Abstract
Recent reports have shown limited anticancer therapeutic efficacy of insulin-like growth factor receptor (IGF-1R)-targeted monoclonal antibodies (mAbs), but the resistance mechanisms have not been completely identified. Because cooperation between epidermal growth factor receptor (EGFR) and IGF-IR could cause resistance to inhibitors of individual RTKs, we investigated the involvement of EGFR signaling in resistance to IGF-1R mAb and the underlying mechanisms of action. Most head and neck squamous cell carcinoma (HNSCC) tissues had co-expression of total and phosphorylated IGF-1R and EGFR at high levels compared to paired adjacent normal tissues. Treatment with cixutumumab (IMC-A12), a fully humanized IgG1 mAb, induced activation of Akt and mammalian target of rapamycin (mTOR), resulting in de novo synthesis of EGFR, Akt1, and survivin proteins and activation of the EGFR pathway in cixutumumab-resistant HNSCC and non-small cell lung cancer (NSCLC) cells. Targeting mTOR and EGFR pathways by treatment with rapamycin and cetuximab (an anti-EGFR mAb), respectively, prevented cixutumumab-induced expression of EGFR, Akt, and survivin and induced synergistic antitumor effects in vitro and in vivo. These data show that resistance to IGF-1R inhibition by mAbs is associated with Akt/mTOR-directed enhanced synthesis of EGFR, Akt1, and survivin. Our findings suggest that Akt/mTOR might be effective targets to overcome the resistance to IGF-1R mAbs in HNSCC and NSCLC.
Keywords: EGFR, IGF-IR, head and neck squamous cell carcinoma, survivin, cixutumumab
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the fifth most common malignancy, and non-small cell lung cancer (NSCLC) is the leading cause of cancer-related death (1). Despite decades of research and treatment advances, the 5-year survival rates for both have improved little, and local and distant metastasis remains significant barriers to disease eradication (2). Recent advances in developing molecularly targeted cancer therapeutic agents that block specific receptors or signaling proteins may lead to promising new treatments for these cancers.
The insulin-like growth factor (IGF) axis plays a pivotal role in regulating tumor cell growth, differentiation, tumor angiogenesis, metastasis, apoptosis, and multidrug resistance (3). The IGF axis is composed of ligands, receptors, and IGF-binding proteins (4). The balance between these molecules’ expression and activity is tightly controlled under normal physiologic conditions; changes in this balance can cause numerous molecular events that can ultimately lead to malignancy (5). Increased IGF-1 receptor (IGF-1R) and circulating IGF-1 expression is associated with an elevated risk for numerous cancer types and rapid disease progression, including of HNSCC and NSCLC (6,7). Increased bioactive IGF-II levels also result from reduced expression of IGF binding protein or inactivation of the type 2 IGF receptor that mediates IGF-II degradation (8,9). These changes can result in high local IGF tissue concentrations. In addition, the binding of IGFs to IGF-IR initiates conformational changes, transmembrane receptor tyrosine kinase (RTK) autophosphorylation, and Ras-Raf-mitogen-activated protein kinase and phosphoinositide 3-kinase (PI3K)/AKT signaling cascade activation, leading to the phosphorylation of several downstream substrates that are involved in cell proliferation, survival and apoptosis, inflammation, genomic instability, and angiogenesis (3). Thus, IGF-1R signaling has been considered as a promising target for cancer therapy. Indeed, IGF-1R inactivation by gene disruption, antisense oligonucleotides, neutralizing antibodies, dominant-negative mutants, small molecule IGF-IR kinase inhibitors, and IGF-binding proteins has resulted in antitumor activity (10). However, several clinical trials with anti-IGF-1R mAb have shown modest therapeutic efficacy in clinical trials and the mechanisms involved in resistance to the drug have not been clearly defined. In a previous study, IGF and EGF stimulation both resulted in a physical association between the two receptors in a TU159 HNSCC cell line protein complex (7). We and others have demonstrated crosstalk between RTKs of EGFR and IGFR, wherein a tyrosine kinase inhibitor (TKI)’s inhibition of one RTK is compensated by enhanced activity of the reciprocal RTK; thus, one suspected IGF-1R resistance mechanism is crosstalk with EGFR or other kinase receptors (11–13). However, to our knowledge, the involvement of the EGFR pathway in resistance to IGF-1R mAb-based anticancer therapy has not been defined. In this article, we report that inhibition of the IGF-1R pathway by cixutumumab (IMC-A12), a fully humanized IgG1 mAb (14), results in stimulation of the Akt/mammalian target of rapamycin (mTOR) pathway through increasing synthesis of EGFR, Akt1, and antiapoptotic survivin proteins. In addition, suppression of mTOR-mediated protein synthesis or inactivation of EGFR renders cixutumumab-resistant cells sensitive to the drug. These results present a drug resistance mechanism of an IGF-1R targeted agent as well as molecular targets to restore its antitumor activity.
Materials and Methods
Cell culture and reagents
All human HNSCC (SqCC/Y1, HN30, LN686, FADU, UMSCC1, UMSCC2, UMSCC4, UMSCC6, UMSCC11A, UMSCC14A, UMSCC38, TR146, and OSC19) were kindly provided by Dr. Jeffrey Myers (MD Anderson Cancer Center, Houston, TX, USA). NSCLC (H226Br, H226B, H596, H460, A549, and H1299) cell lines were kindly provided by Jack Roth or purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). Cells were cultured in DMEM, Ham’s F12 or RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) (Life Technologies, Inc., Gaithersburg, MD, USA). Cells were validated by analysis of their short tandem repeat (STR) profile. We used specific antibodies against the following antigens: phospho-Akt (pAkt, S473), Akt, Akt1, Akt2, Akt3, pIGF-1R (Y1131), pmTOR (S2248), mTOR, pEGFR (Y1068), EGFR, poly (ADP-ribose) polymerase (PARP), cleaved caspase-3, Survivin (Cell Signaling Technology), IGF-1Rβ (C20), pERK (T202/204), and ERK (Santa Cruz). Rapamycin was purchased from MBL International Corporation. Cixutumumab and C225 (cetuximab [Erbitux]) were provided by Imclone Systems, Inc.
Cell viability assay
Poly-HEMA (poly-2-hydroxyethyl methacrylate)-coated plates (PCPs) were prepared as previously described (15). For the cell viability assay, 2 × 103 cells were plated on 96-well PCPs or ultra-low attached plates (UAPs). After 3 days of drug treatment, cell proliferation was measured with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. Six replicate wells were used for each analysis; at least three independent experiments were performed.
