Abstract
The neuronal ceroid lipofuscinoses (NCLs) are a family of devastating pediatric neurodegenerative disorders and currently represent the most common form of pediatric-onset neurodegeneration. Infantile NCL (INCL), the most aggressive of these disorders, is caused by mutations in the CLN1 gene that encodes the enzyme palmitoyl protein thioesterase 1 (PPT1). Previous studies have suggested that glutamatergic neurotransmission may be disrupted in INCL, and therefore, the present study investigates glutamate receptor function in the Ppt1−/− mouse model of INCL by comparing the sensitivity of cultured WT and Ppt1−/− cerebellar granule cells to glutamate receptor-mediated toxicity. Ppt1−/− neurons were significantly less sensitive to AMPA receptor-mediated toxicity but markedly more vulnerable to NMDA receptor-mediated cell death. Since glutamate receptor function is primarily regulated by the surface expression level of the receptor, the surface level of AMPA and NMDA receptor subunits in the cerebella of WT and Ppt1−/− mice was also examined. Western blotting of surface cross-linked cerebellar samples showed a significantly lower surface level of the GluR4 AMPA receptor subunit in Ppt1−/− mice, providing a plausible explanation for the decreased vulnerability of Ppt1−/− cerebellar neurons to AMPA receptor-mediated cell death. The surface expression of the NR1, NR2A and NR2B NMDA receptor subunits was similar in the cerebella of WT and Ppt1−/− mice, indicating that there is another mechanism behind the increased sensitivity of Ppt1−/− cerebellar granule cells to NMDA toxicity. Our results indicate an AMPA receptor hypo- and NMDA receptor hyperfunction phenotype in Ppt1−/− neurons and provide new therapeutic targets for INCL.
Keywords: CLN1, PPT1, AMPA, NMDA, Batten disease
Introduction
The Neuronal Ceroid Lipofuscinoses (NCLs) are the most prevalent pediatric-onset neurodegenerative diseases affecting one out of every 12,500 live births worldwide (Cooper 2003). These autosomal recessive disorders are classified into nine disease variants; each of which is the result of a mutation in one of nine different CLN genes. The clinical phenotypes of the variants vary primarily in age of onset and speed of progression (Jalanko et al. 2005), all following essentially the same course beginning with retinal degeneration leading to blindness followed by neurocognitive decline, seizures, and premature death (Cooper 2003). Morphologically, these diseases are marked by severe neuronal loss and ubiquitous accumulation of autofluorescent storage material. Studies suggest that disruptions in glutamatergic function may play a role in disease progression (Griffin et al. 2002; Hachiya et al. 2006; Kovacs et al. 2006; Pears et al. 2005; Seitz et al. 1998).
Glutamate is the main excitatory neurotransmitter used by the mammalian nervous system (Michaelis 1998), and its activity is facilitated by a family of receptors including metabotropic receptors and ionotropic receptor channels. The ionotropic glutamate receptors are ligand-gated ion channels activated in response to neurotransmitter binding, and are responsible for mediating fast excitatory neurotransmission. This group of receptors is made up of three classes named for their specific agonists: kainate receptors, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors, and N-methyl-d-aspartate (NMDA) receptors (Kew and Kemp 2005). Functional receptors exist as heterotetramers; subunit composition can profoundly affect channel properties (Kew and Kemp 2005).
Of all the NCLs, infantile NCL (INCL) is one of the earliest onset and most rapidly progressing variants. INCL results from mutations in CLN1 that inactivate palmitoyl protein thioesterase 1 [PPT1: (Vesa et al. 1995)]. Although the enzyme has been shown to depalmitoylate a range of substrates in vitro, the in vivo targets have yet to be elucidated. PPT1 is believed to play a role in protein degradation (Lu et al. 1996; Verkruyse and Hofmann 1996) due to its lysosomal localization (Cho and Dawson 1998; Hellsten et al. 1996; Lu et al. 2002; Sleat et al. 1996; Verkruyse and Hofmann 1996) and the fact that proteins must be depalmitoylated before they can be processed by the proteasome (Lu et al. 1996). Although the enzyme is ubiquitously expressed (Chattopadhyay and Pearce 2000) and active in diverse cell types (Camp and Hofmann 1993; Cho and Dawson 1998), the central nervous system is particularly affected by PPT1 loss. The disease manifests between six and eighteen months of age with developmental retardation and decreased cranial growth. Muscular hypotonia, progressive microcephaly, vision loss, and epilepsy follow. Patients are generally blind by the age of two with isoelectric EEGs by three. They ultimately exist in a vegetative state before dying prematurely at about the age of ten (Jalanko et al. 2005).
As with many NCL variants, there is evidence suggesting a disruption of glutamatergic function in INCL (Ahtiainen et al. 2007; Sitter et al. 2004; Suopanki et al. 2002). The Ppt1−/− mouse model has motor coordination defects as measured by latency to fall from a rotarod (Griffey et al. 2006; Macauley et al. 2009). As glutamatergic transmission within granule neurons drives cerebellar control of motor coordination (Hashimoto et al. 1999; Jensen et al. 1999), and attenuation of AMPA receptor function in a mouse model of juvenile NCL improves motor coordination ameliorating the disease phenotype (Kovacs and Pearce 2008), we were drawn to investigate glutamate receptor function within the granule neurons of the Ppt1−/− mouse cerebellum. The findings presented here indicative of a decrease in AMPA receptor function and an increase in NMDA receptor function in Ppt1−/− neurons have unveiled potential new therapeutic approaches for INCL treatment.
Materials and Methods
Chemicals
Neurobasal medium, B-27 neuronal serum replacement, glutamine, and penicillin-streptomycin liquid were purchased from Gibco BRL, Invitrogen Corporation (Grand Island, NY). All glutamate receptor agonists and small molecule treatments used here (N-methyl-d-aspartate (NMDA), MK-801, (RS)-AMPA, CTZ, and CPW-399) are products of Tocris Cookson (Bristol, UK). The clear, polystyrene 48 and 96 well plates used for cell culture, viability assay readout, and determination of protein concentration were obtained from Corning (Corning, NY). Isopropanol used in this study was purchased from JT Baker/Mallinckrodt Baker (Phillipsburg, NJ). The surface cross-linker, bis[sulfosuccinimidyl] suberate (BS3), is a product of Pierce (Rockford, IL). All other chemicals unless stated otherwise were purchased from Sigma Aldrich (St. Louis, MO).
