Abstract
Cancer is a disease of aging and so with the increasing age of the US population, the incidence of cancer is also increasing. Furthermore the global burden of cancer continues to increase largely because of aging and growth of the world population together with increasing smoking rates in economically developing countries. Tumor formation is critically dependent upon two processes – initiation and progression. The initiation step is mediated by DNA damage, which causes activating mutations in proto-oncogenes and inactivation of tumor suppressor genes in many cancers. This is then thought to facilitate tumor progression and metastasis. Cyclooxygenase-2 (COX-2) is up-regulated at an early stage in tumorigenesis and has been implicated as an important mediator of proliferation through the increased formation of bioactive arachidonic acid (AA) metabolites such as prostaglandin (PG) E2. Significantly, we have found that COX-2-mediated AA metabolism also results in the formation of heptanone-etheno (Hε)-DNA-adducts. Furthermore, we showed that the Hε-DNA-adducts arose from the reaction of DNA with the lipid hydroperoxide-derived bifunctional electrophile, 4-oxo-2(E)-nonenal (ONE). Similarly, 5-lipoxoygenase (5-LOX)-mediated arachidonic acid metabolism also results in the formation of ONE-derived DNA-adducts. The resulting Hε-DNA-adducts are highly mutagenic in mammalian cell lines suggesting that these pathways could be (in part) responsible for the somatic mutations observed in tumorigenesis. As approximately 80% of cancers arise from somatic mutations, this provides an additional link between the up-regulation of COX-2 and tumorigenesis.
Keywords: Lipid peroxidation, DNA-adducts, somatic mutations, tumorigenesis
1. Introduction
DNA damage plays a major role in mutagenesis, carcinogenesis, and aging. Damage can occur by a variety of methods, including dietary factors, environmental exposures, and endogenous insults. The effects of exogenous toxins have been extensively studied for their mutagenic potential and a large body of literature exists on how these molecules can lead to DNA damage and ultimately, cancer. Often damage occurs by the reactive oxygen species (ROS) superoxide, peroxide, and hydroxyl radical [1;2]. These are generated constantly from ground state triplet oxygen in vivo by a variety of endogenous processes including normal mitochondrial aerobic respiration, inflammation induced by infection with viruses or bacteria, by cytochromes P-450s [3], and by peroxisomal-mediated degradation of fatty acids [4]. Normally, ROS levels are tightly controlled by an inducible antioxidant program that responds to cellular stressors and is predominantly regulated by the transcription factor Nrf2 (also known as Nfe2l2) and its repressor protein Keap1 [5]. Exposure to environmental toxins such as tobacco smoke and asbestos can lead to increased ROS formation, [6]. ROS are capable of oxidizing DNA and causing single stand and double strand breaks [7] when they escape detoxification by antioxidant defense systems, including superoxide dismutase, catalase, and reduced glutathione (GSH)-dependent peroxidases (POXs) [4]. A state of intracellular oxidative stress can occur when GSH is depleted either through direct GSH-adduct formation, or by providing reducing equivalents to inactivate ROS [8], such as during the metabolism of xenobiotics and endogenous chemicals for example [4;9]. Increased ROS production can overwhelm these endogenous protective mechanisms and initiate breakdown of lipid hydroperoxides formed from polyunsaturated fatty acids (PUFAs) such as linoleic acid (LA) and AA [10] to genotoxic bifunctional electrophiles [11]. This form of endogenous damage from the breakdown of lipid hydroperoxides is not as thoroughly studied as exogenous DNA damage, although a significant amount of research has been conducted in the past decade [4;12;13].
Lipid peroxidation is defined as the oxidation of membrane lipids [14]. It has been implicated in degenerative diseases of aging, in particular cancer, cardiovascular disease, and neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease [15–18]. Lipid structures are very diverse and range in polarity. Polyunsaturated fatty acids (PUFAs) are extremely sensitive to oxidation and therefore provide an excellent source for ROS-derived genotoxins, as well as substrates for endogenous oxidative biotransformations by the actions of COXs and LOXs. Importantly, for COX- and 5-LOX-mediated lipid peroxidation of PUFAs, relevant esterified lipids must first be hydrolyzed, whereas 12- and 15-LOX-mediated lipid peroxidation of PUFAs can occur on intact cell membrane lipids [19;20].
Cancer is a disease of aging and so with the increasing age of the US population, the incidence of cancer is also increasing. Thus, it was estimated that there would be 1,529,560 new cancer cases in 2010 in the US [21] compared with 1,284,900 in 2000 [22]. Furthermore the global burden of cancer continues to increase largely because of aging and growth of the world population together with increasing smoking rates in economically developing countries [23]. Based on the Globocan estimate, there were approximately 12.7 million new cancer cases world-wide in 2008 [23] compared with 10.1 million in 2000 [24]. Some 90 % of cancer genes show somatic mutations in cancer; whereas 20 % show germline mutations and 10 % show both [25]. This suggests that approximately 80 % of cancers arise from somatic mutations. Furthermore, the mutations can take many years to accumulate during tumorigenesis [26;27]. Therefore, understanding the role that COX and LOX-mediated lipid peroxidation and the resulting DNA damage could provide important insight into how these somatic mutations occur.
2 Cyclooxygenases
2.1 Background
The ability of COXs to oxidize PUFAs was recognized over 25 years ago [28]. Two isoforms have been identified, the first of which, COX-1, is constitutively active. The presence of an inducible form of COX was first suggested after experiments showing a transient increase in PGE2 in canine kidney cells upon stimulation with tumor promoters and carcinogens [29;30]. Furthermore, the induction observed was decreased with inhibition of transcription or translation, indicating it was dependent upon de novo COX synthesis. Eventually this isoform of COX, COX-2, was cloned, sequenced, and its expression was found to be inducible in human cells [31]. COX-2 and COX-1 share 60% sequence homology [32].