RT-PCR
Total RNA was isolated and RT-PCR was performed as described elsewhere (12) using the following primer sequences: (sense) 5′-GGAGAA CTGCCAGAAACTGACC-3′ and (antisense) 5′-GCCTGCAGCACACTGGTTG-3′ for EGFR; (sense) 5′-AGCGACGTGGCTATTGTGAAG-3′ and (antisense) 5′-GCCGCCAGGTCTTGATGTAC-3′ for Akt1; and (sense) 5′-CCTCTATGCCAACACAGTGC-3′ and (antisense) 5′-CATCGTACTCCTGCTTGCTG-3′ for (beta-actin). The following thermocycler conditions were used for amplification: 94°C for 6 minutes (hot start), followed by 28–33 cycles of 94°C for 45 seconds, 56–60°C for 45 seconds, and 72°C for 1 minute.
Metabolic labeling
Metabolic labeling was performed as described elsewhere (12). Briefly, LN686 cells were treated with cixutumumab (25 μg/ml) in the presence of rapamycin (1 μM) for 72 hours and incubated with medium lacking methionine and cysteine for 1 hour. The cells were labeled with [35S] methionine-cysteine and cixutumumab and harvested at the indicated time points. Equal amounts of protein were used for immunoprecipitation with antibodies against EGFR, Akt1, Akt2, and Akt3 and the immunoprecipitates were separated by polyacrylamide gel electrophoresis. Laser densitometry was performed to quantify the band intensity.
Western blot analysis
We performed a biochemical analysis of 8 head and neck tumor and 8 healthy adjacent tissue specimens from patients with head and neck cancer who had undergone surgical resection at The University of Texas M. D. Anderson Cancer Center (Houston, Texas). This study was approved by the M. D. Anderson Cancer Center institutional review board. All tissue specimens had frozen in liquid nitrogen immediately after being resected. Total protein isolation and Western blot analysis were performed as described previously (12).
Soft agar assays
An anchorage-independent colony formation assay was performed as previously described (12). To determine the effect of the combined drug treatment, we estimated potentiation by multiplying the percentage of cells remaining (% growth) for each agent. The classification index was calculated as described previously (16).
Caspase-3/CPP32 colorimetric assay
Caspase-3/CPP32 activity was determined as described elsewhere (17) using cells that had been treated with cixutumumab, rapamycin, C225, or their combinations for 3 days. Fold-increase in CPP32 activity was determined by comparing these results with the level of the uninduced control. Six replicate wells were used for each analysis; at least three independent experiments were performed.
In vivo mode
All animal procedures were performed in accordance with a protocol approved by the M. D. Anderson Institutional Animal Care and Use Committee. Xenograft tumors were generated by subcutaneously injecting nude mice with 1 × 106 of LN686 cells. When tumors reached a volume of 80–100 mm3 (termed day 0 for our experiments), we treated control mice with an intraperitoneal injection of sterile PBS and xenografted mice with cixutumumab (10 mg/kg, once a week), C225 (10 mg/kg, once a week), and rapamycin (5 mg/kg, daily); cixutumumab and C225 (10 mg/kg each, once a week); or cixutumumab (10 mg/kg, once a week) and rapamycin (5 mg/kg, daily). Tumor volumes were measured every 3 days.
Statistical analysis
The data acquired from the MTT assay were analyzed using Student’s t test. All means and 95% CI’s from eight samples were calculated using Microsoft Excel software (Microsoft Corporation, Seattle, WA). Statistical significance of differences in tumor growth in the combination treatment group and in the single-agent treatment groups were analyzed by ANOVA. All means from triplicate to eight samples and 95% CI’s were calculated using SAS software (release 8.02; SAS Institute, Cary, NC). In all statistical analyses, two-sided P values of <0.05 were considered statistically significant.
Results
IGF-1R and pIGF-1R expressions in human HNSCC tissue
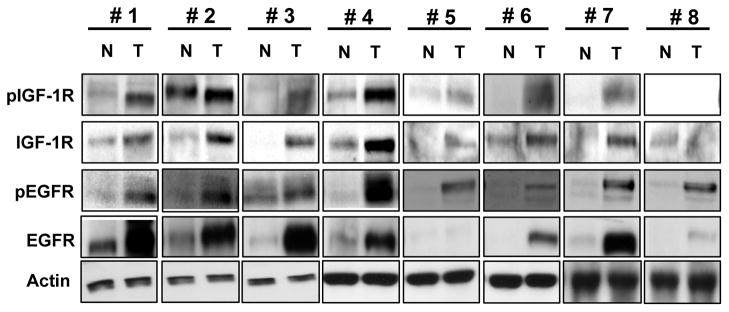
To have the rationale to target both IGF-1R and EGFR signalings, we determined total and phosphorylated IGF-1R and EGFR expression levels in HNSCC tissue. Seven (#1–7) of the eight tumor specimens had high levels of IGF-1R and phosphorylated IGF-1R (pIGF-1R) expression and all of the tumor specimens had high levels of EGFR and phosphorylated EGFR (pEGFR) expression compared to normal tissue specimens from the same patients (Fig. 1). All of the specimens with high levels of IGF-1R and pIGF-1R expressions also had higher levels of pEGFR and EGFR expression than did normal tissue. These findings indicated co-expression and co-activation of IGF-1R and EGFR at high levels in HNSCC, suggesting the potential value of co-targeting the IGF-1R and EGFR pathways.
Figure 1.
The activities and expression of IGF-1R and EGFR in paired squamous cell carcinoma and normal tissue specimens from patients with HNSCC. Proteins were extracted from HNSCC and healthy normal tissue and subjected to Western blot analysis to determine expressions of total and phophorylated IGF-1R and EGFR.
