Abstract
Background
Levels of cannabinoid 1 receptor (CB1R) mRNA and protein, which are expressed most heavily in the cholecystokinin class of GABA neurons, are lower in the dorsolateral prefrontal cortex (DLPFC) in schizophrenia, and the magnitude of these differences is strongly correlated with that for glutamic acid decarboxylase (GAD67) mRNA, a synthesizing enzyme for GABA. However, whether this correlation reflects a cause-effect relationship is unknown.
Methods
Using quantitative in situ hybridization, we measured CB1R, GAD67, and diacylglycerol lipase alpha (DAGLα; the synthesizing enzyme for the endocannabinoid 2-arachidonoylglycerol) mRNA levels in the medial prefrontal cortex of genetically-engineered GAD67 heterozygous (GAD67+/−), CB1R heterozygous (CB1R+/−), CB1R knockout (CB1R−/−), and matched wild-type mice.
Results
In GAD67+/− mice, GAD67 and CB1R mRNA levels were significantly reduced by 37% and 16%, respectively, relative to wild-type mice and were significantly correlated across animals (r=0.61; p=0.01). In contrast, GAD67 mRNA levels were unaltered in CB1R+/− and CB1R−/− mice. Expression of DAGLα mRNA, which is not altered in schizophrenia, was also not altered in any of the genetically-engineered mice.
Conclusions
The findings that reduced GAD67 mRNA expression can induce lower CB1R mRNA expression support the hypothesis that lower cortical levels of CB1Rs in schizophrenia may partially compensate for deficient GAD67-mediated GABA synthesis by reducing endogenous cannabinoid suppression of GABA release.
Keywords: Cannabis, cholecystokinin, cognition, GABA, in situ hybridization, interneurons, mouse model, working memory
Introduction
Alterations in subpopulations of γ-aminobutyric acid (GABA) neurons appear to contribute to dysfunction of the dorsolateral prefrontal cortex (DLPFC) in schizophrenia. Indeed, lower expression of glutamic acid decarboxylase (GAD67), the enzyme responsible for most GABA synthesis, is consistently found in schizophrenia (1). The affected GABA neurons include basket neurons that express both the cannabinoid 1 receptor (CB1R) and the neuropeptide cholecystokinin (CCK) (2–4). For example, CB1R mRNA and protein levels are lower in the DLPFC of subjects with schizophrenia (5–7), and the magnitude of alterations in CB1R mRNA expression is significantly correlated with that for GAD67 mRNA (3,6). These findings suggest that both transcripts are lower in the same population of DLPFC GABA neurons in schizophrenia.
Because CB1R activation suppresses GABA neurotransmission (8), we previously suggested that a lower density of CB1Rs in schizophrenia could be a cell type-specific, homeostatic adaptation to partially compensate for upstream reductions in GAD67-mediated GABA synthesis in CB1R/CCK-containing neurons (5,6). That is, a down-regulation of CB1Rs could reduce the endocanabinoid-mediated block of GABA release from the terminals of CB1R/CCK-containing neurons, thereby enhancing GABA neurotransmission in cells with deficient GABA synthesis (5,6). As a proof-of-concept test of this hypothesis, we assessed GAD67 and CB1R mRNA levels in the medial prefrontal cortex of genetically-engineered GAD67 heterozygous (GAD67+/−), CB1R heterozygous (CB1R+/−), CB1R knockout (CB1R−/−), and matched wild-type (WT) mice. In addition, to test the specificity of the causal relationship between reduced GAD67 mRNA and lower CB1R mRNA levels, we also assessed mRNA levels for diacylglycerol lipase alpha (DAGLα), the synthesizing enzyme for 2-arachidonoylglycerol (2-AG) (the principal endocannabinoid in the DLPFC), which are unaltered in schizophrenia (9).
