Summary
Background
Repair of skin wounds is essential for animals to survive in a harsh environment, yet the signaling pathways initiating wound repair in vivo remain little understood. In C. elegans a p38 MAPK cascade promotes innate immune responses to wounding, but is not required for other aspects of wound healing. We therefore set out to identify additional wound response pathways in C. elegans epidermis.
Results
We show here that wounding the adult C. elegans skin triggers a rapid and sustained rise in epidermal Ca2+ that is critical for survival after wounding. The wound-triggered rise in Ca2+ requires the epidermal TRPM channel GTL-2 and IP3R-stimulated release from internal stores. We identify an epidermal signal transduction pathway that includes the Gαq EGL-30 and its effector PLCβ EGL-8. Loss of function in this pathway impairs survival after wounding. The Gαq-Ca2+ pathway is not required for known innate immune responses to wounding but instead promotes actin-dependent wound closure. Wound closure requires the Cdc42 small GTPase and Arp2/3 dependent actin polymerization, and is negatively regulated by Rho and non-muscle myosin. Finally, we show that the death-associated protein kinase DAPK-1 acts as a negative regulator of wound closure.
Conclusions
Skin wounding in C. elegans triggers a Ca2+-dependent signaling cascade that promotes wound closure, in parallel to the innate immune response to damage. Wound closure requires actin polymerization and is negatively regulated by non-muscle myosin.
Keywords: laser, barrier epithelium, GCaMP, TRP channel, DAP kinase, innate immunity, actin polymerization, nonmuscle myosin, Cdc42, Rho GTPase
Introduction
All organisms must repair damage caused by mechanical injury or environmental pathogens. The skin in particular exhibits a complex sequence of wound healing processes [1]. Understanding the molecular basis of wound healing is of increasing relevance to human health as chronic or non-healing wounds become more common in the elderly or in diabetic individuals [2]. Several vertebrate and invertebrate models are being explored for molecular genetic analyses of wound healing [3–5]. Importantly, transcription factors involved in epidermal wound healing show evolutionary conservation [6, 7], suggesting mechanisms in wound healing could share significant similarity.
Despite extensive efforts in the analysis of wound healing, many questions remain open. It is not well understood how barrier epithelia detect damage, nor how the initial sensation of damage is transmitted to develop a coordinated wound healing response. Studies in Drosophila wound healing have highlighted the role of epidermal tyrosine kinases and ERK signaling in early wound responses [8, 9], whereas mammalian wound responses appear to be initiated by a variety of growth factors and cytokines [10].
The epidermis of the nematode C. elegans is a simple barrier epithelium that generates an external cuticle. The nematode body is under hydrostatic pressure, so puncture wounds can be fatal if not repaired. Such wounds may be common in nature, where nematodes encounter damaging substrates and cuticle-puncturing pathogens [11, 12]. Needle or laser wounding of the epidermis triggers a p38 MAPK/PMK-1 cascade that activates transcription of antimicrobial peptide genes [13]. This innate immune response defends against opportunistic infection at wounds, as p38 MAPK mutants display reduced survival after wounding [14]. However, the p38 MAPK cascade is not required for other wound repair processes such as scar formation [14].
To address how other aspects of C. elegans wound healing are activated we have examined other known wound-triggered pathways. Ca2+ is important for embryonic wound healing [15] and in wound responses of single cells [16]. However the in vivo role of Ca2+ signals in repair of mature barrier epithelia has been less clear. We show here that Ca2+ signals are critical for C. elegans wound healing, acting in parallel to innate immune response pathways to promote actin rearrangement during wound closure. Unlike embryonic or single-cell wounds, which typically involve actomyosin purse-string based contractility [17], we find that wounds in the adult C. elegans skin close by an actin polymerization based mechanism negatively regulated by non-muscle myosin.
Results
Wounding triggers a rapid and sustained rise in epidermal Ca2+
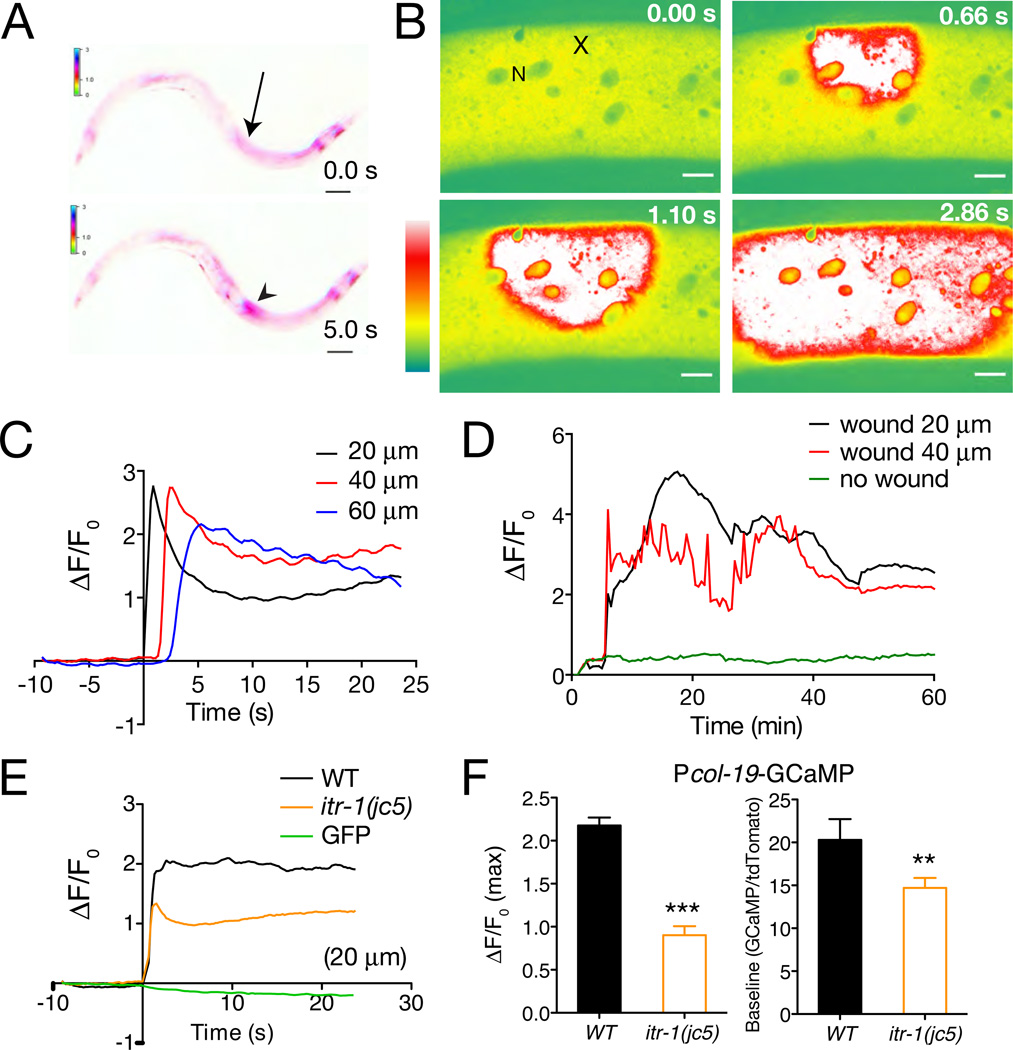
To visualize Ca2+ dynamics in the epidermis after wounding we expressed the Ca2+ sensor GCaMP3 [18] under the control of epidermal-specific promoters (see Experimental Procedures; Table S1). The adult C. elegans epidermis consists of seam cells and multinucleate syncytial cells, of which hyp7 is the largest. Needle wounding of the syncytial hyp7 epidermis in late larvae or adults triggered increases in epidermal GCaMP fluorescence that spread from the site of injury to approximately 1/3 the length of the animal (Movie S1; Figure 1A). To quantitate wound-triggered Ca2+ dynamics we performed localized laser wounding and imaged epidermal GCaMP using spinning-disk microscopy (Movie S2: Figure 1B,C). Laser wounding triggered increases in GCaMP fluorescence similar to those seen after needle wounding; GCaMP waves traveled at 25.6 ± 2 µm s−1 (Figure S1A), a speed consistent with propagation via Ca2+-induced Ca2+ release from internal stores. Thereafter, elevated GCaMP fluorescence underwent small global oscillations but was otherwise stable, and typically persisted for > 1 h before declining (Movie S3; Figure 1D).
