Abstract
Vitamin A and its derivatives (retinoids) are critically important in the development and maintenance of multiple epithelial tissues, including skin, hair, and sebaceous glands, as shown by the detrimental effects of either vitamin A deficiency or toxicity. Thus, precise levels of retinoic acid (RA, active metabolite) are needed. These precise levels of RA are achieved by regulating several steps in the conversion of dietary vitamin A (retinol) to RA and RA catabolism. This review discusses the localization of RA synthesis to specific sites within the hair follicle and sebaceous gland, including their stem cells, during both homeostasis and disease states. It also discusses what is known about the specific roles of RA within the hair follicle and sebaceous gland.
Keywords: Retinoic acid, synthesis, retinoid, hair follicle, stem cells, sebaceous gland
1.1 Introduction
Wolbach and Howe (1925) [1] first found that vitamin A deficiency lead to metaplasia of keratinized epithelia including the hair follicle and atrophy of the sebaceous gland. Follicular hyperkeratosis was also seen in vitamin A deficient humans [2]. Vitamin A deficiency in rodents also leads to a thin hair coat that is frequently seen, but rarely reported [3] (unpublished observation from [4]). Hair loss (alopecia) is a consistent finding during vitamin A toxicity [5–7]. Excess RA also inhibits sebaceous gland function, as is exploited in the treatment of acne [8, 9]. The results from these studies suggest that precise levels of retinoic acid (RA, active metabolite) are needed for optimal function of the hair follicle and sebaceous gland (pilosebaceous unit, PSU). This review will focus on the role of endogenous RA within the PSU.
2.1 Retinoid metabolism in the skin
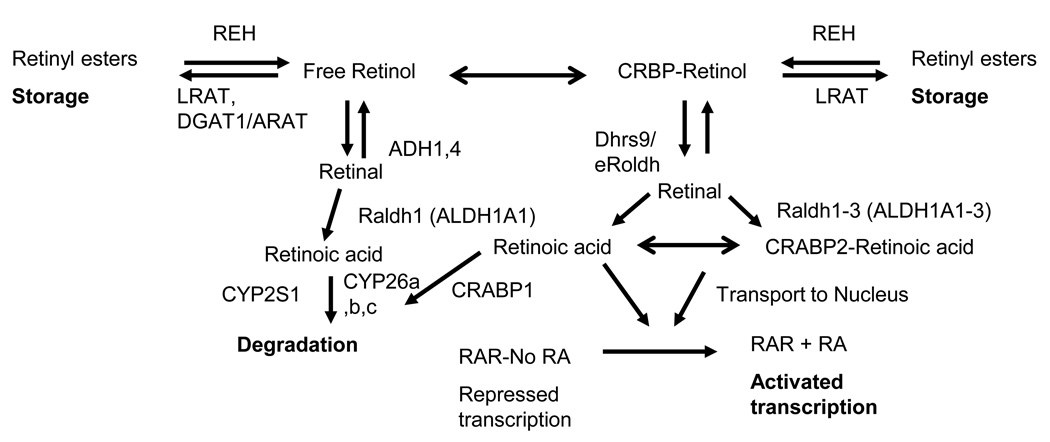
Plasma atRA was demonstrated to contribute little to endogenous atRA levels in numerous organs/sites in the rat [10]. Thus, atRA synthesis occurs at the site of action and precise levels of atRA are achieved by regulating several key steps in vitamin A metabolism (reviewed by Joe Napoli [11] and Greg Duester [12] in this special issue Figure 1). Regulation of retinoid metabolism occurs in part by the regulation of retinoid binding proteins. Vitamin A circulates in the blood in the form of retinol bound to the retinol binding protein (RBP, tentative gene symbol Rbp4) [13] and one level of regulation is to affect RBP4 levels (reviewed in [14]). Stra6 (stimulated by retinoic acid gene 6) was recently shown to bind RBP4 on the surface of the cell and facilitate the cellular uptake of retinol [15]. Within the cell, cellular retinol binding proteins (CRBP I-III, argued gene symbols Rbp1–3) direct retinol to specific enzymes, as only Lecithin: retinol acyltransferase (LRAT) and some retinol dehydrogenases can utilize retinol bound to CRBP as a substrate [16, 17]. Napoli [17] argued that this CRBP bound retinol is what produces atRA for the activation of RA receptors (RARA, B, G). LRAT can esterify either CRBP-retinol or free retinol for storage [16, 17]. This esterification of retinol limits the amount of retinol availabe for RA synthesis, as the majority of retinol that enters primary keratinocytes is converted to retinyl esters and only a small amount is actually oxidized to RA [18]. In addition, Lrat expression and its activity are regulated by atRA in a tissue specific maner, which is one way atRA regulates its own levels [19–21]. Dehydrogenase reductase SDR family member 9 (DHRS9) is one enzyme that catalyzes the oxidation of CRBP-bound, as well as free, retinol to retinal [22] in the epidermis, hair follicle, and sebaceous gland [23, 24]. Retinal is further oxidized to atRA by retinal dehydrogenases 1–3 (ALDH1A1, 2 and 3 [17, 25, 26]). There are three RA binding proteins that direct atRA to different fates. CRABP2 binds atRA, transports atRA to the nucleus, and binds to RARA to achieve efficient channelling of atRA to RARA, which increases its transcriptional efficiency [27–29]. It is assumed that CRABP2 also binds RARB and RARG, but this was not tested. CRABP2 is expressed at many sites of probable atRA synthesis and its presence correlated with times of atRA synthesis [30–32]. When CRABP2 is absent or saturated, atRA was reported to bind fatty acid binding protein 5 (FABP5), which directs atRA to peroxisome proliferators-activated receptor delta (PPARD) [33], although this role of atRA was refuted by two other groups [34, 35]. In contrast, CRABP1 is associated with catabolism of atRA via cytochrome P450 family members CYP26A1, CYP26B1, and CYP26C1 [36–38], although there is still a debate over whether these metabolites are functional or not [39–41]. RARA, RARB and RARG are atRA inducible transcription factors of the nuclear hormone family [42, 43] which regulate the expression of over 500 genes either directly or indirectly [44]. RARs heterodimerize with retinoid X receptor (RXR) [45]. While 9-cis RA (9cRA) was initially reported as the ligand for RXR [46], more recent genetic studies argue against 9cRA being the physiological ligand for RXR [47]. Unsaturated fatty acids also bind and activate RXR [48, 49].
Figure 1. Cellular retinol metabolism in the skin.
Once inside the cell, retinol is either stored as retinyl esters, or oxidized to retinoic acid (RA). Several families of enzymes and binding proteins are involved in this process.
When retinol levels exceed the capacity of CRBP a separate system of enzymes is argued by some to be used to clear the potentially toxic free retinol. Ross et al (1982) [50] found retinol could be esterified with acyl coenzyme A retinol acyltransferase (ARAT) activity and Lrattm1kpal null mice suggested some retinyl esters were stored in the absence of LRAT [51]. DGAT1 was recently shown to esterify retinol to retinyl esters with ARAT activity [52–54]. Since DGAT1 cannot use CRBP bound retinol [55, 56], it was suggested that it only was physiologically relevant when excessive concentrations of retinol were present. Although analysis of Dgat1tm1Far, Lrattm1Kpal, and Rbp2tm1Eli (Crbp2) single and double knockout mice demonstrated that in vivo CRBP2 does not block DGAT1 activity within the intestine when physiological doses of retinol are provided [54]. In addition, these studies revealed that yet another enzyme exists with ARAT activity besides DGAT1. Studies with skin specific Dgat1tm1Far null mice (Dgat1tm2FarTg(KRT14-cre)1AMC) revealed that while retinol and atRA were elevated, there were no differences between null and wild type mice in retinyl ester levels regardless of dietary vitamin A levels, suggesting that LRAT or another ARAT is present within the skin. Both LRAT activity [20] and mRNA [57] were found in cultured keratinocytes. We also see LRAT protein within the epidermis and hair follicle, but have not fully characterized it within the hair cycle (HB Everts, JP Sundberg, DE Ong, unpublished observation). Overall these data suggest that LRAT acts in the skin to store retinyl esters, while DGAT1 acts primarily to clear excess retinol to prevent toxicity (Figure 1). Free retinol can also be oxidized by the medium chain alcohol dehydrogenase family of retinol dehydrogenases (ADH1–4) [58]. Studies with Adh1tm1Gdu and Aldh1a1 tm1Gdu null mice suggest that the clearance of excess retinol in the liver involves the oxidation of retinol to retinal by ADH1 followed by the oxidation of retinal to RA by ALDH1A1 [59–61]. Mice that lack Adh4 tm1Gdu also have decreased retinol oxidation in the kidney [62, 63], but not in the liver [59] when given a toxic retinol dose. ADH1 and ADH4 are present within the skin (unpublished observation and [64]). It is unclear if either of these enzymes is important for clearing free retinol in the skin when CRBP is saturated. In addition, both ADH1 and ADH4 can oxidize other alcohols to aldehydes and may play a role in general alcohol detoxification as well. Combined, the results of these studies show that retinoid metabolism is complex and the regulation of any one of numerous genes can alter RA activity.