Methods and Materials
Animals and Tissue Processing
Generation of GAD67 heterozygous mice
GAD67+/− mice were generated as previously described (10). Exon 2 (the first coding exon) of the Gad1 gene was flanked by loxP sites using gene targeting in embryonic stem cells. Using FLP recombinase, the Sv-NeoR selectable gene was removed to produce Gad1lox/+ mice, which were interbred to generate phenotypically normal Gad1lox/lox mice. Gad1lox/lox mice were bred with Mox2-Cre mice to delete exon 2 in the germ-line (Gad1Δ/+ [GAD67+/−]) mice. Interbreeding of Gad1Δ/+ mice generated some mice that did not survive beyond the perinatal period, consistent with previously described Gad1-null mice (11). Age, sex, and litter-matched (10 males and 4 females) GAD67+/− and WT mice (n=7 per group) were euthanized at eight weeks of age and brains were removed, frozen, and stored at −80°C.
Generation of CB1R heterozygous and knockout mice
CB1R+/− and CB1R−/− mice were generated as previously described (12). The Cnr1 gene was mutated in MPI2 embryonic stem cells by replacing the coding region between amino acids 32 and 448 with PGK-neo. Chimeric mice derived from these cells were bred with C57BL/6J animals. Backcrossing of chimeric and heterozygous animals to C57BL/6J mice and interbreeding of CB1R+/− animals produced CB1R−/− mutants and wild-type mice. Male animals were euthanized at either eight weeks of age (CB1R+/−, CB1R−/−, and WT; n=6 per group) or four weeks of age (CB1R−/− and WT; n=7 per group) and brains were removed, frozen, and stored at −80°C. Fresh frozen brains were provided by Bristol-Myers Squibb.
Coronal sections from all mouse brains were cut on a cryostat at 12 μm, thaw mounted onto SupraFrost slides (Fisher Scientific, Pittsburgh, PA), and stored at −80°C until used.
In situ hybridization
Templates for the synthesis of riboprobes against mouse GAD67, CB1R, and DAGLα mRNA were generated by polymerase chain reaction (see Table). Nucleotide sequencing revealed 100% homology for the amplified template fragments to previously reported sequences. Sense and antisense riboprobes were generated by in vitro transcription in the presence of 35S-CTP using T7 or SP6 RNA polymerase, purified, and reduced to approximately 100 bp by alkaline hydrolysis to increase the effectiveness of tissue penetration (13). Standard hybridization procedures were performed as previously described (13). Following hybridization, sections from all mice for a given comparison were exposed to BioMaxMR film (Kodak, Rochester, NY) for 24 hours (GAD67), 48–72 hours (CB1R), or 36 hours (DAGLα). Tissue sections processed by in situ hybridization for CB1R mRNA were subsequently coated with NTB2 emulsion (Kodak) using a mechanical dipper (Auto-dip Emulsion Coater, Ted Pella, Redding, CA), exposed at 4°C, then developed using D-19 (Kodak), and counterstained with Cresyl violet. Specificity of the hybridization signal produced by each probe was confirmed by the findings that each antisense probe produced the expected distinctive laminar pattern of expression (4,14,15) and by the absence of labeling with sense probes.
Table.
Template characteristics for riboprobe generation
Quantification
Quantification was performed blind to condition and animal number by random coding of slides as previously described (6). Autoradiographic film images of GAD67, CB1R, and DAGLα mRNA were captured using a Microcomputer Imaging Device (MCID) (5.1 μm/pixel resolution) and digitized. All images for slides processed in an experimental run were acquired in the same session under identical room illumination and with the same gain and black levels and flatfield correction. Three sections evenly spaced at ~144 μm intervals containing the medial prefrontal cortex (mPFC; +1.98 to +1.54 bregma (16)), including the cingulate and prelimbic cortices, were selected from each mouse for quantification. For each section, optical density (OD) levels of GAD67, CB1R, and DAGLα mRNA were measured bilaterally from the pial surface to the white matter in the mPFC and expressed as nanocuries per gram of tissue (nCi/g) by reference to carbon-14 standards (ARC Inc., St. Louis, MO) exposed on the same film. All cortical density measures were corrected by subtracting background measured in the white matter.