Animals
Mice used in this study were WT C57BL/6J and Ppt1−/− (Gupta et al. 2001) maintained on the same genetic background and obtained from our in-house breeding colony. All experiments were carried out according to the Animal Welfare Act, NIH policies, and the guidelines developed by the University of Rochester Institutional Animal Care and Use Committee.
Cell Cultures
Primary cerebellar granule cell (CGC) cultures were prepared from seven-day-old pups as previously described (Finn et al. 2010). Briefly, cerebella were cleaned of their meninges and minced with a tissue chopper (McIlwain Tissue Chopper, Brinkmann). Minced tissue was trypsinized and mechanically dissociated. Cultures were then plated into 48 well plates previously coated with poly-l-lysine at a density of 1.5 × 105 cells per well. Cells were cultured in Neurobasal medium (supplemented to include 2% B-27 neuronal serum replacement, 25 mM KCl, 0.5 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin) and maintained in a humidified environment kept at 37 °C in an atmosphere of 5% CO2/95% air. Culturing neurons in a serum free medium eliminates the need to add a mitotic inhibitor; in primary neuronal cultures maintained in Neurobasal/B-27, the rate of GFAP-positive glial cells is 1-2% (Kovacs et al. 2001). Twenty-four hours after plating, the culture medium was replaced completely; approximately every three days for the duration of culture time, half of the culture medium was removed and replaced.
Agonist treatments
After three weeks in culture, cells were incubated for 30 minutes in culture medium lacking B-27 (Neurobasal medium containing 25 mM KCl, 0.5 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin) then exposed to AMPA, CPW-399 or NMDA for two hours in fresh culture medium lacking B-27. As AMPA receptors rapidly desensitize when exposed to AMPA, cyclothiazide (CTZ) was also added to the treatment medium to prevent this desensitization. To prevent transactivation of NMDA receptors, all AMPA receptor agonist treatments (AMPA and CPW-399) were done in the presence of 50 μM MK-801, a specific NMDA receptor blocker.
At the end of the two-hour treatments, the agonists were removed and replaced with Neurobasal medium containing 2% B-27, 25 mM KCl, 0.5 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Twenty-four hours later, the cell viability was determined using the MTT viability assay. Visual inspection of the cells under the microscope preceded quantification of viability by the MTT assay.
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) viability assay
Microscopic inspection of cultures was done to visualize shrinkage due to apoptosis and disintegration due to osmotic lysis and thus determine approximate levels of cell death before viability was quantified using the MTT assay. The assay was carried out in 48 well culture plates as previously described (Kovacs et al. 2006). Briefly, culture medium was removed and replaced with a 0.3 mg/ml solution of MTT dissolved in Neurobasal medium containing 25 mM KCl, 0.5 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. After a one-hour incubation at 37 °C, the medium was aspirated and plates allowed to dry. Isopropanol was added to the wells to lyse cells and dissolve the formazan crystals. Aliquots were transferred to a 96 well plate, and absorbance was read at 562 nm with background subtraction at 690 nm using a SpectraMax M5 multimode microplate reader (Molecular Devices). Viability as presented here is expressed as a percentage of untreated controls.
Surface Cross-linking in acute cerebellar slices
The protocol utilized here for the covalent cross-linking of surface proteins results in the formation of high molecular weight aggregates with no modification of intracellular proteins (Boudreau and Wolf 2005; Conrad et al. 2008; Finn et al. 2010). This cross-linking allows separation of surface and intracellular proteins based on molecular weight and mobility through a SDS-PAGE gel. The cross-linking was carried out as previously described (Finn et al. 2010). Briefly, one-month-old male mice were euthanized and decapitated. The brain was rapidly extracted, and using a brain matrix, a 2-mm horizontal slice was cut from the cerebellum containing the middle part of the region. The slice was put in artificial cerebrospinal fluid (ACSF; 20 mM HEPES, 147 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 1 mM MgCl2 and 10 mM dextrose) and kept on ice while subsequent brains were dissected. The cerebellum was then isolated from the slices and the dissected tissue pieces were cut into 400 μm-wide slices using a tissue chopper (McIlwain Tissue Chopper, Brinkmann) and returned to ice cold ACSF. BS3 was added to a final concentration of 2 mM and the cross-linking reaction was allowed to proceed at 4 °C for 30 minutes before being quenched by the addition of glycine to a final concentration of 100 mM. Samples were then pelleted (200 g, 2 minutes, 4 °C) and resuspended in lysis buffer containing protease and phosphatase inhibitors (25 mM HEPES, pH 7.4, 500 mM NaCl, 2 mM EDTA 20 mM NaF, 1 mM sodium orthovanadate, 0.1% NP-40 substitute, 1X protease inhibitor cocktail, and 1X phosphatase inhibitor cocktail) before being further homogenized by sonication. After determination of protein concentration using the Pierce 660 nm protein assay (Pierce, Rockford, IL), samples were divided into aliquots and stored at −80 °C until analyzed.
Western Blotting
Thirty or sixty μg of total protein was loaded per lane and run through either 5% (NMDA receptor subunits) or 6% (AMPA receptor subunits) Tris-HCl gels. Proteins were transferred to nitrocellulose and membranes blocked for two hours at room temperature in 5% nonfat dry milk in Tris-buffered saline (TBS; 40.7 mM Tris HCl, 9.3 mM Tris base, 0.3 M NaCl) with 0.05% tween-20 (TBS-T). Membranes were then incubated with antibodies recognizing AMPA receptor subunits [rabbit anti-GluR1 (Cat. No. Ab1504), 1:2000, overnight; rabbit anti-GluR2 (Cat. No. Ab1768), 1:1000, 60 hours; rabbit anti-GluR4 (Cat. No. 06-308), 1:1000, 72 hours; all from Millipore (Tremecula, CA)] or those recognizing NMDA receptor subunits [goat anti-NR1 (Cat. No. SC-1467), 1:250, overnight; goat anti-NR2A (Cat. No. SC-1468), 1:1000, 60 hours; goat anti-NR2B (Cat. No. SC-1469), 1:400, 72 hours; all from Santa Cruz Biotechnology (Santa Cruz, CA)] in blocking buffer. Following the primary antibody incubation, membranes were rinsed twice with ultrapure water and washed in TBS-T once for 10 minutes and then three times for five minutes each. Membranes were then incubated for 90 minutes in blocking buffer containing horseradish peroxidase-conjugated secondary antibodies [anti-rabbit IgG (1:5000; GE Healthcare, Piscataway, NY) or anti-goat IgG (1:5000; Santa Cruz Biotechnology, Santa Cruz, CA)]. After the secondary antibody incubation, membranes were again rinsed twice in ultrapure water and washed in TBS-T once for 10 minutes and then four times for five minutes each. Membranes were incubated in Amersham’s ECL Plus Chemiluminescence Detection Reagent (GE Healthcare, Piscataway, NY) for five minutes and imaged using a Biospectrum 500 Imaging System (UVP, Upland, CA). Measurement of band density was done using Image J (NIH). Care was taken to avoid measuring the density of saturated bands; in many cases, figures were made using longer exposure times to enhance signal visibility. Western blot band intensities were normalized to total protein as determined by Ponceau S staining.