2.2 The hair follicle and sebaceous gland (pilosebaceous unit)
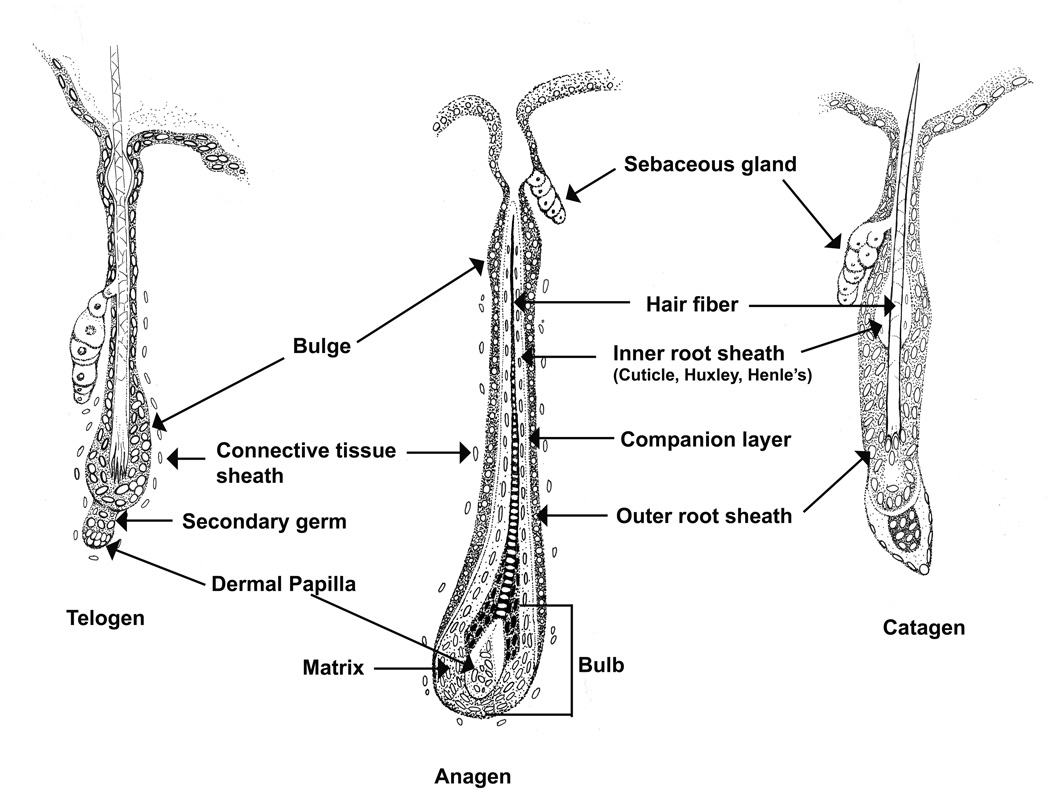
The pilosebaceous unit (PSU) consists of a hair follicle and associated sebaceous gland. Specialized sebaceous glands also exist. The hair follicle contains 5 specific layers of epithelial cells that surround the hair fiber (Figure 2). The outer root sheath is continuous with the basal layer of the epidermis and these layers differentiate as they move inward toward the hair fiber and upward towards the exterior of the skin. The layers include the companion layer, Henle layer of the inner root sheath, Huxley layer of the inner root sheath, and the inner root sheath cuticle. Surrounding the hair follicle is a layer of mesenchymal cells, the connective tissue sheath, which maintains the dermal papilla at the base of the hair follicle. Three types of cells reside in the sebaceous gland (undifferentiated, maturing, and mature sebocytes) [65, 66]. Undifferentiated basal (reserve) cells are located on the entire periphery of the sebaceous gland but only the ones on the bottom are thought to differentiate as they migrate upward (maturing [65]). Lipogenesis occurs as sebocytes differentiate [67]. Mature fully differentiated sebocytes become completely filled with lipid, die, rupture, and release their contents into the hair canal resulting in holocrine secretion of sebum. In cultured cells apoptosis occurred in response to the terminal differentiation marker arachidonic acid [68], suggesting that terminal differentiation and the accumulation of specific lipids may induce apoptosis and aid in the holocrine secretion process. The PSU cycles through four major stages: growth and differentiation (anagen), regression and apoptosis (catagen), rest (telogen), and release of the old hair follicle (exogen) [69].
Figure 2. The hair follicle.
The hair follicle is illustrated with structures labeled as they change during the hair cycle.
Hair follicle stem cells are being tested for use in regenerative medicine allowing a patient to be both the donor of the stem cells and the recipient of these newly differentiated cells [70]. Initial studies localized label retaining cells to the bulge region of the hair follicle [71], Figure 2). These bulge cells are quiescent, marked by CD34 and Keratin 15 (Krt15), and express a unique signature of genes [72, 73]. Results from lineage tracing studies suggest that these bulge stem cells produce all of the cells in the hair follicle during the normal hair cycle, but can repopulate the interfollicular epidermis (IFE) during wound healing (reviewed in [74]). Six additional regions of potential stem cells have since been reported (reviewed in [75]). BLIMP1 marks sebocyte stem cells that sit at the edge of the sebaceous gland duct and give rise to all of the cells of the sebaceous gland, but no other cells in the hair follicle or IFE [76]. Four regions of stem cells were found in the upper isthmus area. Leucine-rich repeats and immunoglobulin-like domain protein 1(LRIG1)+ stem cells localized to the junctional zone between the infundibulum and isthmus [77], while the region just below this (called upper isthmus; UI) contained α6L/CD34−/Sca1− stem cells [78]. Both LRIG+ and UI stem cells also express MTS24, which was independently found to have stem cell like properties [79]. Both LRIG+ and UI cells produced the hair follicle, sebaceous gland, and IFE in skin reconstitution assays; but during homeostasis LRIG+ stem cells only gave rise to sebaceous glands and IFE and UI were not tested. Leucine-rich repeat-containing G protein coupled receptor 6 (LGR6)+ stem cells localized between this region and the bulge [80]. During prenatal development LGR6+ cells gave rise to hair follicle, sebaceous gland, and IFE; but in postnatal and adult skin these stem cells only gave rise to sebaceous gland and IFE cells. Wounding also induced LGR6+ cells to the site of the wound. At the base of the telogen bulge and secondary germ cells sits Leucine-rich repeat-containing G protein coupled receptor 5 (LGR5) stem cells [81]. These LGR5+ cells migrate with the proliferating keratinocytes during early anagen to sit at the base of the bulb and lower outer root sheath. LGR5 localized to the entire outer root sheath during catagen. LGR5+ stem cells produce all of the cells of the hair follicle below the sebaceous gland, including the bulge stem cells. Note that bulge stem cells were also reported to repopulate the secondary germ cells (i.e. LGR5+ cells) at the end of catagen [82]. It was originally believed that the outer root sheath differentiated from the bulge downward, while all other layers of the hair follicle and fiber differentiate from the bulb [83]. The discovery of these LGR5+ stem cells questions this and suggests that the outer root sheath may also differentiate from the bulb. Together the various stem cell populations in the isthmus specify the upper hair follicle and IFE, while the stem cells of the bulge and secondary germ layer specify the cycling portion of the hair follicle during homeostasis. It is still debatable whether all of these cells with stem cell like qualities are truly stem cells or if there is a hierarchy of stem cells like in the hematopoietic system [74, 75]. It was also suggested that all cells of the hair follicle may have stem cell properties in the right environment [84]. When misregulated, these stem cells lead to skin cancers [85].
2.3 Retinoids in the hair follicle
Studies with transgenic mice support a role for RA in the hair follicle. Both a reduction in RA signaling (Krt14 & Krt5 Cre Rxratm4Ipc null mice) and an excess of retinol and atRA (Krt14 Dgat1tm2Far null mice) within the basal epidermis and outer root sheath led to progressive alopecia [6, 86, 87]. Blocking RA signalling (Rxratm4Ipc null mice) delayed anagen initiation, while increasing retinol and atRA (Dgat1tm2Far null mice) accelerated the transition from telogen to anagen. This increased anagen induction and alopecia could be reduced in Dgat1tm2Far null mice by severely reducing dietary vitamin A intake. RXR partners with many nuclear receptors and these effects were originally attributed to its partnering with the vitamin D receptor (VDR), as Vdrtm1Mbd null mice have similar progressive alopecia and anagen inhibition [88, 89]. While it is unlikely that the effects of reduced DGAT1 lead directly to altered vitamin D metabolism or signalling, as they could be reversed by altering dietary vitamin A, an indirect effect of excess vitamin A on vitamin D metabolism or signalling in the hair is possible as interactions between these two vitamins have been seen [90–92]. These Rxratm4Ipc null mice also have elevated proliferation and defective differentiation in IFE and increased inflammation, which were not seen in Vdrtm1Mbd null mice [93], suggesting other RXR partners are involved. Exogenous atRA also induced catagen in cultured hair follicles [94]. In addition, exogenous atRA with BMP directed the differentiation of embryonic [95] and induced pluripotent stem cells into keratinocytes that when grafted into nude mice produced normal epidermis, hair follicles, and sebaceous glands [96]. Transgenic mice that overexpress a dominant negative Rara targeted to the epidermis have aberrant skin, no hair or wrinkles and die shortly after birth [97, 98]. These mice have reduced epidermal barrier function due to defects in lipid metabolism [98, 99]. These effects were not seen in mice that overexpress a dominant negative thyroid receptor [97] or Rxr [100] in the epidermis. In addition, VDR responses were not altered by dominant negative Rara expression, suggesting that these effects are specific to RAR and RA signaling and not other partners of RXR. A similar defect in lipid metabolism and epidermal barrier function was seen in epidermal (Krt14CreERT2) and suprabasal (CMV-CreERT) targeted Rargtm3Ipc null mice [47], although an initial report of epidermal (Krt5) targeted Raratm3Ipc and Rargtm3Ipc double null mice failed to see this effect [101]. No studies have examined epidermal or hair follicle specific Rarb null mice or triple Rar null mice as it was assumed that RARB was not important in the epidermis, as it was not expressed in the nucleus [101] and no skin or hair defects were seen in the original Rarbtm1Ipc null mice [102]. Yet weak expression of RARB was seen in the follicle keratinocytes [103] and we saw cytoplasmic only localization of RARB in the basal epidermis, sebaceous gland, outer root sheath, and hair follicle bulge [24]. In addition, RARs may be important in other cells within the PSU that do not express Krt14 or Krt5. Combined, the results from these studies suggest that atRA alters stem cells to regulate the hair cycle at both the telogen to anagen and anagen to catagen transitions, as well as regulate lipid metabolism in the IFE to maintain epidermal barrier function, but not all of the receptors responsible or specific mechanisms have been elucidated.