Figure 1. Wounding triggers an epidermal Ca2+ response.
(A) Epidermal GCaMP induction after needle wounding; Pcol-19-GCaMP (juIs319). Site of wounding indicated by arrow; GCaMP fluorescence increase, arrowhead (see also Movie S1); inverted color scale. (B) Epidermal GCaMP fluorescence levels after femtosecond laser wounding. Lateral view of epidermis in mid-body; x, site of laser wound; N, epidermal nucleus. Spinning disk confocal, intensity code. See also Movie S2. (C) Quantitation of GCaMP ΔF/F0 at 20, 40, and 60 µm from wound site; representative trace. Note oscillations in GCaMP levels. (D) Prolonged elevation of epidermal GCaMP fluorescence after wounding; representative trace, from Movie S3 at positions 20 and 40 µm from wound site. Unwounded animals display minimal variation in GCaMP signals (green line). (E) itr-1(jc5cs) mutants cultured at 15°C display reduced GCaMP ΔF/F0 compared to WT (average traces); wounding does not affect epidermal GFP fluorescence (green line, E). (F) itr-1 mutants display reduced peak ΔF/F0 after wounding and reduced baseline GCaMP fluorescence, measured as ratio to epidermally expressed tdTomato. n > 20 per genotype Scales, 10 µm.
To test whether release from internal stores contributed to the epidermal Ca2+ wave we tested the IP3 receptor ITR-1. itr-1(jc5cs) mutants displayed significantly reduced epidermal GCaMP responses to injury (Figure 1E,F). To reduce IP3 signaling specifically in the epidermis we overexpressed N-terminal IP3 binding domains (‘IP3 sponges’) [19]. Expression of IP3 sponges in the adult epidermis reduced both baseline and wound-induced increase in GCaMP fluorescence (Figure S1B,C), indicating that wounding triggers Ca2+ release from internal stores in the epidermis.
An epidermal TRPM channel is required for Ca2+ responses
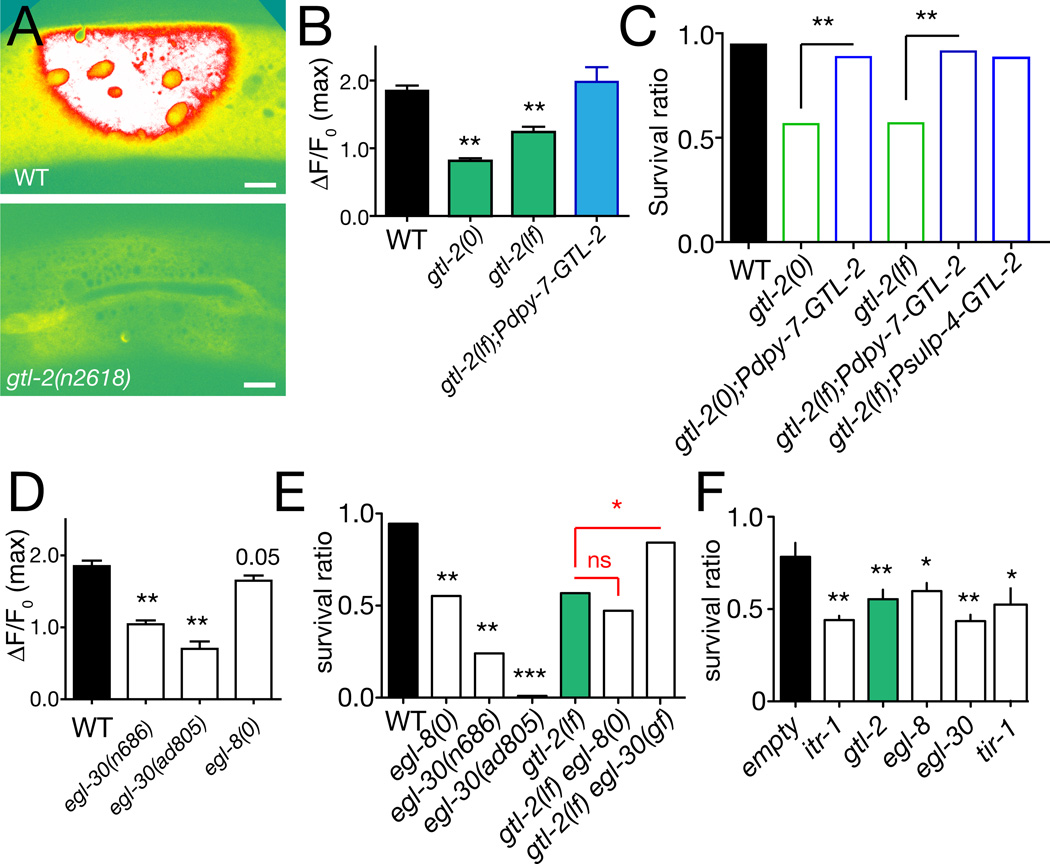
Release of Ca2+ from internal stores could be triggered by an initial Ca2+ influx across the epidermal cell membrane or by other signals that activate the IP3R. To define the sources of epidermal Ca2+ we screened known Ca2+ channels and Ca2+ signaling components (Table S2). C. elegans encodes ~100 Ca2+ channel subunits, including 17 divalent cation channels in the TRP class [20]. Several TRP channels are expressed in the epidermis, including the TRPM-family channel GTL-2 [21]. Of 11 TRP channels tested, only GTL-2 was required for normal epidermal Ca2+ responses (Figure 2A,B). Epidermal expression of GTL-2 rescued gtl-2 Ca2+ defects (Figure 2B), whereas muscle- or neuron-specific expression did not rescue (not shown), suggesting GTL-2 can act cell-autonomously in epidermal Ca2+ homeostasis. GTL-2 is localized to the plasma membrane in the epidermis [21] suggesting GTL-2 may affect initial influx of Ca2+ after wounding rather than subsequent calcium-induced Ca2+ release.
Figure 2. TRPM channel GTL-2 and Gαq/PLCβ signaling are required for epidermal Ca2+ responses and survival after wounding.
(A) Epidermal Ca2+ responses to wounding are reduced in gtl-2 mutants; imaged of Pcol-19-GCaMP(juIs319) (1 s after laser wound). (B) Quantitation of peak ΔF/F0 in gtl-2(0) null mutants (tm1463) and gtl-2(lf) partial loss of function n2618 mutants; rescue by expression of GTL-2 under epidermal control (juEx2893). n > 30 per genotype. (C) gtl-2 mutants display reduced survival after wounding; rescue by GTL-2::GFP expressed under control of epidermal (Pdpy-7, juEx2893) or excretory system (Psulp-4, juEx3216) promoters. GTL-2::GFP signals are weak and do not interfere with GCaMP imaging. Survival ratio calculated as fraction of animals alive 24 h post wounding/number unwounded alive; n > 100 per genotype. (D) Partial loss of function in egl-30 strongly reduces epidermal baseline Ca2+ and ΔF/F0; loss of function in egl-8 reduces baseline GCaMP signal and has a small effect on ΔF/F0; n > 50 per genotype. (E) Reduced survival of egl-8 and egl-30 mutants post wounding; egl-8(0) gtl-2(lf) double mutants are not further enhanced; n > 500 per genotype. Improved survival of gtl-2 egl-30(gf) double mutants. (F) Epidermal-specific RNAi of egl-8 and egl-30 significantly reduces survival post-wounding, comparably to RNAi of itr-1 or gtl-2; n > 200 per RNAi. All bar charts show mean ± SEM. Statistics, t test (B), Mann-Whitney test (D, F), or Fisher exact test (C,E); ***, P < 0.001, **, P < 0.01; *, P < 0.05; ns, not significant.