Resistance to cixutumumab-induced growth inhibition is correlated with EGFR/PI3K/AKT pathway activation in HNSCC and NSCLC cells grown in 3D mimic environment
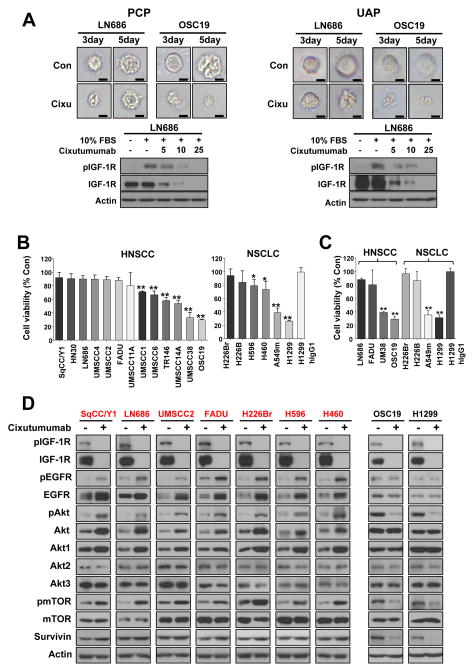
Several studies have reported the difference of cellular responses in a three-dimensional (3D) environment and the higher sensitivities of a number of cancer cell lines to certain anticancer drugs in 3D culture systems compared to the response of the same cell lines grown in monolayers (18–20). Hence, we determined cixutumumab’s effects on HNSCC cells grown on poly-HEMA-coated plates (PCPs) and ultralow attached plates (UAPs), known 3D-mimetic culture systems. Cells cultured under the conditions grew and formed spherical colonies. Representative results from LN686 and OSC19 cells grown in PCPs and UAPs are shown (Fig. 2A). Cixutumumab treatment completely inhibited 10% FBS or IGF-induced, but not insulin-induced, IGF-1R phosphorylation (Fig. 2A, bottom and supplementary Fig. 1), indicating that only IGF-1R-mediated signaling could participate in the cixutumumab’s action. We then performed an MTS assay on 13 HNSCC and 6 NSCLC cell lines in 10% fetal bovine serum (FBS) with or without cixutumumab for 72 h. We observed differential sensitivity of tested cells to cixutumumab treatment, and two HNSCC (UMSCC38 and OSC19) and NSCLC (H1299 and A549m) cell lines had > 60% inhibition in viability (Fig. 2B). Consistent with the results in cells grown on PCPs, cixutumumab treatment strongly suppressed the growth of UMSCC38, OSC19, H1299, and A549m cells in UAPs, whereas the remaining cells demonstrated moderate responses to treatment (Fig. 2C). These results suggest that cixutumumab’s antitumor effects are limited to specific HNSCC and NSCLC cell lines.
Figure 2.
HNSCC and NSCLC cell lines display differential sensitivities to cixutumumab in 3D mimic condition. Indicated HNSCC and NSCLC cells cultured in poly(HEMA)-coated plates (PCPs) and in ultra-low attached plates (UAP) were treated with hIgG1 (25 μg/ml) or IMC-cixutumumab (25 μg/ml) for 3 (A, C, D) or 5 days (B) in the presence of FBS or for 6 hrs in the absence of FBS and then stimulated with 10% FBS for 30 min (A(bottom)). A, Representative morphologies of LN686 and OSC19 cells (Con: control; Cixu: cixutumumab). A(bottom), D, Western blot was performed for the indicated proteins. B, C, Cell viabilities were measured by using MTS assay and were determined as percentages of each control groups. Independent experiments were repeated three times. Bars represent mean ± SD (n=6); *P<0.05 and **P<0.01. The statistical significance was determined by using Student’s t test.
We investigated the mechanisms involved in cixutumumab resistance in HNSCC and NSCLC cells. Since we did not find obvious difference between the results from PCP and UAP, additional studies were performed in PCP, as a representative of 3D-mimic 2D system. We correlated total (supplementary Fig. 2A) and phosphorylated IGF-1R and EGFR (supplementary Fig. 2B) with resistance to cixutumumab and found no obvious correlation between them. Further, IGF-1R mRNA levels were not changed after the drug treatment (data not shown). However, cixutumumab increased phosphorylation of EGFR and its downstream mediators, including Akt and mTOR, in all cixutumumab-resistant HNSCC (SqCC/Y1, LN686, UMSCC2, and FADU) and NSCLC (H226Br, H596, and H460) cell lines but not in cixutumumab-sensitive HNSCC (OSC19) and NSCLC (H1299) cell lines after 3 days of treatment (Fig. 2D). Of note, cixutumumab-resistant cell lines had increased EGFR and Akt1 levels, with no changes in Akt2 and 3, suggesting that activation of the EGFR pathway could have been due to the increased expressions of EGFR and Akt1. Cixutumumab-resistant cells also showed slightly increased level of survivin expression, a member of inhibitor of apoptosis proteins known to decrease the sensitivity of tumor cells to chemotherapeutic drugs (21). In contrast, cixutumumab-sensitive lines showed obviously decreased levels of survivin. These findings suggest that induced expression of EGFR, Akt1, and survivin protein provide cixutumumab-resistant cell lines with ability to proliferate after the drug treatment.
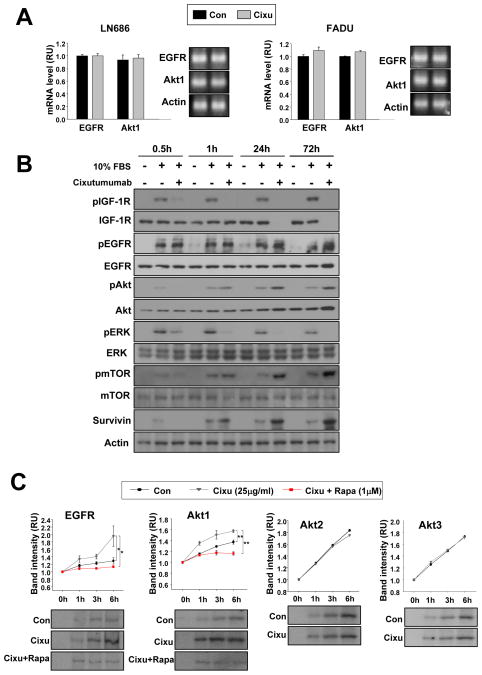
mTOR pathway induces de novo EGFR and Akt protein synthesis
We assessed the mechanisms of cixutumumab-mediated increase in EGFR and Akt1 protein expression using LN686 and FADU cells grown in PCPs. No detectable changes were observed in EGFR and Akt1 mRNA levels (Fig. 3A), suggesting cixutumumab-induced post-transcriptional up-regulation of EGFR and Akt expressions in the drug-resistant cells. Therefore, we monitored the kinetics of cixutumumab-induced phosphorylation of EGFR, Akt, and mTOR in cixutumumab-resistant LN686 cells. Cixutumumab (25 μg/ml) induced decreases in pIGF-1R, pAkt, and pERK1/2 levels as early as 30 minutes after treatment (Fig. 3B). However, pAkt induction was evident after 1 hour of cixutumumab treatment, followed by delayed increases in pEGFR and survivin expressions after 1 day. Obvious increases in EGFR and Akt1 protein expressions were observed after 3 days treatment of the drug. Given the Akt/mTOR pathway’s role in protein synthesis, we determined cixutumumab’s effects on EGFR and Akt1 protein synthesis rates by metabolically labeling LN686 cells with [35S] Met-Cys. As shown in Fig. 3C, the [35S]-labeled EGFR and Akt1 synthesis rate was remarkably higher in cixutumumab-treated LN686 cells than in untreated cells. In contrast, Akt2 and Akt3 protein synthesis was not detectably affected by cixutumumab treatment. We further confirmed cixutumumab-induced de novo synthesis of EGFR and Akt1 proteins was prevented by combined treatment with rapamycin, an mTOR inhibitor. Together, these findings suggest that cixutumumab’s inhibition of IGF-1R signaling resulted in initial activation of the Akt/mTOR pathway followed increased synthesis of EGFR and Akt proteins, leading to activation of the EGFR pathway in cixutumumab-resistant cells.
