Annu Rev Neurosci. 1983;6:217–247. doi: 10.1146/annurev.ne.06.030183.001245. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Barth AL, Wallace H, McKenna M, Silva A, Fox K. Impaired experience-dependent plasticity in barrel cortex of mice lacking the alpha and delta isoforms of CREB. Cereb Cortex. 1999;9:249–256. doi: 10.1093/cercor/9.3.249. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Volkmar FR. Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Exp Neurol. 1973;40:491–504. doi: 10.1016/0014-4886(73)90090-3. [DOI] [PubMed] [Google Scholar]
- Ha S, Redmond L. ERK mediates activity dependent neuronal complexity via sustained activity and CREB-mediated signaling. Dev Neurobio. 2008;68:1565–1579. doi: 10.1002/dneu.20682. [DOI] [PubMed] [Google Scholar]
- Häusser M, Spruston N, Stuart GJ. Diversity and dynamics of dendritic signaling. Science. 2000;290:739–744. doi: 10.1126/science.290.5492.739. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Daza RA, Rubenstein JL, Stunnenberg H, Olavarria JF, Englund C. Beyond laminar fare: toward a molecular classification of cortical projection/pyramidal neurons. Dev Neurosci. 2003;25:139–151. doi: 10.1159/000072263. [DOI] [PubMed] [Google Scholar]
- Hickmott PW, Steen PA. Large-scale changes in dendritic structure during reorganization of adult somatosensory cortex. Nat Neurosci. 2005;8(2):140–142. doi: 10.1038/nn1384. [DOI] [PubMed] [Google Scholar]
- Ho N, Liauw JA, Blaeser F, Wei F, Hanissian S, Muglia LM, Wozniak DF, Nardi A, Arvin KL, Holtzman DM, Linden DJ, Zhuo M, Muglia LJ, Chatila TA. Impaired synaptic plasticity and cAMP responsive element-binding protein activation in Ca2/calmodulin-dependent protein kinase type IV/Gr-deficient mice. J Neurosci. 2000;20:6459–6472. doi: 10.1523/JNEUROSCI.20-17-06459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway RL. Dendritic branching: some preliminary results of training and complexity in rat visual cortex. Brain Res. 1966;2:393–396. doi: 10.1016/0006-8993(66)90009-6. [DOI] [PubMed] [Google Scholar]
- Impey S, Fong AL, Wang Y, Cardinaux JR, Fass DM, Obrietan K, Wayman GA, Storm DR, Soderling TR, Goodman RH. Phosphorylation of CBP mediates transcriptional activation by neural activity and CaM kinase IV. Neuron. 2002;34(2):235–244. doi: 10.1016/s0896-6273(02)00654-2. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Crockett R, Korada S, Abraham WC, Hollmann M, Kalb RG. The AMPA receptor subunit GluR1 regulates dendritic architecture of motor neurons. J Neurosci. 2002;22(18):8042–8051. doi: 10.1523/JNEUROSCI.22-18-08042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KF, Ohmstede CA, Fisher RS, Sahyoun N. Nuclear and axonal localization of Ca 2+/calmodulin-dependent protein kinase type Gr in rat cerebellar cortex. Proc Natl Acad Sci USA. 1991;88:2850–2853. doi: 10.1073/pnas.88.7.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraska JM. The development of pyramidal neurons after eye opening in the visual cortex of hooded rats: A quantitative study. J Comp Neurol. 1982;212:208–212. doi: 10.1002/cne.902120210. [DOI] [PubMed] [Google Scholar]
- Kamata A, Sakagami H, Tokumitsu H, Sanda M, Owada Y, Fukunaga K, Kondo H. Distinct developmental expression of two isoforms of Ca 2+/calmodulin-dependent protein kinase kinases and their involvement in hippocampal dendritic formation. Neurosci Lett. 2007;423:143–148. doi: 10.1016/j.neulet.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Kang H, Sun LD, Atkins CM, Soderling TR, Wilson MA, Tonegawa S. An important role of neural activity-dependent CaMKIV signaling in the consolidation of long-term memory. Cell. 2001;106:771–783. doi: 10.1016/s0092-8674(01)00497-4. [DOI] [PubMed] [Google Scholar]