Statistics
Two-way ANOVA with Bonferroni’s post test for multiple pairwise comparison and unpaired t-test were performed using GraphPad Prism version 4.03 (GraphPad Software, San Diego, CA).
Results
To investigate glutamatergic function in Ppt1−/− and WT neurons, the sensitivity of cultured cerebellar granule cells (CGC) to glutamate-mediated cell death was examined. Glutamate receptor-mediated cell death is widely used as a method of elucidating glutamate receptor function because the extent of neuronal death is determined by a number of receptor properties including expression level, subunit composition, and the kinetic and ion permeability properties of the receptors. The magnitude of cell death was first assessed by microscopic inspection of the cultures and then quantified using the MTT cell viability assay. Cells take up MTT by endocytosis and convert the yellow tetrazolium salt into a purple, insoluble product using cellular enzymes (Liu et al. 1997). This conversion depends upon cellular enzymatic function and may be affected by redox state, but it has been shown to correlate well with cell viability (Aras et al. 2008). The results gleaned from the MTT viability assay correlated well with the preliminary visual inspection results.
Sensitivity to cell death mediated by glutamate receptor agonists
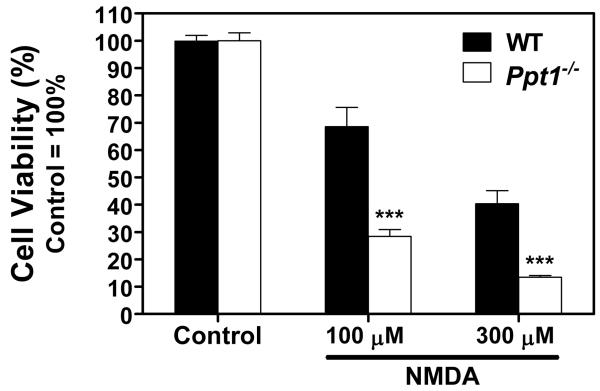
To prevent transactivation of NMDA receptors, all AMPA receptor agonist treatments were done in the presence of 50 μM MK-801, a specific NMDA receptor blocker. As AMPA receptors rapidly desensitize when exposed to AMPA, cyclothiazide (CTZ) was also added to the treatment medium to prevent this desensitization. Treatment with CTZ alone did not affect the two genotypes differently (Fig 1). Overactivation of AMPA-type glutamate receptors with two concentrations of AMPA induced noticeable cell death in WT CGC cultures, but had a significantly smaller effect on Ppt1−/− neurons (Fig. 1; p<0.001 for both AMPA concentrations). As CTZ exhibits selectivity for specific AMPA receptor conformations (Johansen et al. 1995; Partin et al. 1996; Partin et al. 1994) cultures were also treated with CPW-399. CPW-399 is a specific AMPA receptor agonist that elicits a slow or non desensitizing response (Campiani et al. 2001) and thus eliminates the need to treat cells in the presence of CTZ. CPW-399, however, exhibits strong subunit preferences; the compound has a significantly higher affinity for GluR1 and GluR2 that manifests as a 20 fold preference for receptors containing these subunits as opposed to GluR3 and GluR4 (Campiani et al. 2001). CPW-399, applied in three different concentrations, induced a similar extent of cell death in WT and Ppt1−/− CGC cultures (Fig 1). To overactivate NMDA receptors, cultures were exposed to 100 μM or 300 μM NMDA. This treatment resulted in significantly more cell death in Ppt1−/− than in WT CGC cultures (Fig 2; p<0.001 for both concentrations).
Figure 1. Sensitivity of Ppt1−/− cerebellar granule cells (CGC) to AMPA receptor-mediated cell death differs depending upon agonist.
AMPA receptor-mediated cell death was investigated in primary cultures of CGC isolated from WT and Ppt1−/− mice. Cultures were obtained from seven-day-old pups and maintained in Neurobasal medium with B-27 serum replacement. After three weeks of in vitro development, cultures were exposed to either AMPA and cyclothiazide or CPW-399 for two hours in Neurobasal medium without B-27. 100 μM cyclothiazide was added to prevent desensitization of AMPA receptors. To prevent transactivation of NMDA receptors, all treatments were done in the presence of 50 μM MK-801. At the end of the two-hour treatment, medium was replaced with complete, B-27-containing Neurobasal medium. Twenty-four hours later, the cell viability was determined using the MTT viability assay. Results presented here represent mean ± SEM of four separate culture preparations (n = 8-20). Statistical significance was determined by two-way ANOVA with Bonferroni’s post test for multiple comparisons: *** p < 0.001.
Figure 2. Ppt1−/− cerebellar granule cells (CGC) are more sensitive to NMDA-mediated cell death.
NMDA-mediated cell death was investigated in primary cultures of CGC isolated from WT and Ppt1−/− mice. Cultures were obtained from seven-day-old pups and maintained in Neurobasal medium with B-27 serum replacement. After three weeks of in vitro development, cultures were treated with NMDA at the concentrations indicated for two hours in Neurobasal medium without B-27. At the end of the two-hour treatment, medium was replaced with complete, B-27-containing Neurobasal medium. Twenty-four hours later, the cell viability was determined using the MTT viability assay. Results presented here represent mean ± SEM of four separate culture preparations (n = 9-20). Statistical significance was determined by two-way ANOVA with Bonferroni’s post test for multiple comparisons: *** p< 0.001.