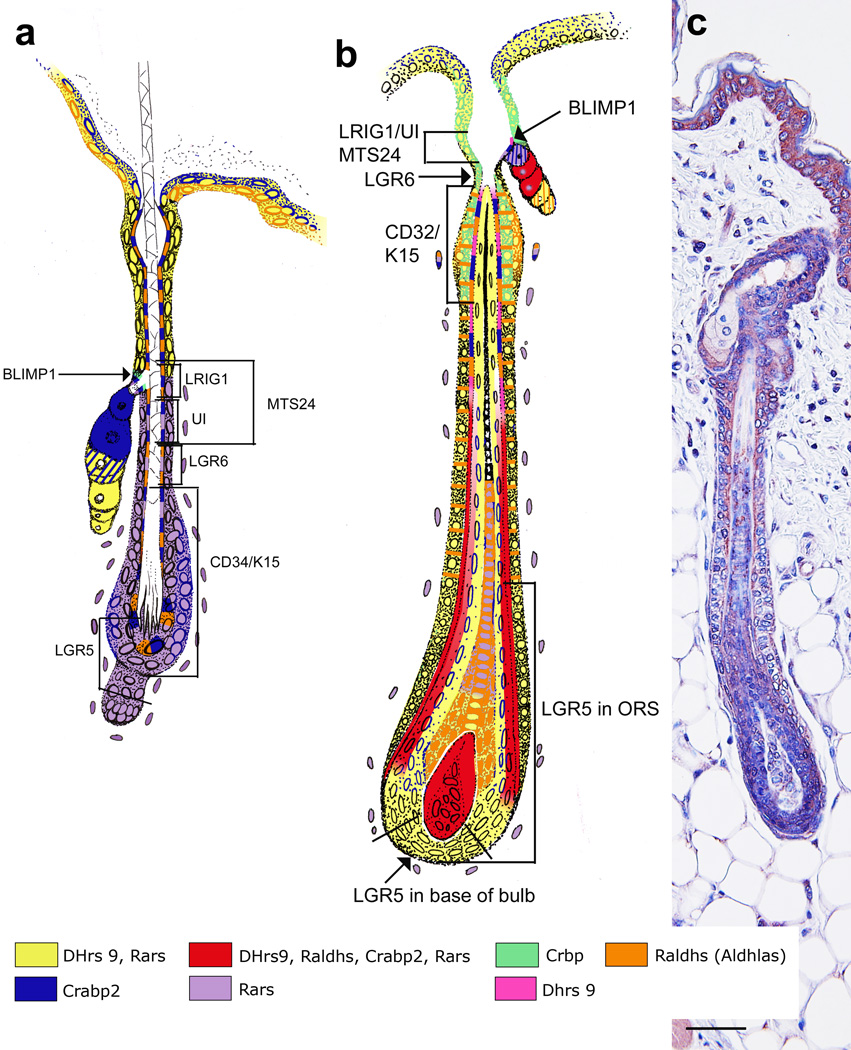
Several reports have localized components of RA synthesis and signaling to the hair follicle in various or unknown stages of the hair cycle [23, 103–106]. To obtain a more complete picture, we localized a whole system of retinoic acid synthesis and signaling proteins to the hair follicle, including all seven stem cell regions (Figure 3, [24, 107]). This localization pattern changed throughout the hair cycle with a peak occurring during mid-anagen through early catagen. We have confirmed this pattern of RA synthesis during mid-anagen using RA reporter mice (Tg(RARE-Hspa1b/lacz)12Jrt/J made by [108]; Figure 3c). RA synthesis was present in the cells of the sebaceous gland duct, the site of BLIMP1+ stem cells, and throughout the whole isthmus, site of MTS24, LRIG+, UI, and LGR6+ stem cells, during mid-anagen (Figure 3b, Red). Topical atRA induced LRIG+ cells to expand and give rise to cells in the sebaceous gland, infundibulum, and IFE [77]. Thus, endogenous RA synthesized within the isthmus may be important for the maintenance of the sebaceous gland, infundibulum, and IFE. In addition, wounds heal faster when hair follicles are in anagen [109], thus the peak of RA synthesis within the isthmus during anagen may also contribute to better wound healing. RA synthesis also localized to the bulge region during mid-anagen (Fig 3c) and some RA synthesis enzymes and binding proteins remained in the bulge throughout the hair cycle ([24], Fig 3a,b). RA synthesis enzymes (DHRS9 and ALDH1A2) and RARA, B, and G localized to proliferating keratinocytes as they migrated downward during early anagen, and RA synthesis was seen in the outer and lower cells of the bulb, where LGR5+ stem cells also localized [24, 81]. During late anagen/early catagen there was a drop in the expression of RARs within the bulb and an increase in CRBP, suggesting a role in catagen induction. These localization patterns suggest that RA may also play a role in the maintenance of hair follicles and its cycle. Together, the results from these studies suggest that endogenous RA may be important for all of the stem cells in the hair follicle and the maintenance of the hair follicle, sebaceous gland, and IFE, although the specific mechanisms of these effects are yet to be determined.
Figure 3. Retinoic acid synthesis in the hair follicle.
Telogen (a) and anagen (b) hair follicles are color coded with retinoic acid synthesis components: DHRS9 plus RARs (yellow), DHRS9 alone (pink), RARs alone (purple), CRABP2 (blue), CRBP (green), ALDH1A1, 2, or 3 (orange), a complete system with DHRS9, ALDH1A1, 2, or 3, CRABP2, and RARa, b,or g (red). Stem cell locations are marked in relation to this localization pattern of RA synthesis components. (c) RA synthesis as determined by immunohistochemistry with an antibody against beta-galactosidase in RA reporter mice (Tg(RARE-Hspa1b/lacz)12Jrt/J). Modified from Everts et al [24].
Excess RA was found to reduce the number and length of hair follicles in vitro [110]. Feather specification and dermal condensations were also inhibited by excess RA, which altered the pattern of Hox gene expression and NCAM in the developing feather, a structure similar to the mammalian hair follicle [111]. In addition, Dgat1tm2FarTg(KRT14-cre)1AMC null mice have cyclical hair loss that is restored by reducing dietary vitamin A, as discussed above [6]. We found increased expression of RA synthesis enzymes and binding proteins in biopsies from patients with several hair loss diseases and skin from their mouse models, including central centrifugal cicatricial alopecia (CCCA) and alopecia areata (AA; HB Everts, LE King Jr, unpublished observation). Flowers et al [112] also found increased retinol, atRA, retinyl esters and mRNA of retinoid binding proteins and target genes in one of these animal models. Primary cicatricial alopecias are a collection of scarring hair loss diseases that result in inflammatory attack of the hair follicle, their stem cells, and permanent hair loss [113]. The cause of this disease is unknown but theories include altered sebaceous gland function with reduced sebum [114], reduced PPARG [115], structural defects in the hair follicle [114], and loss of immune privilege in the hair follicle [116]. Alopecia areata is an autoimmune non-scarring hair loss disease that is mediated by CD8+ T cell attack on the lower cycling hair follicle [117–119] and a loss of immune privilege in the hair follicle [120–122]. We also found that reducing dietary vitamin A prevented cicatricial alopecia and delayed the onset of alopecia areata in mouse models (HB Everts, JP Sundberg, unpublished observation). Future studies are needed to better understand the mechanisms of RA’s function within the hair follicle to produce better treatments for these hair loss diseases.
2.4 Retinoids in the sebaceous gland
Results from early studies suggested a potential therapeutic role for oral retinyl palmitate in the treatment of psoriasis (reviewed in [123]). But this led to severe hypervitaminoisos A and was abandoned. To produce effective results with fewer side effects topical synthetic atRA (tretinoin, Retin-A) and oral synthetic 13-cis RA (13cRA; Accutane) were developed (reviewed in [123]). Retinoids (i.e. Accutane, Retin-A™) are the most effective and first choice for acne treatment [124], but still have significant side effects ranging from teratogenesis [125] to telogen effluvium [7]. Even after 25+ years of use the mechanism of action of retinoids in acne is still unclear. Results from an initial study of acne patients revealed that 13cRA significantly reduced the size of the sebaceous gland and inhibited sebocyte differentiation [126]. Numerous in vitro studies have been performed to better understand this mechanism (reviewed in [8, 9]). Lack of vitamin A in cultured human sebocytes resulted in decreased cell proliferation and lipogenesis that could be partially restored with low doses of 13cRA [127]. At concentrations above 10−7M 13cRA and atRA inhibited proliferation and lipogenesis [127–130]. Thus, while low levels of RA are important for sebaceous gland function, excess RA synthesis within the sebaceous gland could lead to atrophy of the gland, and reduced sebum production.