Figure 3.
Cixutumumab induced-the activities and expression levels of EGFR and Akt is through mTOR-mediated protein synthesis. A, RT-PCR analysis of LN686 and FADU cells grown in PCPs in the presence of vehicle (Con) or cixutumumab (Cixu) for 3 days. B, LN686 cells grown in PCP were treated with cixutumumab in the absence of FBS for indicated time period and then stimulated with 10% FBS for 30min before harvest. The indicated proteins were detected by Western blot analysis. C, LN686 cells grown in PCP in the presence of vehicle (Con) or cixutumumab (Cixu) with or without rapamycin or 3 days were metabolically pulse-labeled with trans 35S-methionine and cysteine and then chased with media containing methionine and cysteine for the indicated time periods. Immunoprecipitation was performed using antibodies against EGFR, Akt1, Akt2, and Akt3. Densitometry was performed to quantify the density of each band compared to that at time 0 h. Student’s t test, average ± SD; n=3 *P<0.05 and **P<0.01. RU: relative unit.
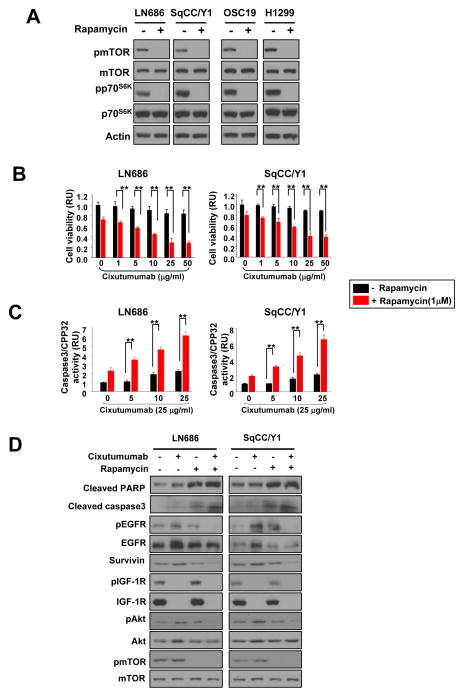
Co-targeting IGF-1R and mTOR or EGFR enhances antitumor activity of cixutumumab in cixutumumab-resistant cells
We next asked whether increased AKT/mTOR activity compensates for loss of IGF-1R signaling by increasing EGFR and Akt1 protein synthesis and thus EGFR signaling activation. To this end, we tested the effects of single or combined treatment with cixutumumab and rapamycin, an mTOR inhibitor on proliferation of cixutumumab-resistant cells grown in PCPs. Rapamycin (1 μM) induced a complete suppression of 10% FBS-induced phosphorylation of mTOR after 6 hrs of treatment (supplementary Fig. 3A) and significant decrease in cell proliferation after 3 days treatment (supplementary Fig. 3B). The rapamycin treatment inhibited mTOR and p70S6K phosphorylation in both cixutumumab-resistant (LN686, SqCC/Y1) and -sensitive (OSC19, H1299) cells (Fig. 4A). Rapamycin is known as an allosteric inhibitor of mTORC1 (22), and p70S6 kinase is a major effector of the of mTOR phosphorylation (Ser2448) (23), suggesting that inactivation of p70S6 kinase by rapamycin via mTOR regulation led to dephsophorylation of mTOR. Synergistic antiproliferative effect was found in cixutumumab-resistant cells treated with cixutumumab and rapamycin combination compared with those treated with each single agent (Fig. 4B and supplementary Table 1). Moreover, the co-treatment showed significantly enhanced caspase-3/CPP32 activity and PARP and caspase-3 cleavages in these cells (Fig. 4C). Treatment with rapamycin also prevented cixutumumab-induced increases in EGFR and Akt. The co-treatment suppressed basal as well as cixutumumab-induced upregulation of pEGFR, survivin, pAkt, and pmTOR expressions with no detectable impact in protein levels of mTOR in these cells (Fig. 4D), suggesting that inactivation of mTOR inhibits cixutumumab-induced activation of Akt/mTOR pathway and de novo EGFR and Akt protein expressions, resulting in restoration of cixutumumab’s apoptotic activity in the drug-resistant cell lines.
Figure 4.
The combination between cixutumumab and rapamycin exhibits synergistic effects on cell viability and caspase3 activity in HNSCC. A, Western blot on the regulation of mTOR and p70S6K by rapamycin treatment in cixutumumab resistant (LN686 and SqCC/Y1) and -sensitive (OSC19 and H1299) cells. Cells were treated with vehicle (Con) or rapamycin for 3 days in PCPs and stimulated with 10% FBS for 30 min. B, C, LN686 and SqCC/Y1 cells grown in PCP were treated with cixutumumab in the presence (red columns) or absence (black columns) of rapamycin for 3 days and then measured cell viability and caspase3/CPP32 activity. Student’s t test, average ± SD; n=6 P<0.05 and P<0.001. RU; relative unit. D, LN686 and SqCC/Y1 cells were treated with cixutumumab, rapamycin, or their combination in PCPs for 3 days and stimulated with 10% FBS. Cell lysates were analyzed by Western blot.