Surface and intracellular expression levels of glutamate receptor subunits
As glutamate receptor function is regulated in part by surface expression, and agonist treatments suggested profound differences in receptor function, differences in surface expression levels of receptor subunits were investigated to determine whether they could be driving the observed differences in receptor function. One-month-old mice were used for this investigation as their age corresponds roughly to the absolute age of the cultures. Cerebellar slices were acutely isolated from male mice and subjected to surface cross-linking by exposure to BS3. BS3 is a water soluble homobifunctional N-hydroxysuccinimide ester that is incapable of crossing cell membranes. The result of this cross-linking is the covalent linkage of any receptor subunits on the cell surface into high molecular weight complexes; intracellular proteins are not cross-linked and thus can be differentiated from surface proteins based on mobility through an SDS-polyacrylamide gel (Boudreau and Wolf 2005; Conrad et al. 2008; Finn et al. 2010). Western blot band intensities were normalized to total protein as determined by Ponceau S staining, a method previously used in studies examining surface and intracellular levels of AMPA and NMDA receptor subunits (Boudreau and Wolf 2005; Conrad et al. 2008; Finn et al. 2010). It has been shown that normalizing to β-tubulin levels gives results identical to those obtained by normalization to total protein levels (Finn et al. 2010). The use of total protein quantification as an alternative to blotting for housekeeping proteins has been investigated (Aldridge et al. 2008), and it was determined that use of total protein stains for normalization of western blots actually results in a lower coefficient of variation.
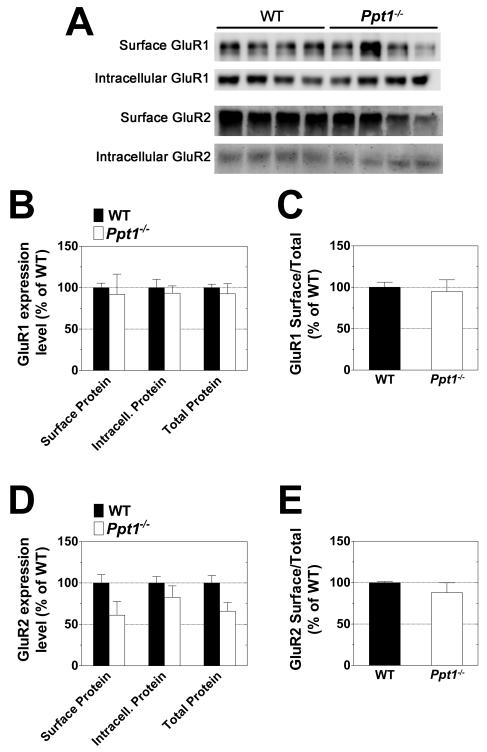
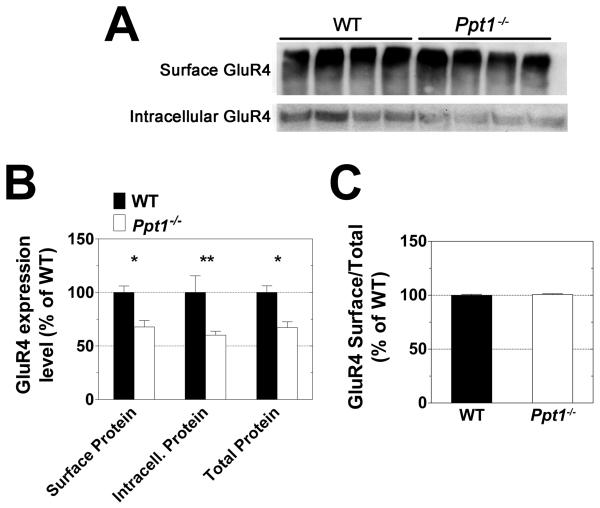
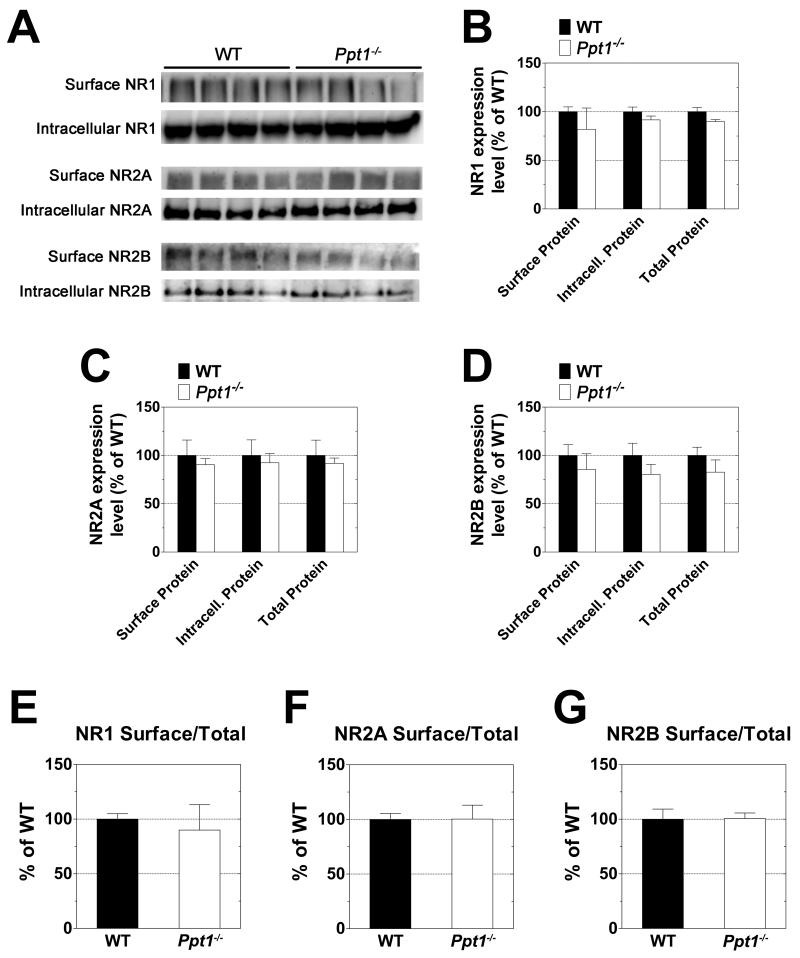
No alterations in the surface expression of the GluR1 or GluR2 AMPA receptor subunits were found in Ppt1−/− cerebella. Likewise, there was no difference between WT and Ppt1−/− with respect to the surface/total ratio of these proteins (Fig 3). Similar investigation of the GluR4 AMPA receptor subunit showed significant differences in expression of the protein in the Ppt1−/− samples (Fig 4). Surface, intracellular, and total (surface + intracellular) levels of GluR4 were found to be decreased by approximately one third in the cerebella of Ppt1−/− mice as compared to WT (Fig 4B). As the decrease in expression appears to be generalized and not limited to either the surface or intracellular population, there was no change in the surface/total ratio of GluR4 in Ppt1−/− mice (Fig 4C). Surface expression levels of the NMDA receptor subunits NR1, NR2A, and NR2B were similar in the WT and Ppt1−/− samples (Fig 5).