Epidermal Ca2+ responses are required for survival of wounding
To address the importance of Ca2+ and GTL-2 in wound healing we analyzed survival post-wounding. We measured survival 24 h after needle wounding, a time when >90% of wounded wild-type animals are healthy [14]. gtl-2 mutants showed drastically reduced survival post wounding, a phenotype rescued by epidermal expression of GTL-2, suggesting epidermal GTL-2 is required for survival after wounding (Figure 2C). Expression of IP3 sponges in the epidermis caused smaller decreases in survival, consistent with their partial reduction in epidermal Ca2+ (Figure S1D). Loss of function in other TRPM family members GON-2 or GTL-1 can compensate for loss of GTL-2 function via systemic ion homeostasis [21, 22], and indeed gtl-2 gon-2 or gtl-2 gtl-1 double mutants showed restored viability after wounding (Figure S2B). These observations further support the model that GTL-2 contributes to epidermal Ca2+ homeostasis required for wound responses.
Reduced survival after wounding could reflect poor overall health rather than a defect in wound repair. To address whether developmental defects might account for reduced survival after wounding we used tissue- and stage-specific RNAi to reduce gene function only in the adult epidermis (see Experimental Procedures). Adult epidermal-specific RNAi of genes acting in the epidermal innate immune response, including the Toll/Interleukin receptor adaptor domain gene tir-1 [23], reduced post-wound survival (Figure 2F), indicating effective gene knockdown in this strain. Adult epidermal-specific RNAi of Ca2+ signaling genes, including itr-1 and gtl-2, significantly reduced post-wound survival (Figure 2F), confirming that ITR-1 and GTL-2 are required cell-autonomously for survival after epidermal wounding.
Gαq signaling is required for the epidermal Ca2+ response to wounding
Among genes required for normal Ca2+ levels in the epidermis we identified egl-8, which encodes phospholipase Cβ and egl-30, encoding the regulatory Gαq protein for EGL-8. egl-8 and egl-30 mutants both displayed reduced epidermal Ca2+ (Figure 2D, S2A) and impaired survival post-wounding (Figure 2E). egl-8 null mutants did not enhance gtl-2(lf) survival phenotypes (Figure 2E), suggesting egl-8 and gtl-2 act in a common pathway. egl-30 null mutants arrest during larval development, precluding firm conclusions from double mutants. However a gain of function mutation, egl-30(js126) significantly suppressed survival defects of gtl-2(lf) mutants, consistent with EGL-30 acting downstream or in parallel to GTL-2. As PLCβ generates IP3 in response to GPCR signals, these results suggest EGL-30 and PLCβ promote epidermal Ca2+ release via ITR-1 after wounding.
EGL-30 is expressed in many tissues, including the epidermis [24], whereas EGL-8 expression has been predominantly observed in neurons. We found that epidermal-specific RNAi of egl-8 or egl-30 significantly reduced post-wounding survival without affecting other behaviors such as egg-laying (Figure 2F; not shown). Conversely, epidermal-specific expression of EGL-8 was sufficient to rescue post-wound survival defects of egl-8 mutants, but did not rescue their Egl phenotypes (not shown). We conclude the EGL-30/EGL-8 pathway functions within the epidermis to promote wound responses.
Ca2+ signals are not involved in the known innate immune responses to wounding
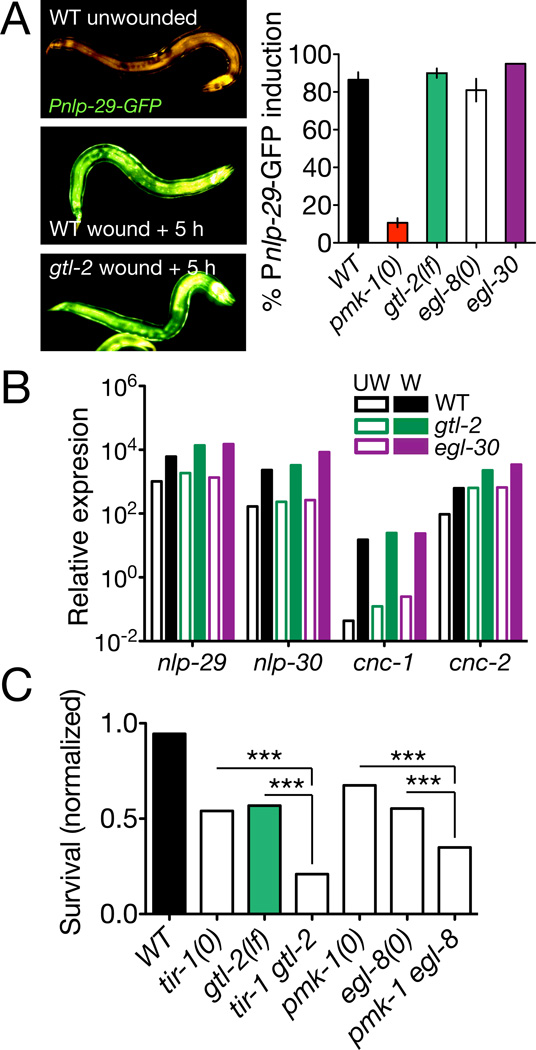
Sterile wounding activates an epidermal TIR-1/PMK-1 p38 MAPK cascade that induces epidermal expression of antimicrobial peptides (AMPs) such as nlp-29 [13]. Wounding also induces p38-independent transcription of caenacin-type (cnc) AMPs via the SMA-6 TGFβ receptor [25]. We tested whether Ca2+ signaling might intersect with these pathways and found that loss of function in gtl-2 or egl-30 had no effect on induction of nlp-29 or nlp-30 (Figure 3A,B). egl-8 null mutants also displayed normal AMP induction, consistent with previous results on egl-8(n488) [26]. gtl-2 and egl-30 mutants also showed normal induction of cnc-1 and cnc-2 after wounding (Figure 3B). These findings suggest Gαq/Ca2+ signals act in parallel to immune responses to wounding. Conversely, loss of function in the TIR-1/PMK-1 pathway or in Gα12/GPA-12 did not affect epidermal Ca2+ (Figure S3A,B). Double mutants between gtl-2 or egl-8 and tir-1 or pmk-1 displayed additive or synergistic reductions in survival after wounding (Figure 3C). Taken together these results suggest that epidermal Ca2+ and innate immune responses act in parallel to promote survival after wounding.
Figure 3. Epidermal Ca2+ signals act in parallel to known innate immune pathways.
(A) Representative images (wild type (WT) unwounded, WT wounded + 5 h and gtl-2 wounded + 5 h) and quantitation of Pnlp-29-GFP(frIs7) induction after needle wounding; n > 100 per genotype. (B) gtl-2(n2618) and egl-30(n686) mutants displayed normal basal levels (UW, empty bars) and wound-induced levels (W, filled bars) of epidermal antimicrobial peptide transcripts; RNA extracted from ~50 animals 6 h after needle wounding. Expression levels are relative to ama-1 in multiple quantitative RT-PCRs. (C) Loss of function in Ca2+ signaling genes and in innate immune response genes has additive or synergistic effects on post-wounding survival. n > 300 per genotype. Statistics, Fisher exact test.