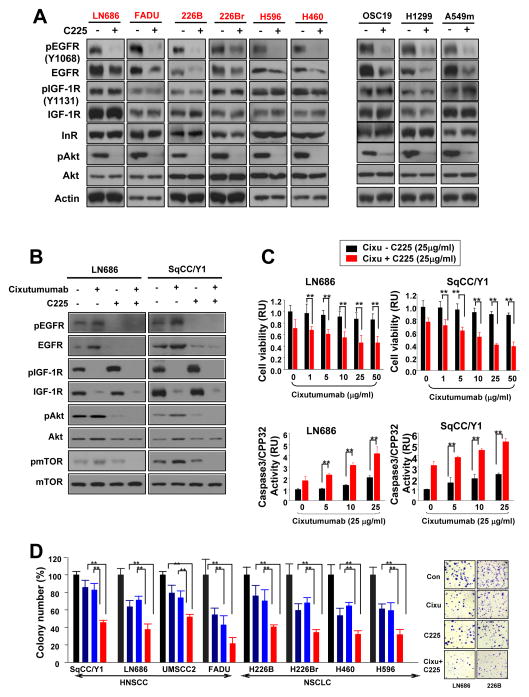
We next tested the effects of single or combined treatment with cixutumumab and C225, an EGFR-neutralizing antibody, on proliferation of cixutumumab-resistant cells grown in PCPs. C225 treatment (25 μg/ml) induced a complete suppression of 10% FBS- or EGF (100 ng/ml)- stimulated EGFR phosphorylation after 6 hrs (supplementary Fig. 4A) and a significant decrease in cell proliferation after 3 days of treatment (supplementary Fig. 4B). The C225 treatment led to decreases in pEGFR, EGFR, and pAkt expressions in both cixutumumab-resistant and -sensitive NSCLC and HNSCC cells with no effects on pIGF-1R, IGF-1R and IR expressions (Fig. 5A). The addition of C225 prevented a cixutumumab-induced increase in EGFR and Akt protein expressions in cixutumumab-resistant cells (Fig. 5B). Further, the C225 treatment completely blocked cixutumumab-induced phosphorylation of EGFR, Akt, and mTOR in the presence of FBS (Fig. 5B) or IGF-1 (supplementary Fig. 5). Combined treatment with cixutumumab and C225 induced synergistically enhanced antiproliferative activities (Fig. 5C, top, supplementary Table 1) with increased apoptosis, as shown by increased caspase-3/CPP32 activity (Fig. 5C, bottom) and PARP cleavage (Fig. 5B, supplementary Fig. 4), indicating that reduced cell viability by the co-treatment was due to increased cell death. We also observed that cixutumumab-resistant cells grown in soft agar showed synergistically increased sensitivity to the co-treatment than to the single treatment (Fig. 5D, supplementary Table 2). Enhanced apoptosis was also observed after co- treatment with cixutumumab with LY294002 (PI3K/Akt inhibitor) or erlotinib (an EGFR TKI) (supplementary Fig. 6). These findings suggest that, when the IGF-1R pathway is inactivated by cixutumumab, the Akt/mTOR pathway-derived EGFR activation by the drug provides an alternative proliferation or survival signaling.
Figure 5.
The co-treatment with cixutumumab and C225 exerts synergistic effects on colony formation and caspase-3 activity. A, B, Western blot on the indicated cixutumumab-resistant and -sensitive cells treated with vehicle (−), C225, cixutumumab, or their combination for 3 days and stimulated with 10% FBS. C, Viability and caspase3/CPP32 activity were measured in the indicated cells grown in PCPs with indicated doses of cixutumumab in the presence (red columns) or absence (black columns) of C225 for 3 days. Student’s t test, average ± SD; n=6 P<0.05 and P<0.001. RU; relative unit. D, The indicated cell lines grown in soft agar were treated with cixutumumab, C225, or their combination. Colonies were stained and then counted. Student’s t test, average ± SD; n=6 *P<0.05 and **P<0.01 (Left panel). Representative images are shown in the right panel.
Effects of cixutumumab, C225, rapamycin, and their combinations on the growth of cixutumumab resistant HNSCC xenograft tumors
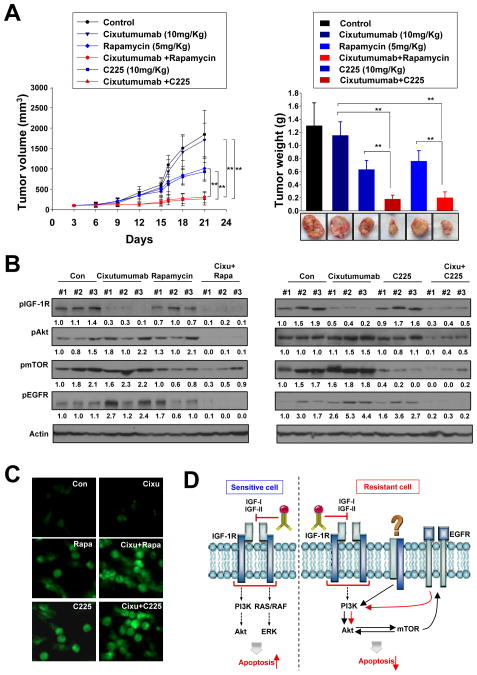
To determine whether EGFR and mTOR signaling inhibition enhances cixutumumab’s antitumor activity in vivo, we tested the effects of cixutumumab, rapamycin, and C225 alone or in combination on the growth of cixutumumab-resistant LN686 xenograft tumors established in nude mice. Single treatment of cixutumumab with 10 mg/kg (once a week) (Fig. 6A, left) or with higher doses (25 or 50 mg/kg, twice a week) (data not shown) showed modest effects on the tumor growth. Significant smaller tumors were found in mice treated with cixutumumab and rapamycin or C225 than those in control mice and in mice treated with single agent alone (Fig. 6A). Cixutumumab treatment alone or in combination with rapamycin did not exhibit significant toxic effects, including weight loss (data not shown). Western blot analysis on the tumor tissues revealed that Akt, mTOR, and EGFR activity was effectively blocked by combined treatment with cixutumumab and rapamycin or with cixutumumab and C225 (Fig. 6B). In addition, cixutumumab and C225 or rapamycin led to increased levels of terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL) staining (Fig. 6C). These findings suggest that combined treatment with cixutumumab and rapamycin or C225 enhances in vivo antitumor activity by decreasing cixutumumab-induced Akt, mTOR, and EGFR activity and by inducing apoptosis.