More recent studies suggest that 13cRA treats acne by both RAR dependent and independent mechanisms. 13cRA does not bind to RARs, but was shown to isomerizes to atRA in culture [131] and RARB and/or RARG were reported to mediate the antiproliferative and antidifferentiative affects of retinoids [132]. 13cRA induced apoptosis after 48 and 72 hours independent of RARs [133]. This long time frame suggests an indirect mechanism. In addition to inhibiting genes involved in lipid metabolism [134], 13cRA also inhibits androgen synthesis [135], FGFR2 signaling (reviewed in [136]), and genes involved in arachidonic acid generation [134, 137], which have all been implicated in acne pathogenesis. In contrast, 13cRA induces genes encoding extracellular matrix proteins involved in wound healing and tissue remodeling including collagens, fibronectin, neutrophil gelatinase associated lipocalin, and matrix metalloproteinases [134, 137, 138]. Thus, 13cRA uses multiple mechanisms to reduce sebum and treat acne, future studies will focus on altering these downstream effects of retinoids directly to treat acne with fewer side effects than current retinoid therapies, although an additional option would be to directly regulate RA synthesis within the sebocytes.
We localized RA synthesizing enzymes (DHRS9 and ALDH1a1–3) and cytoplasmic RARB to the basal and early maturing cells with decreased intensity as the sebocytes matured (Figure 3) [24]. Cytoplasmic localization of CRABP2 and RARA were seen in the early maturing sebocytes, while these proteins were nuclear localized in the most differentiated mature cells. This suggests something blocked nuclear localization of CRABP2 and RARA in basal sebocytes, or RA synthesis in the presence of enzymes DHRS9, ALDH1A1, and ALDH1A3. In testis cell lines, PPARA agonist inhibited nuclear localization of RARA, while promoting nuclear localization of PPARA [139]. PPARA, PPARB/D, and PPARG are all expressed in sebocytes [140] and PPARG localized to the basal sebocytes in adult scalp skin [141]. Collins and Watt (2008) [142] also saw nuclear localization of CRABP2 in mature sebocytes, as well as FABP5 nuclear localization in the maturing sebocytes. This would suggest that RA made in the maturing sebocytes could activate PPARD. But we also saw cytoplasmic RARB in these RA synthesizing cells suggesting a potential non-genomic effect, as seen in other tissues (reviewed in [143]). Wnt signaling is normally low in the sebaceous gland and ectopic expression of Wnt signaling mediator beta-catenin inhibited CRABP2 and FABP5 expression in the sebaceous gland [142]. The localization of RA synthesis to the sebaceous gland may also be a way to send CRABP2 bound RA to the surface of the epidermis. Vitamin E was delivered to the stratum corneum of the epidermis by this mechanism of holocrine sebum secretion [144]. All the enzymes necessary for Vitamin D metabolism were also found in the sebaceous gland [145]. Thus, sebum may contain numerous fat-soluble vitamins and the secretion of sebum may deliver all of these vitamins to the surface of the skin and help in barrier function. Future studies are needed to distinguish these potential mechanisms of endogenous RA synthesis within the sebaceous gland. Combined, the results from these studies suggest that precise levels of RA are required for optimal sebum production and that this may be achieved by endogenous synthesis of RA within the sebocyte, as all of the necessary enzymes are present.
3.1 Summary and Future Studies
The PSU is a complex mini-organ that is regulated by the same signaling factors that regulate development of other organs. Similar to organogenesis in other sites, RA synthesis enzymes, binding proteins, receptors, and RA were seen in a specific temporal and spatial pattern. This localization pattern and the fact that both vitamin A deficiency and toxicity lead to defects in the PSU suggest that RA is important for its function. Older work in the field laid the groundwork, but the specific mechanisms by which RA regulates the PSU are just beginning to be dissected. Future studies are needed to determine the mechanisms by which: RA alters all of the stems cells in the PSU, RA regulates differentiation of the PSU, and RA regulates sebaceous gland function. In addition, future studies are needed to determine what signaling factors regulate RA synthesis and how modulating RA synthesis can be used to treat various diseases of the PSU such as CCCA, AA, and acne.
Highlights.
The pilosebaceous unit consists of a hair follicle and sebaceous gland.
The pilosebaceous unit requires precise levels of retinoic acid.
Retinoic acid synthesis localized to the pilosebaceous unit, including stem cells.
Mechanisms of action of retinoic acid within the pilosebaceous unit are emerging.
But much more information has yet to be discovered.
Acknowledgements
The author thanks Ingrid Sundberg (www.sundbergstudio.com) for drawing the hair follicle illustrations. This work was supported by grants from the National Institutes of Health (AR052009), the National Alopecia Areata Foundation, and the North American Hair Research Society.
Abbreviations
- RA
retinoic acid
- atRA
all-trans retinoic acid
- 9cRA
9-cis retinoic acid
- 13cRA
13-cis retinoic acid
- PSU
pilosebaceous unit
- IFE
interfollicular epidermis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Wolbach SB, Howe PR. Tissue changes following deprivation of fat-soluble A vitamin. J. Exp. Med. 1925;42:753–777. doi: 10.1084/jem.42.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girard C, Dereure O, Blatiere V, Guillot B, Bessis D. Vitamin A deficiency phrynoderma associated with chronic giardiasis. Pediatr. Dermatol. 2006;23:346–349. doi: 10.1111/j.1525-1470.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- 3.Anzano MA, Lamb AJ, Olson JA. Growth, appetite, sequence of pathological signs and survival following the induction of rapid, synchronous vitamin A deficiency in the rat. J. Nutr. 1979;109:1419–1431. doi: 10.1093/jn/109.8.1419. [DOI] [PubMed] [Google Scholar]
- 4.Everts HB, Berdanier CD. Nutrient-gene interactions in mitochondrial function: vitamin A needs are increased in BHE/Cdb rats. IUBMB Life. 2002;53:289–294. doi: 10.1080/15216540213464. [DOI] [PubMed] [Google Scholar]
- 5.Ries G, Hess R. Retinol: Safety considerations for its use in cosmetic products. J Toxicol., Cutaneous Ocul. Toxicol. 1999;18:169–185. [Google Scholar]
- 6.Shih MYS, Kane MA, Zhou P, Yen CLE, Streeper RS, Napoli JL, Farese RV. Retinol Esterification by DGAT1 Is Essential for Retinoid Homeostasis in Murine Skin. J. Biol. Chem. 2009;284:4292–4299. doi: 10.1074/jbc.M807503200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruzicka T, Sommerburg C, Goerz G, Kind P, Mensing H. Treatment of cutaneous lupus-erythematosus with acitretin and hydroxychloroquine. Br. J. Dermatol. 1992;127:513–518. doi: 10.1111/j.1365-2133.1992.tb14851.x. [DOI] [PubMed] [Google Scholar]
- 8.Zouboulis CC, Schagen S, Alestas T. The sebocyte culture: a model to study the pathophysiology of the sebaceous gland in sebostasis, seborrhoea and acne. Arch. Dermatol. Res. 2008;300:397–413. doi: 10.1007/s00403-008-0879-5. [DOI] [PubMed] [Google Scholar]
- 9.Smith KR, Thiboutot DM. Sebaceous gland lipids: friend or foe? J. Lipid Res. 2008;49:271–281. doi: 10.1194/jlr.R700015-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Kurlandsky SB, Gamble MV, Ramakrishnan R, Blaner WS. Plasma delivery of retinoic acid to tissues in the rat. J. Biol. Chem. 1995;270:17850–17857. doi: 10.1074/jbc.270.30.17850. [DOI] [PubMed] [Google Scholar]
- 11.Napoli JL. Physiological insights into all-trans-retinoic acid biosynthesis. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids. 2011 doi: 10.1016/j.bbalip.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar S, Sandell LL, Trainer PA, Koentgen F, Duester G. Alcohol and aldehyde dehydrogenases: Retinoid metabolic effects in mouse knockout models. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids. 2011 doi: 10.1016/j.bbalip.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blaner WS, Gamble MV, Vogel S, Piantedosi R, Paik J, Gottesman ME. Retinol-binding protein (RBP): Essential physiologic functions. J. Nutr. 2002;132:2979S–2979S. [Google Scholar]