Ca2+ signals trigger formation of actin rings at wounds
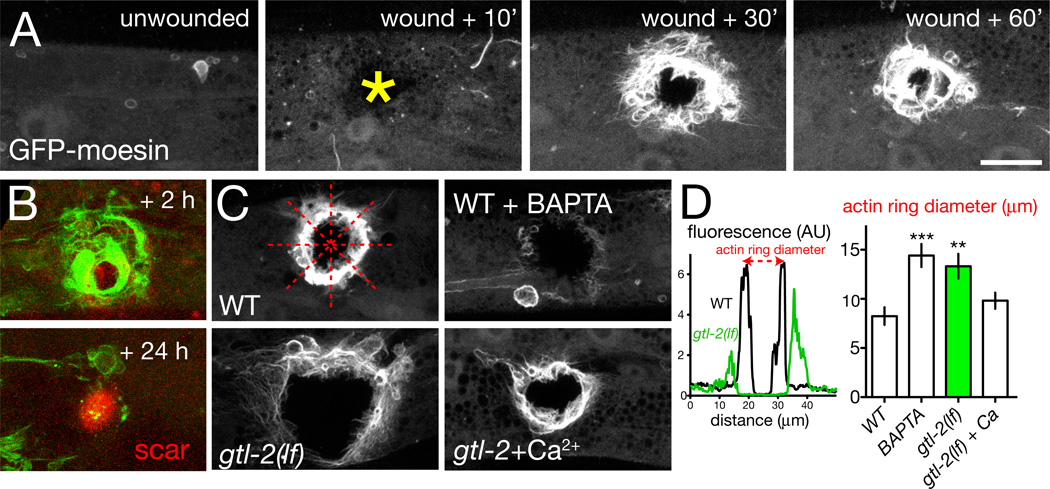
We next asked whether the need for Ca2+ signaling in survival of wounding reflected defects in specific repair processes. Healing of adult wounds typically involves cell proliferation, migration and re-epithelialization by cell crawling, and remodeling of ECM. In contrast, wound closure in embryonic epithelia is characterized by contractile actomyosin cables (‘purse-strings’) [27, 28]. Further, actin [29]cable formation in Xenopus oocyte wound closure is Ca2+-dependent [30]. We therefore focused on whether Ca2+ signals promoted cytoskeletal dynamics in wound closure. To visualize the epidermal actin cytoskeleton we expressed the F-actin marker GFP-moesin [31] in the epidermis. GFP-moesin distribution in the unwounded epidermis was diffuse (Figure 4A). Within minutes of needle wounding a dense ring of filamentous GFP indicative of actin filaments formed at the site of damage (Movie S4; Figure 4A). In most cases the actin ring gradually closed over the next 1–2 h; a minority failed to close over this time period, possibly reflecting variability in needle wounding or in closure dynamics. After 24 h the actin rings were completely closed (Figure 4B), and colocalized with autofluorescent scar material [13]. These findings suggest that C. elegans adult wound closure involves a dynamic rearrangement of the actin cytoskeleton.
Figure 4. Wounding triggers Ca2+-dependent formation of actin rings.
(A) Epidermal needle wounds trigger formation of actin rings at the wound margin, visualized with Pcol-19-GFP-moesin (juIs352); frames from Movie S4. Scales in A–C, 10 µm. (B) In wild type animals actin rings close by 24 h; an autofluorescent scar (red channel) remains, marking the wound. (C,D) GFP intensity along line scans of representative WT and gtl-2(n2618) mutants; arbitrary units. Quantitation of actin ring diameter from line scans, n > 20 per genotype; 4 line scans per animal (dotted red lines in panel C, WT); Mann-Whitney test. Actin ring assembly and closure is inhibited by incubation in BAPTA-AM and in gtl-2 mutants. In ~42% of BAPTA-treated animals and 29% of gtl-2 mutants the GFP-moesin ring never forms; ring diameters were quantitated in remaining animals. The gtl-2 actin closure defect is partly suppressed by incubation in buffer containing 2 mM Ca2+ (P = 0.05).
To quantitate the dynamics of actin during wound closure we used line scans of confocal images to measure the GFP-moesin ring diameter at 1 h after wounding, a time intermediate in the closure process (Figure 4C,D). We first tested whether Ca2+ signals promote closure by incubating wounded worms in Ca2+ chelators such as BAPTA-AM, and found that these treatments severely compromised actin ring formation (Figure 4C). Incubation in Ca2+ chelators also reduced survival post-wounding (Figure S1E). gtl-2 mutants displayed reduced actin accumulation after wounding, and impaired closure of actin rings (Figure 4C,D; Movie S4). Incubation of gtl-2 mutants in media containing increased external Ca2+ significantly restored closure (Figure 4C,D), suggesting the closure defects of gtl-2 mutants result from reduced epidermal Ca2+. Conversely, mutants defective in innate immune responses (tir-1, pmk-1) displayed normal actin ring formation (Figure S3C). The reduced survival of immunocompromised mutants such as tir-1 can be partly suppressed by incubation with antibiotics or DNA replication blockers such as FUDR [14]. In contrast, impaired survival of gtl-2 or egl-8 mutants was not suppressed by FUDR (Figure S2C), consistent with a defect in physical closure of wounds.
Wound closure involves local actin polymerization and is inhibited by non-muscle myosin
The dynamics of actin rings after wounding suggest wound closure involves the actin cytoskeleton, but do not distinguish whether this is driven by actomyosin-based contractility or some other form of actin dynamics. Time-lapse analysis of actin wounding suggested at least two processes contribute to actin dynamics: first, recruitment of F-actin to the ring, and second, closure of the ring. To address the role of actin we first tested actin polymerization inhibitors. Incubation of animals in the actin polymerization inhibitor Latrunculin A (LatA) completely blocked recruitment of actin to wounds (Figure S4A) and significantly reduced post-wounding survival (Figure S4B), indicating that actin polymerization is essential for formation of actin rings.
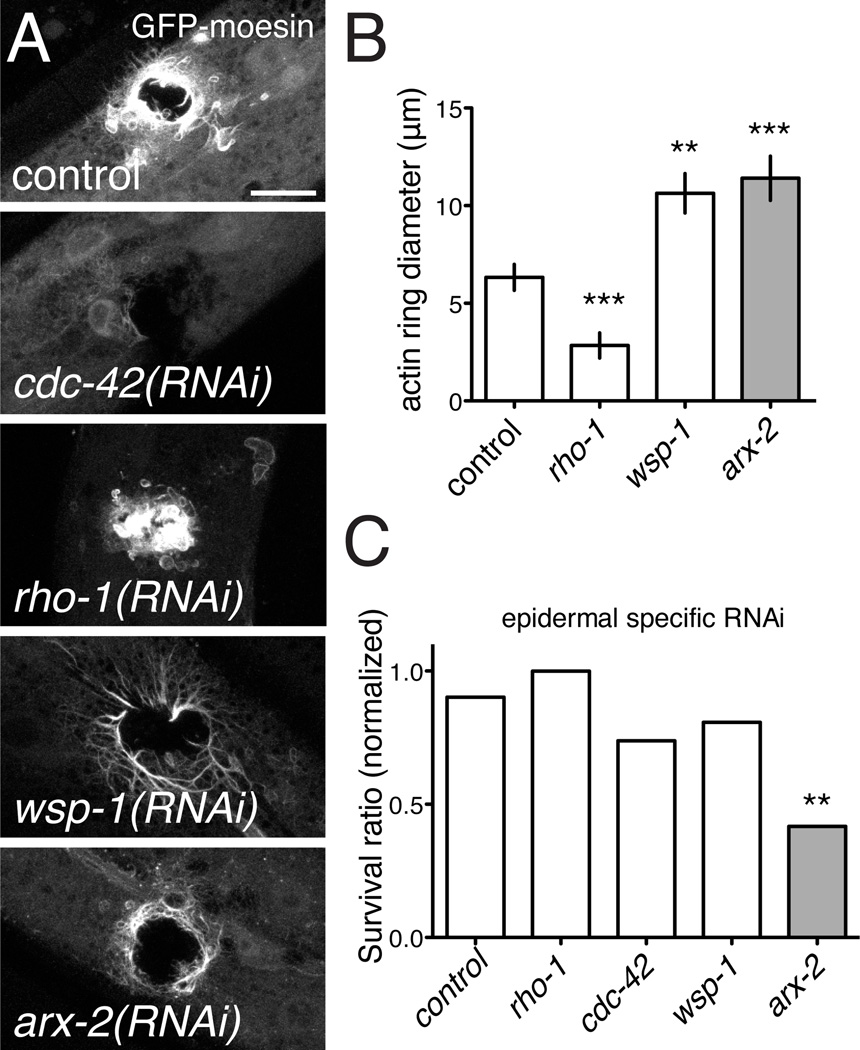
We next used RNAi to test the roles of known regulators of actin polymerization, including Rho-family GTPases and a set of actin binding or nucleating proteins. Among small GTPases, Rho is required for actin cable formation in wound closure [32], whereas Cdc42 is essential for actin assembly and disassembly in several contexts, including filopodial protrusions [33]. We found that cdc-42(RNAi) abolished actin recruitment to wound sites, whereas loss of function in rho-1 caused actin rings to close more rapidly than in the wild type. In contrast, loss of function in the Rac genes (rac-2, ced-10, mig-2) had little effect on actin recruitment or survival (not shown). Knockdown of the actin nucleation factors WSP-1/WASP or ARX-2/Arp2/3 complex reduced but did not abolish ring closure (Figure 5A,B); arx-2(RNAi) strongly reduced survival post-wounding (Figure 5C). Taken together these results suggest that Cdc42-dependent actin polymerization is important for wound closure and survival.