Figure 6.
The co-targeting IGF-1R and mTOR or EGFR exhibits potent antitumor properties in vivo in HNSCC. A, mice bearing LN686 xenograft tumors were administrated with vehicle (control), cixutumumab, C225, rapamycin or their combination. Tumors were measured every three days. Results are expressed as mean tumor volume (calculated from 8 mice) relative to the tumor volume at day 0. Student’s t test, average ± SE; n=8 P<0.05 and P<0.001. B, Western blot analysis on the expression of pAkt, pEGFR, pmTOR, and actin. C, TUNEL staining of the xenografts tissues. D, schematic model of resistance mechanism to cixutumumab.
Discussion
In the present study, we show that: 1) blocking IGF-1R signaling by cixutumumab induces activation of EGFR signaling in cixutumumab-resistant HNSCC and NSCLC cells through Akt/mTOR-mediated de novo synthesis of EGFR and Akt1, leading to activation of the EGFR pathway; 2) activation of the Akt/mTOR pathway also results in induction of survivin protein expression, contributing to increase in antiapoptotic potential in the cixutumumab-resistant cells; and 3) blocking the mTOR or EGFR signaling pathway restores cixutumumab’s pro-apoptotic activity in HNSCC cells both in vitro and in vivo (Fig. 6D). These results provide a first mechanistic evidence for a crosstalk between the IGF-1R and the EGFR signaling pathways as a consequence of cixutumumab-mediated inactivation of the IGF-1R signaling. Overall, these findings suggest that Akt/mTOR-mediated synthesis of proteins involved in cell proliferation and survival is involved in HNSCC and NSCLC cells’ resistance to anti-IGF-IR mAbs, indicating the potential clinical utility of co-targeting IGF-IR and mTOR as well as co-targeting IGF-1R and EGFR in patients with HNSCC or NSCLC.
IGF-1R- and IGF-1R/IR-targeting drug candidates, which are mainly composed of anti-IGF-1R mAbs and small molecule inhibitors, have demonstrated a variety of antitumor activities in several preclinical studies (24,25). However, the clinical response rates to IGF-1R mAbs, alone and with chemotherapeutic agents, have been lower than expected (26). To develop effective anticancer therapeutic strategies with anti-IGF-1R mAbs, we determined the mechanisms that induce primary resistance to the anti-IGF-1R mAb cixutumumab, a fully humanized IgG1 mAb that is being clinically evaluated for the treatment of several cancers, including HNSCC and NSCLC (14,26). It has been suggested that activation of the IGF-IR pathway after EGFR TKI treatment counteracted the drugs’ antitumor activity in several cancer cell types (11–13). Conversely, in a recent report, IGF-IR inhibition by TKI promoted EGFR activation (27). Given the interplay and considerable functional similarities between EGFR’s and IGF-1R’s functions, we hypothesized that switching to EGFR signaling allows cells to resist cixutumumab treatment. Our data showed that cixutumumab induced EGFR, Akt, and mTOR phosphorylation, which was well correlated with HNSCC and NSCLC cells’ resistance to cixutumumab treatment. Hence, we sought to identify the pathways involved in the activation of the EGFR pathway in HNSCC and NSCLC cells by cixutumumab treatment.
Resistance to anticancer drugs has been associated with genetic alterations, quantitative protein changes, truncation, posttranslational modification(s), and subcellular localization of selected proteins (28,29). For example, EGFR T790M mutation, c-MET and K-Ras gene amplification, loss of PTEN expression, and c-MET expression and phosphorylation have been suggested to cause resistance to TKIs of EGFR or MET (30–33). However, activation mutation and amplification of IGF-1R have not been reported, and we observed no detectable changes in IGF-1R mRNA levels after drug treatment. Our in vitro kinetic study show that cixutumumab treatment induced initial activation of the Akt/mTOR pathway followed by increase in EGFR, Akt1, and survivin protein levels and EGFR phosphorylation in drug-resistant cells. The induced activation of the Akt/mTOR pathway appeared to increase survivin expression in cixutumumab-resistant cells. The Akt/mTOR pathway plays a major role in regulating the translation of mRNA subsets, many of which encode for proteins involved in cell proliferation, growth, and angiogenesis (34). We previously demonstrated that treatment with EGFR TKIs results in mTOR-mediated de novo synthesis of EGFR and survivin proteins, protecting NSCLC cells from EGFR TKIs’ anti-proliferative effects (12). It is plausible that cixutumumab-induced increase in Akt/mTOR activities could have contributed to resistance to the drug through increased expression of EGFR signaling components and anti-apoptotic protein, compensating for loss of the IGF-1R pathway. Indeed, blocking mTOR activity suppressed synthesis of these proteins and restored cixutumumab’s apoptotic activity in cixutumumab-resistant HNSCC cells both in vitro and in vivo. These findings suggest that the ability of HNSCC and NSCLC cells to resist EGFR- and IGF-1R-targeting agents and adapt to a stressful environment is at least in part from their capacity to stimulate mTOR-mediated protein synthesis involved in cell proliferation and survival. In this study, we did not determine the mechanism by which cixutumumab treatment induces initial activation of the Akt/mTOR pathway. Given that the insulin receptor (IR) has been implicated in acquired resistance to anti-IGF-1R therapeutic agents, IR signaling may be one such pathway. In cell cultures, IR downregulation suppressed cancer cell proliferation and metastasis and reversed cixutumumab resistance, and inhibition of IR’s function was required for cixutumumab’s anti-tumor activity in a mouse neuroendocrine tumor model (35,36). Active investigations are underway to determine whether activation of IR signaling or other pathways are involved in cixutumumab-mediated initial activation of the Akt/mTOR pathway.