- 14.Blomhoff R, Green MH, Green JB, Berg T, Norum KR. Vitamin-A metabolism - New perspectives on absorption, transport, and storage. Physiol. Rev. 1991;71:951–990. doi: 10.1152/physrev.1991.71.4.951. [DOI] [PubMed] [Google Scholar]
- 15.Kawaguchi R, Yu JM, Honda J, Hu J, Whitelegge J, Ping PP, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald PN, Ong DE. A lecithin:retinol acyltransferase activity in human and rat liver. Biochem. Biophys. Res. Commun. 1988;156:157–163. doi: 10.1016/s0006-291x(88)80818-0. [DOI] [PubMed] [Google Scholar]
- 17.Napoli JL. Interactions of retinoid binding proteins and enzymes in retinoid metabolism. Biochim. Biophys. Acta. 1999;1440:139–162. doi: 10.1016/s1388-1981(99)00117-1. [DOI] [PubMed] [Google Scholar]
- 18.Kurlandsky SB, Xiao JH, Duell EA, Voorhees JJ, Fisher GJ. Biological activity of all-trans retinol requires metabolic conversion to all-trans retinoic acid and is mediated through activation of nuclear retinoid receptors in human keratinocytes. J. Biol. Chem. 1994;269:32821–32827. [PubMed] [Google Scholar]
- 19.Matsuura T, Ross AC. Regulation of hepatic lecithin: retinol acyltransferase activity by retinoic acid. Arch. Biochem. Biophys. 1993;301:221–227. doi: 10.1006/abbi.1993.1137. [DOI] [PubMed] [Google Scholar]
- 20.Kurlandsky SB, Duell EA, Kang S, Voorhees JJ, Fisher GJ. Auto-regulation of retinoic acid biosynthesis through regulation of retinol esterification in human keratinocytes. J. Biol. Chem. 1996;271:15346–15352. doi: 10.1074/jbc.271.26.15346. [DOI] [PubMed] [Google Scholar]
- 21.Ross AC. Retinoid production and catabolism: role of diet in regulating retinol esterification and retinoic Acid oxidation. J. Nutr. 2003;133:291S–296S. doi: 10.1093/jn/133.1.291S. [DOI] [PubMed] [Google Scholar]
- 22.Rexer BN, Ong DE. A novel short-chain alcohol dehydrogenase from rats with retinol dehydrogenase activity, cyclically expressed in uterine epithelium. Biol. Reprod. 2002;67:1555–1564. doi: 10.1095/biolreprod.102.007021. [DOI] [PubMed] [Google Scholar]
- 23.Markova NG, Pinkas-Sarafova A, Karaman-Jurukovska N, Jurukovski V, Simon M. Expression pattern and biochemical characteristics of a major epidermal retinol dehydrogenase. Mol. Genet. Metab. 2003;78:119–135. doi: 10.1016/s1096-7192(02)00226-3. [DOI] [PubMed] [Google Scholar]
- 24.Everts HB, Sundberg JP, King LE, Jr, Ong DE. Immunolocalization of enzymes, binding proteins, and receptors sufficient for retinoic acid synthesis and signaling during the hair cycle. J. Invest. Dermatol. 2007;127:1593–1604. doi: 10.1038/sj.jid.5700753. [DOI] [PubMed] [Google Scholar]
- 25.Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- 26.Dupe V, Matt N, Garnier JM, Chambon P, Mark M, Ghyselinck NB. A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc. Natl. Acad. Sci. U. S. A. 2003;100:14036–14041. doi: 10.1073/pnas.2336223100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong D, Ruuska SE, Levinthal DJ, Noy N. Distinct roles for cellular retinoic acid-binding proteins I and II in regulating signaling by retinoic acid. J. Biol. Chem. 1999;274:23695–23698. doi: 10.1074/jbc.274.34.23695. [DOI] [PubMed] [Google Scholar]
- 28.Budhu AS, Noy N. Direct channeling of retinoic acid between cellular retinoic acid-binding protein II and retinoic acid receptor sensitizes mammary carcinoma cells to retinoic acid-induced growth arrest. Mol. Cell. Biol. 2002;22:2632–2641. doi: 10.1128/MCB.22.8.2632-2641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sessler RJ, Noy N. A ligand-activated nuclear localization signal in cellular retinoic acid binding protein-II. Mol. Cell. 2005;18:343–353. doi: 10.1016/j.molcel.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 30.Bucco RA, Zheng WL, Davis JT, Sierra-Rivera E, Osteen KG, Chaudhary AK, Ong DE. Cellular retinoic acid-binding protein (II) presence in rat uterine epithelial cells correlates with their synthesis of retinoic acid. Biochemistry. 1997;36:4009–4014. doi: 10.1021/bi962094o. [DOI] [PubMed] [Google Scholar]
- 31.Zheng WL, Bucco RA, Sierra-Rievera E, Osteen KG, Melner MH, Ong DE. Synthesis of retinoic acid by rat ovarian cells that express cellular retinoic acid-binding protein-II. Biol. Reprod. 1999;60:110–114. doi: 10.1095/biolreprod60.1.110. [DOI] [PubMed] [Google Scholar]
- 32.Everts HB, Sundberg JP, Ong DE. Immunolocalization of retinoic acid biosynthesis systems in selected sites in rat. Exp. Cell Res. 2005;308:309–319. doi: 10.1016/j.yexcr.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 33.Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rieck M, Meissner W, Ries S, Mueller-Brusselbach S, Muller R. Ligand-Mediated Regulation of Peroxisome Proliferator-Activated Receptor (PPAR) beta/delta: A Comparative Analysis of PPAR-Selective Agonists and All-trans Retinoic Acid. Mol. Pharmacol. 2008;74:1269–1277. doi: 10.1124/mol.108.050625. [DOI] [PubMed] [Google Scholar]
- 35.Borland MG, Foreman JE, Girroir EE, Zolfaghari R, Sharma AK, Amin S, Gonzalez FJ, Ross AC, Peters JM. Ligand Activation of Peroxisome Proliferator-Activated Receptor-beta/delta Inhibits Cell Proliferation in Human HaCaT Keratinocytes. Mol. Pharmacol. 2008;74:1429–1442. doi: 10.1124/mol.108.050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fiorella PD, Napoli JL. Microsomal retinoic acid metabolism. Effects of cellular retinoic acid-binding protein (type I) and C18-hydroxylation as an initial step. J. Biol. Chem. 1994;269:10538–10544. [PubMed] [Google Scholar]
- 37.Boylan JF, Gudas LJ. The level of Crabp-I expression influences the amounts and types of all-trans-retinoic acid metabolites in F9 teratocarcinoma stem-cells. J. Biol. Chem. 1992;267:21486–21491. [PubMed] [Google Scholar]
- 38.Chen AC, Yu K, Lane MA, Gudas LJ. Homozygous deletion of the CRABPI gene in AB1 embryonic stem cells results in increased CRABPII gene expression and decreased intracellular retinoic acid concentration. Arch. Biochem. Biophys. 2003;411:159–173. doi: 10.1016/s0003-9861(02)00732-4. [DOI] [PubMed] [Google Scholar]
- 39.Baron JM, Heise R, Blaner WS, Neis M, Joussen S, Dreuw A, Marquardt Y, Saurat JH, Merk HF, Bickers DR, Jugert FK. Retinoic acid and its 4-oxo metabolites are functionally active in human skin cells in vitro. J. Invest. Dermatol. 2005;125:143–153. doi: 10.1111/j.0022-202X.2005.23791.x. [DOI] [PubMed] [Google Scholar]
- 40.Niederreither K, Abu-Abed S, Schuhbaur B, Petkovich M, Chambon P, Dolle P. Genetic evidence that oxidative derivatives of retinoic acid are not involved in retinoid signaling during mouse development. Nat. Genet. 2002;31:84–88. doi: 10.1038/ng876. [DOI] [PubMed] [Google Scholar]
- 41.Sorg O, Tran C, Carraux P, Grand D, Barraclough C, Arrighi J-F, Descombes P, Piguet V, Saurat J-H. Metabolism and biological activities of topical 4-oxoretinoids in mouse skin. J. Invest. Dermatol. 2008;128:999–1008. doi: 10.1038/sj.jid.5701106. [DOI] [PubMed] [Google Scholar]
- 42.Petkovich M, Brand NJ, Krust A, Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987;330:444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- 43.Chambon P. A decade of molecular biology of retinoic acid receptors. Faseb J. 1996;10:940–954. [PubMed] [Google Scholar]
- 44.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J. Lipid Res. 2002;43:1773–1808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- 45.Mangelsdorf DJ, Ong ES, Dyck JA, Evans RM. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990;345:224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- 46.Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, Thaller C. 9-cis retinoic acid is a high-affinity ligand for the retinoid-X receptor. Cell. 1992;68:397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- 47.Calleja C, Messaddeq N, Chapellier B, Yang HY, Krezel W, Li M, Metzger D, Mascrez B, Ohta K, Kagechika H, Endo Y, Mark M, Ghyselinck NB, Chambon P. Genetic and pharmacological evidence that a retinoic acid cannot be the RXR-activating ligand in mouse epidermis keratinocytes. Genes Dev. 2006;20:1525–1538. doi: 10.1101/gad.368706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lengqvist J, de Urquiza AM, Bergman AC, Willson TM, Sjovall J, Perlmann T, Griffiths WJ. Polyunsaturated fatty acids including docosahexaenoic and arachidonic acid bind to the retinoid X receptor alpha ligand-binding domain. Mol. Cell. Proteomics. 2004;3:692–703. doi: 10.1074/mcp.M400003-MCP200. [DOI] [PubMed] [Google Scholar]