Quantification of CB1R mRNA at the cellular level was performed as previously described (13) for the 5 pairs of GAD67 WT and heterozygous mice with available emulsion-dipped, Nissl-counterstained sections. Using the MCID software and a Nikon microscope with a motorized stage, sampling boxes (120 × 170 μm) were systematically tiled from the pial surface to the layer 6 - white matter border in both hemispheres of the mPFC. Sampling circles with a fixed diameter of 16 μm (15) were placed over CB1R silver grain clusters, and the number of silver grains per circle was quantified. Background signal, determined for each tissue section by quantifying grains in a 120 × 170 μm sampling box placed in the white matter, was subtracted from each grain cluster before analysis. Examination of individual grain cluster counts revealed 13 clusters that were ≥ 2SD away from the mean, and these clusters were excluded from analyses as outliers. A total of 119 and 118 CB1R grain clusters were analyzed for GAD67 WT and heterozygous mice, respectively.
Statistics
T tests (GAD67+/− and WT mice) or analysis of variance (CB1R+/−, CB1R−/−, and WT mice) were performed to test the effect of genetic condition on OD measures using mean values across all of the sections from each animal, and two-tailed paired t-tests were used to assess group differences in grain density measures. One-tailed Pearson correlation analysis was performed to test the a priori hypothesis that GAD67 mRNA levels positively predict CB1R mRNA levels (6).
Results
Transcript levels in GAD67 heterozygous mice
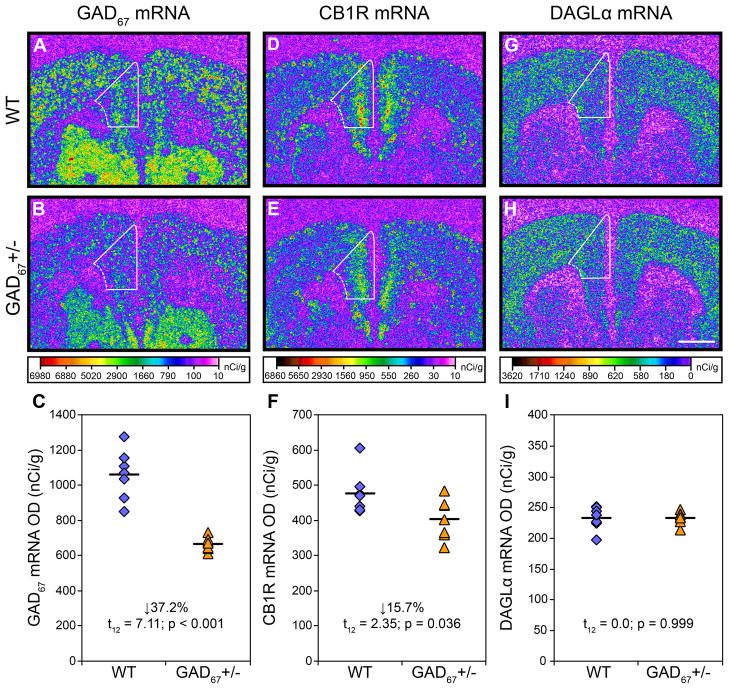
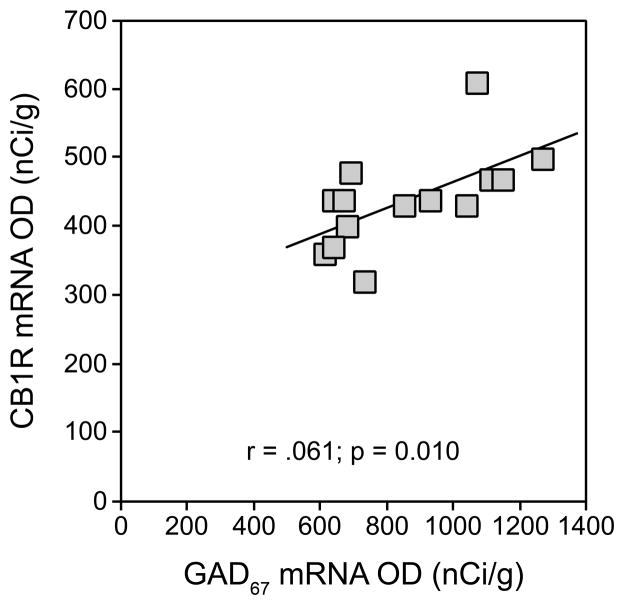
Mean (±SD) GAD67 mRNA levels were significantly 37.2% lower (t12=7.11; p<0.001) in GAD67+/− mice (664.9 ± 39.0 nCi/g) relative to WT mice (1059.4 ± 141.4 nCi/g) (Figure 1A–C). Mean CB1R mRNA levels were also significantly 15.7% lower (t12=2.35; p=0.036) in GAD67+/− mice (402.1 ± 56.8 nCi/g) relative to WT mice (477.0 ± 62.4 nCi/g) (Figure 1D–F). Furthermore, GAD67 and CB1R mRNA levels were positively correlated across all mice (r=0.61; p=0.010; Figure 2). In contrast, DAGLα mRNA levels did not differ (t12=0.0; p=0.999) between groups (Figure 1G–I).
