Abstract
Age-related adiposity has been linked to chronic inflammatory diseases in late-life. To date, the studies on adipose tissue leukocytes and aging have not taken into account the heterogeneity of adipose tissue macrophages (ATMs), nor have they examined how age impacts other leukocytes such as T cell in fat. Therefore, we have performed a detailed examination of ATM subtypes in young and old mice using state of the art techniques. Our results demonstrate qualitative changes in ATMs with aging that generate a decrease in resident Type 2 (M2) ATMs. The profile of ATMs in old fat shifts towards a pro-inflammatory environment with increased numbers of CD206-CD11c- (double negative) ATMs. The mechanism of this aging-induced shift in the phenotypic profile of ATMs was found to be related to a decrease in PPARγ expression in ATMs and alterations in chemokine/chemokine receptor expression profiles. Furthermore, we have revealed a profound and unexpected expansion of adipose tissue T (ATT) cells in visceral fat with aging that includes a significant induction of regulatory T cells (Tregs) in fat. Our findings demonstrate a unique inflammatory cell signature in the physiologic context of aging adipose tissue that differs from those induced in setting of diet-induced obesity.
Introduction
Aging is increasingly recognized as being associated with a pro-inflammatory state that plays an important role in the development of chronic diseases(1). Thus, although a healthy acute inflammation response is a crucial part of the body's defense against foreign pathogens, chronic low grade inflammation may be detrimental to health. To date, published works have largely focused on correlating serum level of pro-inflammatory cytokines with aging or aging syndromes in cross-sectional analyses. For example, significant positive correlation between human frailty and serum IL-6, C-reactive protein, hemoglobin, T cell CCR5 expression, peripheral blood monocyte number and total white blood cell count have all been reported(2-4). However, the source of the pro-inflammatory cytokines in aging has not been definitively determined.
Advancing age is also accompanied by general obesity (increased total body fat percentage) and in particular central obesity that contributes to a number of important health problems such as insulin resistance, cardiovascular disease, sarcopenia and disability (5-8). White adipose tissue has been proposed to be a key regulator of lifespan. In several model organisms, genetic manipulations that modify fat mass also impact on life expectancy, in part through sirtuin 1 (SIRT1) and suppression of the nuclear receptor protein peroxisome proliferator-activated receptors gamma (PPARγ)(9-11).
While the underlying mechanism for the association between age-related obesity and disease is incompletely understood, adipose tissue inflammation has been shown to be a critical regulator of the systemic inflammatory phenotype in diet-induced and genetic obesity models(12, 13). In this context there is a renewed appreciation that adipose tissue is composed of a diverse cell populations besides adipocytes that contribute to metabolism and adipocyte function. The non-adipocyte cells can be purified from fat after collagenase digestion and are known as the stromal vascular fraction (SVF). The dominant cells in the SVF areleukocytes (e.g. macrophages and lymphocytes) and adipose tissue stromal cells (ATSC) that include preadipocytes and fibroblasts. The coordinated inflammatory response to obesity is complex and involved numerous leukocytes such as T cells, B cells, mast cells, and NKT cells in communication with adipocytes and stromal cells(14-17). Of these adipose tissue leukocytes, adipose tissue macrophages (ATMs) are the most abundant and have been shown to be a critical link between obesity and metabolic dysfunction and disease(18). Based on this role, ATMs have been proposed to play a potential pathogenic role in “inflamm-aging” (19, 20).
ATMs are present in multiple fat depots in lean states and obesity induces both quantitative and qualitative changes in ATMs that impact the inflammatory state in fat(21-23). A resident population of ATMs (Type 2) express multiple markers of an alternative activation state (M2) with anti-inflammatory properties that include CD206, CD301, and increased production of IL-10(21, 23, 24). Maintenance of Type 2 ATMs is dependent on local IL-4 production in adipose tissue in lean animals(25) and the activity of lipid nuclear receptors such as PPARγ and PPARδ(26-28). Obesity is associated with the accumulation of a distinct ATM subtype (Type 1) that expresses CD11c and is associated with dead and dying adipocytes(29, 30). These ATMs have a gene expression profile that resemblesa classical or M1 activation state induced by LPS and IFNγ stimulation which may relate to the ability of free fatty acids to activate TLR4(31, 32). At least two other ATM subtypes have been described with a mix of M1 and M2 gene expression profiles (21)and include CD206-CD11c- double negative ATMs (DN; Type 4)which are induced with obesity(33).
Overall, current data suggest that the balance between ATM subtypes regulates the global inflammatory environment in fat. Obesity induces the activation and recruitment of pro-inflammatory ATMs(21, 34) which overwhelm the homeostatic function of the resident Type 2 ATMs. Impairment of M2 activation tilts the balance towards a pro-inflammatory environment that can directly negatively influence nutrient metabolism(25, 35). Such heterogeneity in ATMs has been observed in human obesity as well(22) and the balance of ATMs is shifted away from inflammatory ATMs by weight loss(36).
Our understanding of how aging-induced obesity influences adipose tissue leukocytes is incomplete. Macrophage content in subcutaneous fat increases with age in humans (37), but there is limited data on the inflammatory events in visceral adipose tissue ATMs which may drive much of the systemic changes in inflammatory mediators with age (38). In rat models, there is evidence of depot specific changes in ATMs (39) and an increase in ATM content with age (40). In mice, Wu and colleagues (41)showed that total ATM content was not altered with age although inflammatory cytokine expression was different between ATMs from young and old mice. Despite this, they argued that adipocytes, and not adipose tissue stromal cells, had the most profound alterations in inflammatory cytokine expression with age.
Given the interest in the intersection between aging, inflammation, and adipose tissue, we investigated the hypothesis that aging would lead to qualitative changes in ATMs that would resemble those seen in obesity models. Using mouse models of aging, we performed a comprehensive evaluation of ATM subsets, distribution, and inflammatory function in visceral adipose tissue. These studies demonstrate that while there are few quantitative changes in ATMs in visceral fat with age, there are significant qualitative changes in the ratio of ATM subtypes, chemokine expression, and chemokine receptor expression consistent with a pro-inflammatory environment in old fat. The mechanisms behind these changes are related to an age dependent down-regulation of PPARγ expression in ATMs that bias the system away from an M2 profile. Furthermore, we observed that adipose tissue T cells are significantly and specifically induced in visceral fat with aging which may influence ATM phenotypes. Overall, our studies suggest a complex regulation of adipose tissue leukocytes with age that has unique features compared to the events that occur with obesity.
Materials and Methods
Mice
Young (3–4 mo) and old(18–22mo) C57BL/6mice were obtained from the National Institute on Aging aged rodent colonies through Harlan Sprague Dawley(Indianapolis, IN). All mice were maintained in a pathogen-free environment provided by the Unit for Laboratory Animal Medicine at the University of Michigan(Ann Arbor, MI) until they were used. All the experimental research in the current study has been approved by the University of Michigan University Committee on Use and Care of Animals (UCUCA).