- Kokubo M, Nishio M, Ribar TJ, Anderson KA, West AE, Means AR. BDNF-mediated cerebellar granule cell development is impaired in mice null for CaMKK2 or CaMKIV. J Neurosci. 2009;29(28):8901–8913. doi: 10.1523/JNEUROSCI.0040-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Gross CG, Kopil C, Battaglia L, McBreen M, Stranahan AM, Gould E. Experience induces structural and biochemical changes in the adult primate brain. Proc Natl Acad Sci USA. 2005;102(48):17478–17482. doi: 10.1073/pnas.0508817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Huang H, Feng G, Sanes JR, Brown EN, So PT, Nedivi E. Dynamic remodeling of dendritic arbors in GABAergic interneurons of adult visual cortex. PLoS Biol. 2006;4(2):e29. doi: 10.1371/journal.pbio.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maravall M, Koh IYY, Lindquist WB, Svoboda K. Experience-dependent changes in basal dendritic branching of Layer 2/3 pyramidal neurons during a critical period for developmental plasticity in rat barrel cortex. Cereb Cortex. 2004;14(6):655–664. doi: 10.1093/cercor/bhh026. [DOI] [PubMed] [Google Scholar]
- Marín-Padilla M. Ontogenesis of the pyramidal cell of the mammalian neocortex and developmental cytoarchitectonics: A unifying theory. J Comp Neurol. 1992;321:223–240. doi: 10.1002/cne.903210205. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophin regulation of cortical dendrite growth requires activity. Neuron. 1996;17:1057–1064. doi: 10.1016/s0896-6273(00)80239-1. [DOI] [PubMed] [Google Scholar]
- Miller M. Maturation of rat visual cortex. I. A quantitative study of Golgi-impregnated pyramidal neurons. J Neurocyt. 1981;10:859–878. doi: 10.1007/BF01262658. [DOI] [PubMed] [Google Scholar]
- Miller M, Peters A. Maturation of rat visual cortex. II. A combined Golgi-electron microscope study of pyramidal neurons. J Comp Neurol. 1981;203(4):555–573. doi: 10.1002/cne.902030402. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Okuno S, Sato F, Fujisawa H. An immunohisotchemical study of Ca2+/calmodulin-dependent protein kinase IV in the rat central nervous system: Light and electron microscopic observations. Neurosci. 1995;68(1):181–194. doi: 10.1016/0306-4522(95)00092-w. [DOI] [PubMed] [Google Scholar]
- Rajan I, Cline HT. Glutamate receptor activity is required for normal development of tectal cell dendrites in vivo. J Neurosci. 1998;18:7836–7846. doi: 10.1523/JNEUROSCI.18-19-07836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond L, Kashani AH, Ghosh A. Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription. Neuron. 2002;34:999–1010. doi: 10.1016/s0896-6273(02)00737-7. [DOI] [PubMed] [Google Scholar]
- Redmond L. Translating neuronal activity into dendrite elaboration: signaling to the nucleus. Neurosignals. 2008;16(2–3):194–208. doi: 10.1159/000111563. [DOI] [PubMed] [Google Scholar]
- Rubinstein JH. Broad thumb-hallux (Rubinstein-Taybi) syndrome 1957–1988. Am J Med Genet Suppl. 1990;6:3–16. doi: 10.1002/ajmg.1320370603. [DOI] [PubMed] [Google Scholar]
- Scott EK, Lou L. How do dendrites take their shape? Nat Neurosci. 2001;4:359–365. doi: 10.1038/86006. [DOI] [PubMed] [Google Scholar]
- Sík A, Hájos N, Gulácsi A, Mody I, Freund TF. The absence of a major Ca2+ signaling pathway in GABAergic neurons of the hippocampus. Proc Natl Acad Sci USA. 1998;95(6):3245–3250. doi: 10.1073/pnas.95.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soekarman D, Fryns JP. Corpus callosum agenesis in Coffin-Lowry syndrome. Genet Couns. 1994;5:77–80. [PubMed] [Google Scholar]