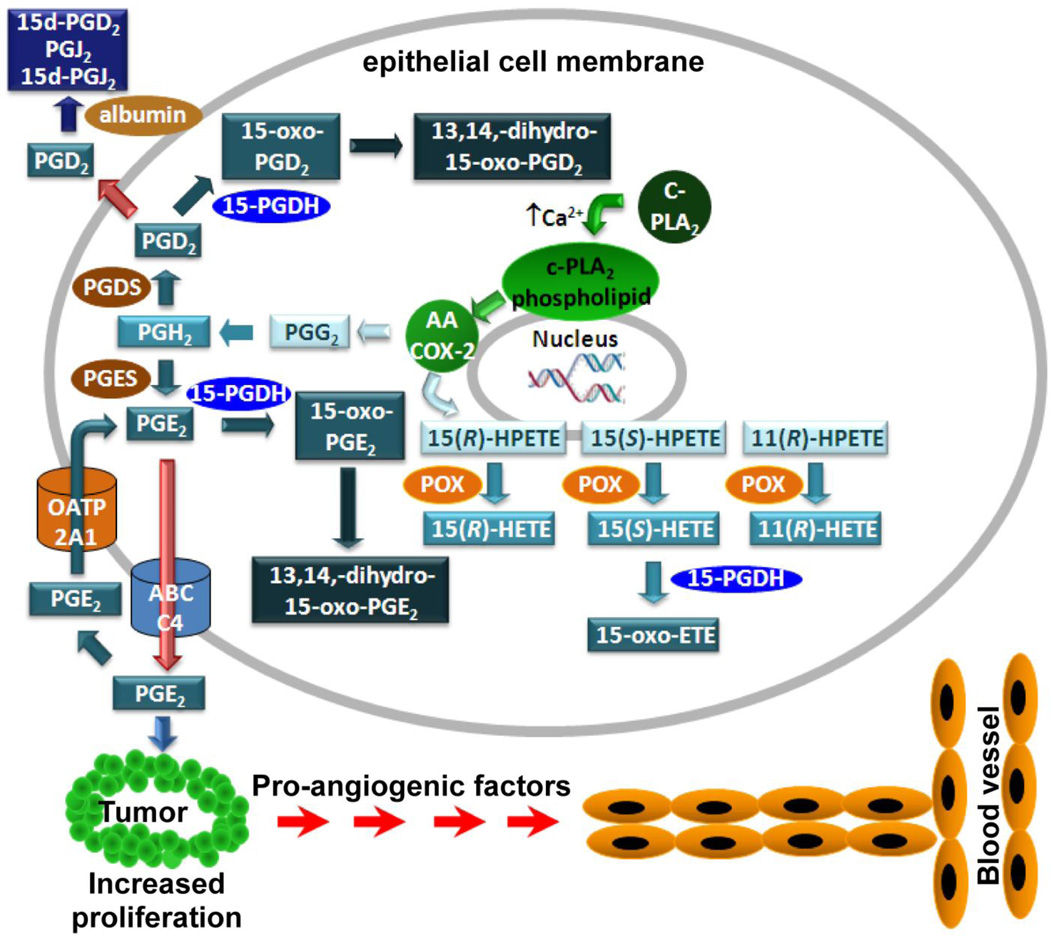
Both COXs are capable of producing PGs, which are important signaling molecules that are formed by the oxidative metabolism of AA [28]. As such, it is the key regulatory enzyme of PG signaling, promoting the conversion of AA to the hydroperoxy-endoperoxide PGG2, and subsequently converts it to the hydroxy-endoperoxide PGH2 via the enzyme’s POX activity (Fig.1) [33]. A variety of eicosanoids are produced from PGH2, varying in function from regulating inflammation, blood clotting, ovulation, initiation of labor, bone metabolism, nerve growth and development, kidney function, and blood vessel tone, As such, changes to COX-2 expression help to regulate diverse functions in several tissues. It is not surprising then that alterations in normal COX-2 activity are seen in several disease states, ranging from cardiovascular disease to cancer [28]. Initial reports indicated elevated COX-2 expression in colorectal cancer [34], and further work showed several other epithelial cancers to be associated with elevated COX-2 as well [35–37]. In fact, the presence of COX-2 activity in cancer correlates to a more aggressive phenotype [38;39]. For example, breast cancers found to express COX-2 saw increased incidence of recurrence, metastasis, and worse clinical prognosis and survival rate [40;41]. Many of these adverse effects have been ascribed to increased production of pro-proliferative COX-2-derived PGE2 [28]. The activity of PGE2 is regulated by conversion to inactive 15-oxo-PGE2 by 15-hydroxyprostaglandin dehydrogenase (15-PGDH) [42] and subsequent further reductase-mediated inactivation to 13,14-dihydro-15-oxo-PGE2 (Fig.1) [43]. 15-PGDH-mediated PGE2 metabolism is controlled by cellular influx through the organic anion transporter polypeptide (OATP) 2A1 (also known as the PG transporter to solute 2A1) [44;45]. Intriguingly both 15-PGDH [45–47] and OATP2A1 [48] are down-regulated in colon cancer
Figure 1.
Metabolism of AA by COX-2 to eicosanoids.
2.2 COX inhibition
Some of the most widely-used drugs, including aspirin and other non-steroidal anti-inflammatory (NSAIDs) drugs such as ibuprofen, act to block both isoforms of COX [49]. Importantly, these drugs appear to have chemoprotective effects, particularly against colorectal cancer [49]. Unfortunately side-effects of NSAIDs over long term use include unacceptable gastrointestinal (GI) bleeding thought to arise from the inhibition COX-1 [50]. Therefore, it was reasoned that a COX-2 specific inhibitor would avoid these side-effects and reduce inflammation associated with up-regulation of COX-2 expression [50]. In fact, rofecoxib (Vioxx) did significantly reduce the GI side effects when compared with naproxen [51] as well as when compared with other NSAIDs [52]. However, Vioxx also caused a dose-dependent increase in cardiovascular events [53] which resulted in it being withdrawn from the market. This has been ascribed to inhibition of vascular COX-2-mediated PGI2 biosynthesis [50]. In support of this concept, similar side-effects were observed with the COX-2 inhibitor celecoxib (Celebrex) [54] which has resulted in significant restrictions in its use. Overall, these studies have revealed that COX-2 inhibitors cannot be used for long term treatment in disease prevention.
2.3 COX-mediated formation of lipid hydroperoxides
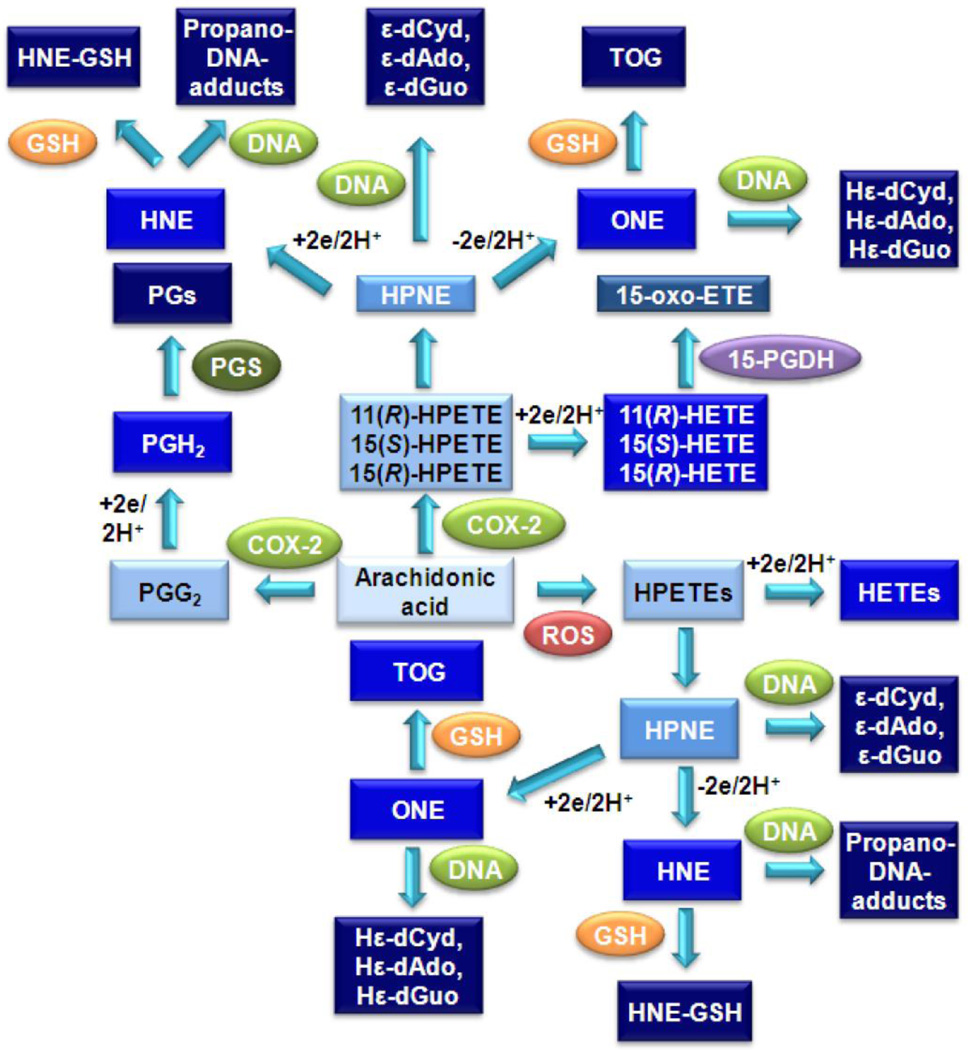
In addition to PG production, both COX-1 and COX-2 can abstract the pro 13(S)-hydrogen from AA, followed by double bond rearrangements and oxygen insertion to form a mixture of 11(R)-hydroperoxyeicosatetraenoic acid (HPETE), 15(R)-HPETE, and 15(S)-HPETE [55] (Fig. 1). COX-2 also converts LA primarily to 9(R)-hydroperoxyoctadecadienoic acid (HPODE) and 13(R)-HPODE. The HPETEs and HODEs are reduced to the corresponding hydroxyeicosatetraenoic acids (HETEs) or hydroxyoctadecadienoic acids (HODEs). 15(S)-HETE is subsequently converted to 15-oxo-ETE by 15-PGDH [56] (Fig. 1). However, the HPETEs can also decompose to form highly reactive α,β-unsaturated aldehydes, a process that can be initiated by transition metal ions or vitamin C [57]. 15-HPETE is converted into 4-hydroxyperoxy-2(E)-nonenal (HPNE), which then forms 4-hydroxy-2(E)-nonenal (HNE) and 4-oxo-2-nonenal (ONE) (Fig. 2) [58]. Similarly, 11-HPETE is converted to HPNE, which is in turn converted to HNE and ONE (Blair IA unpublished). As indicated earlier in this article, the by-products of lipid peroxidation can be reactive, and as such, there are cellular systems in place to detoxify these molecules. In particular, GSH will form adducts with the decomposition products of hydroperoxides, such as HNE and ONE, thus rendering them unable to react with cellular macromolecules [8]. HNE forms a mixture of GSH-adduct diastereomers; whereas ONE forms the unique thiadiazabicyclo-ONE-GSH-adduct (TOG) (Fig. 2) [59;60]. However, if the cell is already under oxidative stress, so that GSH is depleted, covalent modifications to cellular proteins and DNA can occur [55].