Figure 5. Wound closure requires actin polymerization factors and Cdc42, and is inhibited by Rho.
(A,B) Ring formation is abolished in cdc-42(RNAi) animals and promoted in rho-1(RNAi) animals (control, L4440 empty vector). Of the three Rac genes, only mig-2 showed a weak requirement for ring formation (not shown). Ring closure was reduced after RNAi for wsp-1/WASP and arx-2/Arp2/3. Scale, 10 µm (A). (C) Survival post-wounding is unaffected by rho-1(RNAi), slightly impaired by cdc-42(RNAi), and strongly reduced by arx-2(RNAi). Statistics, t test (C) or Fisher exact test (B).
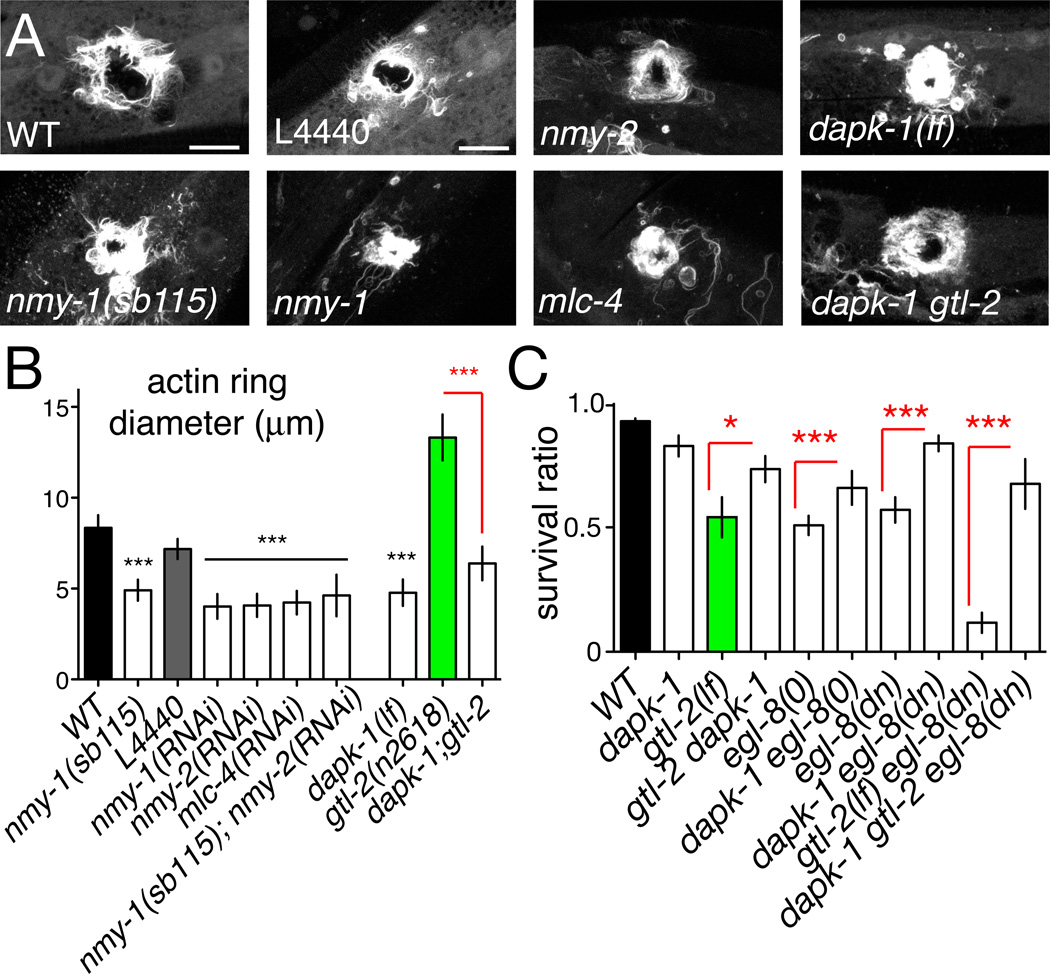
Finally, to test whether C. elegans wound closure required actomyosin contractility we reduced the function of non-muscle myosin. C. elegans encodes two non-muscle myosin heavy chains, NMY-1 and NMY-2, with partly redundant functions in epidermal development [34]. Unexpectedly, loss of function in either nmy gene significantly promoted actin ring closure (Figure 6A,B; Movie S5), as did RNAi of the epidermally expressed myosin light chain mlc-4. nmy-2(RNAi) did not enhance nmy-1 mutant phenotypes (Figure 6B), suggesting the nmy genes may be non-redundant in wound closure. Knockdown of non-muscle myosin light chain mlc-4(RNAi) also promoted ring closure(Figure 6A,B). Inhibition of non-muscle-myosin did not affect post-wounding survival (not shown). Time-lapse analysis of actin dynamics in nmy-1(sb115) mutants (Movie S5) suggested that wound closure was accelerated after knockdown of non-muscle myosin. Taken together, these results suggest C. elegans adult epidermal wounds do not close by a ‘purse-string’ mechanism. Instead, local actin polymerization related to filopodial protrusions appears to be critical.
Figure 6. Negative regulation of wound closure by non-muscle myosin and DAP kinase.
(A) Loss of function in non-muscle myosin and dapk-1 promotes actin ring closure. GFP-moesin (juIs352) imaged at 1 h post wounding in nmy-1(sb115) mutants, and after RNAi for nmy-1, nmy-2, mlc-4 (control: L4440). Survival post-wounding was unaffected under these conditions (not shown). Actin ring assembly is enhanced in dapk-1(ju4) and in dapk-1 gtl-2 double mutants (compared gtl-2 in Figure 4C). Confocal projections of z-stacks, 1 h post needle wound. (B) Quantitation of actin ring diameters as in Figure 4. (C) dapk-1 suppresses the post-wound survival defects of gtl-2, egl-8, and of gtl-2 egl-8 double mutants. n > 100 per genotype; Fisher exact test.