Figure 3. Expression levels of the GluR1 and GluR2 AMPA receptor subunits are similar in the cerebellum of Ppt1−/− and WT mice.
The surface and intracellular expression levels of the GluR1 and GluR2 AMPA receptor subunits were investigated using acutely isolated slices from the cerebella of one-month-old WT and Ppt1−/− mice. Slices were subjected to surface cross-linking and then analyzed by Western blot for GluR1 and GluR2. A: Western blots show separation of surface (cross-linked) and intracellular receptor subunits. B-E: Subunit expression levels were normalized to total protein as determined by Ponceau S staining. All measurements for Ppt1−/− samples are expressed as a percentage of WT. B and D show quantification of surface, intracellular, and total (surface + intracellular) subunit expression levels. C and E compare relative surface/total subunit expression ratios. Four mice were analyzed from each genotype; bars represent mean ± SEM. Statistical significance was determined by two-way ANOVA with Bonferroni’s post test for multiple comparisons.
Figure 4. Expression level of the GluR4 AMPA receptor subunit is significantly lower in the cerebellum of Ppt1−/− mice.
The surface and intracellular expression levels of the GluR4 AMPA receptor subunit were investigated using acutely isolated slices from the cerebella of one-month-old WT and Ppt1−/− mice. Slices were subjected to surface cross-linking and then analyzed by Western blot for GluR4. A: Western blots show separation of surface (cross-linked) and intracellular receptor subunit. B and C: GluR4 expression levels were normalized to total protein as determined by Ponceau S staining. All measurements for Ppt1−/− samples are expressed as a percentage of WT. B shows quantification of surface, intracellular, and total (surface + intracellular) subunit expression levels. C compares relative surface/total subunit expression ratios. Four mice were analyzed from each genotype; bars represent mean ± SEM. Statistical significance was determined by two-way ANOVA with Bonferroni’s post test for multiple comparisons: *p<0.05, **p<0.01.
Figure 5. Expression levels of NR1, NR2A and NR2B NMDA receptor subunits are similar in the cerebellum of Ppt1−/− and WT mice.
The surface and intracellular expression levels of the NR1, NR2A and NR2B NMDA receptor subunits were investigated using acutely isolated slices from the cerebella of one-month-old WT and Ppt1−/− mice. Slices were subjected to surface cross-linking and then analyzed by Western blot for NR1, NR2A and NR2B. A: Western blots show separation of surface (cross-linked) and intracellular receptor subunits. B-G: Subunit expression levels were normalized to total protein as determined by Ponceau S staining. All measurements for Ppt1−/− samples are expressed as a percentage of wild type. B-D show quantification of surface, intracellular, and total (surface + intracellular) subunit expression levels. E-G compare relative surface/total subunit expression ratios. Four mice were analyzed from each genotype; bars represent mean ± SEM. Statistical significance was determined by two-way ANOVA with Bonferroni’s post test for multiple comparisons.
Discussion
Previous studies have suggested a possible disruption of glutamatergic function in INCL (Ahtiainen et al. 2007; Sitter et al. 2004; Suopanki et al. 2002). A motor coordination defect measured by latency to fall from a rotarod (Griffey et al. 2006; Macauley et al. 2009) further supports the hypothesis that there may be a disruption in glutamatergic function in Ppt1−/− mice. Excitatory signals mediated by glutamate receptors in the cerebellum drive motor coordination (Hashimoto et al. 1999; Jensen et al. 1999; Kadotani et al. 1996; Watanabe et al. 1998) and the link between juvenile onset NCL, motor coordination, and glutamate receptors has previously been demonstrated (Kovacs and Pearce 2008; Kovacs et al. 2006). Based on these existing bodies of evidence, we were drawn to examine the function of glutamate receptors in the Ppt1−/− murine model of INCL.
It should be noted that the observed motor coordination phenotypes in Ppt1−/− mice don’t manifest to significance until the mice reach four or five months of age (Macauley et al. 2009). Here, younger (one-month-old) animals were examined so as to determine whether the development of the observable phenotypes is preceded by changes on a biochemical level. A recent study has shown that significant molecular evidence of dysfunction precedes symptomatic evidence of pathology in the cortex and thalamus of Ppt1−/−mice (Kielar et al. 2009). It is likely that a similar time line occurs in the cerebellum with biochemical changes in glutamate receptors manifesting before the development of a behavioral phenotype.
