Abstract
Renal ischemia/reperfusion (I/R) injury is a major clinical problem where main metabolic pathways are compromised and cellular homeostasis crashes after ATP depletion. Fatty acids are major energy source in the kidneys. Carnitine palmitoyltransferase I (CPT1), a mitochondrial membrane enzyme, utilizes carnitine to transport fatty acids to mitochondria for the process of β-oxidation and ATP generation. In addition, CPT1 activity is indirectly regulated by adenosine monophosphate (AMP)-activated protein kinase, which can be activated by 5-aminoimidazole-4-carboxyamide ribonucleoside (AICAR). We hypothesized that administration of carnitine and AICAR could reestablish the energetic balance after reperfusion and ameliorate renal I/R injury. Male adult rats were subjected to renal I/R by bilateral renal pedicle clamping for 60 min, followed by administration of saline (vehicle), carnitine (250 mg/kg BW), AICAR (30 mg/kg BW), or combination of both drugs. Blood and renal tissues were collected 24 h after reperfusion for various measurements. Renal carnitine levels decreased 53% after I/R. The combined treatment significantly increased CPT1 activity and ATP levels, and lowered renal malondialdehyde and serum TNF-α levels against the vehicle group. It led to improvement in renal morphology and histological damage score associated with diminution in serum creatinine, blood urea nitrogen, and aspartate aminotransferase levels. Moreover, the combined treatment significantly improved the survival rate in comparison to the vehicle group. In contrast, administration of either drug alone did not show a significant improvement in most of the measurements. In conclusion, enhancing energy metabolism by combination of carnitine and AICAR provides a novel modality to treat renal I/R injury.
Keywords: kidney, ischemia/reperfusion, carnitine, AICAR, CPT1, energy metabolism
INTRODUCTION
Acute renal failure (ARF) which remains a major clinical problem usually results from ischemia/reperfusion (I/R) injury. A strong association of ARF with increased length of hospital stay and mortality rate denoting adjusted odds ratios of 4.1 has been reported (1). A recent multinational/multicenter study shows that major surgeries and septic shock attributed to 34.3% and 47.5%, respectively, among all the surveyed contributors for the development of ARF (2). Moreover, in renal transplantation, I/R injury is a common cause of delayed graft function, which occurs in 20–80% of cadaveric kidney recipients (3). Despite advances in preventive strategies, no effective treatment is available for I/R injury and its care is still mainly supportive. Therefore, development of therapeutic interventions to treat renal I/R injury remains the focus of research.
Renal I/R injury is initiated by energy depletion due to the lack of oxygen and nutrients at the ischemic stage. At the reperfusion stage, a complex series of events cause additional tissue damage, such as cytoskeletal disruption, alteration of cellular ionic homeostasis, induction of proteolytic and phospholipolytic pathways, acidosis, production of reactive oxygen/nitrogen species, generation of inflammatory mediators, leukocyte infiltration, microvascular reactivity and cell death via apoptosis or necrosis (4). Various agents that can block these deteriorated events at the reperfusion stage have shown some degrees of beneficial effects on alleviating renal I/R injury. However, enhancing the recovery of kidney from energy depletion as treatment strategy has not been well explored.
Fatty acids are the major energy source in the kidneys (5). Upon uptake by renal epithelial cells, fatty acids are activated to form fatty acyl-CoA in the cytosol and then transported to mitochondria where the process of fatty acid oxidation or β-oxidation takes place for ATP generation (6). The transport of fatty acyl-CoA to mitochondria is catalyzed by carnitine palmitoyltransferase I (CPT1) localized in the outer mitochondrial membrane with the aid of carnitine as a carrier (7). Carnitine is a quaternary ammonium compound biosynthesized from lysine and methionine (8). The main sites of carnitine synthesis are the kidneys, liver, and brain (9). CPT1 is believed to be the rate-limiting step in fatty acid oxidation (10). In addition to the availability of substrates, CPT1 is inhibited by malonyl-CoA. The synthesis and degradation of malonyl-CoA are controlled by acetyl-CoA carboxylase (ACC) and malonyl-CoA decarboxylase (MCD), respectively. Activation of adenosine monophosphate (AMP)-activated protein kinase (AMPK) can inhibit ACC activity and stimulate MCD activity, which results in increased CPT1 activity (11). 5-Aminoimidazole-4-carboxyamide ribonucleoside (AICAR) is a cell permeable compound that serves as a pharmacological activator of AMPK (12).
In this study, we tested the hypothesis that an adequate stimulation of the cellular energy-generating metabolism ameliorates the renal I/R injury. We used a rat model to examine whether the administration of carnitine in combination with AICAR at the reperfusion stage could reduce tissue damage and improve renal function after I/R. We examined the effect of the combined treatment on CPT1 activity and ATP production in the kidneys after I/R. The productions of lipid peroxidation and pro-inflammatory cytokine TNF-α were also monitored. The treatment with individual drugs in contributing to modulate the outcomes of renal I/R injury was evaluated as well.
MATERIALS AND METHODS
Experimental animals
Male Sprague-Dawley rats (250 ~ 300g) were purchased from Charles River Laboratories (Wilmington, MA). The rats were housed in a temperature controlled room and on a 12-h light/dark cycle. The rats were fed a standard Purina rat chow diet and allowed water ad libitum. Animal experimentation was carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources). This project was approved by the Institutional Animal Care and Use Committee at the Feinstein Institute for Medical Research.