Loss of DAP kinase function accelerates wound closure and suppresses the effects of Ca2+ signaling mutants
The Ca2+-calmodulin regulated kinase DAPK/DAPK-1 has been shown to negatively regulate epidermal innate immune responses to damage [14] and is a known regulator of non-muscle myosin [35]. We therefore tested whether DAPK/dapk-1 might also negatively regulate cytoskeletal dynamics in wound closure. In dapk-1 single mutants GFP-moesin rings were smaller than in the wild type and closed more rapidly, paralleling the rho-1 and nmy-1 phenotypes (Movie S5). To address whether such mutations could overcome the effects of reduced Ca2+ signals we constructed double mutants between dapk-1 and gtl-2, egl-8, and egl-30. We found that gtl-2 dapk-1 double mutants displayed significantly faster ring closure than did gtl-2 mutants (Figure 6A,B). dapk-1 mutants displayed normal epidermal Ca2+ dynamics (not shown). Finally, the low post-wound survival of egl-30, egl-8, or gtl-2 single and compound mutants was significantly suppressed by dapk-1(lf) (Figure 6C). These results support DAPK-1 acting as a negative regulator of wound closure dynamics, either downstream or in parallel to Ca2+ signals.
Discussion
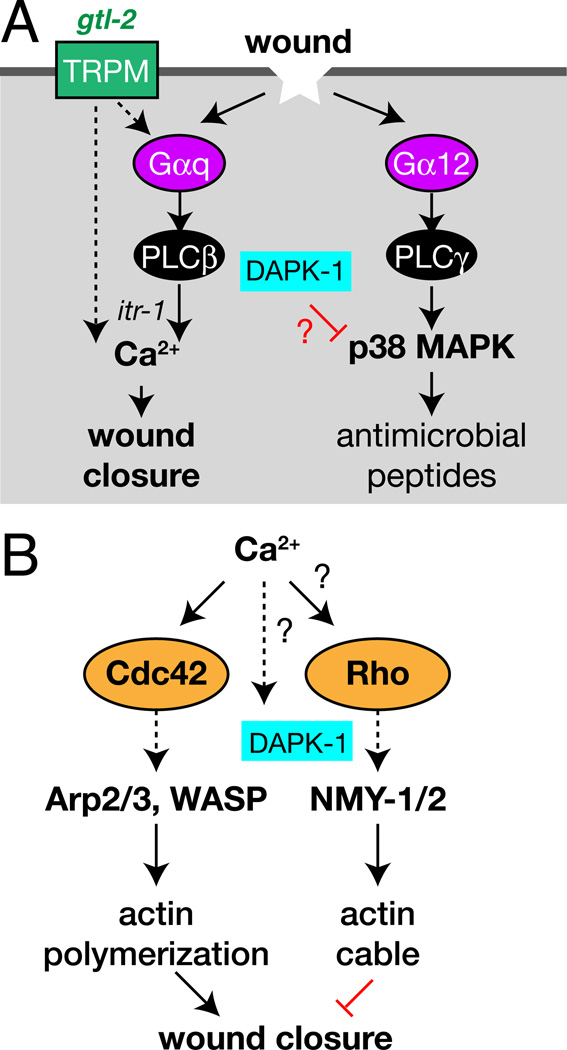
In conclusion, we have identified a G-protein/Ca2+ based wound closure pathway in the adult C. elegans epidermis. Our results link single-cell and embryonic wound models to physiologically relevant outcomes in repair of a mature barrier epithelium. Previous work has shown epidermal immune responses to wounding and fungal infection depend on Gα12/GPA-12 [26]. We find that Gαq/EGL-30 and Ca2+ act in parallel to promote epidermal wound closure via actin polymerization (Figure 7A,B). Although the Gαq-Ca2+ pathway described here is not required for upregulation of antimicrobial peptides after wounding, it could play other roles in antimicrobial defense. For example, Gαq and PLCβ regulate insulin secretion and PMK-1 in intestinal innate immunity in C. elegans [36]. Clearly, an important future goal is to identify the activators and effectors of Gαq in the epidermis.
Figure 7. Wound response pathways in the adult C. elegans epidermis.
(A) Model for the relationship of the innate immunity and wound closure pathways in C. elegans epidermal wound repair. (B) Possible mechanism of small GTPase regulation in wound healing.
Our analysis suggests at least two sources contribute to epidermal Ca2+, possibly in sequence. The TRPM channel GTL-2 is important for epidermal Ca2+ responses, suggesting wounding triggers an influx of Ca2+ via GTL-2. As several studies indicate GTL-2 localizes to cell plasma membranes rather than internal compartments [21, 22], it might directly sense wound-triggered changes in membrane tension. Alternatively, like other mechanosensitive TRP channels, GTL-2 might be indirectly gated by GPCR signaling [37]. The mechanism of gating of GTL-2 remains an open question. The EGL-30/EGL-8 pathway may be activated by an initial GTL-2-dependent Ca2+ influx to trigger further ITR-1-dependent release of Ca2+ from intracellular stores. Mammalian epidermal cells express a variety of TRP channels, some of which are implicated in epidermal wound healing [38]. Indeed, application of TRPM agonists can promote barrier reformation after wounding [39], suggesting some roles of TRPM channels in wound healing may be conserved.
Our results suggest a model for how Ca2+ signals trigger wound closure in the adult C. elegans epidermis (Figure 7B). CDC-42 is essential for actin rings to form, consistent with findings that Cdc42 is important in Drosophila larval wound closure [29]. As Cdc42 is activated by Ca2+ after wounding in Xenopus oocytes [40], it may act downstream of the Ca2+ signal in the C. elegans epidermis. Why does actin accumulate locally at wound sites when the Ca2+ signal is delocalized? This may be explained if Ca2+ acts with a local signal, possibly generated by cellular ‘compartment mixing’ [41]. In contrast, Rho acts antagonistically to CDC-42 in C. elegans wound responses. Rho and CDC-42 might directly antagonize, as in Xenopus oocyte wounding [42]. Alternatively, the enhanced closure seen after inhibition of Rho or non-muscle myosin might be an indirect consequence of reduced actin cable formation. Future studies should address whether and how Rho and CDC-42 are locally activated at wounds. Nevertheless, our results suggest that, unlike embryonic epithelia in which purse-string actomyosin contractility has a major role, wound closure in the adult C. elegans epidermis is driven by directed actin polymerization. The unexpected negative role of non-muscle myosin suggests actin cable formation may compete with actin polymerization in wound closure. C. elegans wound closure may more resemble that found in other adult epithelia, in which closure is driven by filopodial protrusion at the epithelial leading edge [43]. Most of our wounds affect the large syncytial epidermal cell hyp7, distant from their contacts with other epidermal cells such as seam cells. We therefore believe that actin structures formed after wounding are predominantly generated by hyp7, although contributions from other epidermal cells have not yet been examined.
Death Associated Protein Kinase DAPK-1 is a negative regulator of the innate immune response to wounding [14]. As DAPK is regulated by Ca2+ [44], DAPK-1 is an excellent candidate to be regulated by Ca2+ signals reported here. The inhibitory role of DAPK-1 in closure, together with previous evidence that DAPK-1 inhibits the innate immune response to damage, indicate DAPK-1 acts as a coordinate negative regulator of wound responses. If this inhibitory role for DAPK is conserved, pharmacological inhibition of DAPK could be a promising avenue for acceleration of wound healing.
Experimental Procedures
C. elegans genetics
All strains were maintained at 20–22.5 °C on NGM agar plates with E. coli OP50 as food source. Strains were constructed using standard procedures, and genotypes confirmed by PCR or sequencing. Mutations used include: gtl-2[n2618(lf), tm1463(0)], egl-8[n488(dn), sa47(0)], egl-30(n686, ad805, js126gf), itr-1(jc5cs), dapk-1(ju4, gk219), rde-1(ne219), pmk-1(km25), tir-1(tm3036), tpa-1(k501, k530), gpa-12(pk322), gon-2(q365), gtl-1(dx171), nmy-1(sb115). New transgenic strains are listed in Table S1.