Isolation of adipose tissue cell fractions
Careful inspection was done to exclude aged animals with cancer orlymphoma. Mice were euthanized by CO2 asphyxiation or cervical dislocation. Visceral fat from mice was excised under sterile conditions and gonadal/epididymal, mesenteric, and renal fat depots weighed as a group to determine visceral fat weight. Epididymal adipose tissue was then fractionated into adipocyte and stromal vascular fraction (SVF)as described (24).For chemokine studies, epididymal adipose tissue from several mice was pooled together (7-10 mice for young and 3-5 for old), weighed and minced into small pieces. For flow cytometry analysis, individual mice were assessed (n=5) as independent data points. Three adipose tissue cell populations: adipocytes, adipose tissue macrophages (ATM) and CD11b-adipose tissue stromal cells(ATSC)were purified. CD11b+ ATMs were positively selected from the total SVF using the MACS Microbeadstechnology (MiltenyiBiotec, Bergisch-Gladbach, Germany) according to the manufacturer's instructions. Briefly, the macrophages were magnetically labeled with the CD11b Microbeads (10 μl/107 total cells) and passed through the MS+ separation column while placed in the magnetic field of a MidiMACS separator (MiltenyiBiotec). The columns were then washed 3 times with 1ml MACS buffer and the CD11b-ATSCs were collected. The CD11b+ cells were removed from the column by washing two times with 2 ml of MACS buffer away from the magnetic field. Purity of the isolated cells was determined by staining with the FITC-conjugated anti-CD11b and PE-conjugated anti-F4/80 (eBioscience, San Diego, CA) and was consistently between 94–99%.
Isolation of splenic monocytes
Spleens were removed from young and old mice. A single cell suspension was prepared by passing the spleens through a 40μm Cell Strainer filter (BD Falcon). CD11b+ monocytes were then isolated by the MACS Microbeadstechnology (MiltenyiBiotec, Bergisch-Gladbach, Germany) according to the manufacturer's instructions. CD11b+ cells were positively selected using CD11b+Microbeads. Purity of the isolated cells was determined by staining with the FITC-conjugated anti-CD11b and PE-conjugated anti-CD14, and the cells were confirmed to be greater than 90% CD11b and CD14 double positive.
RNA extraction and real-time RT-PCR
ATMs, the ATSC(CD11b-) and splenic monocytes were placed directly in RNAlysate buffer and RNA was extracted using the RNeasy kit (Qiagen). The adipocytes were added to the DIAzollysis reagent (Qiagen) and RNA was extracted using RNeasy Lipid Tissue Midi Kit (Qiagen). The following PCR primers were designed using the online software Primer3 (v. 0.4.0)(42), CCR1: 5′-AGG GCC CGA ACT GTT ACT TT-3′(forward), 5′-TAT AAG CCA GGC ATG GAA GC-3′(reverse); CCR2: 5′-AGG CTC ATC TTT GCC ATC AT-3′(forward), 5′-AAG GAT TCC TGG AAG GTG GT-3′(reverse); CCR3: 5′-GGA ACA CAC TCT CCT CAT AAT GC (forward), 5′-CAC CTG GAC TTC TCA ATA CAG ATG-3′ (reverse); CCR4: 5′-GCT GCC CAC ATC CAC TTA TT-3′(forward), 5′-CAT TAA CTT GGG GCA GGA AA-3′(reverse); CCR5: 5′-TAG ATG AGG GCT GTT TCC ATA G-3′(forward), 5′-CTT CCA GAG ATG ATG ACT GCT AAG-3′ (reverse); CCR7: 5′-GAC GGA TAC CTA CCT GCT CAA C-3′(forward), 5′-TGC CAA AGA TGC CCT TAC AC-3′ (reverse); CCR8: 5′-TTC CTG CCT CGA TGG ATT AC-3′(forward), 5′-GAG GAG GAA CTC TGC GTC AC-3′(reverse), CCR9: 5′-TGG TCA ATG GAT GTT CCA GA-3′(forward), 5′-TGC ACA TGA TGA GAA GCA CA-3′(reverse); CXCR2: 5′-AGC AGA GGA TGG CCT AGT CA-3′(forward), 5′-TCC ACC TAC TCC CAT TCC TG-3′(reverse); CXCR3: 5′-CCA TGC CCT ATC TTG CTG TT-3′(forward), 5′-ACA CAG GGA TGG CTG AGT TC-3′(reverse); CXCR4: 5′-GAA ACT GCT GGC TGA AAA GG-3′(forward), 5′-CTG TCA TCC CCC TGA CTG AT-3′(reverse), CXCR5: 5′-CCA AGC AGA AAG CTG AAA CC-3′(forward), 5′-CTT CTG GAA CTT GCC CTC AG-3′(reverse), CX3CR1: 5′-GTG GTG CCT TCA TCC ATT CT-3′(forward), 5′-CCA GCT CCA TTT CTC AGA GG-3′(reverse);MIP-1α: 5′-GTG TAG AGC AGG GGC TTG AG-3′ (forward), 5′-AGA GTC CCT CGA TGT GGC TA-3′(reverse); MIP-1β: 5′-GAG CCC TGG GTC ACT GAG TA-3′ (forward), 5′-GAG GAG GCC TCT CCT GAA GT-3′(reverse); PPAR-γ: 5′-GAT GGA AGA CCA CTC GCA TT-3′(forward), 5′-AAC CAT TGG GTC AGC TCT TG-3′(reverse); guanine nucleotide binding protein (G protein), beta polypeptide 2 like 1 (GNB2L1):5′-GGACAAGCTGGTCAAGGTGT-3′ (forward), 5′-GGGATCCATCTGGAGAGACA-3′ (reverse). Real time PCR was performed using the QuantiTect SYBR Green RT-PCR kit (Qiagen) according to the manufacture's protocol. Relative expression of inflammatory cytokine/chmokine and PPAR-γ was assessed and normalized with mouse house keeping gene GNB2L1.
FACS staining and flow cytometry
Before antibody staining, all the cells were incubated with Mouse BD Fc Block™ (BD PharMingen, San Diego, CA). To detect macrophage infiltration in adipose tissue, total SVF cells were double-stained with macrophage specific PE-conjugatedanti-mouse F4/80 (eBioscience) and FITC-conjugated anti-mouse CD11b (e-Bioscience) or the appropriate isotype controls. To detect CCR5 chemokine receptor cell surface expression, total SVF cells were double-stained with PE-conjugatedanti-mouse CCR5 (e-bioscience) and FITC-conjugated anti-mouse F4/80(e-bioscience). Labeled cells were then washed twice with FACS buffer (1% bovine serum albumin and0.1% sodium azide in 1×PBS), fixed in 1% paraformaldehyde in PBS, and analyzed for fluorescence on a FACSCaliburor Canto II (Becton Dickinson, San Jose, CA). Intracellular staining was performed after fixation and permeabilization (Becton Dickinson, San Jose, CA). Data analyses were done based on examination of 30,000 cells per sample and performed using FCS express (De novo software) or Weasel (Walter and Eliza Hall Institute of Medical Research). The following antibodies were used for flow cytometry for ATM subset and ATT cell analysis: Anti-CD3 APC-Alexa780, Anti-CD4 PE-Cy7, Anti-CD8 FITC, Anti-FoxP3 APC, Anti-CD11c PE-Cy7, Anti-CD11b APC-Alexa780, Anti-F4/80 PE, Anti-7/4 PE, Anti-CD115 APC, Anti-Ly6c PerCP5.5, and Anti-CD206 Alexa647 (All antibodies were from eBioscience except Anti-CD206 from AbdSerotec).