- 49.de Urquiza AM, Liu SY, Sjoberg M, Zetterstrom RH, Griffiths W, Sjovall J, Perlmann T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 50.Ross AC. Retinol esterification by rat-liver microsomes - evidence for a fatty acyl coenzyme-A-retinol acyltransferase. J. Biol. Chem. 1982;257:2453–2459. [PubMed] [Google Scholar]
- 51.O'Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, Palczewski K, Blaner WS. Retinoid absorption and storage is impaired in mice lacking lecithin : retinol acyltransferase (LRAT) J. Biol. Chem. 2005;280:35647–35657. doi: 10.1074/jbc.M507924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orland MD, Anwar K, Cromley D, Chu CH, Chen LP, Billheimer JT, Hussain MM, Cheng D. Acyl coenzyme A dependent retinol esterification by acyl coenzyme A : diacylglycerol acyltransferase 1. Biochim. Biophys. Acta. 2005;1737:76–82. doi: 10.1016/j.bbalip.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Yen CLE, Monetti M, Burri BJ, Farese RV. The triacylglycerol synthesis enzyme DGAT1 also catalyzes the synthesis of diacylglycerols, waxes, and retinyl esters. J. Lipid Res. 2005;46:1502–1511. doi: 10.1194/jlr.M500036-JLR200. [DOI] [PubMed] [Google Scholar]
- 54.Wongsiriroj N, Piantedosi R, Palczewski K, Goldberg IJ, Johnston TP, Li E, Blaner WS. The molecular basis of retinoid absorption - A genetic dissection. J. Biol. Chem. 2008;283:13510–13519. doi: 10.1074/jbc.M800777200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ong DE, MacDonald PN, Gubitosi AM. Esterification of retinol in rat liver. Possible participation by cellular retinol-binding protein and cellular retinol-binding protein II. J. Biol. Chem. 1988;263:5789–5796. [PubMed] [Google Scholar]
- 56.Herr FM, Ong DE. Differential interaction of lecithin-retinol acyltransferase with cellular retinol binding proteins. Biochemistry. 1992;31:6748–6755. doi: 10.1021/bi00144a014. [DOI] [PubMed] [Google Scholar]
- 57.Lorie EP, Li H, Vahlquist A, Torma H. The involvement of cytochrome p450 (CYP) 26 in the retinoic acid metabolism of human epidermal keratinocytes. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids. 2009;1791:220–228. doi: 10.1016/j.bbalip.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Duester G. Genetic dissection of retinoid dehydrogenases. Chem.-Biol. Interact. 2001;130:469–480. doi: 10.1016/s0009-2797(00)00292-1. [DOI] [PubMed] [Google Scholar]
- 59.Molotkov A, Fan X, Duester G. Excessive vitamin A toxicity in mice genetically deficient in either alcohol dehydrogenase Adh1 or Adh3. Eur. J. Biochem. 2002;269:2607–2612. doi: 10.1046/j.1432-1033.2002.02935.x. [DOI] [PubMed] [Google Scholar]
- 60.Fan X, Molotkov A, Manabe S, Donmoyer CM, Deltour L, Foglio MH, Cuenca AE, Blaner WS, Lipton SA, Duester G. Targeted disruption of Aldh1a1 (Raldh1) provides evidence for a complex mechanism of retinoic acid synthesis in the developing retina. Mol. Cell. Biol. 2003;23:4637–4648. doi: 10.1128/MCB.23.13.4637-4648.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Molotkov A, Duester G. Genetic evidence that retinaldehyde dehydrogenase Raldh1 (Aldh1a1) functions downstream of alcohol dehydrogenase Adh1 in metabolism of retinol to retinoic acid. J. Biol. Chem. 2003;278:36085–36090. doi: 10.1074/jbc.M303709200. [DOI] [PubMed] [Google Scholar]
- 62.Deltour L, Foglio MH, Duester G. Impaired retinol utilization in Adh4 alcohol dehydrogenase mutant mice. Dev. Genet. 1999;25:1–10. doi: 10.1002/(SICI)1520-6408(1999)25:1<1::AID-DVG1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 63.Deltour L, Foglio MH, Duester G. Metabolic deficiencies in alcohol dehydrogenase Adh1, Adh3, and Adh4 null mutant mice. Overlapping roles of Adh1 and Adh4 in ethanol clearance and metabolism of retinol to retinoic acid. J. Biol. Chem. 1999;274:16796–16801. doi: 10.1074/jbc.274.24.16796. [DOI] [PubMed] [Google Scholar]
- 64.Haselbeck RJ, Ang HL, Duester G. Class IV alcohol/retinol dehydrogenase localization in epidermal basal layer: Potential site of retinoic acid synthesis during skin development. Dev. Dyn. 1997;208:447–453. doi: 10.1002/(SICI)1097-0177(199704)208:4<447::AID-AJA1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 65.Jenkinson DM, Elder HY, Montgomery I, Moss VA. Comparative studies of the ultrastructure of the sebaceous gland. Tissue & Cell. 1985;17:683–698. doi: 10.1016/0040-8166(85)90004-7. [DOI] [PubMed] [Google Scholar]
- 66.Thody AJ, Shuster S. Control and Function of Sebaceous Glands. Physiol. Rev. 1989;69:383–416. doi: 10.1152/physrev.1989.69.2.383. [DOI] [PubMed] [Google Scholar]
- 67.Tosti A. Comparison of Histodynamics of Sebaceous Glands and Epidermis in Man - Microanatomic and Morphometric Study. J. Invest. Dermatol. 1974;62:147–152. doi: 10.1111/1523-1747.ep12676779. [DOI] [PubMed] [Google Scholar]
- 68.Wrobel A, Seltmann H, Fimmel S, Muller-Decker K, Tsukada M, Bogdanoff B, Mandt N, Blume-Peytavi U, Orfanos CE, Zouboulis CC. Differentiation and apoptosis in human immortalized sebocytes. J. Invest. Dermatol. 2003;120:175–181. doi: 10.1046/j.1523-1747.2003.12029.x. [DOI] [PubMed] [Google Scholar]
- 69.Schneider MR, Schmidt-Ullrich R, Paus R. The Hair Follicle as a Dynamic Miniorgan. Curr. Biol. 2009;19:R132–R142. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 70.Amoh Y, Katsuoka K, Hoffman RM. The advantages of hair follicle pluripotent stem cells over embryonic stem cells and induced pluripotent stem cells for regenerative medicine. J. Dermatol. Sci. 2010;60:131–137. doi: 10.1016/j.jdermsci.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 71.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 72.Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 73.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cotsarelis G. Epithelial stem cells: A folliculocentric view. J. Invest. Dermatol. 2006;126:1459–1468. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- 75.Jaks V, Kasper M, Toftgard R. The hair follicle-a stem cell zoo. Exp. Cell Res. 2010;316:1422–1428. doi: 10.1016/j.yexcr.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 76.Horsley V, O'Carroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T, Nussenzweig M, Tarakhovsky A, Fuchs E. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, Watt FM. Lrig1 Expression Defines a Distinct Multipotent Stem Cell Population in Mammalian Epidermis. Cell Stem Cell. 2009;4:427–439. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jensen UB, Yan X, Triel C, Woo SH, Christensen R, Owens DM. A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. J. Cell Sci. 2008;121:609–617. doi: 10.1242/jcs.025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nijhof JGW, Braun KM, Giangreco A, van Pelt C, Kawamoto H, Boyd RL, Willemze R, Mullenders LHF, Watt FM, de Gruijl FR, van Ewijk W. The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development. 2006;133:3027–3037. doi: 10.1242/dev.02443. [DOI] [PubMed] [Google Scholar]
- 80.Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG, Stange DE, Toftgard R, Clevers H. Lgr6 Marks Stem Cells in the Hair Follicle That Generate All Cell Lineages of the Skin. Science. 2010;327:1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- 81.Jaks V, Barker N, Kasper M, Van Es JH, Snippert HJ, Clevers H, Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 82.Ito M, Kizawa K, Hamada K, Cotsarelis G. Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation. 2004;72:548–557. doi: 10.1111/j.1432-0436.2004.07209008.x. [DOI] [PubMed] [Google Scholar]
- 83.Legue E, Nicolas JF. Hair follicle renewal: organization of stem cells in the matrix and the role of stereotyped lineages and behaviors. Development. 2005;132:4143–4154. doi: 10.1242/dev.01975. [DOI] [PubMed] [Google Scholar]
- 84.Watt FM, Jensen KB. Epidermal stem cell diversity and quiescence. Embo Mol. Med. 2009;1:260–267. doi: 10.1002/emmm.200900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim DJ, Kataoka K, Rao D, Kiguchi K, Cotsarelis G, DiGiovanni J. Targeted Disruption of Stat3 Reveals a Major Role for Follicular Stem Cells in Skin Tumor Initiation. Cancer Res. 2009;69:7587–7594. doi: 10.1158/0008-5472.CAN-09-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li M, Indra AK, Warot X, Brocard J, Messaddeq N, Kato S, Metzger D, Chambon P. Skin abnormalities generated by temporally controlled RXRalpha mutations in mouse epidermis. Nature. 2000;407:633–636. doi: 10.1038/35036595. [DOI] [PubMed] [Google Scholar]