Figure 2.
Lipid hydroperoxide-mediated formation of bifunctional electrophiles, GSH-adducts and DNA-adducts.
3 Lipoxygenases
3.1 Background
LOXs are also able to convert PUFAs such as AA into lipid hydroperoxides [61–63] that can decompose into reactive bifunctional electrophiles such as ONE and HNE. They also produce a variety of signaling molecules, including leukotrienes (LTs), HETEs, and lipoxins [19]. These are important mediators of several cellular functions, but are perhaps most well known for their roles in inflammation. 12-LOX and 15-LOX can act on esterified phospholipids in cellular membranes directly and produce lipid hydroperoxides [64]. These are reduced and hydrolyzed by phospholipase A2 and secreted as HETEs [65]. 5-LOX acts on AA to produce 5(S)-HPETE, which is either reduced to 5(S)-HETE or serves as a precursor to the formation of LTs [66]. ROS also produces HETEs from free or esterified AA, but does so in a non-stereospecific manner [14]. Therefore, analysis of the chirality of HPETEs and HETEs makes it possible to determine whether they were formed enzymatically or from the action of ROS [67]. The conversion of AA to 5(S)-HPETE by 5-LOX is critically dependent upon the presence of 5-LOX-acitvating protein (FLAP) [68]. 5-LOX and FLAP are expressed primarily in inflammatory cells such as polymorphonuclear leukocytes, monocytes, macrophages, and mast cells [66;69–71]. Therefore, 5-LOX-derived eicosanoids are thought to play a critical role in inflammation, and allergic disorders [72–75]. A number of studies have also implicated 5-LOX-derived AA metabolites as mediators of atherogenesis and heart disease [66;76;77]. Furthermore, the 5-LOX pathway of AA metabolism has also been proposed to play a role in prostate and pancreatic cancer [78–80].
3.2 Cellular model for 5-LOX expression
CESS, a human lymphoblastic cell line, has been reported to express both 5-LOX and FLAP mRNA. In addition, 5-HETE was identified as one of the major AA metabolites formed in CESS cells upon stimulation with calcium ionophore [81;82]. This made the CESS cells an ideal model to examine the effects of lipid hydroperoxide-derived DNA damage. A targeted chiral lipidomics approach was used to examine the endogenously produced lipid peroxidation products secreted from the cells into the culture medium [83;84]. This revealed that there was a substantial and stereoselective secretion of 5(S)-HETE upon stimulation of the CESS cells with calcium ionophore A23187 and confirmed the presence of a functional 5-LOX activity. [61]. The concomitant secretion of substantial amounts of LTB4 provided additional confirmation of the 5-LOX activity. Interestingly, almost equimolar amounts of 5(S)-HETE and LTB4 were generated. This suggested that sufficient 5(S)-HPETE could have been generated to undergo homolytic decomposition to ONE [85]. MK886, a FLAP inhibitor, reduced 5(S)-HETE production by 97 % and LTB4 production by 99 % confirming that these eicosanoids were both derived exclusively from 5-LOX [61].
4 Lipid hydroperoxide-mediated formation of DNA-adducts
4.1 Lipid hydroperoxide-derived bifunctional electrophiles
Development of stable-isotope dilution LC-MS/MS methods for analysis of lipid hydroperoxides, their decomposition products, and DNA adducts has allowed for a better understanding of endogenous DNA damage. As discussed, lipid hydroperoxides can undergo decomposition in the presence of metal ions [86]. Lee and colleagues first showed in 2001 that vitamin C was also capable of converting lipid hydroperoxides into endogenous genotoxins [57]. The major decomposition products were identified as the bifunctional electrophiles, HPNE, ONE, HNE, 4,5-epoxy-2(E)-decenal (EDE), and 9,12-dioxo-10(E)-dodecenoic acid (DODE) [58;87;88]. Each of the bifunctional electrophiles is capable of forming DNA-adducts and therefore has mutagenic potential [55].
4.2 Etheno-DNA-adducts
HPNE, the precursor to ONE and HNE, forms unsubstituted-ε-DNA adducts [89]. EDE is also capable of forming these adducts [87], but HPNE has been shown to be much more reactive with 2-deoxyguanosine (dGuo) [87], and as such is likely the major source of endogenous unsubstituted-ε-DNA adducts. These adducts can also be formed by environmental toxins such as vinyl chloride, vinyl fluoride, and chloroethylene oxide [90;91]. As such, measurements of unsubstituted-ε-DNA-adducts cannot account solely for the amount of COX and LOX mediated damage occurring to DNA, but rather a sum of endogenous and exogenous damage. Importantly, ε-dGuo is mutagenic in human cells, leading to A to T transversions [92]; therefore its formation is significant.
4.3. Propano-DNA-adducts
HNE itself will react with DNA to form two distinct pairs of hydroxypropano-DNA-adducts [93]. This reaction occurs at a low rate, therefore the propano-DNA-adducts are not readily detectable in cellular DNA [93]. HNE is more reactive towards amino acids and can covalently modify lysine, histidine, and cysteine residues [94].
4.4. Carboxylate-containing DNA-adducts
Degradation of LA-derived 13(S)-HPODE results in the formation of DODE [88]. This reactive molecule can bind to DNA, forming caboxynonanone-ε-DNA-adducts. In initial reactions with 13(S)-HPODE and calf thymus DNA, this adduct was found to be the most abundant adduct when compared to unsubstituted-εdGuo and HεdGuo [88]. In contrast, treatment of calf thymus DNA with 15(S)-HPETE did not show any carboxynonanone-εdGuo adducts, therefore it appears this forms only through LA decomposition [88]. Importantly, the structure of DODE indicates it might not cross the nuclear membrane [88], although carboxylate-containing DNA adducts have been identified [95].