Adipose tissue cells and cytokine production
Adipose tissue from young and old mice was fractioned into adipocytes, ATSCs(CD11b-) and ATMs (CD11b+) as stated above. ATMs and ATSCs(CD11b-) were plated into24-well plates at the density of 1.0 × 106 cells/well and incubatedat 37°C, 5% CO2 for 24 hours. Equal volume of young or old adipocytes (200 μl) were cultured in 1 ml DMEM plus 10% FBS in 24 well plates separately for 24 hours. For ATMs, the supernatant were directly collected after 24 hours; for SVF, the cell suspensions were collected after 24 hours and centrifugedat 360 × g at 4°C for 5 min to pellet the floating cells and the supernatants were collected. For adipocytes, the culture media below the floating cells were collected. All the supernatants were stored at −80°C until use. The supernatants were simultaneously assayed for IL-6, IL-10, IL12-p70, MCP-1, TNF-α, and IFN-γ cytokines using the mouse inflammatory Cytometric BeadArray (CBA) kit (BD Biosciences), following the manufacturer' sinstructions. This assay kit provides a mixture of six microbeads with distinct fluorescence intensity that are precoated with capturing antibodies specific for the inflammatory proteins. When the beads were incubated with the corresponding PE-conjugated detection antibodies and the test sample, sandwich complexes were formed. The fluorescence produced by the beads was measured using a FACSCalibur flow cytometer.
Incubation of peritoneal macrophages with conditioned medium (CM)
To obtain peritoneal macrophages, young and old mice were euthanized by CO2 asphyxiation and peritoneal cells were immediately collected by injecting and washing the peritoneal cavity with 2 × 5ml ice-cold PBS. The peritoneal cells were spun down, washed twice with DMEM containing 10% FBS and resuspended in DMEM with 10% FBS. The cells were then seededon 96-well plates at a density of 0.3 × 106 cells/well, and incubated for2 h at 37°C/5% CO2. Floating cells were removed by gentle washing with DMEM + 10% FBS, the adherent cells are peritoneal macrophages. ATM-CM was made by culturing 1×106 freshly isolated young and old ATMs for 24 hours at 37°C, 5% CO2 and the supernatants were obtained as ATM-CM. ATSC-CM was made by culturing 1×106 freshly isolated young and old ATSC for 24 hours at 37°C, 5% CO2 respectively and the supernatants were obtained as ATSC-CM. The CM were stored at −80°C until use. ATM-CM and ATSC-CM from young or old mice was then added to the young or old peritoneal macrophages and incubated for additional 24 h. The culture media were used for analysis of IL-6, TNF-α, MCP-1, IL-12, and IL-10 by mouse inflammatory CBA kit.
Treatment of old mice with PPAR-γ specific agonist Rosiglitazone
Old mice were injected i.p. with 2mg/kg/day with Rosiglitazone (potassium salt, Cayman Chemical) or vehicle (sterile ddH2O) for 2 weeks and ATMs were harvested from the mice. RNAs were extracted from the isolated ATMs and real time PCR performed to compare cellular markers between Rosiglitazone- and vehicle-treated groups.
Immunofluorescence Microscopy
Immunofluorescence microscopy was performed as described (30). Antibodies included Anti-MGL1, Anti-CD4 with species specific secondary antibodies (Jackson Immuno Research Inc.). Alexa568 conjugated Isolectin (Molecular Probes) was used to highlight blood vessels. FALC and adipocyte sizing was performed using ImageJ software.
Statistical analysis
Results are expressed as means ± standard error of the mean (SEM). Statistical analyses were performed using Student's t test, and p<0.05 was considered to be statistically significant.
Results
The non-adipocyte micro environment contributes to the age-related adipose tissue inflammation
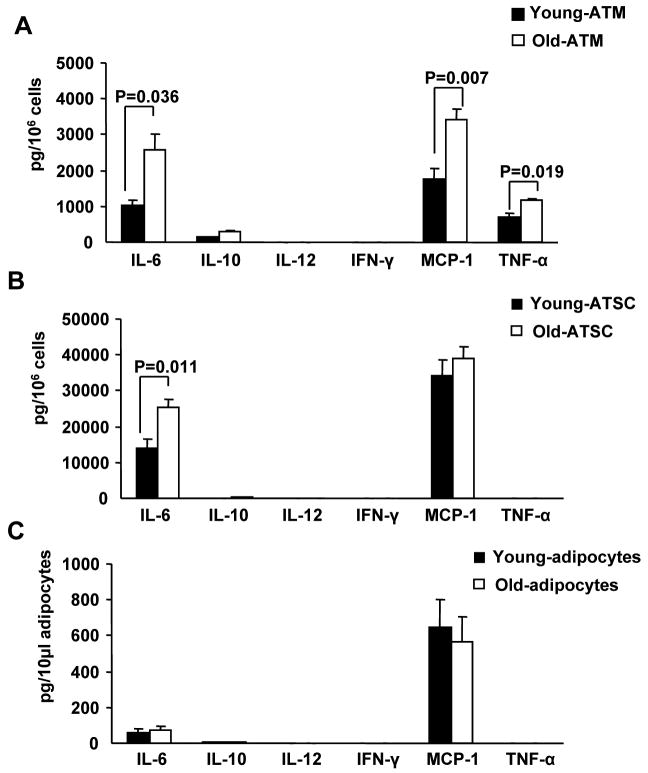
In obesity, the majority of pro-inflammatory mediators are produced by the SVF, but it is unclear if this is also true in aging. To examine this directly, the mouse inflammatory CBA assay was used to quantify inflammatory cytokines produced by ATMs (CD11b+), non-macrophage adipose tissue stromal cells (ATSC; CD11b-) and adipocytes (Figure 1). Comparing the different adipose tissue cell types, ATMs were found to be the dominant source for TNF-α and the source for about 10% of IL-6 production (Figure 1A), while ATSC are the primary source for IL-6 (representing 90% of the cytokine production) (Figure 1B). With age, there was a significant increase in TNF-α, IL-6 and MCP-1 production in ATMs as well as a significant increase in IL-6 production in ATSC. In contrast, we did not find any significant changes in the cytokines/chemokines production by adipocytes from young and old mice (Figure 1C). These results indicate that, similar to that seen in diet-induced obesity, non-fat cells including ATMs and ATSC, but not adipocytes are the major sources of proinflammatory mediators that promote age-related adipose tissue inflammation. In addition, it suggests that age shifts the inflammatory cytokine production towards aproinflammatoryM1 profile with enhanced TNFα and IL-6 production. IL12 and IFNγ are both part of the commercial CBA kit. Our results show that murine visceral ATMs express very low levels of IL12 and IFNγ, and there is no age difference detected.
Figure 1. Cytokine production by adipose tissue cells.
SVFs and adipocytes from young and old mice were cultured for 24 hours. The conditioned medium was collected to measure cytokine release using the Mouse Inflammatory Cytometric Bead Array (BD Bioscience). Cytokine release from (A) total ATMs (CD11b+), (B) ATSC (CD11b-), and (C) adipocytes. The results represent the mean ± SEM; n=4-6 experiments, representing a total of 15 old and 40 young mice.