Needle wounding, survival assay, and drug experiments
We wounded animals with single stabs of a microinjection needle to the posterior body 24 h after L4 stage, as described [13]. We transferred wounded animals to new plates and scored viability every 24 h; we defined death as failure to respond to touch. In some assays we prevented bacterial proliferation and egg-laying by transferring animals to plates containing 50 mg/mL FUDR immediately before wounding. Calcium chelators or Latrunculin A were dissolved in DMSO or M9 then diluted in M9. We incubated wounded worms in drug solution (containing E. coli OP50) and checked survival every 12 h; control animals were incubated in M9 or DMSO. In experiments where unwounded strains displayed similar survival 24 h after L4 we calculated normalized survival (fraction surviving 24 h after wounding)/(fraction surviving unwounded). To image GCaMP after needle wounding on plates we used a Zeiss Discovery V12 dissecting stereomicroscope and a Nikon DS Qi-1 monochrome camera.
Adult epidermal-specific RNAi
To knock down gene expression in the adult epidermis we expressed the wild type RDE-1 gene under the control of Pcol-19 (juIs346) in an rde-1(ne219) mutant background. rde-1 mutants are resistant to RNAi; as the col-19 promoter is expressed only in late L4 and adult epidermis, transgenic animals are resistant to RNAi in all tissues except the adult epidermis. We transferred rde-1; Pcol-19-RDE-1(juIs346) as L1s to bacteria expressing appropriate dsRNA, wounded the animals as day 1 adults, and assayed survival after 24 h. Other (tissue-nonspecific) RNAi experiments were performed in a similar way, with animals grown from the L1 stage on RNAi bacteria and wounded as young adults. Feeding RNAi clones were obtained from the Ahringer library and confirmed by sequencing, or made by PCR from cDNA or genomic templates; a complete list of RNAi clones is available on request.
Laser wounding and imaging
To analyze epidermal Ca2+ we wounded animals using femtosecond laser irradiation, essentially as described [45] except with 2 × 200 ms pulses. We imaged GCaMP fluorescence using spinning disk confocal microscopy, as described [45]. For short movies (<30 s) we acquired a single channel in burst mode; for longer time-lapse series we acquired two channels every 30–60 s. To measure peak ΔF/F0 we imaged 10 frames using a 100× objective (Zeiss Planapo, NA 1.46). We measured average fluorescence in ten equivalent regions of interest (ROI), five centered on the epidermal cell and five in the background. Baseline fluorescence (F0) was obtained by averaging fluorescence in 5 ROIs in the epidermis then subtracting the average of 5 ROIs in the background before injury. GCaMP fluorescence was normalized to an internal control, Pcol-19-tdTomato; tdTomato or GFP levels did not change after wounding (Figure S1B). The change in fluorescence ΔF was expressed as the ratio of change with respect to the baseline [(Ft-F0)/F0]. To follow ΔF/F0 over time at different distances from the injury site we imaged using the 63× objective and drew 2 µm ROIs at intervals of 5 µm. As the GCaMP transient extended 10 µm from the injury site within 230 ms of wounding, we selected a 10 µm long ROI to compare initial ΔF/F0 between conditions.
To quantitate epidermal GFP-moesin (juIs352) we used confocal microscopy to acquire z-stacks (13 × 0.5 µm) of GFP-moesin rings after wounding. We generated maximum intensity projections of each z-stack. For each image we used 4 line scans (oriented at 45° to each other) over the GFP-moesin ring and defined actin ring diameter as the average peak to peak distance. In some conditions (e.g. BAPTA-AM, gtl-2) a significant fraction of animals did not assemble GFP-moesin rings after wounding; such animals were not included in quantitation. In cases where the actin ring had closed by 1 h we defined the diameter as 0. In embryonic epidermis GFP–moesin localized to circumferential bundles (not shown), consistent with known distribution of F-actin.
Statistical analysis
All statistical analyses used GraphPad Prism (La Jolla, CA). Two-way comparisons used the Student t-test, Mann-Whitney test, or the Fisher exact test for proportions.
Highlights.
Wounding the C. elegans skin triggers epidermal Ca2+ responses
Wound triggered Ca2+ responses require the TRPM channel GTL-2 and PLCβ/Gαq signals.
Ca2+ responses regulate actin polymerization dependent wound closure
DAPK is a coordinate negative regulator of wound responses
Supplementary Material
Acknowledgments
We thank Amy Tong for initial work on the epidermal calcium sensor, Tiffany Hsiao for help with transgene integration and mapping, Zilu Wu for assistance with the femtosecond laser, Claudiu Giurumescu for help with confocal imaging, and Anindya Ghosh-Roy for constructs and advice on GCaMP imaging. We thank Loren Looger for GCaMP3, Arshad Desai and Fabio Piano for the moesin clone, Emily Troemel for use of her real-time PCR machine, and Tamara Stawicki and Yishi Jin for sharing information and reagents prior to publication. We thank Yishi Jin, Bill McGinnis, Karen Oegema, Emily Troemel, Nathalie Pujol, Jonathan Ewbank, and members of the Jin and Chisholm labs for discussions and comments on this manuscript. Supported by NIH R01 GM54657 to A.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 2.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 4.Schafer M, Werner S. Transcriptional control of wound repair. Annu Rev Cell Dev Biol. 2007;23:69–92. doi: 10.1146/annurev.cellbio.23.090506.123609. [DOI] [PubMed] [Google Scholar]
- 5.Galko MJ, Krasnow MA. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2004;2:E239. doi: 10.1371/journal.pbio.0020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mace KA, Pearson JC, McGinnis W. An epidermal barrier wound repair pathway in Drosophila is mediated by grainy head. Science. 2005;308:381–385. doi: 10.1126/science.1107573. [DOI] [PubMed] [Google Scholar]
- 7.Ting SB, Caddy J, Hislop N, Wilanowski T, Auden A, Zhao LL, Ellis S, Kaur P, Uchida Y, Holleran WM, et al. A homolog of Drosophila grainy head is essential for epidermal integrity in mice. Science. 2005;308:411–413. doi: 10.1126/science.1107511. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Tsarouhas V, Xylourgidis N, Sabri N, Tiklova K, Nautiyal N, Gallio M, Samakovlis C. The tyrosine kinase Stitcher activates Grainy head and epidermal wound healing in Drosophila. Nat Cell Biol. 2009;11:890–895. doi: 10.1038/ncb1898. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Brock AR, Wang Y, Fujitani K, Ueda R, Galko MJ. A blood-borne PDGF/VEGF-like ligand initiates wound-induced epidermal cell migration in Drosophila larvae. Curr Biol. 2009;19:1473–1477. doi: 10.1016/j.cub.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 11.Luo H, Liu Y, Fang L, Li X, Tang N, Zhang K. Coprinus comatus damages nematode cuticles mechanically with spiny balls and produces potent toxins to immobilize nematodes. Appl Environ Microbiol. 2007;73:3916–3923. doi: 10.1128/AEM.02770-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glockling SL, Beakes GW. An ultrastructural study of sporidium formation during infection of a rhabditid nematode by large gun cells of Haptoglossa heteromorpha. J Invertebr Pathol. 2000;76:208–215. doi: 10.1006/jipa.2000.4967. [DOI] [PubMed] [Google Scholar]