- 87.Li M, Chiba H, Warot X, Messaddeq N, Gerard C, Chambon P, Metzger D. RXR alpha ablation in skin keratinocytes results in alopecia and epidermal alterations. Development. 2001;128:675–688. doi: 10.1242/dev.128.5.675. [DOI] [PubMed] [Google Scholar]
- 88.Li YC, Pirro AE, Amling M, Delling G, Baroni R, Bronson R, DeMay MB. Targeted ablation of the vitamin D receptor: An animal model of vitamin D-dependent rickets type II with alopecia. Proc. Nat. Acad. Sci. U.S.A. 1997;94:9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bikle DD, Elalieh H, Chang S, Xie Z, Sundberg JP. Development and progression of alopecia in the vitamin D receptor null mouse. J. Cell Physiol. 2006;207:340–353. doi: 10.1002/jcp.20578. [DOI] [PubMed] [Google Scholar]
- 90.Cao X, Teitelbaum SL, Zhu HJ, Zhang L, Feng X, Ross FP. Competition for a unique response element mediates retinoic acid inhibition of vitamin D3-stimulated transcription. J. Biol. Chem. 1996;271:20650–20654. doi: 10.1074/jbc.271.34.20650. [DOI] [PubMed] [Google Scholar]
- 91.Jugert FK, Roos TC, Notzon I, Merk HF. Vitamin D3 and its synthetic analogue secocholestra-trien-1,2, 24-triol influence the metabolism and the isomerization of retinoic acid in human keratinocytes. Skin Pharmacol. Appl. Skin Physiol. 1998;11:161–165. doi: 10.1159/000029822. [DOI] [PubMed] [Google Scholar]
- 92.Koszewski NJ, Herberth J, Malluche HH. Retinoic acid receptor gamma 2 interactions with vitamin D response elements. J. Steroid Bioch. Mol. Biol. 2010;120:200–207. doi: 10.1016/j.jsbmb.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 93.Sakai Y, Demay MB. Evaluation of keratinocyte proliferation and differentiation in vitamin D receptor knockout mice. Endocrinology. 2000;141:2043–2049. doi: 10.1210/endo.141.6.7515. [DOI] [PubMed] [Google Scholar]
- 94.Foitzik K, Spexard T, Nakamura M, Halsner U, Paus R. Towards dissecting the pathogenesis of retinoid-induced hair loss: All-trans retinoic acid induces premature hair follicle regression (catagen) by upregulation of transforming growth factor-beta 2 in the dermal papilla. J. Invest. Dermatol. 2005;124:1119–1126. doi: 10.1111/j.0022-202X.2005.23686.x. [DOI] [PubMed] [Google Scholar]
- 95.Metallo CM, Ji L, De Pablo JJ, Palecek SP. Retinoic acid and bone morphogenetic protein signaling synergize to efficiently direct epithelial differentiation of human embryonic stem cells. Stem Cells. 2008;26:372–380. doi: 10.1634/stemcells.2007-0501. [DOI] [PubMed] [Google Scholar]
- 96.Bilousova G, Chen JA, Roop DR. Differentiation of Mouse Induced Pluripotent Stem Cells into a Multipotent Keratinocyte Lineage. J. Invest. Dermatol. 2011;131:857–864. doi: 10.1038/jid.2010.364. [DOI] [PubMed] [Google Scholar]
- 97.Saitou M, Sugai S, Tanaka T, Shimouchi K, Fuchs E, Narumiya S, Kakizuka A. Inhibition of Skin Development by Targeted Expression of a Dominant-Negative Retinole Acid Receptor. Nature. 1995;374:159–162. doi: 10.1038/374159a0. [DOI] [PubMed] [Google Scholar]
- 98.Imakado S, Bickenbach JR, Bundman DS, Rothnagel JA, Attar PS, Wang XJ, Walczak VR, Wisniewski S, Pote J, Gordon JS, Heyman RA, Evans RM, Roop DR. Targeting Expression of a Dominant-Negative Retinoic Acid Receptor Mutant in the Epidermis of Transgenic Mice Results in Loss of Barrier Function. Genes Dev. 1995;9:317–329. doi: 10.1101/gad.9.3.317. [DOI] [PubMed] [Google Scholar]
- 99.Attar PS, Wertz PW, McArthur M, Imakado S, Bickenbach JR, Roop DR. Inhibition of retinoid signaling in transgenic mice alters lipid processing and disrupts epidermal barrier function. Mol. Endocrinol. 1997;11:792–800. doi: 10.1210/mend.11.6.0010. [DOI] [PubMed] [Google Scholar]
- 100.Feng X, Peng ZH, Di W, Li XY, RochetteEgly C, Chambon P, Voorhees JJ, Xiao JH. Suprabasal expression of a dominant-negative RXR alpha mutant in transgenic mouse epidermis impairs regulation of gene transcription and basal keratinocyte proliferation by RAR-selective retinoids. Genes Dev. 1997;11:59–71. doi: 10.1101/gad.11.1.59. [DOI] [PubMed] [Google Scholar]
- 101.Chapellier B, Mark M, Messaddeq N, Calleja C, Warot X, Brocard J, Gerard C, Li M, Metzger D, Ghyselinck NB, Chambon P. Physiological and retinoid-induced proliferations of epidermis basal keratinocytes are differently controlled. Embo J. 2002;21:3402–3413. doi: 10.1093/emboj/cdf331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ghyselinck NB, Dupe V, Dierich A, Messaddeq N, Garnier JM, Rochette-Egly C, Chambon P, Mark M. Role of the retinoic acid receptor beta (RARbeta) during mouse development. Int. J. Dev. Biol. 1997;41:425–447. [PubMed] [Google Scholar]
- 103.Reichrath J, Mittmann M, Kamradt J, Muller SM. Expression of retinoid-X receptors (- alpha,-beta,-gamma) and retinoic acid receptors (-alpha,-beta,-gamma) in normal human skin: an immunohistological evaluation. Histochem. J. 1997;29:127–133. doi: 10.1023/a:1026481205135. [DOI] [PubMed] [Google Scholar]
- 104.Billoni N, Gautier B, Mahe YF, Bernard BA. Expression of retinoid nuclear receptor superfamily members in human hair follicles and its implication in hair growth. Acta Derm.-Venereol. 1997;77:350–355. doi: 10.2340/0001555577350355. [DOI] [PubMed] [Google Scholar]
- 105.Viallet JP, Dhouailly D. Retinoic Acid and Mouse Skin Morphogenesis .1. Expression Pattern of Retinoic Acid Receptor Genes During Hair Vibrissa Follicle, Plantar, and Nasal Gland Development. J. Invest. Dermatol. 1994;103:116–121. doi: 10.1111/1523-1747.ep12391880. [DOI] [PubMed] [Google Scholar]
- 106.Tsou HC, Si SP, Lee XH, Gonzalezserva A, Peacocke M. A Beta-2rare-Lacz Transgene Identifies Retinoic Acid-Mediated Transcriptional Activation in Distinct Cutaneous Sites. Exp. Cell Res. 1994;214:27–34. doi: 10.1006/excr.1994.1230. [DOI] [PubMed] [Google Scholar]
- 107.Everts HB, King LE, Jr, Sundberg JP, Ong DE. Hair cycle-specific immunolocalization of retinoic acid synthesizing enzymes Aldh1a2 and Aldh1a3 indicate complex regulation. J. Invest. Dermatol. 2004;123:258–263. doi: 10.1111/j.0022-202X.2004.23223.x. [DOI] [PubMed] [Google Scholar]
- 108.Rossant J, Zirngibl R, Cado D, Shago M, Giguere V. Expression of a Retinoic Acid Response Element-Hsplacz Transgene Defines Specific Domains of Transcriptional Activity During Mouse Embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- 109.Ansell DM, Kloepper JE, Thomason HA, Paus R, Hardman MJ. Exploring the "Hair Growth-Wound Healing Connection": Anagen Phase Promotes Wound Re-Epithelialization. J. Invest. Dermatol. 2011;131:518–528. doi: 10.1038/jid.2010.291. [DOI] [PubMed] [Google Scholar]
- 110.Viallet JP, Dhouailly D. Retinoic Acid and Mouse Skin Morphogenesis .2. Role of Epidermal Competence in Hair Glandular Metaplasia. Dev. Biol. 1994;166:277–288. doi: 10.1006/dbio.1994.1314. [DOI] [PubMed] [Google Scholar]
- 111.Chuong CM, Ting SA, Widelitz RB, Lee YS. Mechanism of skin morphogenesis 2. retinoic acid modulates axis orientation and phenotypes of skin appendages. Development. 1992;115:839–852. doi: 10.1242/dev.115.3.839. [DOI] [PubMed] [Google Scholar]
- 112.Flowers MT, Paton CM, O'Byrne SM, Schiesser K, Dawson JA, Blaner WS, Kendziorski C, Ntambi JM. Metabolic Changes in Skin Caused by Scd1 Deficiency: A Focus on Retinol Metabolism. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.King LE, McElwee KJ, Sundberg JP. Alopecia Areata. In: Nickoloff BJ, Nestle FO, editors. Dermatologic Immunity, Curr Dir Autoimmun. Karger: Basel; 2008. pp. 280–312. [DOI] [PubMed] [Google Scholar]
- 114.Sundberg JP, Boggess D, Sundberg BA, Eilertsen K, Parimoo S, Filippi M, Stenn K. Asebia-2J (Scd1ab2J): A new allele and a model for scarring alopecia. Am. J. Pathol. 2000;156:2067–2075. doi: 10.1016/S0002-9440(10)65078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Karnik P, Tekeste Z, McCormick TS, Gilliam AC, Price VH, Cooper KD, Mirmirani P. Hair follicle stem cell-specific PPARg deletion causes scarring alopecia. J. Invest. Dermatol. 2009;129:1243–1257. doi: 10.1038/jid.2008.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Harries MJ, Meyer KC, Paus R. Hair loss as a result of cutaneous autoimmunity: Frontiers in the immunopathogenesis of primary cicatricial alopecia. Autoimmun. Rev. 2009;8:478–483. doi: 10.1016/j.autrev.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 117.McElwee KJ, Hoffmann R, Freyschmidt-Paul P, Wenzel E, Kissling S, Sundberg JP, Zoller M. Resistance to alopecia areata in C3H/HeJ mice is associated with increased expression of regulatory cytokines and a failure to recruit CD4+ and CD8+ cells. J. Invest. Dermatol. 2002;119:1426–1433. doi: 10.1046/j.1523-1747.2002.19620.x. [DOI] [PubMed] [Google Scholar]