Total ATM content is unchanged in old mice
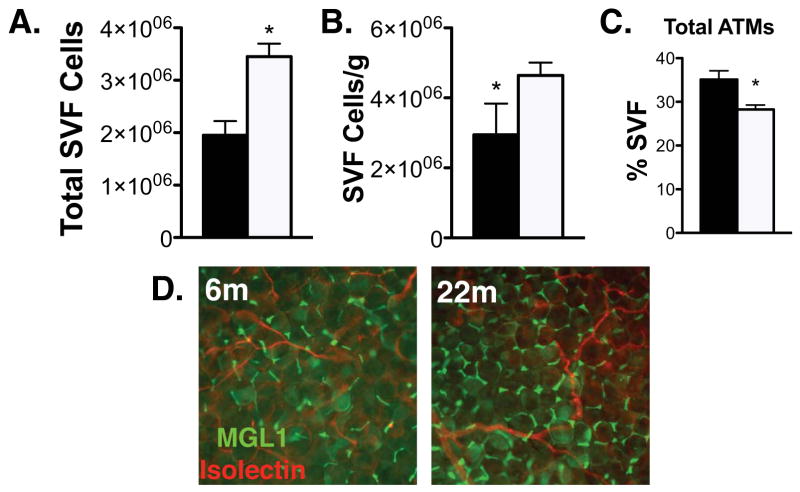
To better assess the macrophage derived changes in adipose tissue with age, we isolated ATMs from the epididymal fat tissue from young and old mice and found that, consistent with other reports, aging is associated with increased total visceral fat content (gonadal, mesenteric, and renal depots) and bodyweight (Table 1). Total number of SVF cells per gonadal fat pad were increased in old mice and persisted even when normalized for fat pad weight. (Figure 2A and 2B). CD11b+F4/80+ ATMs were quantified by flow cytometry and were found to represent a smaller proportion of the SVF in old mice when expressed as a percentage of total cells (Figure 2C). However, when normalized to fat mass, total ATMs content (cells/g) did not change with age in contrast to what is observed with high fat diet and genetic obesity models(23). These results were supported by immunofluorecence microscopy which did not demonstrate any significant qualitative differences in the distribution or density of ATMs in fat comparing young and old mice (Figure 2D).
Table 1. Old mice have greater amount of visceral fat.
p<0.01 vs. young mice; the results represent the mean ± SEM; N = 5 experiment.
Figure 2. Analysis of ATM content in old and young mice.
(A) Quantitation of total SVF cells isolated from epididymal fat pads from 6m (black bars)and 22m old (white bars)C57 mice. (B) SVF cells normalized to fat pad mass. (C) ATMs (F4/80+CD11b+) were quantified by flow cytometry. (D) Immunofluorescence localization of resident Type 2 ATMs (MGL1+; green) in young and old mice. Vasculature labeled with isolectin (red). The results represent the mean ± SEM; n=3-5 experiments, representing a total of 12 old and 35 young mice.*p-value<0.05.
Aging alters the balance of ATM subsets towards a pro-inflammatory (M1) macrophage profile
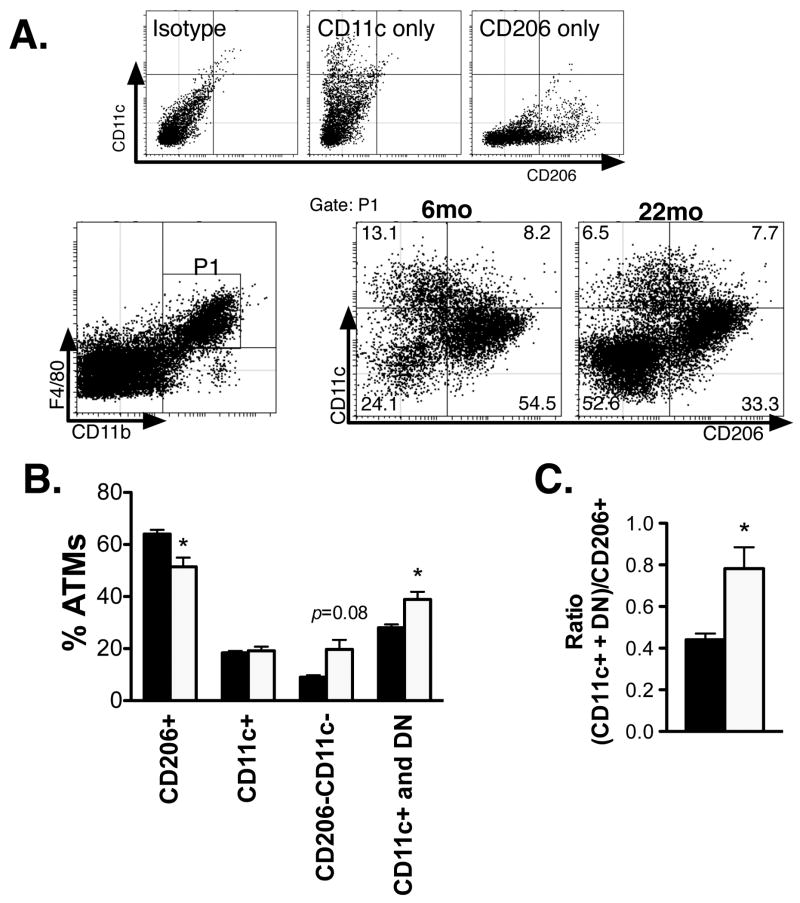
Since ATMs exist in many distinct subtypes with differential surface marker expression, we next proceeded to examine the possibility that the proportion of ATM subtypes may change with age independent of differences in total ATM content. We were able to delineate three main subsets of CD11b+F4/80+ ATMs in epididymal fat pads from young and old male mice that parallel prior studies: resident Type 2 ATMs (CD206+CD11c-), inflammatory Type 1 ATMs (CD206-CD11c+), and double negative ATMs(DN; CD206-CD11c-). When the proportion of these ATM subsets were enumerated, there was a significant decrease in the percentage of Type 2 ATMs with age, no change in Type 1 ATMs, and a trend towards an increase in DN ATMs with age (Figure 3A and 3B). The appearance of DN ATMs was substantial in some old mice and was surprising as these ATMs are typically induced with obesity and express pro-inflammatory genes such as Ccr2, Ccr5, and Il1b(33).
Figure 3. Alterations in ATM subtypes with age.
(A) Flow cytometry delineation of ATM subtypes. Definition of control gates is shown (upper panel). After gating for ATMs (F4/80+CD11b+; P1), subtypes were differentiated by CD11c and CD206 staining in young and old mice (representative plots shown in lower panel). (B) ATM subtypes quantified as a percentage of the total ATM population in 6mo (black bars) and 22mo mice (white bars). (C) Ratio of CD11c+ and DN ATMs relative to resident CD206+ ATMs. *p-value<0.05.
With age, the ratio of pro-inflammatory (Type 1 and DN ATMs) to resident Type 2 (M2) ATMs was significantly increased demonstratingage-induced qualitative changes in ATMs that are similar to the proportional changes in ATM subtypes seen in diet-induced obesity (Figure 3C). This data supported the cytokine data (Figure 1)and suggests that aging generates a shift in the properties of ATMs towards an M1 profile due to the accumulation of DN ATMs and a loss of resident Type 2 (M2 polarized) ATMs. This altered ratio occurred in the absence of any significant changes in the distribution or quantity of crown-like structures (Figure 2D) that are hallmarks of high fat diet induced obesity.
Paracrine signals from old ATM and ATSC are sufficient to induce the M1 macrophage polarization
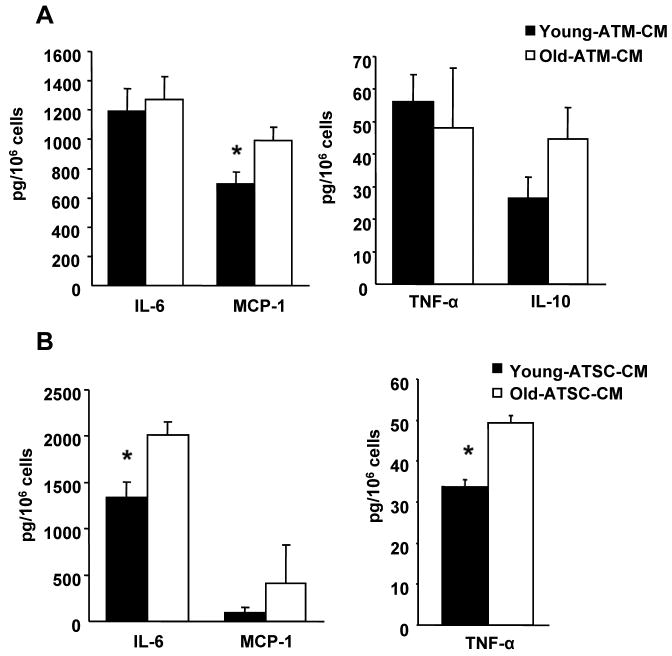
Since ATMs and ATSC from old animals have significantly increased production of M1 promoting cytokines, we next determined if these soluble factors are capable of altering macrophage phenotypes via paracrine action. To test this, we incubated peritoneal macrophages (PM) from young and old mice with conditioned media (CM) from ATMs and ATSC and found that ATM-CM from old animals induced significantly greater CCL2(MCP-1) production in PM compared to ATM-CM from young mice (Figure A). Additionally, ATSC-CM induced higher IL-6 and TNF-α production in PM (Figure 4B), indicating that paracrine signals derived from both ATMs and ATSC are capable of influencing the polarization state of ATMs.