4.5 Heptanone-etheno DNA-adducts
ONE shares a similar structure to HNE except it contains a C-4 oxo group instead of a C-4 hydroxyl group. In vitro, ONE was able to form adducts with dGuo [96], 2’-deoxyadenosine (dAdo) [97;98], and 2’-deoxycytidine (dCyd) [99]. 15(S)-HPETE or 13(S)-HPODE have also been shown to form these adducts from an in vitro reaction with calf thymus DNA [96–98]. To establish the genotoxicity of these adducts, Pollack and colleagues produced a site-specific HεdCyd-containing oligonucleotide that was incorporated into a shuttle vector. This vector was then transfected into E. coli cells and the human cell lines XPA and GM637 [100]. The HεdCyd has an exocyclic ring and a bulky heptanone side chain, so it was predicted it would strongly block DNA synthesis. In fact, analysis of the progeny showed that HεdCyd did in fact block synthesis. Specialized DNA polymerases exist to replicate DNA around lesions, for they have a roomier active site that accommodates a modified base and new nucleotide [101]. Due to the lesion remaining, correct base pairing is forsaken. These polymerases exist to continue DNA synthesis when DNA is damaged and not repaired properly, known as translesional synthesis [102]. With synthesis blocked, miscoding around the heptanone adducts was observed. In E. coli, the miscoding frequency was 40–50%, and dGuo and dCyd were almost exclusively inserted opposite the lesion. In human cells, the miscoding frequency was more than 90%, indicating that these adducts are highly mutagenic. In contrast to E. coli, human cells inserted dAdo and thymidine exclusively across from the lesion [100]. Using a similar plasmid as described above in mouse fibroblasts, it was shown that insertion of thymidine across from HεdCyd was dependent upon translesional polymerase ζ and REV1, where as insertion of dAdo was not dependent upon either of these polymerases [101]. To date it is not known which polymerases are responsible for dAdo incorporation. These findings demonstrate two distinct translesional pathways at work to replicate DNA around the same adduct, and overall demonstrate that Hε-DNA-adducts are highly genotoxic. As such, understanding their formation is important to preventing mutations, genomic instability, and the development and progression of cancer.
5 COX-2-mediated DNA damage
5.1. COX-2 and cancer
As discussed earlier, both COXs and LOXs are capable of forming mutagenic Hε-DNA-adducts via ONE formation. Several diseases have been linked to elevated expression of these enzymes, which potentially could increase the formation of DNA adducts as well. Important to the topic of this review, elevated COX expression is often seen in cancer, in fact this increase was first documented in colon cancer [33]. In addition, drugs that target COX, namely aspirin and other NSAIDs, were found to reduce the risk for colorectal cancer by 40–50% [103]. However, whether this effect is solely due to inhibition of COX-mediated PG biosynthesis remains to be determined. With the ability of COX-2 to assist in the production of genotoxic bifunctional electrophiles that damage DNA, it is possible that elevated production of COX-2 seen in cancer might contribute to the genomic instability associated with tumorigenesis.
5.2. COX-2-derived DNA-adducts in cell lines
Initial experiments used mammalian cell lines to examine the role of elevated COX-2 in Hε-DNA-adduct formation. In many tissues, COX-2 expression is very low unless stimulated. To get around this, rat intestinal epithelial cells that stably expressed COX-2 (RIES cells) were created [104]. In these cells, HεdGuo was observed as a major DNA-adduct. These cells were then treated with increasing amounts of vitamin C for 24 hours, and the levels of HεdGuo increased in a vitamin C concentration-dependent manner. Furthermore, the formation of adducts in RIES cells was blocked with treatment by aspirin and with a COX-2 specific inhibitor, NS-398, also in a concentration dependent manner [104]. Interestingly, carboxynonanone-ε-dGuo was not detected [104]. Overall this study indicates that elevated COX-2 levels do contribute to DNA damage, specifically Hε-DNA-adducts, in mammalian intestinal epithelial cells, and showed that further studies on the formation of these adducts in colon cancer and in other disease states with elevated COX-2 was warranted.
5.3. COX-2-derived DNA-adducts in animal models
Colorectal cancer is the third most common cancer and the fourth most frequent cause of cancer-related deaths [105]. Two genetic forms of colorectal cancer are known, hereditary nonpolyposis colorectal cancer (HNPCC), which occurs due to germline mutations in mismatch repair genes, and familial adenomatous polyposis (FAP) which is characterized by a mutation in the apc gene. In FAP, mutation of apc leads to dysfunctional β-catenin signaling and increased transcription of several genes, including COX-2 [106]. COX-2 mediated DNA damage and its contribution to colon cancer, therefore, were explored further by using the Min mouse, a FAP model where the apc gene is knocked out. These mice form many large polyps spontaneously throughout the intestine. The link between apc, COX-2, and colorectal cancer was firmly established in Min mice by Oshima and colleagues. They examined APC −/− mice in a wild type background and in a COX-2 null background. As mentioned earlier, apc −/− mice (Min mice) readily form intestinal adenomas, but those altered to a COX-2 null background saw a significant reduction in polyp formation [107]. Their conclusions were that apc −/− mediated changes to COX-2 expression is a critical component for the development of intestinal adenomas. Therefore, Min mice provide an excellent model to explore COX-2 mediated DNA damage. When intestinal tissue from wild type and Min mouse were examined, Hε-DNA-adducts, specifically HεdCyd and HεdGuo, were found in both genotypes [108]. However, the Min mice had significantly higher levels of both adducts compared to wild type mice [108]. The authors also looked for carboxynonanone-ε- and unsubstituted-ε-DNA-adducts, but did not find any in either genotype, demonstrating that Hε-DNA-adducts were the major DNA lesions formed in vivo [108]. This work also demonstrated that DNA damage is elevated in a disease model of colorectal cancer where COX-2 expression is up-regulated.
Now the question becomes if these findings extend to humans, which is being examined currently. One recent study looked at several human tissues by LC-MS/MS for Hε-DNA-adducts. Such adducts were found among several tissue types, including colon, heart, kidney, lung, pancreas, and small intestine [109]. The authors of this study also examined the levels of 8-oxo-dGuo, the most commonly found adduct, and found that there was no correlation to the levels of 8-oxo-dGuo compared to HεdCyd [109]. This indicates that the two DNA-adducts develop from different sources. Since 8-oxo-dGuo often forms from exogenous insults to cells, and Hε-DNA-adducts result from endogenous means, this finding indicates that damage to DNA that occurs throughout life results not only from exposure to carcinogens, but also from our own cellular enzymatic activities. Along with the known mutagenic properties of Hε-DNA-adducts, this work further supports the importance of understanding the formation of endogenous DNA-adducts in tissues. The knowledge that Hε-DNA-adducts are found in human tissues, that they can form from COX-2 action, and that elevated COX-2 activity correlates to a more aggressive cancer phenotype, all indicate that this damage may contribute to the somatic mutations observed in cancer progression, helping to promote cancer development and metastases [26;27;110].
6. Lipoxygenase-mediated DNA damage
Fewer studies have been conducted on LOX-mediated DNA damage when compared with COX-mediated damage. However, the formation of DNA adducts by 5-LOX has been confirmed in the cultured human lymphoblastoid CESS cell line. Jian and colleagues demonstrated that levels of ONE and HεdGuo increased with 5-LOX-stimulation by calcium ionophore A23187 [61]. These cells expressed COX-1, but not COX-2 or 15-LOX. To verify that adduct formation was 5-LOX mediated, cells were treated with the FLAP inhibitor MK886. This reduced adduct formation in a dose-dependent manner, confirming 5-LOX mediated formation of HεdGuo [61]. Furthermore, vitamin C increased DNA-adduct formation in a dose-dependent manner, as seen in the COX-2 study with RIES cells. 5-LOX is linked to inflammatory diseases such as cancer and atherosclerosis [111]. It is also found to be up-regulated in several cancer cell lines, and is over-expressed in prostate and pancreatic cancer tissue [78–80]. As with COX, LOX-mediated DNA damage is a potential contributor to cancer progression. Therefore, therapies designed to block excess 5-LOX activity could be useful in cancer treatment and warrant further investigation.