- 118.McElwee KJ, Spiers EM, Oliver RF. In vivo depletion of CD8+ T cells restores hair growth in the DEBR model for alopecia areata. Br. J. Dermatol. 1996;135:211–217. [PubMed] [Google Scholar]
- 119.Gilhar A, Landau M, Assy B, Shalaginov R, Serafimovich S, Kalish RS. Mediation of alopecia areata by cooperation between CD4(+) and CD8(+) T lymphocytes - Transfer to human scalp explants on Prkd(cscid) mice. Arch. Dermatol. 2002;138:916–922. doi: 10.1001/archderm.138.7.916. [DOI] [PubMed] [Google Scholar]
- 120.Paus R, Ito N, Takigawa M, Ito T. The hair follicle and immune privilege. J. Invest. Dermatol. Symp. Proc. 2003;8:188–194. doi: 10.1046/j.1087-0024.2003.00807.x. [DOI] [PubMed] [Google Scholar]
- 121.Gilhar A, Kam Y, Assy B, Kalish RS. Alopecia areata induced in C3H/HeJ mice by interferon-gamma: Evidence for loss of immune privilege. J. Invest. Dermatol. 2005;124:288–289. doi: 10.1111/j.0022-202X.2004.23580.x. [DOI] [PubMed] [Google Scholar]
- 122.Ito T, Ito N, Saatoff M, Hashizume H, Fukamizu H, Nickoloff BJ, Takigawa M, Paus R. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J. Invest. Dermatol. 2008;128:1196–1206. doi: 10.1038/sj.jid.5701183. [DOI] [PubMed] [Google Scholar]
- 123.Stuttgen G. Historical perspectives of tretinoin. J. Am. Acad. Dermatol. 1986;15:735–740. doi: 10.1016/s0190-9622(86)70228-4. [DOI] [PubMed] [Google Scholar]
- 124.Thiboutot D, Gollnick H, Bettoli V, Dreno B, Kang SW, Leyden JJ, Shalita AR, Lozada V, Berson D, Finlay A, Goh CL, Herane MI, Kaminsky A, Kubba R, Layton A, Miyachi Y, Perez M, Martin JP, Ramos-e-Silva M, See JA, Shear N, Wolf J. New insights into the management of acne: An update from the Global Alliance to Improve Outcomes in Acne Group. J. Am. Acad. Dermatol. 2009;60:S1–S50. doi: 10.1016/j.jaad.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 125.Tzimas G, Nau H. The role of metabolism and toxicokinetics in retinoid teratogenesis. Curr. Pharm. Des. 2001;7:803–831. doi: 10.2174/1381612013397708. [DOI] [PubMed] [Google Scholar]
- 126.Landthaler M, Kummermehr J, Wagner A, Plewig G. Inhibitory effects of 13-cis retinoic acid on human sebaceous glands. Arch. Dermatol. Res. 1980;269:297–309. doi: 10.1007/BF00406424. [DOI] [PubMed] [Google Scholar]
- 127.Zouboulis CC, Korge BP, Mischke D, Orfanos CE. Altered proliferation, synthetic activity, and differentiation of cultured human sebocytes in the absence of vitamin-A and their modulation by synthetic retinoids. J. Invest. Dermatol. 1993;101:628–633. doi: 10.1111/1523-1747.ep12366092. [DOI] [PubMed] [Google Scholar]
- 128.Zouboulis CC, Korge B, Akamatsu H, Xia LQ, Schiller S, Gollnick H, Orfanos CE. Effects of 13-cis-retinoic acid, all-trans-retinoic acid, and acitretin on the proliferation, lipid-synthesis and keratin expression of cultured human sebocytes in vitro. J. Invest. Dermatol. 1991;96:792–797. doi: 10.1111/1523-1747.ep12471782. [DOI] [PubMed] [Google Scholar]
- 129.Sato T, Imai N, Akimoto N, Sakiguchi T, Kitamura K, Ito A. Epidermal growth factor and 1 alpha,25-dihydroxyvitamin D-3 suppress lipogenesis in hamster sebaceous gland cells in vitro. J. Invest. Dermatol. 2001;117:965–970. doi: 10.1046/j.0022-202x.2001.01516.x. [DOI] [PubMed] [Google Scholar]
- 130.Gomez EC. Differential effect of 13-cis-retinoic acid and an aromatic retinoid (Ro-10-9359) on the sebaceous glands of the hamster flank organ. J. Invest. Dermatol. 1981;76:68–69. doi: 10.1111/1523-1747.ep12524899. [DOI] [PubMed] [Google Scholar]
- 131.Tsukada M, Schroder M, Roos TC, Chandraratna RA, Reichert U, Merk HF, Orfanos CE, Zouboulis CC. 13-cis retinoic acid exerts its specific activity on human sebocytes through selective intracellular isomerization to all-trans retinoic acid and binding to retinoid acid receptors. J. Invest. Dermatol. 2000;115:321–327. doi: 10.1046/j.1523-1747.2000.00066.x. [DOI] [PubMed] [Google Scholar]
- 132.Kim MJ, Ciletti N, Michel S, Reichert U, Rosenfield RL. The role of specific retinoid receptors in sebocyte growth and differentiation in culture. J. Invest. Dermatol. 2000;114:349–353. doi: 10.1046/j.1523-1747.2000.00868.x. [DOI] [PubMed] [Google Scholar]
- 133.Nelson AM, Gilliland KL, Cong ZY, Thiboutot DM. 13-cis retinoic acid induces apoptosis and cell cycle arrest in human SEB-1 sebocytes. J. Invest. Dermatol. 2006;126:2178–2189. doi: 10.1038/sj.jid.5700289. [DOI] [PubMed] [Google Scholar]
- 134.Nelson AM, Zhao W, Gilliland KL, Zaenglein AL, Liu WL, Thiboutot DM. Isotretinoin Temporally Regulates Distinct Sets of Genes in Patient Skin. J. Invest. Dermatol. 2009;129:1038–1042. doi: 10.1038/jid.2008.338. [DOI] [PubMed] [Google Scholar]
- 135.Karlsson T, Vahlquist A, Kedishvili N, Torma H. 13-cis-Retinoic acid competitively inhibits 3 alpha-hydroxysteroid oxidation by retinol dehydrogenase RoDH-4: a mechanism for its anti-androgenic effects in sebaceous glands? Biochem. Biophys. Res. Commun. 2003;303:273–278. doi: 10.1016/s0006-291x(03)00332-2. [DOI] [PubMed] [Google Scholar]
- 136.Melnik BC, Schmitz G, Zouboulis CC. Anti-Acne Agents Attenuate FGFR2 Signal Transduction in Acne. J. Invest. Dermatol. 2009;129:1868–1877. doi: 10.1038/jid.2009.8. [DOI] [PubMed] [Google Scholar]
- 137.Nelson AM, Zhao W, Gilliland KL, Zaenglein AL, Liu WL, Thiboutot DM. Neutrophil gelatinase-associated lipocalin mediates 13-cis retinoic acid-induced apoptosis of human sebaceous gland cells. J. Clin. Invest. 2008;118:1468–1478. doi: 10.1172/JCI33869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Papakonstantinou E, Aletras AJ, Glass E, Tsogas P, Dionyssopoulos A, Adjaye J, Fimmel S, Gouvousis P, Herwig R, Lehrach H, Zouboulis CC, Karakiulakis G. Matrix metalloproteinases of epithelial origin in facial sebum of patients with acne and their regulation by isotretinoin. J. Invest. Dermatol. 2005;125:673–684. doi: 10.1111/j.0022-202X.2005.23848.x. [DOI] [PubMed] [Google Scholar]
- 139.Dufour JM, Vo MN, Bhattacharya N, Okita J, Okita R, Kim KH. Peroxisorne proliferators disrupt retinoic acid receptor alpha signaling in the testis. Biol. Reprod. 2003;68:1215–1224. doi: 10.1095/biolreprod.102.010488. [DOI] [PubMed] [Google Scholar]
- 140.Kim MJ, Deplewski D, Ciletti N, Michel S, Reichert U, Rosenfield RL. Limited cooperation between peroxisome proliferator-activated receptors and retinoid X receptor agonists in sebocyte growth and development. Mol. Genet. Metab. 2001;74:362–369. doi: 10.1006/mgme.2001.3242. [DOI] [PubMed] [Google Scholar]
- 141.Chen WC, Yang CC, Sheu HM, Seltmann H, Zouboulis CC. Expression of peroxisome proliferator-activated receptor and CCAAT/enhancer binding protein transcription factors in cultured human sebocytes. J. Invest. Dermatol. 2003;121:441–447. doi: 10.1046/j.1523-1747.2003.12411.x. [DOI] [PubMed] [Google Scholar]
- 142.Collins CA, Watt FM. Dynamic regulation of retinoic acid-binding proteins in developing, adult and neoplastic skin reveals roles for beta-catenin and Notch signalling. Dev. Biol. 2008;324:55–67. doi: 10.1016/j.ydbio.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 143.Theodosiou M, Laudet V, Schubert M. From carrot to clinic: an overview of the retinoic acid signaling pathway. Cell. Mol. Life Sci. 2010;67:1423–1445. doi: 10.1007/s00018-010-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Thiele JJ, Weber SU, Packer L. Sebaceous gland secretion is a major physiologic route of vitamin E delivery to skin. J. Invest. Dermatol. 1999;113:1006–1010. doi: 10.1046/j.1523-1747.1999.00794.x. [DOI] [PubMed] [Google Scholar]
- 145.Kramer C, Seltmann H, Seifert M, Tilgen W, Zouboulis CC, Reichrath J. Characterization of the vitamin D endocrine system in human sebocytes in vitro. J. Steroid Bioch. Mol. Biol. 2009;113:9–16. doi: 10.1016/j.jsbmb.2008.10.010. [DOI] [PubMed] [Google Scholar]