Figure 4. Paracrine signals from ATM and ATSC.
Conditioned medium from young and old ATM (A) and ATSC (CD11b-) (B) was incubated with peritoneal macrophages for 24 hours and the cytokines released were measured by CBA. The results represent the mean ± SEM; n=4 experiments, representing a total of 20 old and 28 young mice.
Increase in selected chemokine and chemokine receptor gene expression in ATMs
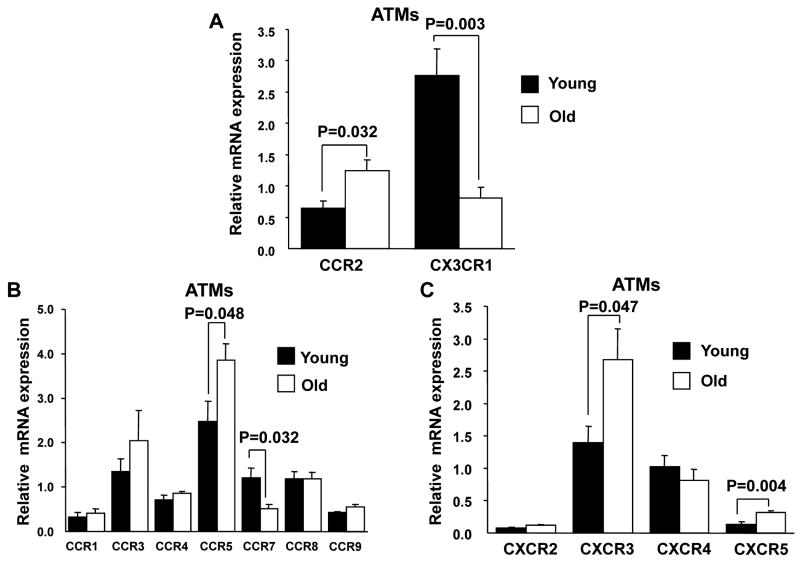
Multiple studies have identified a critical role of the CCR2/CCL2 chemokine axis in the recruitment of inflammatory ATMs to fat in obesity(43, 44). The data above suggest that CCR2 dependent chemokine pathways may also be involved the unique pattern of ATM recruitment observed with age. Consistent with this ATMs from aged animals expressed twice the level of CCR2 (Figure 5A)a finding consistent with the increase in DN ATMs which have high CCR2 expression. In contrast, the expression of the fractalkine receptor CX3CR1 in ATMs was decreased which differs from data demonstrating an induction of CX3CR1 in adipose tissue with obesity (45). ATMs from old mice also express greater amount of CCR5, CXCR3 and CXCR5, but lower amount of CCR7 (Figure 5B and 5C). In contrast, young and old splenic monocytes had similar CCR2 and CX3CR1 expression (Supplemental Figure 2) suggesting that these changes are specific to the adipose tissue compartment.
Figure 5. Young and old ATM chemokine receptor expression.
(A) Real time PCR was performed to compare the expression of chemokine receptor CCR2,and CX3CR1expression in young and old ATMs. Other CCR (B) and CXCR (C) chemokine receptors were also examined. The results represent the mean ± SEM; n=4-5 experiments, representing a total of 25 old and 40 young mice.
In diet-induced obesity, the increase in CCR2 expression in fat is related to an increase in the quantity and the trafficking of inflammatory CCR2+Ly6chi monocytes from the circulation(43, 46). Therefore, we hypothesized that the age-induced imbalance between ATM subsets may relate to alterations in the circulating pools of Ly6chi and Ly6clo monocytes. CD115+ monocytes were profiled in the bone marrow and blood of young and old male mice by flow cytometry(Supplemental Figure 1).There was no change in total CD115+ monocytes with age. In addition, no significant alterations in the ratio or quantity of Ly6chi and Ly6clo monocytes were observed within the CD115+ monocyte pool.
PPAR-γ controls alternative activation of adipose tissue macrophage in aging
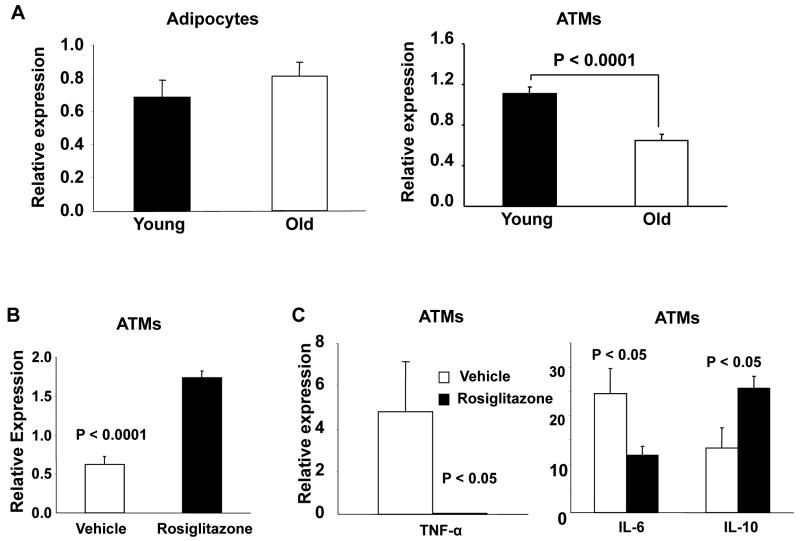
The maintenance of an alternative M2 activation state of Type 2 ATMs is linked to the activity of nuclear receptors important in lipid metabolism that include PPARγ and PPARδ(26, 47). Decreased PPARγ expression has been reported in the aging kidney(48), brain (49)and spleen(50). Since aging led to a suppression of Type 2 (CD206+) ATMs, we examined if the observed changes in ATMs with old age are associated with altered PPAR-γ expression. PPAR-γ expression was significantly decreased in old ATMs (total) compared with those from younger animals (Figure 6A) consistent with the decrease in Type 2 ATMs (CD206+). Treatment with the PPARγ agonist rosiglitazone has been shown to promote Type 2 ATMs in diet-induced obesity in mice (51), but the drug's effect in aging ATMs is unknown. Old mice were treated for 14 days with rosiglitazone and ATMs isolated for gene expression analysis. As expected, rosiglitazone increased PPARγ expression in ATMs from old mice (Figure 6B). Expression of M1 cytokines Tnfa and Il6 were decreased while Il10 expression was increased in isolated ATMs (Figure 6C). These effects were observed to be independent of any changes in body weight or total ATM content with rosiglitazone treatment.
Figure 6. Effects of rosiglitazone on PPARγ gene expression by PCR.