The results presented here suggest that there are significant differences in glutamate receptor function between Ppt1−/−and WT granule cell cultures that have been maintained in culture for 21 days. The finding that Ppt1−/− CGC, as compared to their WT counterparts, are less sensitive to AMPA receptor-mediated toxicity but markedly more sensitive to NMDA receptor-mediated cell death indicates that the vulnerability of Ppt1−/− neurons is specific to NMDA receptor activation and is not a result of a general vulnerability to any toxic insults. When exposed to AMPA, Ppt1−/− CGC cultures were found to be significantly less sensitive to excitotoxic cell death. This change in sensitivity does not correspond to a decrease in GluR1 or GluR2 AMPA receptor subunit surface expression or protein levels. There was, however a significant decrease in the surface and overall expression level of the GluR4 AMPA receptor subunit. Neurons expressing GluR4 are particularly sensitive to AMPA-induced excitotoxicity (Page and Everitt 1995), and homomeric GluR4 channels are highly permeable to Ca2+ ions (Iizuka et al. 2000). WT and Ppt1−/− CGC cultures were equally sensitive to the AMPA receptor agonist, CPW-399 (see Fig. 1). As CPW-399 exhibits strong subunit preference for GluR1 and GluR2 as opposed to GluR3 and GluR4 (Campiani et al. 2001), it is likely that the decreased sensitivity of Ppt1−/− granule neurons to AMPA-mediated cell death is due to the lower expression levels of the GluR4 subunit. Investigation of sensitivity to NMDA receptor-mediated cell death also exposed significant differences between Ppt1−/− and WT cells. Ppt1−/− CGC were strikingly more vulnerable to NMDA-induced cell death than their WT counterparts. Although no differences were found in the expression of the NR1, NR2A and NR2B NMDA receptor subunits (either total expression or relative surface expression), the possibility that lack of the PPT1 enzyme affects the expression levels of the NR2C and NR3 NMDA receptor subunits cannot be excluded. There are also a number of other ways that NMDA receptor function is regulated. Phosphorylation of receptors (Chen and Roche 2007; Collingridge et al. 2004; Wang et al. 2006), interacting proteins (Sornarajah et al. 2008), and spatial distribution of ion channels (Kohr 2006; Leveille et al. 2008) all regulate NMDA receptor function. In addition, palmitoylation of NMDA receptor subunits has been shown to affect phosphorylation and trafficking (Hayashi et al. 2009). Alterations in any of these could explain the observed difference between WT and Ppt1−/− cells with respect to NMDA-mediated cell death.
PPT1 was originally identified based on its ability to depalmitoylate ras in vitro (Camp and Hofmann 1993; Camp et al. 1994), but the enzyme’s in vivo substrates have yet to be elucidated. Based on the particularly deleterious effect that loss of PPT1 has on the nervous system, we hypothesize that PPT1 has a neuron-specific substrate. Palmitoylation has been shown to impact a number of proteins involved in synaptic vesicle fusion and other aspects of neurotransmitter function; both AMPA and NMDA receptors are palmitoylated and this modification affects surface expression and protein-protein interactions (Sen and Snyder 2010). PSD95 is also subject to dynamic palmitoylation which plays a role in regulating glutamate receptor surface expression (Sen and Snyder 2010). In addition to a lysosomal localization, PPT1 has also been found in synaptosomes and synaptic vesicles (Lehtovirta et al. 2001) where it could play a role in depalmitoylating either glutamate receptor subunits or interacting proteins. If glutamate receptor subunits are substrates of PPT1, loss of the enzyme’s depalmitoylating activity could lead to aberrant receptor function and subcellular localization. Alternatively, loss of PPT1 activity could also result in a build-up of receptor subunits as proteins must be depalmitoylated before they can be degraded by the proteasome.
The present study indicates notable functional differences between Ppt1−/− and WT glutamate receptors, although further studies are required to determine the precise molecular mechanisms driving these changes. These findings suggest that obliteration of PPT1 enzyme activity has a significant effect on glutamatergic function. Results demonstrating AMPA receptor hypo- and NMDA receptor hyperfunction in Ppt1−/− neurons provide new therapeutic targets for INCL. Positive allosteric modulators of AMPA receptors and NMDA receptor antagonists are available, and future studies in Ppt1−/− mice will determine the therapeutic effectiveness of these drugs administered alone or in combination.
Acknowledgements
We thank Tim Curran and Andrew Cardillo for technical assistance.
This work was supported in part by NIH R01 NS043310 and Hayden’s Hope
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate
- CGC
cerebellar granule cell
- CTZ
cyclothiazide
- INCL
infantile neuronal ceroid lipofuscinosis
- NCL
neuronal ceroid lipofuscinosis
- NMDA
N-methyl-D-aspartate
- PPT1
palmitoyl protein thioesterase 1
References
- Ahtiainen L, Kolikova J, Mutka AL, Luiro K, Gentile M, Ikonen E, Khiroug L, Jalanko A, Kopra O. Palmitoyl protein thioesterase 1 (Ppt1)-deficient mouse neurons show alterations in cholesterol metabolism and calcium homeostasis prior to synaptic dysfunction. Neurobiol Dis. 2007;28(1):52–64. doi: 10.1016/j.nbd.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J Neurosci Methods. 2008;172(2):250–254. doi: 10.1016/j.jneumeth.2008.05.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aras MA, Hartnett KA, Aizenman E. Assessment of cell viability in primary neuronal cultures. Curr Protoc Neurosci Chapter 7. 2008 doi: 10.1002/0471142301.ns0718s44. Unit 7 18. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25(40):9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp LA, Hofmann SL. Purification and properties of a palmitoyl-protein thioesterase that cleaves palmitate from H-Ras. J Biol Chem. 1993;268(30):22566–22574. [PubMed] [Google Scholar]
- Camp LA, Verkruyse LA, Afendis SJ, Slaughter CA, Hofmann SL. Molecular cloning and expression of palmitoyl-protein thioesterase. J Biol Chem. 1994;269(37):23212–23219. [PubMed] [Google Scholar]