- 13.Pujol N, Cypowyj S, Ziegler K, Millet A, Astrain A, Goncharov A, Jin Y, Chisholm AD, Ewbank JJ. Distinct innate immune responses to infection and wounding in the C. elegans epidermis. Curr Biol. 2008;18:481–489. doi: 10.1016/j.cub.2008.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong A, Lynn G, Ngo V, Wong D, Moseley SL, Ewbank JJ, Goncharov A, Wu YC, Pujol N, Chisholm AD. Negative regulation of Caenorhabditis elegans epidermal damage responses by death-associated protein kinase. Proc Natl Acad Sci U S A. 2009;106:1457–1461. doi: 10.1073/pnas.0809339106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanisstreet M. Calcium and wound healing in Xenopus early embryos. J Embryol Exp Morphol. 1982;67:195–205. [PubMed] [Google Scholar]
- 16.McNeil PL, Steinhardt RA. Plasma membrane disruption: repair, prevention, adaptation. Annu Rev Cell Dev Biol. 2003;19:697–731. doi: 10.1146/annurev.cellbio.19.111301.140101. [DOI] [PubMed] [Google Scholar]
- 17.Nodder S, Martin P. Wound healing in embryos: a review. Anat Embryol (Berl) 1997;195:215–228. doi: 10.1007/s004290050041. [DOI] [PubMed] [Google Scholar]
- 18.Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker DS, Gower NJ, Ly S, Bradley GL, Baylis HA. Regulated disruption of inositol 1,4,5-trisphosphate signaling in Caenorhabditis elegans reveals new functions in feeding and embryogenesis. Mol Biol Cell. 2002;13:1329–1337. doi: 10.1091/mbc.01-08-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahn-Kirby AH, Bargmann CI. TRP channels in C elegans. Annu Rev Physiol. 2006;68:719–736. doi: 10.1146/annurev.physiol.68.040204.100715. [DOI] [PubMed] [Google Scholar]
- 21.Stawicki T, Zhou K, Yochem J, Chen L, Jin Y. TRPM channels modulate epileptic-like convulsions via systemic ion homeostasis. Curr Biol. 2011;21:883–888. doi: 10.1016/j.cub.2011.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teramoto T, Sternick LA, Kage-Nakadai E, Sajjadi S, Siembida J, Mitani S, Iwasaki K, Lambie EJ. Magnesium excretion in C elegans requires the activity of the GTL-2 TRPM channel. PLoS One. 2010;5:e9589. doi: 10.1371/journal.pone.0009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couillault C, Pujol N, Reboul J, Sabatier L, Guichou JF, Kohara Y, Ewbank JJ. TLR-independent control of innate immunity in Caenorhabditis elegans by the TIR domain adaptor protein TIR-1, an ortholog of human SARM. Nat Immunol. 2004;5:488–494. doi: 10.1038/ni1060. [DOI] [PubMed] [Google Scholar]
- 24.Bastiani CA, Gharib S, Simon MI, Sternberg PW. Caenorhabditis elegans Gαq regulates egg-laying behavior via a PLCβ-independent and serotonin-dependent signaling pathway and likely functions both in the nervous system and in muscle. Genetics. 2003;165:1805–1822. doi: 10.1093/genetics/165.4.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zugasti O, Ewbank JJ. Neuroimmune regulation of antimicrobial peptide expression by a noncanonical TGF-β signaling pathway in Caenorhabditis elegans epidermis. Nat Immunol. 2009;10:249–256. doi: 10.1038/ni.1700. [DOI] [PubMed] [Google Scholar]
- 26.Ziegler K, Kurz CL, Cypowyj S, Couillault C, Pophillat M, Pujol N, Ewbank JJ. Antifungal innate immunity in C. elegans: PKCδ links G protein signaling and a conserved p38 MAPK cascade. Cell Host Microbe. 2009;5:341–352. doi: 10.1016/j.chom.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Martin P, Lewis J. Actin cables and epidermal movement in embryonic wound healing. Nature. 1992;360:179–183. doi: 10.1038/360179a0. [DOI] [PubMed] [Google Scholar]
- 28.Wood W, Jacinto A, Grose R, Woolner S, Gale J, Wilson C, Martin P. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol. 2002;4:907–912. doi: 10.1038/ncb875. [DOI] [PubMed] [Google Scholar]
- 29.Lesch C, Jo J, Wu Y, Fish GS, Galko MJ. A targeted UAS-RNAi screen in Drosophila larvae identifies wound closure genes regulating distinct cellular processes. Genetics. 2010;186:943–957. doi: 10.1534/genetics.110.121822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark AG, Miller AL, Vaughan E, Yu HY, Penkert R, Bement WM. Integration of single and multicellular wound responses. Curr Biol. 2009;19:1389–1395. doi: 10.1016/j.cub.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards KA, Demsky M, Montague RA, Weymouth N, Kiehart DP. GFP-moesin illuminates actin cytoskeleton dynamics in living tissue and demonstrates cell shape changes during morphogenesis in Drosophila. Dev Biol. 1997;191:103–117. doi: 10.1006/dbio.1997.8707. [DOI] [PubMed] [Google Scholar]
- 32.Brock J, Midwinter K, Lewis J, Martin P. Healing of incisional wounds in the embryonic chick wing bud: characterization of the actin purse-string and demonstration of a requirement for Rho activation. J Cell Biol. 1996;135:1097–1107. doi: 10.1083/jcb.135.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 34.Piekny AJ, Johnson JL, Cham GD, Mains PE. The Caenorhabditis elegans nonmuscle myosin genes nmy-1 and nmy-2 function as redundant components of the let-502/Rho-binding kinase and mel-11/myosin phosphatase pathway during embryonic morphogenesis. Development. 2003;130:5695–5704. doi: 10.1242/dev.00807. [DOI] [PubMed] [Google Scholar]
- 35.Bialik S, Bresnick AR, Kimchi A. DAP-kinase-mediated morphological changes are localization dependent and involve myosin-II phosphorylation. Cell Death Differ. 2004;11:631–644. doi: 10.1038/sj.cdd.4401386. [DOI] [PubMed] [Google Scholar]
- 36.Kawli T, Wu C, Tan MW. Systemic and cell intrinsic roles of Gqα signaling in the regulation of innate immunity, oxidative stress, and longevity in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2010;107:13788–13793. doi: 10.1073/pnas.0914715107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci. 2007;8:510–521. doi: 10.1038/nrn2149. [DOI] [PubMed] [Google Scholar]
- 38.Yamada T, Ueda T, Ugawa S, Ishida Y, Imayasu M, Koyama S, Shimada S. Functional expression of transient receptor potential vanilloid 3 (TRPV3) in corneal epithelial cells: involvement in thermosensation and wound healing. Exp Eye Res. 2010;90:121–129. doi: 10.1016/j.exer.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 39.Denda M, Tsutsumi M, Denda S. Topical application of TRPM8 agonists accelerates skin permeability barrier recovery and reduces epidermal proliferation induced by barrier insult: role of cold-sensitive TRP receptors in epidermal permeability barrier homoeostasis. Exp Dermatol. 2010;19:791–795. doi: 10.1111/j.1600-0625.2010.01154.x. [DOI] [PubMed] [Google Scholar]
- 40.Benink HA, Bement WM. Concentric zones of active RhoA and Cdc42 around single cell wounds. J Cell Biol. 2005;168:429–439. doi: 10.1083/jcb.200411109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bement WM, Yu HY, Burkel BM, Vaughan EM, Clark AG. Rehabilitation and the single cell. Curr Opin Cell Biol. 2007;19:95–100. doi: 10.1016/j.ceb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaughan EM, Miller AL, Yu HY, Bement WM. Control of local Rho GTPase crosstalk by Abr. Curr Biol. 2011;21:270–277. doi: 10.1016/j.cub.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sonnemann KJ, Bement WM. Wound Repair: Toward Understanding and Integration of Single-Cell and Multicellular Wound Responses. Annu Rev Cell Dev Biol. 2010;27:237–263. doi: 10.1146/annurev-cellbio-092910-154251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bialik S, Kimchi A. The death-associated protein kinases: structure, function, and beyond. Annu Rev Biochem. 2006;75:189–210. doi: 10.1146/annurev.biochem.75.103004.142615. [DOI] [PubMed] [Google Scholar]
- 45.Ghosh-Roy A, Wu Z, Goncharov A, Jin Y, Chisholm AD. Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J Neurosci. 2010;30:3175–3183. doi: 10.1523/JNEUROSCI.5464-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.