Real time RT-PCR analysis of Pparg expression, relative to the control gene guanine nucleotide binding protein (G protein), beta polypeptide 2 like 1 (GNB2L1). (A) Decreased PPARγ expression is seen in old ATMs but not adipocytes. (B) Short term (14 days) treatment of rosiglitazone increased PPARγ expression in old ATMs. (C) Rosiglitazone treatment increased Il10 and reduced Tnfa and Il6 expression in old ATMs. The results represent the mean ± SEM; n=7-10 experiments, representing a total of 30 old and 49 young mice.
Aging is associated with a significant induction of adipose tissue T (ATT) cells in visceral fat depots
Current models of adipose tissue inflammation suggest that alterations in ATMs are coordinated with alterations in other adipose tissue leukocytes such as T cells. To date, no studies have examined the effect of old age on adipose tissue T (ATT) cells. We hypothesized that old age may modify T cell content in fat and performed an analysis of ATT cells by flow cytometry. Total lymphocytes (CD3+) invisceral (epididymal) fat were increased approximately two fold in old mice even when normalized for fat weight (Figure 7A). This finding was unique to adipose tissue as CD3+ cells were unchanged in the spleen with age (Figure 7D).
Figure 7. – Increase in ATT cells with age.
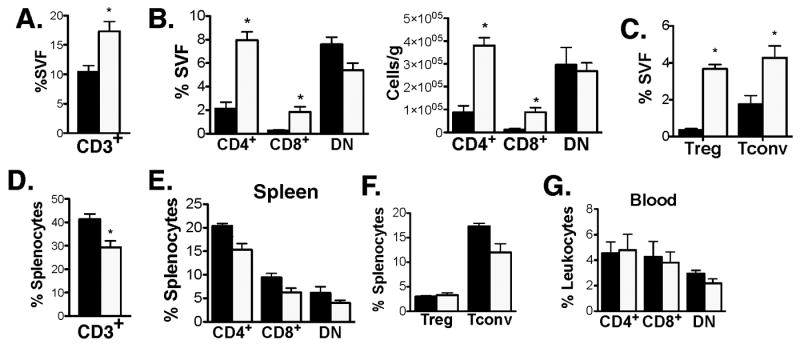
Flow cytometry analysis of ATT cells from SVF of epididymal fat from 6mo (black bars) and 22mo mice (white bars). (A) Percent CD3+ cells in fat. (B) Percentage of T cell subtypes in adipose tissue. Cells were gated on SSClowCD3+ cells in adipose tissue prior to analysis. (C) Quantitation of adipose tissue Tregs. CD3+CD4+FoxP3+ (Treg) and CD3+CD4+FoxP3- (Tconv) were quantitated in young and old mice. (D) Splenic CD3+ cells. (E) T cell subsets and (F)Treg content in spleens of young and old mice. (G) Blood T cell subsets (gated on CD3+ cells). Results represent the mean ± SEM, n=5 per group. *p-value <0.05.
Within the CD3+ ATT cell population, CD4+, CD8+, and double negative (DN; CD4- CD8-) CD3+ cells were quantified (Figure 7B). No change in DN cells were observed however there was a significant increase in the percentage of both CD4+ (4-fold) and CD8+ (7-fold) cells in adipose tissue in old mice. Further analysis of the CD4+ T cell population indicated that adipose tissue Tregs (CD3+CD4+FoxP3+) were increased 11-fold while Tconv (CD3+CD4+FoxP3-) were increased 2-fold with age (Figure 7C). This increase in CD4+ and CD8+ T cells were specific to visceral fat as there was no significant changes in CD4+ and CD8+ cells in the spleen or blood with old age (Figure 7D).
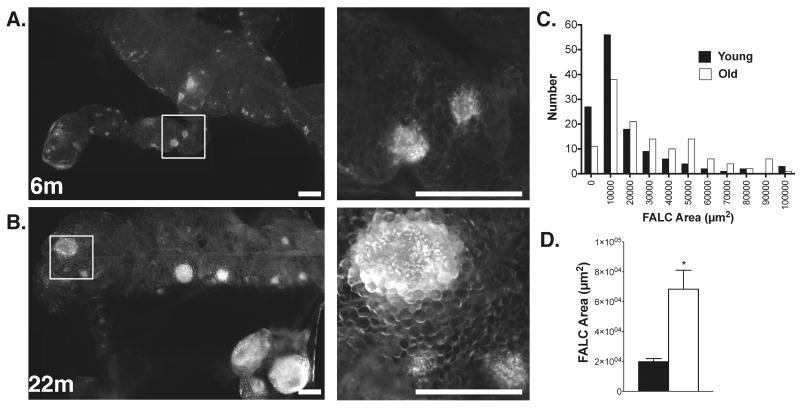
The location of T cells in adipose tissue in non-obese states is unclear. Recently, fat associated lymphoid clusters (FALCs) have been identified as adipose tissue regions that are enriched for lymphocytes in multiple fat depots(52). To examine if the increase in ATT cell was related to alterations in the architecture of FALCs, we examined milky spots in omental fat pads identified byCD4+ T cell content. FALCs were found in young and old mice in similar density in the omental fat pads (Figure 8A and 8B). However, many of the FALCs in old mice were significantly larger than those seen in young mice. Sizing of the FALCs confirmed a shift in the distribution of FALC size in omental fat towards larger CD4+FALCs with old age (Figure 8C and 8D). This indicates that the expansion of CD4+ T cells in adipose tissue with age relates to an increase in the size of FALCs.
Figure 8. – Enlargement of fat associated lymphoid clusters with age.
Omental fat pads were dissected and stained with anti-CD4 antibodies to identify FALCs/milky spots. Images from representative samples from young (A) and old (B) mice. Low (left panels) and high power (right) images are shown. Bar=500μm. Right panels are enlargements of the boxed area. Similar results obtained from 5 independent samples. (C) FALC size frequency distribution in omental fat with age. CD4+ FALCs were sized from 4 mice. (D) FALC size represented as mean +/- SEM. *p-value<0.001.
Discussion
Our data demonstrate that aging induces a unique profile of ATMs and ATT cells in visceral adipose tissue that differs significantly from what is observed with high fat diet induced and genetic models of obesity. The key observations in this study are: 1) that aging induces a decrease in the resident CD206+ ATM population in visceral fat that leads to a decrease in the ratio between these cells and two inflammatory ATM subtypes (CD11c+ and CD206- CD11c-double negative); 2) that non-macrophage stromal cells from old mice are able to activate macrophages via paracrine action; 3) that a decrease in PPARγ expression in ATMs are associated with the change in the balance between inflammatory and non-inflammatory ATMs with age; and 4) that aging is associated with a robust expansion of CD4+ and CD8+ T cells in adipose tissue that correlates with the enlargement of fat associated lymphoid clusters (FALCs).
Compared to the report by Wu and colleagues(41),we saw similar decreases in ATMs as a percentage of the SVF with age without an overall change in ATM numbers per gram due to an increase in the total number of SVF cells with age. In agreement with his study, we found qualitative changes in the inflammatory profile of old ATMs that fall in line with our current knowledge about ATM subtypes derived from obesity models. Interestingly, we have found that aging decreases the proportion of resident CD206+ ATMs. Since CD206 is a marker of alternatively activated M2 macrophages, our data that PPARγ is coordinately downregulated in ATMs with age is consistent with this observation. Current evidence suggests that defining ATMs along a simple M1/M2 dichotomy is imprecise (53)and our data support this as a prominent double negative (DN; CD206- CD11c-) ATM population was identified as increased in old mice. DN ATMs have been shown to be induced with obesity and express genes prominent in both M1 and M2 macrophage activation(33). Importantly, along with Type 1 CD11c+ ATMs, DN ATMs express high levels of CCR2 andCCR5 which matches our observation of the induction of these gene in ATMs with aging. The net result of these changes is an alteration in the balance between resident M2 polarized ATMs and the other subtypes with age biasing against an M2 environment with age. The importance of this balance is well established in obesity-associated adipose tissue inflammation (25) and this is the first evidence that aging can alter ATM biology in a similar manner.