- Campiani G, Morelli E, Nacci V, Fattorusso C, Ramunno A, Novellino E, Greenwood J, Liljefors T, Griffiths R, Sinclair C, Reavy H, Kristensen AS, Pickering DS, Schousboe A, Cagnotto A, Fumagalli E, Mennini T. Characterization of the 1H-cyclopentapyrimidine-2,4(1H,3H)-dione derivative (S)-CPW399 as a novel, potent, and subtype-selective AMPA receptor full agonist with partial desensitization properties. J Med Chem. 2001;44(26):4501–4504. doi: 10.1021/jm015552m. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Pearce DA. Neural and extraneural expression of the neuronal ceroid lipofuscinoses genes CLN1, CLN2, and CLN3: functional implications for CLN3. Mol Genet Metab. 2000;71(1-2):207–211. doi: 10.1006/mgme.2000.3056. [DOI] [PubMed] [Google Scholar]
- Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53(3):362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Dawson G. Enzymatic and molecular biological analysis of palmitoyl protein thioesterase deficiency in infantile neuronal ceroid lipofuscinosis. J Neurochem. 1998;71(1):323–329. doi: 10.1046/j.1471-4159.1998.71010323.x. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5(12):952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454(7200):118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JD. Progress towards understanding the neurobiology of Batten disease or neuronal ceroid lipofuscinosis. Curr Opin Neurol. 2003;16(2):121–128. doi: 10.1097/01.wco.0000063762.15877.9b. [DOI] [PubMed] [Google Scholar]
- Finn R, Kovacs AD, Pearce DA. Altered sensitivity to excitotoxic cell death and glutamate receptor expression between two commonly studied mouse strains. J Neurosci Res. 2010;88(12):2648–2660. doi: 10.1002/jnr.22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffey MA, Wozniak D, Wong M, Bible E, Johnson K, Rothman SM, Wentz AE, Cooper JD, Sands MS. CNS-directed AAV2-mediated gene therapy ameliorates functional deficits in a murine model of infantile neuronal ceroid lipofuscinosis. Mol Ther. 2006;13(3):538–547. doi: 10.1016/j.ymthe.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Griffin JL, Muller D, Woograsingh R, Jowatt V, Hindmarsh A, Nicholson JK, Martin JE. Vitamin E deficiency and metabolic deficits in neuronal ceroid lipofuscinosis described by bioinformatics. Physiol Genomics. 2002;11(3):195–203. doi: 10.1152/physiolgenomics.00100.2002. [DOI] [PubMed] [Google Scholar]
- Gupta P, Soyombo AA, Atashband A, Wisniewski KE, Shelton JM, Richardson JA, Hammer RE, Hofmann SL. Disruption of PPT1 or PPT2 causes neuronal ceroid lipofuscinosis in knockout mice. Proc Natl Acad Sci U S A. 2001;98(24):13566–13571. doi: 10.1073/pnas.251485198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiya Y, Hayashi M, Kumada S, Uchiyama A, Tsuchiya K, Kurata K. Mechanisms of neurodegeneration in neuronal ceroid-lipofuscinoses. Acta Neuropathol. 2006;111(2):168–177. doi: 10.1007/s00401-005-0024-x. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fukaya M, Qiao X, Sakimura K, Watanabe M, Kano M. Impairment of AMPA receptor function in cerebellar granule cells of ataxic mutant mouse stargazer. J Neurosci. 1999;19(14):6027–6036. doi: 10.1523/JNEUROSCI.19-14-06027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Thomas GM, Huganir RL. Dual palmitoylation of NR2 subunits regulates NMDA receptor trafficking. Neuron. 2009;64(2):213–226. doi: 10.1016/j.neuron.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten E, Vesa J, Olkkonen VM, Jalanko A, Peltonen L. Human palmitoyl protein thioesterase: evidence for lysosomal targeting of the enzyme and disturbed cellular routing in infantile neuronal ceroid lipofuscinosis. EMBO J. 1996;15(19):5240–5245. [PMC free article] [PubMed] [Google Scholar]
- Iizuka M, Nishimura S, Wakamori M, Akiba I, Imoto K, Barsoumian EL. The lethal expression of the GluR2flip/GluR4flip AMPA receptor in HEK293 cells. Eur J Neurosci. 2000;12(11):3900–3908. doi: 10.1046/j.1460-9568.2000.00270.x. [DOI] [PubMed] [Google Scholar]
- Jalanko A, Vesa J, Manninen T, von Schantz C, Minye H, Fabritius AL, Salonen T, Rapola J, Gentile M, Kopra O, Peltonen L. Mice with Ppt1Deltaex4 mutation replicate the INCL phenotype and show an inflammation-associated loss of interneurons. Neurobiol Dis. 2005;18(1):226–241. doi: 10.1016/j.nbd.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Jensen P, Surmeier DJ, Goldowitz D. Rescue of cerebellar granule cells from death in weaver NR1 double mutants. J Neurosci. 1999;19(18):7991–7998. doi: 10.1523/JNEUROSCI.19-18-07991.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen TH, Chaudhary A, Verdoorn TA. Interactions among GYKI-52466, cyclothiazide, and aniracetam at recombinant AMPA and kainate receptors. Mol Pharmacol. 1995;48(5):946–955. [PubMed] [Google Scholar]
- Kadotani H, Hirano T, Masugi M, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Motor discoordination results from combined gene disruption of the NMDA receptor NR2A and NR2C subunits, but not from single disruption of the NR2A or NR2C subunit. J Neurosci. 1996;16(24):7859–7867. doi: 10.1523/JNEUROSCI.16-24-07859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew JN, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology (Berl) 2005;179(1):4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- Kielar C, Wishart TM, Palmer A, Dihanich S, Wong AM, Macauley SL, Chan CH, Sands MS, Pearce DA, Cooper JD, Gillingwater TH. Molecular correlates of axonal and synaptic pathology in mouse models of Batten disease. Hum Mol Genet. 2009;18(21):4066–4080. doi: 10.1093/hmg/ddp355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohr G. NMDA receptor function: subunit composition versus spatial distribution. Cell Tissue Res. 2006;326(2):439–446. doi: 10.1007/s00441-006-0273-6. [DOI] [PubMed] [Google Scholar]
- Kovacs AD, Cebers G, Cebere A, Moreira T, Liljequist S. Cortical and striatal neuronal cultures of the same embryonic origin show intrinsic differences in glutamate receptor expression and vulnerability to excitotoxicity. Exp Neurol. 2001;168(1):47–62. doi: 10.1006/exnr.2000.7576. [DOI] [PubMed] [Google Scholar]