7. Heptanone-etheno DNA-adduct formation and repair
When not repaired, Hε-DNA adducts (specifically HεdCyd), were found to be mutagenic [100;101]. As with any bulky adduct, the first defense the cell has is to remove the base prior to DNA replication. Excised adducts can be found in biofluids such as blood plasma and urine. Their existence in these fluids could prove useful to identify the amount of endogenous DNA damage that may be occurring in a patient. Screening for increased Hε-DNA-adducts could possibly point to early adenoma formation in the colon, for example, and a test for such adducts in urine might detect this lesion. It is the potential of technologies such as these that make the understanding of endogenous COX and 5-LOX-mediated DNA damage important. Further studies are needed to test this possibility of using urinary Hε-DNA-adducts as biomarkers of endogenous DNA damage.
It is noteworthy that COX-2 could contribute to cancer progression through several mechanisms. As discussed earlier, it can induce DNA damage through degradation of its AA-derived lipid peroxidation products to DNA-reactive bifunctional electrophiles, but it also produces pro-proliferative PGs. PG signaling is clearly an important aspect of COX-2-dependent proliferation (Fig. 1); however, its ability to promote mutagenesis and genomic instability, adds another layer of complexity to the involvement of COX-2 during cancer development. With studies linking many cancers to early up-regulation of COX-2, understanding the effects this enzyme has on the biology of the cell is critical. Initial studies demonstrating the effectiveness of NSAIDs in reducing polyp formation led to the suggestion that inhibition of PG production was responsible [50;112]. Undoubtedly, this is an important aspect of the activity of COX-2 in causing polyps, but its ability to cause DNA damage might also be important and as such could provide an alternative strategy for developing new therapeutic approaches [55]. This is particularly important in view of the adverse cardiovascular side-effects observed with conventional COX-2 inhibitors [53;54]. 5-LOX activation can also contribute to DNA damage and its elevated expression is also linked to cancer [78;79]. Therefore, both COX-2 and 5-LOX pathways could provide drug targets to modulate the mutagenesis and genomic instability that are associated with tumor formation and metastases. In addition, repair of COX and LOX-mediated DNA lesions might provide useful urinary biomarkers to monitor cancer progression.
8 Conclusion
Lipid hydroperoxides undergo homolytic decomposition to the highly reactive bifunctional electrophile ONE. This results in the formation of HεdGuo, HεdAdo, and HεdCyd as well as the GSH-adduct, TOG (Fig. 2). ONE was found to arise from vitamin C or transition ion-mediated decomposition of lipid hydroperoxides through the intermediate formation of HPNE. The Hε-DNA-adducts were also shown to also arise from COX-2- and 5-LOX-mediated AA metabolism and, importantly, Hε-DNA-adduct formation was increased in a mouse model of intestinal cancer in which COX-2 is up-regulated. Furthermore, HεdCyd was found to be highly mutagenic in mammalian cell lines suggesting that this DNA lesion can result in somatic mutations. Significantly, a large number of mutations at dCyd have been found to arise during the evolution of pancreatic cancer [27]. Up-regulation of COX-2 is an early event in tumorigenesis; whereas down-regulation of 15-PGDH occurs during the progression of many types of cancer. This means that COX-2-derived pro-proliferative eicosanoids such as PGE2 are not inactivated. Therefore, during the progression of tumorigenesis there is a “perfect storm” in which COX-2-dependent DNA damage can cause mutations in proto-oncogenes and/or inactivating mutations in tumor suppressor genes. This is coupled with reduced inactivation of COX-2-derived pro-proliferative eicosanoids through down-regulation of the metabolizing enzyme 15-PGDH and the influx transporter OATP2A1. There is also substantial evidence that 5-LOX is up-regulated in cancer. Therefore, 5-LOX-derived Hε-DNA-adducts could also be involved in tumorigenesis.
Acknowledgements
This work was supported by NIH grants RO1CA1091016, P30ES013508, and R25CA101871.
References
- 1.Mangal D, Vudathala DK, Park JH, Lee SH, Penning TM, Blair IA. Analysis of 7,8-dihydro-8-oxo-2'-deoxyguanosine in cellular DNA during oxidative stress. Chemical Research in Toxicology. 2009;22:788–797. doi: 10.1021/tx800343c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JH, Mangal D, Tacka KA, Quinn AM, Harvey RG, Blair IA, et al. Evidence for the aldo-keto reductase pathway of polycyclic aromatic trans-dihydrodiol activation in human lung A549 cells. Proceedings of the National Academy of Sciences USA. 2008;105:6846–6851. doi: 10.1073/pnas.0802776105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez FJ. Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutation Research. 2005;569:101–110. doi: 10.1016/j.mrfmmm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 4.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proceedings of the National Academy of Sciences USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Churg A. Interactions of exogenous or evoked agents and particles: the role of reactive oxygen species. Free Radical Biology and Medicine. 2003;34:1230–1235. doi: 10.1016/s0891-5849(03)00175-8. [DOI] [PubMed] [Google Scholar]
- 7.Cadet J, Delatour T, Douki T, Gasparutto D, Pouget JP, Ravanat JL, et al. Hydroxyl radicals and DNA base damage. Mutation Research. 1999;424:9–21. doi: 10.1016/s0027-5107(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 8.Zhu P, Oe T, Blair IA. Determination of cellular redox status by stable isotope dilution liquid chromatography/mass spectrometry analysis of glutathione and glutathione disulfide. Rapid Communications in Mass Spectrometry. 2008;22:432–440. doi: 10.1002/rcm.3380. [DOI] [PubMed] [Google Scholar]
- 9.Watson WP, Mutti A. Role of biomarkers in monitoring exposures to chemicals: present position, future prospects. Biomarkers. 2004;9:211–242. doi: 10.1080/13547500400015642. [DOI] [PubMed] [Google Scholar]
- 10.Porter NA, Caldwell SE, Mills KA. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- 11.Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361–370. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 12.Marnett LJ, Riggins JN, West JD. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. Journal of Clinical Investigation. 2003;111:583–593. doi: 10.1172/JCI18022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marnett LJ. Oxy radicals, lipid peroxidation and DNA damage. Toxicology. 2002;181–182:219–222. doi: 10.1016/s0300-483x(02)00448-1. [DOI] [PubMed] [Google Scholar]
- 14.Horton AA, Fairhurst S. Lipid peroxidation and mechanisms of toxicity. Critical Reviews in Toxicology. 1987;18:27–79. doi: 10.3109/10408448709089856. [DOI] [PubMed] [Google Scholar]