Figure 1.
Transcript levels in the mPFC of adult wild-type and GAD67+/− mice. Representative film autoradiograms illustrating the expression of GAD67 (A, B), CB1R (D, E), and DAGLα (G, H) mRNAs. The density of hybridization signal for each transcript is presented in pseudocolor according to the calibration bars below B, E, and H. Expression of GAD67 and CB1R mRNA in GAD67+/− mice (B, E) appears lower than in wild-type mice (A, D), whereas DAGLα (G, H) does not appear to differ across the two conditions. Note that CB1R mRNA signal is most pronounced in the superficial cortical layers, consistent with the laminar distribution of CCK-containing GABA neurons that heavily express CB1R mRNA and that are the principal CB1R mRNA expressing neuron type in the cortex(4). White contours denote the quantified region of the mPFC. Comparison of cortical GAD67 (C), CB1R (F), and DAGLα (I) mRNA levels by film optical density (OD) in wild-type (diamonds) and GAD67+/− (triangles) mice. Mean values for each genetic condition are indicated by hash marks. Scale bar (1mm) in H applies to all panels.
Figure 2.
Positive correlation between levels of CB1R and GAD67 mRNAs in adult wild-type and GAD67+/− mice. These findings suggest that changes in CB1R mRNA expression parallel changes in GAD67 mRNA expression.
We next sought to determine whether lower CB1R mRNA levels were specific to mPFC in GAD67+/− mice or were also found in other cortical brain regions, such as the supplementary motor area. First, as expected (17), in WT mice CB1R expression was lower in supplementary motor area (402.5 ± 51.9 nCi/g) than in mPFC (477.0 ± 62.4 nCi/g) and CB1R mRNA levels were highly correlated between these two cortical regions (r=0.86, p<0.001). Similar to mPFC, mean CB1R mRNA levels were 11.0% lower in GAD67+/− mice (358.3 ± 47.7 nCi/g) relative to WT mice (402.5 ± 51.9 nCi/g), although this difference did not reach statistical significance (t12=1.66; p=0.123). The reduced strength of the finding in the supplementary motor area relative to mPFC may reflect the lower baseline levels of CB1R expression in motor areas (17); indeed, we previously suggested that reduced CB1R mRNA is likely to be an effective compensatory response to a deficit in GAD67 expression only in regions, like the PFC, with high levels of CB1R expression (6).
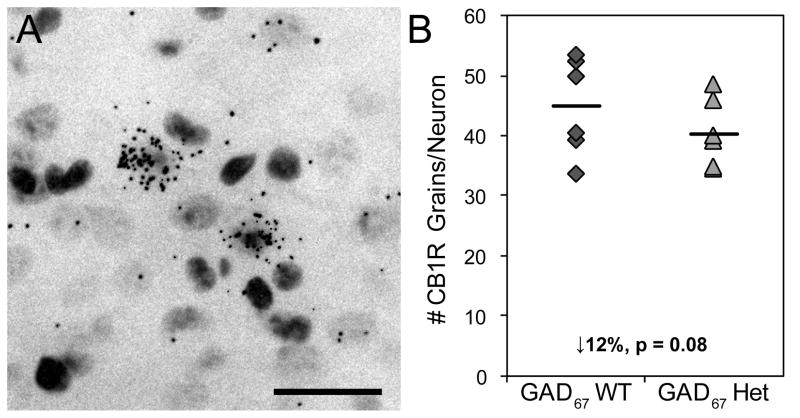
Grain counting analyses (Figure 3A) revealed a similar effect of reduced GAD67 expression on cellular CB1R mRNA levels. The mean number of CB1R grains per neuron was 12% lower (t10=1.79, p=0.08) in GAD67+/− mice (40.8 ± 5.1) relative to WT mice (36.0 ± 3.7) (Figure 3B).