Our findings also suggest a role for the stromal vascular fraction cells in the induction of the inflammatory ATM phenotype in aging. The altered ratio of inflammatory to non-inflammatory ATMs with occurred independent of any significant differences in the number of crown-like structures that are a hallmark of inflamed obese fat although a moderate increase in adipocyte size was observed with age. Further work will have to be done to assess if the properties of these ATMs subtypes defined by surface marker expression are identical to or different from the ATMs induced with high fat diet. Our observations suggest a different architecture of ATMs with age-induced adipocyte hypertrophy.
Given the evidence showing that T cell activation occurs in adipose tissue with high fat diet induced obesity which may alter ATM phenotype(54, 55),we examined the dominant ATT cell populations in visceral fat with age. This led to the striking observation of an age dependent accumulation of CD4+(Tconv and Treg) and CD8+ T cells in visceral fat. Despite this drastic induction, there was only a modest change in ATMs. This does not fully support the model that T cell accumulation is sufficient to drive ATM recruitment to fat. However, it is important to realize that the changes in adipose tissue leukocytes in physiologic contexts (e.g. fasting and aging) are likely to be divergent from those induced with non-physiologic disease states such as obesity. The role that ATT cells play in regulating ATM function in age-associated obesity is currently unclear, and will need to be addressed in future research.
Based on the obesity literature, the massive induction of CD8+ and Tconv (CD4+) cells in fat would be expected to promote adipose tissue inflammation and the accumulation of pro-inflammatory ATMs(54, 55). This may in fact explain some of ATM alterations observed with age. However, we also observed a parallel increase in Tregs in fat with age that would be predicted to suppress pro-inflammatory stimuli and maintain a low inflammation state(56, 57). These findings are consistent with recent reports demonstrating age-related increase in Tregs number and function in central and peripheral T cell compartments (58-60). Expansion of ATT appears to be due to an age-dependent increase in the size of FALCs which are highly specialized structures enriched for T cells, B cells, and macrophages(52). These lymphoid structures are not well characterized but appear to be a site of immune surveillance in the peritoneal cavity(61). Further work will be required to detail the exact mechanism of this expansion and what it may mean for systemic immune challenges especially in the visceral compartment. Our results also highlight the fact that for T cells, adipose tissue represents a unique environment with different regulatory controls compared to secondary lymphoid organs such as the spleen.
We have much to learn and clarify about the function of leukocytes in various setting and that care much be taken to differentiate ATT cell and ATM responses in different contexts. Much of what we know about adipose tissue inflammation is driven by the examination of non-physiologic disease states such as obesity and insulin resistance. It is important to understand that the presence of a resident pool of macrophages and lymphocytes in fat provides the means for innate immune components to sense and respond to metabolic cues in many physiologic contexts. Recent studies have highlighted an important contribution of ATMs to the response to fasting and lipolysis which are critical adipocyte function required to preserve metabolic homeostasis(62). Our study provides crucial details about the physiologic changes induced in fat with aging that have broad implications to many disease states.
Supplementary Material
Footnotes
This work was supported in part by the National Institute of Health grants RO1AG020628 (R.Y.), RO1AG028268 (R.Y.), RO1AR042525 (R.Y.), R01DK090262 (C.N.L), and K08DK078851 (C.N.L), Veteran Affairs Merit Review grant (R.Y.), the Ann Arbor VA GRECC (R.Y.), and funds from the University of Michigan Claude D. Pepper OAIC (NIA P30AG024824) (R.Y.), Nathan Shock Center (NIA AG013283) (R.Y.), and UM-P30 Core Center (NIEHS P30ES017885) (R.Y.).
References
- 1.Desai A, Grolleau-Julius A, Yung R. Leukocyte function in the aging immune system. J Leukocyte Biol. 2010;87:1001–1009. doi: 10.1189/jlb.0809542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Fanis U, Wang GC, Fedarko NS, Walston JD, Casolaro V, Leng SX. T-lymphocytes expressing CC chemokine receptor-5 are increased in frail older adults. J Am Geriatr Soc. 2008;56:904–908. doi: 10.1111/j.1532-5415.2008.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leng SX, Yang H, Walston JD. Decreased cell proliferation and altered cytokine production in frail older adults. Aging Clin Exp Res. 2004;16:249–252. doi: 10.1007/BF03327392. [DOI] [PubMed] [Google Scholar]
- 4.Walston J, Fedarko N, Yang H, Leng S, Beamer B, Espinoza S, Lipton A, Zheng H, Becker K. The physical and biological characterization of a frail mouse model. J Gerontol A Biol Sci Med Sci. 2008;63:391–398. doi: 10.1093/gerona/63.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horber FF, Gruber B, Thomi F, Jensen EX, Jaeger P. Effect of sex and age on bone mass, body composition and fuel metabolism in humans. Nutrition. 1997;13:524–534. doi: 10.1016/s0899-9007(97)00031-2. [DOI] [PubMed] [Google Scholar]
- 6.Heymsfield SB, Gallagher D, Poehlman ET, Wolper C, Nonas K, Nelson D, Wang ZM. Menopausal changes in body composition and energy expenditure. Exp Gerontol. 1994;29:377–389. doi: 10.1016/0531-5565(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 7.Zamboni M, Armellini F, Harris T, Turcato E, Micciolo R, Bergamo-Andreis IA, Bosello O. Effects of age on body fat distribution and cardiovascular risk factors in women. Am J Clin Nutr. 1997;66:111–115. doi: 10.1093/ajcn/66.1.111. [DOI] [PubMed] [Google Scholar]
- 8.Pascot A, Lemieux S, Lemieux I, Prud'homme D, Tremblay A, Bouchard C, Nadeau A, Couillard C, Tchernof A, Bergeron J, Despres JP. Age-related increase in visceral adipose tissue and body fat and the metabolic risk profile of premenopausal women. Diabetes Care. 1999;22:1471–1478. doi: 10.2337/diacare.22.9.1471. [DOI] [PubMed] [Google Scholar]
- 9.Argmann C, Dobrin R, Heikkinen S, Auburtin A, Pouilly L, Cock TA, Koutnikova H, Zhu J, Schadt EE, Auwerx J. Ppargamma2 is a key driver of longevity in the mouse. PLoS Genet. 2009;5:e1000752. doi: 10.1371/journal.pgen.1000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howroyd P, Swanson C, Dunn C, Cattley RC, Corton JC. Decreased longevity and enhancement of age-dependent lesions in mice lacking the nuclear receptor peroxisome proliferator-activated receptor alpha (PPARalpha) Toxicol Pathol. 2004;32:591–599. doi: 10.1080/01926230490515283. [DOI] [PubMed] [Google Scholar]
- 11.Picard F, Guarente L. Molecular links between aging and adipose tissue. Int J Obes (Lond) 2005;29 1:S36–39. doi: 10.1038/sj.ijo.0802912. [DOI] [PubMed] [Google Scholar]
- 12.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 13.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. The Journal of clinical investigation. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lumeng CN, Maillard I, Saltiel AR. T-ing up inflammation in fat. Nat Med. 2009;15:846–847. doi: 10.1038/nm0809-846. [DOI] [PubMed] [Google Scholar]