- Steffen H, Van der Loos H. Early lesions of mouse vibrissal follicles: their influence on dendritic orientation in the cortical barrelfield. Exp Brain Res. 1980;40:410–431. doi: 10.1007/BF00236150. [DOI] [PubMed] [Google Scholar]
- Sun P, Enslen H, Myung PS, Maurer RA. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 1994;8(21):2527–2539. doi: 10.1101/gad.8.21.2527. [DOI] [PubMed] [Google Scholar]
- Tai Y, Feng S, Ge R, Du W, Zhang X, He Z, Wany Y. TRPC6 channels promote dendritic growth via the CaMKIV-CREB pathway. J Cell Sci. 2008;121(14):2301–2307. doi: 10.1242/jcs.026906. [DOI] [PubMed] [Google Scholar]
- Takao K, Tanda K, Nakamura K, Kasahara J, Nakao K, Katsuki M, Nakanishi K, Yamasaki N, Toyama K, Adachi M, Umeda M, Araki T, Fukunaga K, Kondo H, Sakagami H, Miyakawa T. Comprehensive behavioral analysis of calcium/calmodulin-dependent protein kinase IV knockout mice. PLoS One. 2010;5(3):e9460. doi: 10.1371/journal.pone.0009460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant AR, Zanassi P, Walsh GS, Aumont A, Alonso A, Miller FD. Signaling mechanisms underlying reversible, activity-dependent dendrite formation. Neuron. 2002;34:985–998. doi: 10.1016/s0896-6273(02)00717-1. [DOI] [PubMed] [Google Scholar]
- Volkmar FR, Greenough WT. Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science. 1972;176:1145–1147. doi: 10.1126/science.176.4042.1445. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Kaech S, Grant WF, Davare M, Impey S, Tokumitsu H, Nazaki N, Banker G, Soderling TR. Regulation of axonal extension and growth cone motility by calmodulin-dependent protein kinase I. J Neurosci. 2004;24:3786–3794. doi: 10.1523/JNEUROSCI.3294-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR. Activity dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron. 2006;50:897–909. doi: 10.1016/j.neuron.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Wei F, Qiu CH, Liauw J, Robinson DA, Ho N, Chatila T, Zhuo M. Calucium-calmodulin-dependent protein kinase IV is required for fear memory. Nat Neurosci. 2002;5:573–579. doi: 10.1038/nn0602-855. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Effects of visual deprivation on morphology and physiology of cells in the cat’s lateral geniculate body. J Neurophysiol. 1963;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Wise SP, Fleshman JW, Jr, Jones EG. Maturation of pyramidal cell form in relation to developing afferent and efferent connections of rat somatic sensory cortex. Neuroscience. 1979;4:1275–1297. doi: 10.1016/0306-4522(79)90157-x. [DOI] [PubMed] [Google Scholar]
- Wong ROL, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nat Rev Neurosci. 2002;3:803–812. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]
- Yacoubian TA, Lo DC. Truncated and full-length TrkB receptors regulate distinct modes of dendritic growth. Nat Neurosci. 2000;3:342–349. doi: 10.1038/73911. [DOI] [PubMed] [Google Scholar]
- Yoneshima H, Yamasaki S, Voelker CC, Molnár Z, Christophe E, Audinat E, Takemoto M, Nishiwaki M, Tsuji S, Fujita I, Yamamoto N. Er81 is expressed in a subpopulation of layer 5 neurons in rodent and primate neocortices. Neuroscience. 2006;137(2):401–412. doi: 10.1016/j.neuroscience.2005.08.075. [DOI] [PubMed] [Google Scholar]
- Yu X, Malenka RC. Beta-catenin is critical for dendritic morphogenesis. Nat Neurosci. 2003;6:1169–1177. doi: 10.1038/nn1132. [DOI] [PubMed] [Google Scholar]