- 15.Völkel W, Sicilia T, Pahler A, Gsell W, Tatschner T, Jellinger K, et al. Increased brain levels of 4-hydroxy-2-nonenal glutathione conjugates in severe Alzheimer's disease. Neurochemistry International. 2006;48:679–686. doi: 10.1016/j.neuint.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Jenner P. Oxidative stress in Parkinson's disease. Annals of Neurology. 2003;53 Suppl 3:S26–S36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 17.Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radical Biology and Medicine. 2002;32:1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 18.Heinecke JW. Oxidized amino acids: culprits in human atherosclerosis and indicators of oxidative stress. Free Radical Biology and Medicine. 2002;32:1090–1101. doi: 10.1016/s0891-5849(02)00792-x. [DOI] [PubMed] [Google Scholar]
- 19.Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. Journal of Biological Chemistry. 1999;274:23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 20.Laneuville O, Breuer DK, Xu N, Huang ZH, Gage DA, Watson JT, et al. Fatty acid substrate specificities of human prostaglandin-endoperoxide H synthase-1 and -2. Formation of 12-hydroxy-(9Z, 13E/Z, 15Z)- octadecatrienoic acids from alpha-linolenic acid. Journal of Biological Chemistry. 1995;270:19330–19336. doi: 10.1074/jbc.270.33.19330. [DOI] [PubMed] [Google Scholar]
- 21.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: A Cancer Journal for Clinicians. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 22.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA: A Cancer Journal for Clinicians. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 23.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: A Cancer Journal for Clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 24.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. International Journal of Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 25.Futreal PA, Coin L, Marshall M, Down T, Hubbard T, Wooster R, et al. A census of human cancer genes. Nature Reviews Cancer. 2004;4:177–183. doi: 10.1038/nrc1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nature Medicine. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 27.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, van de Putte LB, et al. Cyclooxygenase in biology and disease. The FASEB Journal. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 29.Pong SS, Hong SL, Levine L. Prostaglandin production by methylcholanthrene-transformed mouse BALB/3T3. Requirement for protein synthesis. Journal of Biological Chemistry. 1977;252:1408–1413. [PubMed] [Google Scholar]
- 30.Hassid A, Levine L. Induction of fatty acid cyclooxygenase activity in canine kidney cells (MDCK) by benzo(a)pyrene. Journal of Biological Chemistry. 1977;252:6591–6593. [PubMed] [Google Scholar]
- 31.Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proceedings of the National Academy of Sciences USA. 1992;89:7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tazawa R, Xu XM, Wu KK, Wang LH. Characterization of the genomic structure, chromosomal location and promoter of human prostaglandin H synthase-2 gene. Biochemical and Biophysical Research Communications. 1994;203:190–199. doi: 10.1006/bbrc.1994.2167. [DOI] [PubMed] [Google Scholar]
- 33.Rizzo MT. Cyclooxygenase-2 in oncogenesis. Clinica Chimica Acta. 2011;412:671–687. doi: 10.1016/j.cca.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 34.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, Dubois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 35.Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schror K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Research. 1999;59:198–204. [PubMed] [Google Scholar]
- 36.Goulet AC, Einsphar JG, Alberts DS, Beas A, Burk C, Bhattacharyya A, et al. Analysis of cyclooxygenase 2 (COX-2) expression during malignant melanoma progression. Cancer Biology and Therapy. 2003;2:713–718. [PubMed] [Google Scholar]
- 37.Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, et al. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Research. 1999;59:987–990. [PubMed] [Google Scholar]
- 38.Richardsen E, Uglehus RD, Due J, Busch C, Busund LT. COX-2 is overexpressed in primary prostate cancer with metastatic potential and may predict survival. A comparison study between COX-2, TGF-beta, IL-10 and Ki67. Cancer Epidemiology. 2010;34:316–322. doi: 10.1016/j.canep.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 39.Mrena J, Wiksten JP, Kokkola A, Nordling S, Ristimaki A, Haglund C. COX-2 is associated with proliferation and apoptosis markers and serves as an independent prognostic factor in gastric cancer. Tumor Biology. 2010;31:1–7. doi: 10.1007/s13277-009-0001-4. [DOI] [PubMed] [Google Scholar]
- 40.Denkert C, Winzer KJ, Hauptmann S. Prognostic impact of cyclooxygenase-2 in breast cancer. Clinical Breast Cancer. 2004;4:428–433. doi: 10.3816/cbc.2004.n.006. [DOI] [PubMed] [Google Scholar]
- 41.Denkert C, Winzer KJ, Muller BM, Weichert W, Pest S, Kobel M, et al. Elevated expression of cyclooxygenase-2 is a negative prognostic factor for disease free survival and overall survival in patients with breast carcinoma. Cancer. 2003;97:2978–2987. doi: 10.1002/cncr.11437. [DOI] [PubMed] [Google Scholar]
- 42.Yan M, Rerko RM, Platzer P, Dawson D, Willis J, Tong M, et al. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-beta-induced suppressor of human gastrointestinal cancers. Proceedings of the National Academy of Sciences USA. 2004;101:17468–17473. doi: 10.1073/pnas.0406142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chou WL, Chuang LM, Chou CC, Wang AH, Lawson JA, FitzGerald GA, et al. Identification of a novel prostaglandin reductase reveals the involvement of prostaglandin E2 catabolism in regulation of peroxisome proliferator-activated receptor gamma activation. Journal of Biological Chemistry. 2007;282:18162–18172. doi: 10.1074/jbc.M702289200. [DOI] [PubMed] [Google Scholar]
- 44.Schuster VL. Molecular mechanisms of prostaglandin transport. Annual Review of Physiology. 1998;60:221–242. doi: 10.1146/annurev.physiol.60.1.221. [DOI] [PubMed] [Google Scholar]
- 45.Nomura T, Lu R, Pucci ML, Schuster VL. The two-step model of prostaglandin signal termination: in vitro reconstitution with the prostaglandin transporter and prostaglandin 15 dehydrogenase. Molecular Pharmacology. 2004;65:973–978. doi: 10.1124/mol.65.4.973. [DOI] [PubMed] [Google Scholar]
- 46.Backlund MG, Mann JR, Holla VR, Shi Q, Daikoku T, Dey SK, et al. Repression of 15-hydroxyprostaglandin dehydrogenase involves histone deacetylase 2 and snail in colorectal cancer. Cancer Research. 2008;68:9331–9337. doi: 10.1158/0008-5472.CAN-08-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. New England Journal of Medicine. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holla VR, Backlund MG, Yang P, Newman RA, Dubois RN. Regulation of prostaglandin transporters in colorectal neoplasia. Cancer Prevention Research. 2008;1:93–99. doi: 10.1158/1940-6207.CAPR-07-0009. [DOI] [PubMed] [Google Scholar]
- 49.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. Journal of the American Medical Asoociation. 2005;294:914–923. doi: 10.1001/jama.294.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. Journal of Clinical Investigation. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. New England Journal of Medicine. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. 2. [DOI] [PubMed] [Google Scholar]