Although additional mechanisms underlying activation of EGFR signaling by cixutumumab should be explored (such as whether upregulation of ligand, availability of adaptor proteins, or changes in EGFR confirmation with other EGFR family members), our in vitro and in vivo results provide a mechanistic model in which cixutumumab stimulates PI3K/Akt, resulting in mTOR-mediated de novo protein expression of EGFR and Akt1 proteins. Increased expressions of EGFR and Akt1 could have been involved in stimulation of the EGFR pathway, and induced expression of survivin protein could have protected HNSCC and NSCLC cells from apoptosis. This newly identified resistance mechanism against IGF-1R mAbs could provide new avenues for therapeutic strategy. Firstly, combination regimens of EGFR inhibitors and IGF-1R mAbs may be effective if the IGF-1R-overexpressing tumors have high levels of EGFR. Indeed, inhibition of EGFR activation by treatment with C225, an anti-EGFR mAb, abolished resistance to cixutumumab and induced apoptosis in cixutumumab-resistant cells in vitro and in vivo. Secondly, a combined treatment with mTOR inhibitor seems to benefit IGF-1R mAb–resistant patients. It is well known that mTOR inhibition activates PI3-K/Akt by up-regulating IGF-1R signaling, and therapeutic inhibition of the IGF-1R pathway as a strategy to overcome resistance to mTOR inhibitor has been suggested in a variety of cancers, including HNSCC (37,38), in which mTOR overexpression has been observed (39). Although the rationale for co-targeting mTOR and IGF-1R/Akt is different, the previous findings and our current results support the hypothesis that combination regimens of mTOR and IGF-1R inhibitors could be better therapeutically for the treatment of IGF-1R-overexpressing tumors with high levels of mTOR. In light of this notion, we found that combined treatment with cixutumumab and rapamycin suppressed EGFR, Akt and survivin expression, decreased proliferative activities, and induced apoptosis in cixutumumab-resistant cells in vitro and in vivo.
In conclusion, we have described for the first time that the Akt/mTOR pathway has a specific role in inducing cell survival against anti-IGF-1R mAb, cixutumumab. Further investigations are warranted to validate mTOR expression as a prognostic marker or predictor of resistance to IGF-1R mAb-based therapy and to determine the detailed mechanism by which cixutumumab mediates Akt/mTOR activation. In addition, clinical trials are needed to determine whether cixutumumab in combination with an mTOR inhibitor would enhance objective response and survival rates in HNSCC patients.
Supplementary Material
Acknowledgments
Financial support: This research was supported by the National Research Foundation of Korea (NRF) grand funded by the Korea government (MEST)(No. 2011-0017639) (H-Y. Lee), National Institutes of Health grant R01 CA100816 (H.-Y. Lee) and P50 CA907007 (S. Lippman).
Footnotes
Conflicts of Interest: The authors declare no conflict of interest.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Chin D, Boyle GM, Porceddu S, Theile DR, Parsons PG, Coman WB. Head and neck cancer: past, present and future. Expert Rev Anticancer Ther. 2006;6:1111–8. doi: 10.1586/14737140.6.7.1111. [DOI] [PubMed] [Google Scholar]
- 3.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 4.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–18. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 5.Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev. 2000;21:215–44. doi: 10.1210/edrv.21.3.0399. [DOI] [PubMed] [Google Scholar]
- 6.Yu H, Spitz MR, Mistry J, Gu J, Hong WK, Wu X. Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J Natl Cancer Inst. 1999;91:151–6. doi: 10.1093/jnci/91.2.151. [DOI] [PubMed] [Google Scholar]
- 7.Barnes CJ, Ohshiro K, Rayala SK, El-Naggar AK, Kumar R. Insulin-like Growth Factor Receptor as a Therapeutic Target in Head and Neck Cancer. Clin Cancer Res. 2007;13:4291–9. doi: 10.1158/1078-0432.CCR-06-2040. [DOI] [PubMed] [Google Scholar]
- 8.Zhan S, Shapiro D, Zhang L, Hirschfeld S, Elassal J, Helman LJ. Concordant loss of imprinting of the human insulin-like growth factor II gene promoters in cancer. J Biol Chem. 1995;270:27983–6. doi: 10.1074/jbc.270.47.27983. [DOI] [PubMed] [Google Scholar]
- 9.Jamieson TA, Brizel DM, Killian JK, Oka Y, Jang HS, Fu X, et al. M6P/IGF2R loss of heterozygosity in head and neck cancer associated with poor patient prognosis. BMC Cancer. 2003;3:4. doi: 10.1186/1471-2407-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahr C, Groner B. The insulin like growth factor-1 receptor (IGF-1R) as a drug target: novel approaches to cancer therapy. Growth Horm IGF Res. 2004;14:287–95. doi: 10.1016/j.ghir.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Adams TE, McKern NM, Ward CW. Signalling by the type 1 insulin-like growth factor receptor: interplay with the epidermal growth factor receptor. Growth Factors. 2004;22:89–95. doi: 10.1080/08977190410001700998. [DOI] [PubMed] [Google Scholar]
- 12.Morgillo F, Woo JK, Kim ES, Hong WK, Lee HY. Heterodimerization of insulin-like growth factor receptor/epidermal growth factor receptor and induction of survivin expression counteract the antitumor action of erlotinib. Cancer Res. 2006;66:10100–11. doi: 10.1158/0008-5472.CAN-06-1684. [DOI] [PubMed] [Google Scholar]
- 13.Morgillo F, Kim WY, Kim ES, Ciardiello F, Hong WK, Lee HY. Implication of the insulin-like growth factor-IR pathway in the resistance of non-small cell lung cancer cells to treatment with gefitinib. Clin Cancer Res. 2007;13:2795–803. doi: 10.1158/1078-0432.CCR-06-2077. [DOI] [PubMed] [Google Scholar]
- 14.Rowinsky EK, Youssoufian H, Tonra JR, Solomon P, Burtrum D, Ludwig DL. IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor. Clin Cancer Res. 2007;13:5549s–55s. doi: 10.1158/1078-0432.CCR-07-1109. [DOI] [PubMed] [Google Scholar]
- 15.Fukazawa H, Mizuno S, Uehara Y. A microplate assay for quantitation of anchorage-independent growth of transformed cells. Anal Biochem. 1995;228:83–90. doi: 10.1006/abio.1995.1318. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein D, Bushmeyer SM, Witt PL, Jordan VC, Borden EC. Effects of type I and II interferons on cultured human breast cells: interaction with estrogen receptors and with tamoxifen. Cancer Res. 1989;49:2698–702. [PubMed] [Google Scholar]