- Kovacs AD, Pearce DA. Attenuation of AMPA receptor activity improves motor skills in a mouse model of juvenile Batten disease. Exp Neurol. 2008;209(1):288–291. doi: 10.1016/j.expneurol.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs AD, Weimer JM, Pearce DA. Selectively increased sensitivity of cerebellar granule cells to AMPA receptor-mediated excitotoxicity in a mouse model of Batten disease. Neurobiol Dis. 2006;22(3):575–585. doi: 10.1016/j.nbd.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Lehtovirta M, Kyttala A, Eskelinen EL, Hess M, Heinonen O, Jalanko A. Palmitoyl protein thioesterase (PPT) localizes into synaptosomes and synaptic vesicles in neurons: implications for infantile neuronal ceroid lipofuscinosis (INCL) Hum Mol Genet. 2001;10(1):69–75. doi: 10.1093/hmg/10.1.69. [DOI] [PubMed] [Google Scholar]
- Leveille F, El Gaamouch F, Gouix E, Lecocq M, Lobner D, Nicole O, Buisson A. Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. FASEB J. 2008;22(12):4258–4271. doi: 10.1096/fj.08-107268. [DOI] [PubMed] [Google Scholar]
- Liu Y, Peterson DA, Kimura H, Schubert D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem. 1997;69(2):581–593. doi: 10.1046/j.1471-4159.1997.69020581.x. [DOI] [PubMed] [Google Scholar]
- Lu JY, Verkruyse LA, Hofmann SL. Lipid thioesters derived from acylated proteins accumulate in infantile neuronal ceroid lipofuscinosis: correction of the defect in lymphoblasts by recombinant palmitoyl-protein thioesterase. Proc Natl Acad Sci U S A. 1996;93(19):10046–10050. doi: 10.1073/pnas.93.19.10046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JY, Verkruyse LA, Hofmann SL. The effects of lysosomotropic agents on normal and INCL cells provide further evidence for the lysosomal nature of palmitoyl-protein thioesterase function. Biochim Biophys Acta. 2002;1583(1):35–44. doi: 10.1016/s1388-1981(02)00158-0. [DOI] [PubMed] [Google Scholar]
- Macauley SL, Wozniak DF, Kielar C, Tan Y, Cooper JD, Sands MS. Cerebellar pathology and motor deficits in the palmitoyl protein thioesterase 1-deficient mouse. Exp Neurol. 2009;217(1):124–135. doi: 10.1016/j.expneurol.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis EK. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog Neurobiol. 1998;54(4):369–415. doi: 10.1016/s0301-0082(97)00055-5. [DOI] [PubMed] [Google Scholar]
- Page KJ, Everitt BJ. The distribution of neurons coexpressing immunoreactivity to AMPA-sensitive glutamate receptor subtypes (GluR1-4) and nerve growth factor receptor in the rat basal forebrain. Eur J Neurosci. 1995;7(5):1022–1033. doi: 10.1111/j.1460-9568.1995.tb01090.x. [DOI] [PubMed] [Google Scholar]
- Partin KM, Fleck MW, Mayer ML. AMPA receptor flip/flop mutants affecting deactivation, desensitization, and modulation by cyclothiazide, aniracetam, and thiocyanate. J Neurosci. 1996;16(21):6634–6647. doi: 10.1523/JNEUROSCI.16-21-06634.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Mayer ML. Cyclothiazide differentially modulates desensitization of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor splice variants. Mol Pharmacol. 1994;46(1):129–138. [PubMed] [Google Scholar]
- Pears MR, Cooper JD, Mitchison HM, Mortishire-Smith RJ, Pearce DA, Griffin JL. High resolution 1H NMR-based metabolomics indicates a neurotransmitter cycling deficit in cerebral tissue from a mouse model of Batten disease. J Biol Chem. 2005;280(52):42508–42514. doi: 10.1074/jbc.M507380200. [DOI] [PubMed] [Google Scholar]
- Seitz D, Grodd W, Schwab A, Seeger U, Klose U, Nagele T. MR imaging and localized proton MR spectroscopy in late infantile neuronal ceroid lipofuscinosis. AJNR Am J Neuroradiol. 1998;19(7):1373–1377. [PMC free article] [PubMed] [Google Scholar]
- Sen N, Snyder SH. Protein modifications involved in neurotransmitter and gasotransmitter signaling. Trends Neurosci. 2010;33(11):493–502. doi: 10.1016/j.tins.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitter B, Autti T, Tyynela J, Sonnewald U, Bathen TF, Puranen J, Santavuori P, Haltia MJ, Paetau A, Polvikoski T, Gribbestad IS, Hakkinen AM. High-resolution magic angle spinning and 1H magnetic resonance spectroscopy reveal significantly altered neuronal metabolite profiles in CLN1 but not in CLN3. J Neurosci Res. 2004;77(5):762–769. doi: 10.1002/jnr.20123. [DOI] [PubMed] [Google Scholar]
- Sleat DE, Sohar I, Lackland H, Majercak J, Lobel P. Rat brain contains high levels of mannose-6-phosphorylated glycoproteins including lysosomal enzymes and palmitoyl-protein thioesterase, an enzyme implicated in infantile neuronal lipofuscinosis. J Biol Chem. 1996;271(32):19191–19198. doi: 10.1074/jbc.271.32.19191. [DOI] [PubMed] [Google Scholar]
- Sornarajah L, Vasuta OC, Zhang L, Sutton C, Li B, El-Husseini A, Raymond LA. NMDA receptor desensitization regulated by direct binding to PDZ1-2 domains of PSD-95. J Neurophysiol. 2008;99(6):3052–3062. doi: 10.1152/jn.90301.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suopanki J, Lintunen M, Lahtinen H, Haltia M, Panula P, Baumann M, Tyynela J. Status epilepticus induces changes in the expression and localization of endogenous palmitoyl-protein thioesterase 1. Neurobiol Dis. 2002;10(3):247–257. doi: 10.1006/nbdi.2002.0503. [DOI] [PubMed] [Google Scholar]
- Verkruyse LA, Hofmann SL. Lysosomal targeting of palmitoyl-protein thioesterase. J Biol Chem. 1996;271(26):15831–15836. doi: 10.1074/jbc.271.26.15831. [DOI] [PubMed] [Google Scholar]
- Vesa J, Hellsten E, Verkruyse LA, Camp LA, Rapola J, Santavuori P, Hofmann SL, Peltonen L. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature. 1995;376(6541):584–587. doi: 10.1038/376584a0. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Liu X, Zhang G, Parelkar NK, Arora A, Haines M, Fibuch EE, Mao L. Phosphorylation of glutamate receptors: a potential mechanism for the regulation of receptor function and psychostimulant action. J Neurosci Res. 2006;84(8):1621–1629. doi: 10.1002/jnr.21050. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Inokawa H, Hashimoto K, Suzuki N, Kano M, Shigemoto R, Hirano T, Toyama K, Kaneko S, Yokoi M, Moriyoshi K, Suzuki M, Kobayashi K, Nagatsu T, Kreitman RJ, Pastan I, Nakanishi S. Ablation of cerebellar Golgi cells disrupts synaptic integration involving GABA inhibition and NMDA receptor activation in motor coordination. Cell. 1998;95(1):17–27. doi: 10.1016/s0092-8674(00)81779-1. [DOI] [PubMed] [Google Scholar]