Figure 3.
Cellular expression of CB1R mRNA. Representative photomicrograph of Nissl-counterstained, emulsion-exposed tissue section showing silver grains representing CB1R mRNA clustered over a subset of neuronal cell bodies (A). Scale bar = 30 μm. Expression of CB1R mRNA is lower in GAD67+/− mice relative to wild-type mice (B).
Transcript levels in CB1R heterozygous and knockout mice
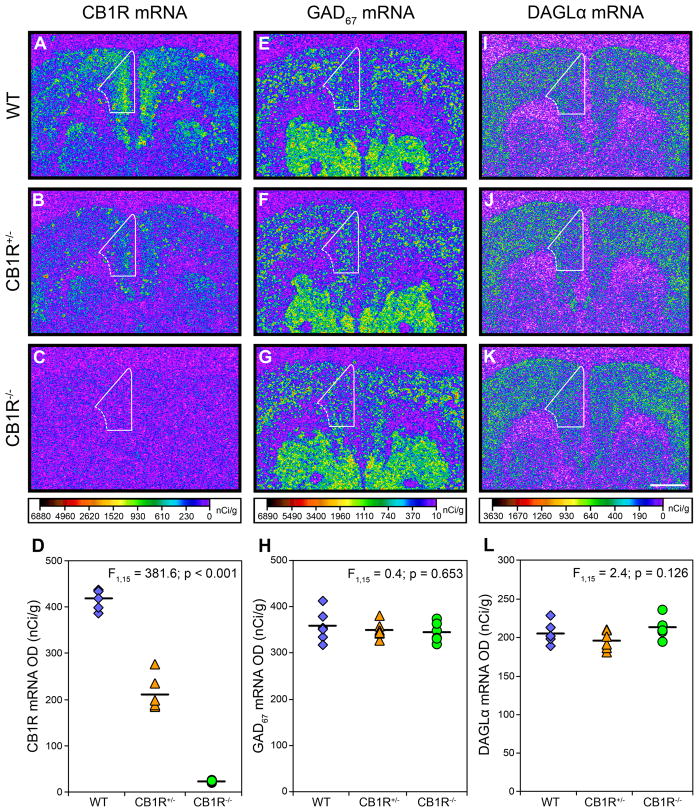
Manipulation of the Cnr1 gene produced the expected gene dose-dependent effect (F1,15=381.6; p<0.001) on CB1R mRNA expression (Figure 4A–D). Post-hoc analysis demonstrated that mean CB1R mRNA levels were significantly 49.6% lower in CB1R+/− mice (210.9 ± 37.2) compared to WT mice (418.9 ± 21.7) and 94.6% lower in CB1R−/− mice (22.6 ± 2.2). However, neither GAD67 (F1,15=0.4; p=0.653; Figure 4E–H) nor DAGLα (F1,15=2.4; p=0.126; Figure 3I–L) mRNA levels were altered in CB1R+/− or CB1R−/− mice relative to WT mice.
Figure 4.
Transcript levels in the mPFC of adult wild-type, CB1R+/−, and CB1R−/− mice. Representative film autoradiograms illustrating the expression of CB1R (A, B, C), GAD67 (E, F, G), and DAGLα (I, J, K) mRNA. The density of hybridization signal for each transcript is presented in pseudocolor according to the calibration bars below C, G, and K. Expression of CB1R mRNA is markedly reduced in CB1R+/− mice (B) and nearly undetectable in CB1R−/− mice (C) compared to wild-type mice (A). Expression of GAD67 (E, F, G) and DAGLα (I, J, K) mRNA appear unaltered in either genetic condition compared to wild-type mice. White contours denote the quantified region of the mPFC. Comparison of cortical CB1R (D), GAD67 (H), and DAGLα (L) mRNA levels by film optical density (OD) in wild-type (diamonds), CB1R+/− (triangles), and CB1R−/− (circles) mice. Mean values for each condition are indicated by hash marks. Scale bar (1mm) in K applies to all panels.