- 15.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, Doria A, Libby P, Blumberg RS, Kahn BB, Hotamisligil GS, Shi GP. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, Leong HX, Glassford A, Caimol M, Kenkel JA, Tedder TF, McLaughlin T, Miklos DB, Dosch HM, Engleman EG. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 19.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 20.Salvioli S, Capri M, Valensin S, Tieri P, Monti D, Ottaviani E, Franceschi C. Inflamm-aging, cytokines and aging: state of the art, new hypotheses on the role of mitochondria and new perspectives from systems biology. Curr Pharm Des. 2006;12:3161–3171. doi: 10.2174/138161206777947470. [DOI] [PubMed] [Google Scholar]
- 21.Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-Like Remodeling Phenotypes of CD11c+ Adipose Tissue Macrophages During High-Fat Diet-Induced Obesity in Mice. Diabetes. 2010;59:1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, O'Brien PE, Harrison LC. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westcott DJ, Delproposto JB, Geletka LM, Wang T, Singer K, Saltiel AR, Lumeng CN. MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J Exp Med. 2009;206:3143–3156. doi: 10.1084/jem.20091333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, Jouihan H, Morel CR, Heredia JE, Mukundan L, Wu D, Locksley RM, Chawla A. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2010;107:22617–22622. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Eagle AR, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, Lee CH. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saberi M, Woods NB, de Luca C, Schenk S, Lu JC, Bandyopadhyay G, Verma IM, Olefsky JM. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419–429. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeyda M, Gollinger K, Kriehuber E, Kiefer FW, Neuhofer A, Stulnig TM. Newly identified adipose tissue macrophage populations in obesity with distinct chemokine and chemokine receptor expression. Int J Obes (Lond) 2010;34:1684–1694. doi: 10.1038/ijo.2010.103. [DOI] [PubMed] [Google Scholar]
- 34.Wu H, Perrard XD, Wang Q, Perrard JL, Polsani VR, Jones PH, Smith CW, Ballantyne CM. CD11c expression in adipose tissue and blood and its role in diet-induced obesity. Atertio Thromb Vasc Biol. 2010;30:186–192. doi: 10.1161/ATVBAHA.109.198044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan H, Bando JK, Chawla A, Locksley RM. Eosinophils Sustain Adipose Alternatively Activated Macrophages Associated with Glucose Homeostasis. Science. 2011 doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aron-Wisnewsky J, Tordjman J, Poitou C, Darakhshan F, Hugol D, Basdevant A, Aissat A, Guerre-Millo M, Clement K. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab. 2009;94:4619–4623. doi: 10.1210/jc.2009-0925. [DOI] [PubMed] [Google Scholar]
- 37.Ortega Martinez de Victoria E, Xu X, Koska J, Francisco AM, Scalise M, Ferrante AW, Jr, Krakoff J. Macrophage content in subcutaneous adipose tissue: associations with adiposity, age, inflammatory markers, and whole-body insulin action in healthy Pima Indians. Diabetes. 2009;58:385–393. doi: 10.2337/db08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cartier A, Cote M, Lemieux I, Perusse L, Tremblay A, Bouchard C, Despres JP. Age-related differences in inflammatory markers in men: contribution of visceral adiposity. Metabolism. 2009;58:1452–1458. doi: 10.1016/j.metabol.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 39.Jerschow E, Anwar S, Barzilai N, Rosenstreich D. Macrophages Accumulation in Visceral and Subcutaneous Adipose Tissue Correlates with Age. The Journal of allergy and clinical immunology. 2007;119:S179. [Google Scholar]
- 40.Einstein FH, Huffman DM, Fishman S, Jerschow E, Heo HJ, Atzmon G, Schechter C, Barzilai N, Muzumdar RH. Aging per se increases the susceptibility to free fatty acid-induced insulin resistance. The journals of gerontology Series A, Biological sciences and medical sciences. 2010;65:800–808. doi: 10.1093/gerona/glq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill AH, Meydani SN. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol. 2007;179:4829–4839. doi: 10.4049/jimmunol.179.7.4829. [DOI] [PubMed] [Google Scholar]
- 42.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 43.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2005;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah R, Hinkle CC, Ferguson JF, Mehta NN, Li M, Qu L, Lu Y, Putt ME, Ahima RS, Reilly MP. Fractalkine is a novel human adipochemokine associated with type 2 diabetes. Diabetes. 2011;60:1512–1518. doi: 10.2337/db10-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 48.Sung B, Park S, Yu BP, Chung HY. Modulation of PPAR in aging, inflammation, and calorie restriction. J Gerontol A Biol Sci Med Sci. 2004;59:997–1006. doi: 10.1093/gerona/59.10.b997. [DOI] [PubMed] [Google Scholar]
- 49.Sastre M, Dewachter I, Rossner S, Bogdanovic N, Rosen E, Borghgraef P, Evert BO, Dumitrescu-Ozimek L, Thal DR, Landreth G, Walter J, Klockgether T, van Leuven F, Heneka MT. Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proc Natl Acad Sci U S A. 2006;103:443–448. doi: 10.1073/pnas.0503839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gelinas DS, McLaurin J. PPAR-alpha expression inversely correlates with inflammatory cytokines IL-1beta and TNF-alpha in aging rats. Neurochem Res. 2005;30:1369–1375. doi: 10.1007/s11064-005-8341-y. [DOI] [PubMed] [Google Scholar]
- 51.Stienstra R, Duval C, Keshtkar S, van der Laak J, Kersten S, Muller M. Peroxisome proliferator-activated receptor gamma activation promotes infiltration of alternatively activated macrophages into adipose tissue. J Biol Chem. 2008;283:22620–22627. doi: 10.1074/jbc.M710314200. [DOI] [PubMed] [Google Scholar]
- 52.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 53.Morris DL, Singer K, Lumeng CN. Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states. Current Opinion in Clinical Nutrition and Metabolic Care. 2011;14:341–346. doi: 10.1097/MCO.0b013e328347970b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 55.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker D, Endleman E, Winer D, Dosch HM. Normalization of Obesity-Associated Insulin Resistance through Immunotherapy: CD4+ T Cells Control Glucose Homeostasis. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deiuliis J, Shah Z, Shah N, Needleman B, Mikami D, Narula V, Perry K, Hazey J, Kampfrath T, Kollengode M, Sun Q, Satoskar AR, Lumeng C, Moffatt-Bruce S, Rajagopalan S. Visceral adipose inflammation in obesity is associated with critical alterations in tregulatory cell numbers. PLoS One. 2011;6:e16376. doi: 10.1371/journal.pone.0016376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hwang KA, Kim HR, Kang I. Aging and human CD4(+) regulatory T cells. Mech Ageing Dev. 2009;130:509–517. doi: 10.1016/j.mad.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma S, Dominguez AL, Lustgarten J. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J Immunol. 2006;177:8348–8355. doi: 10.4049/jimmunol.177.12.8348. [DOI] [PubMed] [Google Scholar]
- 60.Zhao L, Sun L, Wang H, Ma H, Liu G, Zhao Y. Changes of CD4+CD25+Foxp3+ regulatory T cells in aged Balb/c mice. J Leukocyte Biol. 2007;81:1386–1394. doi: 10.1189/jlb.0506364. [DOI] [PubMed] [Google Scholar]
- 61.Rangel-Moreno J, Moyron-Quiroz JE, Carragher DM, Kusser K, Hartson L, Moquin A, Randall TD. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity. 2009;30:731–743. doi: 10.1016/j.immuni.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW., Jr Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.