- 17.Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–40. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Mizushima H, Wang X, Miyamoto S, Mekada E. Integrin signal masks growth-promotion activity of HB-EGF in monolayer cell cultures. J Cell Sci. 2009;122:4277–86. doi: 10.1242/jcs.054551. [DOI] [PubMed] [Google Scholar]
- 19.Pickl M, Ries CH. Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene. 2009;28:461–8. doi: 10.1038/onc.2008.394. [DOI] [PubMed] [Google Scholar]
- 20.Hirschhaeuser F, Leidig T, Rodday B, Lindemann C, Mueller-Klieser W. Test system for trifunctional antibodies in 3D MCTS culture. J Biomol Screen. 2009;14:980–90. doi: 10.1177/1087057109341766. [DOI] [PubMed] [Google Scholar]
- 21.Altieri DC. Survivin in apoptosis control and cell cycle regulation in cancer. Prog Cell Cycle Res. 2003;5:447–52. [PubMed] [Google Scholar]
- 22.Choo AY, Blenis J. Not all substrates are treated equally: Implications for mTOR, rapamycin-resistance and cancer therapy. Cell Cycle. 2009;8:567–72. doi: 10.4161/cc.8.4.7659. [DOI] [PubMed] [Google Scholar]
- 23.Chiang GG, Abraham RT. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem. 2005;280:25485–90. doi: 10.1074/jbc.M501707200. [DOI] [PubMed] [Google Scholar]
- 24.Allen G, Armstrong E, Modhia F, Ludwig D, Hicklin D, Harari P. Inhibition of insulin-like growth factor-1 receptor signaling impairs proliferation of head and neck, lung, prostate and breast cancer cells. Proc Amer Assoc Cancer Res. 2005;46:5041. [Google Scholar]
- 25.Gualberto A, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions. Oncogene. 2009;28:3009–3021. doi: 10.1038/onc.2009.172. [DOI] [PubMed] [Google Scholar]
- 26.Reidy DL, Vakiani E, Fakih MG, Sanif MW, Hecht JR, Goodman-Davis N, et al. Randomized, phase II study of the insulin-like growth factor-1 receptor inhibitor IMC-A12, with or without cetuximab, in patients with cetuximab- or panitumumab-refractory metastatic colorectal cancer. J Clin Oncol. 2010;28:4240–4246. doi: 10.1200/JCO.2010.30.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buck E, Eyzaguirre A, Rosenfeld-Franklin M, Thomson S, Mulvihill M, Barr S, et al. Feedback mechanisms promote cooperativity for small molecule inhibitors of epidermal and insulin-like growth factor receptors. Cancer Res. 2008;68:8322–32. doi: 10.1158/0008-5472.CAN-07-6720. [DOI] [PubMed] [Google Scholar]
- 28.Janne PA, Gray N, Settleman J. Factors underlying sensitivity of cancers to small-molecule kinase inhibitors. Nat Rev Drug Discov. 2009;8:709–23. doi: 10.1038/nrd2871. [DOI] [PubMed] [Google Scholar]
- 29.Onitsuka T, Uramoto H, Nose N, Takenoyama M, Hanagiri T, Sugio K, et al. Acquired resistance to gefitinib: the contribution of mechanisms other than the T790M, MET, and HGF status. Lung Cancer. 2010;68:198–203. doi: 10.1016/j.lungcan.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Bianco R, Shin I, Ritter CA, Yakes FM, Basso A, Rosen N, et al. Loss of PTEN/MMAC1/TEP in EGF receptor-expressing tumor cells counteracts the antitumor action of EGFR tyrosine kinase inhibitors. Oncogene. 2003;22:2812–22. doi: 10.1038/sj.onc.1206388. [DOI] [PubMed] [Google Scholar]
- 31.Cepero V, Sierra JR, Corso S, Ghiso E, Casorzo L, Perera T, et al. MET and KRAS gene amplification mediates acquired resistance to MET tyrosine kinase inhibitors. Cancer Res. 2010;70:7580–90. doi: 10.1158/0008-5472.CAN-10-0436. [DOI] [PubMed] [Google Scholar]
- 32.Skalnikova H, Martinkova J, Hrabakova R, Halada P, Dziechciarkova M, Hajduch M, et al. Cancer drug-resistance and a look at specific proteins: Rho GDP-Dissociation Inhibitor 2, Y-Box Binding Protein 1 and HSP70/90 Organising Protein in proteomics clinical application. J Proteome Res. 2011;10:404–15. doi: 10.1021/pr100468w. [DOI] [PubMed] [Google Scholar]
- 33.Benedettini E, Sholl LM, Peyton M, Reilly J, Ware C, Davis L, et al. Met activation in non-small cell lung cancer is associated with de novo resistance to EGFR inhibitors and the development of brain metastasis. Am J Pathol. 2010;177:415–23. doi: 10.2353/ajpath.2010.090863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao JS, Gondi C, Chetty C, Chittivelu S, Joseph PA, Lakka SS. Inhibition of invasion, angiogenesis, tumor growth, and metastasis by adenovirus-mediated transfer of antisense uPAR and MMP-9 in non-small cell lung cancer cells. Mol Cancer Ther. 2005;4:1399–408. doi: 10.1158/1535-7163.MCT-05-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunn SE, Ehrlich M, Sharp NJ, Reiss K, Solomon G, Hawkins R, et al. A dominant negative mutant of the insulin-like growth factor-I receptor inhibits the adhesion, invasion, and metastasis of breast cancer. Cancer Res. 1998;58:3353–61. [PubMed] [Google Scholar]
- 36.Sachdev D, Singh R, Fujita-Yamaguchi Y, Yee D. Down-regulation of insulin receptor by antibodies against the type I insulin-like growth factor receptor: implications for anti-insulin-like growth factor therapy in breast cancer. Cancer Res. 2006;66:2391–402. doi: 10.1158/0008-5472.CAN-05-3126. [DOI] [PubMed] [Google Scholar]
- 37.Clark C, Shah S, Herman-Ferdinandez L, Ekshyyan O, Abreo F, Rong X, et al. Teasing out the best molecular marker in the AKT/mTOR pathway in head and neck squamous cell cancer patients. Laryngoscope. 2010;120:1159–65. doi: 10.1002/lary.20917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu FY, Zhao ZJ, Li P, Ding X, Zong ZH, Sun CF. Mammalian target of rapamycin (mTOR) is involved in the survival of cells mediated by chemokine receptor 7 through PI3K/Akt in metastatic squamous cell carcinoma of the head and neck. Br J Oral Maxillofac Surg. 2010;48:291–6. doi: 10.1016/j.bjoms.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Stadler ME, Patel MR, Couch ME, Hayes DN. Molecular biology of head and neck cancer: risks and pathways. Hematol Oncol Clin North Am. 2008;22:1099–124. vii.s. doi: 10.1016/j.hoc.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.