To determine if age-related compensations in GAD67 mRNA expression occur during development, we assessed GAD67 mRNA levels in four week old CB1R−/− and WT mice. In these mice, cortical GAD67 mRNA levels did not differ (F1,12=0.2; p=0.671) between CB1R−/− (536.7 ± 41.4 nCi/g) and WT mice (549.4 ± 64.7 nCi/g).
Discussion
Because deficits in GAD67 and CB1R mRNA levels are strongly correlated in the PFC in schizophrenia (6), we used genetically-engineered mice to investigate the plausibility of the hypothesis that a deficiency in GAD67 mRNA expression induces a corresponding reduction in CB1R mRNA, versus the alternative hypothesis that lower CB1R mRNA leads to a reduction in GAD67 expression. We found that GAD67+/− mice with a mean 37% decrease in GAD67 mRNA in the mPFC had CB1R mRNA levels that were significantly 16% lower than WT mice, and that GAD67 and CB1R mRNA levels were positively correlated across animals. Together, these data demonstrate that reduced GAD67 mRNA expression in mice is sufficient to produce lower levels of CB1R mRNA in the mPFC. In contrast, GAD67 mRNA levels were not changed in either peripubertal or adult mice with reduced CB1R mRNA expression, demonstrating that reduced CB1R mRNA expression does not affect GAD67 mRNA levels. In concert with our previous finding that alterations in CB1R and GAD67 mRNA expression in schizophrenia are strongly correlated (r=0.64; p=0.001) (6), these data support the hypothesis that reduced GAD67 mRNA expression may drive lower CB1R mRNA expression in the DLPFC of subjects with schizophrenia, whereas deficient CB1R mRNA expression is unlikely to be a cause of lower GAD67 mRNA in the disorder.
Consistent with this interpretation, it is noteworthy that the relative reductions in GAD67 and CB1R mRNA expression in the mPFC of GAD67+/− mice are similar to those observed in schizophrenia; mean GAD67 and CB1R mRNA levels are significantly ~28–37% (18,19) and 15% (6) lower, respectively, in the DLPFC of subjects with schizophrenia. In addition, the GAD67+/− mice have lower GAD67 expression across the cortical mantle, consistent with the observations that GAD67 mRNA levels are lower to a similar degree in multiple cortical regions in the same subjects with schizophrenia (19). However, GAD67 mRNA expression is reduced from early prenatal life in the GAD67+/− mice and whether this time course matches that of the GAD67 mRNA deficit in schizophrenia is unknown.
The deficit in GAD67 expression in the GAD67+/− mice was only about three-quarters of the 50% predicted reduction for a heterozygote, perhaps reflecting a compensatory increase in transcription from the remaining allele. On the other hand, the deficits in CB1R mRNA expression in the CB1R+/− mice (49.6%) and CB1R−/− mice (94.6%) were nearly exactly those predicted for heterozygotes and knockout animals, respectively. These comparisons suggest that the methods employed were sensitive to detect real differences in gene expression, and the absence of any differences in DAGLα mRNA expression also indicates that the CB1R mRNA deficit observed in the GAD67+/− mice is unlikely to be a false positive finding.
The mechanism through which deficient GAD67 expression results in reduced CB1R expression in the GAD67+/− mice remains to be determined. Lower GAD67 expression could cause alterations in cortical circuitry that produce increased 2-AG levels and subsequently CB1R down-regulation. However, the expression of DAGLα mRNA, which synthesizes 2-AG in pyramidal neurons postsynaptic to CB1R/CCK-containing axon terminals, was not altered in GAD67+/− mice. Alternatively, the effect of decreased GAD67 expression on CB1R expression may occur through a cell autonomous mechanism within CB1R/CCK-containing GABA neurons. Consistent with this idea, DAGLα mRNA expression was not altered in CB1R−/− mice suggesting that alterations in CB1R expression can occur independently of, and without alterations in, other components of the endocannabinoid system. Alternatively, because GAD67 is critical for the development of perisomatic axon terminals (10), deficient GAD67 expression may result in fewer CB1R-containing axon terminals and thus a reduced need for CB1R mRNA expression.