- 52.Langman MJ, Jensen DM, Watson DJ, Harper SE, Zhao PL, Quan H, et al. Adverse upper gastrointestinal effects of rofecoxib compared with NSAIDs. Journal of the American Medical Asoociation. 1999;282:1929–1933. doi: 10.1001/jama.282.20.1929. [DOI] [PubMed] [Google Scholar]
- 53.Baron JA, Sandler RS, Bresalier RS, Quan H, Riddell R, Lanas A, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131:1674–1682. doi: 10.1053/j.gastro.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 54.Solomon SD, Pfeffer MA, McMurray JJ, Fowler R, Finn P, Levin B, et al. Effect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomas. Circulation. 2006;114:1028–1035. doi: 10.1161/CIRCULATIONAHA.106.636746. [DOI] [PubMed] [Google Scholar]
- 55.Blair IA. DNA-adducts with lipid peroxidation products. Journal of Biological Chemistry. 2008;283:15545–15549. doi: 10.1074/jbc.R700051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee SH, Rangiah K, Williams MV, Wehr AY, Dubois RN, Blair IA. Cyclooxygenase-2-mediated metabolism of arachidonic acid to 15-oxo-eicosatetraenoic acid by rat intestinal epithelial cells. Chemical Research in Toxicology. 2007;20:665–1675. doi: 10.1021/tx700130p. [DOI] [PubMed] [Google Scholar]
- 57.Lee SH, Oe T, Blair IA. Vitamin C-induced decomposition of lipid hydroperoxides to endogenous genotoxins. Science. 2001;292:2083–2086. doi: 10.1126/science.1059501. [DOI] [PubMed] [Google Scholar]
- 58.Williams MV, Lee SH, Blair IA. Liquid chromatography/mass spectrometry analysis of bifunctional electrophiles and DNA adducts from vitamin C mediated decomposition of 15-hydroperoxyeicosatetraenoic acid. Rapid Communications in Mass Spectrometry. 2005;19:849–858. doi: 10.1002/rcm.1854. [DOI] [PubMed] [Google Scholar]
- 59.Jian W, Lee SH, Mesaros C, Oe T, Silva Elipe MV, Blair IA. A novel 4-oxo-2(E)-nonenal-derived endogenous thiadiazabicyclo glutathione adduct formed during cellular oxidative stress. Chemical Research in Toxicology. 2007;20:1008–1018. doi: 10.1021/tx700001t. [DOI] [PubMed] [Google Scholar]
- 60.Zhu P, Jian W, Blair IA. A 4-oxo-2(E)-nonenal-derived glutathione adduct from 15-lipoxygenase-1-mediated oxidation of cytosolic and esterified arachidonic acid. Free Radical Biology and Medicine. 2009;47:953–961. doi: 10.1016/j.freeradbiomed.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jian W, Lee SH, Williams MV, Blair IA. 5-Lipoxygenase-mediated endogenous DNA damage. Journal of Biological Chemistry. 2009;284:16799–16807. doi: 10.1074/jbc.M109.011841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jian WY, Lee SH, Blair IA. Lipoxygenase-mediated endogenous DNA damage. Chemical Research in Toxicology. 2004;17:1759. [Google Scholar]
- 63.Blair IA. Lipid hydroperoxide-mediated DNA damage. Experimental Gerontology. 2001;36:1473–1481. doi: 10.1016/s0531-5565(01)00133-4. [DOI] [PubMed] [Google Scholar]
- 64.Kühn H, Walther M, Kuban RJ. Mammalian arachidonate 15-lipoxygenases structure, function, and biological implications. Prostaglandins and Other Lipid Mediators. 2002;68–69:263–290. doi: 10.1016/s0090-6980(02)00035-7. [DOI] [PubMed] [Google Scholar]
- 65.Chaitidis P, Schewe T, Sutherland M, Kühn H, Nigam S. 15-Lipoxygenation of phospholipids may precede the sn-2 cleavage by phospholipases A2: reaction specificities of secretory and cytosolic phospholipases A2 towards native and 15-lipoxygenated arachidonoyl phospholipids. Federation of European Biochemical Societies Letters. 1998;434:437–441. doi: 10.1016/s0014-5793(98)01024-2. [DOI] [PubMed] [Google Scholar]
- 66.Lotzer K, Funk CD, Habenicht AJ. The 5-lipoxygenase pathway in arterial wall biology and atherosclerosis. Biochimica et Biophysica Acta. 2005;1736:30–37. doi: 10.1016/j.bbalip.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 67.Lee SH, Williams MV, Dubois RN, Blair IA. Targeted lipidomics using electron capture atmospheric pressure chemical ionization mass spectrometry. Rapid Communications in Mass Spectrometry. 2003;17:2168–2176. doi: 10.1002/rcm.1170. [DOI] [PubMed] [Google Scholar]
- 68.Woods JW, Evans JF, Ethier D, Scott S, Vickers PJ, Hearn L, et al. 5-lipoxygenase and 5-lipoxygenase-activating protein are localized in the nuclear envelope of activated human leukocytes. Journal of Experimental Medicine. 1993;178:1935–1946. doi: 10.1084/jem.178.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peters-Golden M, Henderson WR., Jr. The role of leukotrienes in allergic rhinitis. Annals of Allergy, Asthma and Immunology. 2005;94:609–618. doi: 10.1016/S1081-1206(10)61317-8. [DOI] [PubMed] [Google Scholar]
- 70.Murphy RC, Gijon MA. Biosynthesis and metabolism of leukotrienes. Biochemical Journal. 2007;405:379–395. doi: 10.1042/BJ20070289. [DOI] [PubMed] [Google Scholar]
- 71.Werz O. 5-lipoxygenase: cellular biology and molecular pharmacology. Current Drug Targets Inflammation Allergy. 2002;1:23–44. doi: 10.2174/1568010023344959. [DOI] [PubMed] [Google Scholar]
- 72.Sharma JN, Mohammed LA. The role of leukotrienes in the pathophysiology of inflammatory disorders: is there a case for revisiting leukotrienes as therapeutic targets? Inflammopharmacology. 2006;14:10–16. doi: 10.1007/s10787-006-1496-6. [DOI] [PubMed] [Google Scholar]
- 73.Hicks A, Monkarsh SP, Hoffman AF, Goodnow R., Jr. Leukotriene B4 receptor antagonists as therapeutics for inflammatory disease: preclinical and clinical developments. Expert Opinion on Investigational Drugs. 2007;16:1909–1920. doi: 10.1517/13543784.16.12.1909. [DOI] [PubMed] [Google Scholar]
- 74.Wymann MP, Schneiter R. Lipid signalling in disease. Nature Reviews Molecular and Cell Biology. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 75.Peters-Golden M. Expanding roles for leukotrienes in airway inflammation. Current Allergy and Asthma Reports. 2008;8:367–373. doi: 10.1007/s11882-008-0057-z. [DOI] [PubMed] [Google Scholar]
- 76.Zhao L, Funk CD. Lipoxygenase pathways in atherogenesis. Trends in Cardiovascular Medicine. 2004;14:191–195. doi: 10.1016/j.tcm.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 77.Fairweather D, Frisancho-Kiss S. Mast cells and inflammatory heart disease: potential drug targets. Cardiovascular and Hematological Disorders Drug Targets. 2008;8:80–90. doi: 10.2174/187152908783884957. [DOI] [PubMed] [Google Scholar]
- 78.Gupta S, Srivastava M, Ahmad N, Sakamoto K, Bostwick DG, Mukhtar H. Lipoxygenase-5 is overexpressed in prostate adenocarcinoma. Cancer. 2001;91:737–743. doi: 10.1002/1097-0142(20010215)91:4<737::aid-cncr1059>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 79.Hennig R, Ding XZ, Tong WG, Schneider MB, Standop J, Friess H, et al. 5-Lipoxygenase and leukotriene B(4) receptor are expressed in human pancreatic cancers but not in pancreatic ducts in normal tissue. American Journal of Pathology. 2002;161:421–428. doi: 10.1016/S0002-9440(10)64198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen X, Sood S, Yang CS, Li N, Sun Z. Five-lipoxygenase pathway of arachidonic acid metabolism in carcinogenesis and cancer chemoprevention. Current Cancer Drug Targets. 2006;6:613–622. doi: 10.2174/156800906778742451. [DOI] [PubMed] [Google Scholar]
- 81.Schulam PG, Shearer WT. Evidence for 5-lipoxygenase activity in human B cell lines. A possible role for arachidonic acid metabolites during B cell signal transduction. Journal of Immunology. 1990;144:2696–2701. [PubMed] [Google Scholar]