Together, these findings support the hypothesis that in schizophrenia, a lower density of CB1Rs could be an adaptation that partially compensates for upstream reductions in GAD67-mediated GABA synthesis (5,6) by reducing the 2-AG-mediated block of GABA release from the terminals of CB1R/CCK-containing neurons. By enhancing GABA release specifically from the terminals of those neurons, this homeostatic adaptation could contribute to a partial, albeit insufficient, normalization of neural network activity necessary for working memory function (20). However, although GAD67+/− mice nicely model the magnitude of reduced PFC GAD67 mRNA levels in schizophrenia, this illness is not defined by a single gene heterozygous null mutation and, consequently, other potential pathogenetic processes must be considered. For example, deficits in GABA-related transcripts in schizophrenia may alternatively reflect impaired development of specific classes of GABA neurons due to other upstream pathogenetic sources (15) or perhaps a compensatory downregulation of inhibitory signaling mechanisms in response to deficient excitation in the disorder (21). Furthermore, the extent to which the genetic manipulation of GAD67 expression in mice recapitulates the disease process of schizophrenia requires knowledge of other factors, such as when in development the deficit in GAD67 arises in schizophrenia.
Acknowledgments
This work was supported by National Institutes of Health grants NIH grants MH043784 and DA023109 and a Bristol-Myers Squibb Research Grant (DAL) and the G. Harold and Leila Y. Mathers Foundation (ZJH).
The authors thank Nestor Barrezueta for kindly providing CB1R heterozygous and knockout mice brains and Mary Brady for assistance with the graphics.
Footnotes
Financial Disclosures
Dr. David A. Lewis currently receives investigator-initiated research support from the BMS Foundation, Bristol-Myers Squibb, Curridium Ltd and Pfizer and in 200-2011 served as a consultant in the areas of target identification and validation and new compound development to AstraZeneca, Bristol-Myers Squibb, Hoffman-Roche, Lilly, Merck, and SK Life Science. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Rev. 2006;52:293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Eggan SM, Melchitzky DS, Sesack SR, Fish KN, Lewis DA. Relationship of cannabinoid CB1 receptor and cholecystokinin immunoreactivity in monkey dorsolateral prefrontal cortex. Neuroscience. 2010;169:1651–1661. doi: 10.1016/j.neuroscience.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- 5.Eggan SM, Stoyak SR, Verrico CD, Lewis DA. Cannabinoid CB1 receptor immunoreactivity in the prefrontal cortex: Comparison of schizophrenia and major depressive disorder. Neuropsychopharm. 2010;35:2060–2071. doi: 10.1038/npp.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eggan SM, Hashimoto T, Lewis DA. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch Gen Psychiatry. 2008;65:772–784. doi: 10.1001/archpsyc.65.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uriguen L, Garcia-Fuster MJ, Callado LF, Morentin B, La Harpe R, Casado V, et al. Immunodensity and mRNA expression of A2A adenosine, D2 dopamine, and CB1 cannabinoid receptors in postmortem frontal cortex of subjects with schizophrenia: effect of antipsychotic treatment. Psychopharmacology (Berl) 2009;206:313–324. doi: 10.1007/s00213-009-1608-2. [DOI] [PubMed] [Google Scholar]
- 8.Pistis M, Ferraro L, Pira L, Flore G, Tanganelli S, Gessa GL, et al. Delta(9)-tetrahydrocannabinol decreases extracellular GABA and increases extracellular glutamate and dopamine levels in the rat prefrontal cortex: an in vivo microdialysis study. Brain Res. 2002;948:155–158. doi: 10.1016/s0006-8993(02)03055-x. [DOI] [PubMed] [Google Scholar]
- 9.Volk DW, Eggan SM, Lewis DA. Alterations in metabotropic glutamate receptor 1alpha and regulator of G protein signaling 4 in the prefrontal cortex in schizophrenia. Am J Psychiatry. 2010;167:1489–1498. doi: 10.1176/appi.ajp.2010.10030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y, et al. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54:889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, et al. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, et al. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, et al. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25:372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. 2. San Diego: Academic Press; 2001. [Google Scholar]
- 17.Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17:175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- 18.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 21.Glausier JR, Lewis DA. Selective pyramidal cell reduction of GABA(A) receptor alpha1 subunit messenger RNA expression in schizophrenia. Neuropsychopharm. 2011;36:2103–2110. doi: 10.1038/npp.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]