- 82.el Makhour-Hojeij Y, Baclet MC, Chable-Rabinovitch H, Beneytout JL, Cook J. Expression of 5-lipoxygenase in lymphoblastoid B and T cells. Prostaglandins. 1994;48:21–29. doi: 10.1016/0090-6980(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 83.Lee SH, Williams MV, Blair IA. Targeted chiral lipidomics analysis. Prostaglandins and Other Lipid Mediators. 2005;77:141–157. doi: 10.1016/j.prostaglandins.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 84.Lee SH, Blair IA. Targeted chiral lipidomics analysis by liquid chromatography electron capture atmospheric pressure chemical ionization mass spectrometry (LC-ECAPCI/MS) Methods in Enzymology. 2007;433:159–174. doi: 10.1016/S0076-6879(07)33009-7. [DOI] [PubMed] [Google Scholar]
- 85.Jian W, Lee SH, Arora JS, Silva Elipe MV, Blair IA. Unexpected formation of etheno-2'-deoxyguanosine adducts from 5(S)-hydroperoxyeicosatetraenoic acid: evidence for a bis-hydroperoxide intermediate. Chemical Research in Toxicology. 2005;18:599–610. doi: 10.1021/tx049693d. [DOI] [PubMed] [Google Scholar]
- 86.Lee SH, Blair IA. Characterization of 4-oxo-2-nonenal as a novel product of lipid peroxidation. Chemical Research in Toxicology. 2000;13:698–702. doi: 10.1021/tx000101a. [DOI] [PubMed] [Google Scholar]
- 87.Lee SH, Oe T, Blair IA. 4,5-Epoxy-2(E)-decenal-induced formation of 1,N(6)-etheno-2'-deoxyadenosine and 1,N(2)-etheno-2'-deoxyguanosine adducts. Chemical Research in Toxicology. 2002;15:300–304. doi: 10.1021/tx010147j. [DOI] [PubMed] [Google Scholar]
- 88.Lee SH, Elipe MVS, Arora JS, Blair IA. Dioxododecenoic acid: A lipid hydroperoxide-derived bifunctional electrophile responsible for etheno DNA adduct formation. Chemical Research in Toxicology. 2005;18:566–578. doi: 10.1021/tx049716o. [DOI] [PubMed] [Google Scholar]
- 89.Lee SH, Arora JA, Oe T, Blair IA. 4-Hydroperoxy-2-nonenal-induced formation of 1,N2-etheno-2'-deoxyguanosine adducts. Chemical Research in Toxicology. 2005;18:780–786. doi: 10.1021/tx0497088. [DOI] [PubMed] [Google Scholar]
- 90.Swenberg JA, Bogdanffy MS, Ham A, Holt S, Kim A, Morinello EJ, et al. Formation and repair of DNA adducts in vinyl chloride- and vinyl fluoride-induced carcinogenesis. International Agency for Research on Cancer Scientific Publication. 1999;150:29–43. [PubMed] [Google Scholar]
- 91.Barbin A, Bartsch H. Mutagenic and promutagenic properties of DNA adducts formed by vinyl chloride metabolites. International Agency for Research on Cancer Scientific Publication. 1986;70:345–358. [PubMed] [Google Scholar]
- 92.Akasaka S, Guengerich FP. Mutagenicity of site-specifically located 1,N2-ethenoguanine in Chinese hamster ovary cell chromosomal DNA. Chemical Research in Toxicology. 1999;12:501–507. doi: 10.1021/tx980259j. [DOI] [PubMed] [Google Scholar]
- 93.Winter CK, Segall HJ, Haddon WF. Formation of cyclic adducts of deoxyguanosine with the aldehydes trans-4-hydroxy-2-hexenal and trans-4-hydroxy-2-nonenal in vitro. Cancer Research. 1986;46:5682–5686. [PubMed] [Google Scholar]
- 94.Poli G, Biasi F, Leonarduzzi G. 4-Hydroxynonenal-protein adducts: A reliable biomarker of lipid oxidation in liver diseases. Molecular Aspects of Medicine. 2008;29:67–71. doi: 10.1016/j.mam.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 95.Hankin JA, Jones DN, Murphy RC. Covalent binding of leukotriene A4 to DNA and RNA. Chemical Research in Toxicology. 2003;16:551–561. doi: 10.1021/tx034018+. [DOI] [PubMed] [Google Scholar]
- 96.Rindgen D, Nakajima M, Wehrli S, Xu K, Blair IA. Covalent modifications to 2'-deoxyguanosine by 4-oxo-2-nonenal, a novel product of lipid peroxidation. Chemical Research in Toxicology. 1999;12:1195–1204. doi: 10.1021/tx990034o. [DOI] [PubMed] [Google Scholar]
- 97.Lee SH, Rindgen D, Bible RH, Jr., Hajdu E, Blair IA. Characterization of 2'-deoxyadenosine adducts derived from 4-oxo-2-nonenal, a novel product of lipid peroxidation. Chemical Research in Toxicology. 2000;13:565–574. doi: 10.1021/tx000057z. [DOI] [PubMed] [Google Scholar]
- 98.Rindgen D, Lee SH, Nakajima M, Blair IA. Formation of a substituted 1,N(6)-etheno-2'-deoxyadenosine adduct by lipid hydroperoxide-mediated generation of 4-oxo-2-nonenal. Chemical Research in Toxicology. 2000;13:846–852. doi: 10.1021/tx0000771. [DOI] [PubMed] [Google Scholar]
- 99.Pollack M, Oe T, Lee SH, Silva Elipe MV, Arison BH, Blair IA. Characterization of 2'-deoxycytidine adducts derived from 4-oxo-2-nonenal, a novel lipid peroxidation product. Chemical Research in Toxicology. 2003;16:893–900. doi: 10.1021/tx030009p. [DOI] [PubMed] [Google Scholar]
- 100.Pollack M, Yang IY, Kim HY, Blair IA, Moriya M. Translesion DNA Synthesis across the heptanone - etheno-2'-deoxycytidine adduct in cells. Chemical Research in Toxicology. 2006;19:1074–1079. doi: 10.1021/tx0600503. [DOI] [PubMed] [Google Scholar]
- 101.Yang IY, Hashimoto K, de WN, Blair IA, Moriya M. Two distinct translesion synthesis pathways across a lipid peroxidation-derived DNA adduct in mammalian cells. Journal of Biological Chemistry. 2009;284:191–198. doi: 10.1074/jbc.M806414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ohmori H, Friedberg EC, Fuchs RP, Goodman MF, Hanaoka F, Hinkle D, et al. The Y-family of DNA polymerases. Molecular Cell. 2001;8:7–8. doi: 10.1016/s1097-2765(01)00278-7. [DOI] [PubMed] [Google Scholar]
- 103.Dubois RN, Smalley WE. Cyclooxygenase, NSAIDs, and colorectal cancer. Journal of Gastroenterology. 1996;31:898–906. doi: 10.1007/BF02358623. [DOI] [PubMed] [Google Scholar]
- 104.Lee SH, Williams MV, Dubois RN, Blair IA. Cyclooxygenase-2-mediated DNA damage. Journal of Biological Chemistry. 2005;280:28337–28346. doi: 10.1074/jbc.M504178200. [DOI] [PubMed] [Google Scholar]
- 105.Brown JR, Dubois RN. COX-2: a molecular target for colorectal cancer prevention. Journal of Clinical Oncology. 2005;23:2840–2855. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 106.Williams CS, Luongo C, Radhika A, Zhang T, Lamps LW, Nanney LB, et al. Elevated cyclooxygenase-2 levels in Min mouse adenomas. Gastroenterology. 1996;111:1134–1140. doi: 10.1016/s0016-5085(96)70083-5. [DOI] [PubMed] [Google Scholar]
- 107.Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 108.Williams MV, Lee SH, Pollack M, Blair IA. Endogenous lipid hydroperoxide-mediated DNA-adduct formation in min mice. Journal of Biological Chemistry. 2006;281:10127–10133. doi: 10.1074/jbc.M600178200. [DOI] [PubMed] [Google Scholar]
- 109.Chou PH, Kageyama S, Matsuda S, Kanemoto K, Sasada Y, Oka M, et al. Detection of lipid peroxidation-induced DNA adducts caused by 4-oxo-2(E)-nonenal and 4-oxo-2(E)-hexenal in human autopsy tissues. Chemical Research in Toxicology. 2010;23:1442–1448. doi: 10.1021/tx100047d. [DOI] [PubMed] [Google Scholar]
- 110.Blackford A, Serrano OK, Wolfgang CL, Parmigiani G, Jones S, Zhang X, et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clinical Cancer Research. 2009;15:4674–4679. doi: 10.1158/1078-0432.CCR-09-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 112.Waddell WR, Ganser GF, Cerise EJ, Loughry RW. Sulindac for polyposis of the colon. The American Journal of Surgery. 1989;157:175–179. doi: 10.1016/0002-9610(89)90442-x. [DOI] [PubMed] [Google Scholar]