Animal model of renal I/R injury
Prior to surgery, rats were fasted overnight, but water was given ad libitum. Rats were anesthetized with isoflurane inhalation. A midline laparotomy incision was performed to expose the abdomen. Intestines were covered with warm, moist gauze and retracted to the right to expose the left renal pedicle. An atraumatic microvascular clamp was placed around the left renal vascular pedicle (artery and vein). Cessation of blood flow to the kidney was judged by the blanching of the kidney. Same procedure was conducted to the right kidney. The total clamp time was 60 min based on the previous study in our laboratory (13). Restoration of blood flow into the kidneys was judged visually. The incision was closed in layers and the animals were returned to their cages with food and water for recovery. At 24 h after reperfusion, the animals were euthanized and blood and tissue samples were harvested for further analyses.
Experimental groups
All drugs were administered via a femoral venous catheter in 5 ml saline over a period of 30 min, immediately followed by the removal of the microvascular clamps. Group 1, sham-operated animals underwent a midline laparotomy incision and the kidneys were isolated, but neither clamping nor infusion was performed. Group 2, renal I/R rats were treated with saline as vehicle. Group 3, renal I/R rats treated with L-carnitine (Sigma-Aldrich, St. Louis, MO) at 250 mg/kg BW. Group 4, renal I/R rats were treated with AICAR (EMD Biosciences, Gibbstown, NJ) at 30 mg/kg BW. Group 5, renal I/R rats were treated with L-carnitine and AICAR at 250 mg/kg and 30 mg/kg BW, respectively. The doses of carnitine and AICAR were based on previous studies shown effectiveness to rats without generating toxicity (14–18).
Determination of tissue carnitine, ATP, and malondialdehyde (MDA) levels
Twenty-four hours after reperfusion, the right and left kidneys were cut by sagittal sections. Each half of the kidney was immediately frozen in liquid nitrogen. Later, the frozen right and left kidneys were pulverized together with mortar immersed liquid nitrogen. Kidney tissue (25 mg) was homogenized in 100 µl assay buffer and centrifuged to remove insoluble materials at 13,000g for 10 min. The supernatant was deproteinized by perchloric acid precipitation followed by KOH neutralization before subjecting to carnitine and ATP assay kits from BioVision (Mountain View, CA). For measuring MDA levels, kidney tissue was homogenized in lysis buffer (10 mM Tris-HCl pH 7.5, 100 mM NaCl, 50 mM EDTA, 50 mM EGTA, and 1% Triton X-100) and sonicated for 15 sec at 40V over ice. The solution was centrifuged at 1,600g for 10 min at 4°C and the supernatant was subjected to an assay kit from Cayman Chemical (Ann Arbor, MI).
Determination of CPT activity
CPT activity was analyzed as described (19). Briefly, 200 mg of frozen kidney tissue was minced and suspended in homogenization buffer (0.25 M sucrose, 1 mM EDTA, 0.1% ethanol, and protease inhibitors) at a ratio of 1:5 (w/v). The suspension was then homogenized on ice and centrifuged at 300g for 10 min at 4°C. The precleared supernatant was transferred to a new tube and centrifuged at 12,000g for a further 5 min at 4°C.
CPT activity was assayed in these supernatants spectrophotometrically by following the release of CoA-SH from palmitoyl-CoA (Sigma-Aldrich) using the general thiol reagent DTNB (Sigma-Aldrich); 175 µl Tris-HCl–DTNB buffer (116 mM Tris-HCl pH 8.0, 2.5 mM EDTA, 2 mM DTNB, and 0.2% Triton X-100). 10 µl homogenization buffer and 10 µl cleared supernatant was added to a 96-well plate. After 5 min preincubation at 30°C, 10 µl palmitoyl-CoA (1 mM dissolved in distilled water) was added. The reaction was then started by adding 2 µl L-carnitine solution (1.2 mM dissolved in 1 M Tris-HCl pH 8.0), immediately followed by photometric measurement at 412 nm for 15 min. Activity was defined as nmol CoA-SH released/min/mg protein or U/mg protein. Protein concentration was determined by DC protein assay (Bio-Rad, Hercules, CA).
Determination of serum levels of injury markers
Blood samples were centrifuged at 2,000g for 15 min to collect serum, and then stored at −80°C for measuring the levels of creatinine, blood urea nitrogen (BUN) and aspartate aminotransferase (AST) by using assay kits from Pointe Scientific (Canton, MI).
Histopathological analysis
The right kidney was harvested, cut by sagittal section into two portions, and fixed by formalin. Tissue blocks were cut in 4-µm sections, mounted on glass, followed by hematoxylineosin (H&E) staining for light microscopy analysis. Morphological changes were analyzed by an experienced renal pathologist (blinded to the experimental groups). The extent of right renal damage was graded with a modified schema of Kelly, et al (20). The percentage of morphologic alterations (dilatation of Bowman’s space, flattened tubular epithelium, interstitial inflammation, loss of tubular brush borders and casts) in the outer medulla and corticomedullary junction were estimated and scored as follows: 0, none; 1+, < 10%; 2+, 10–25%; 3+, 26–75%; and 4+, > 75%.
Determination of serum levels of TNF-α
The concentration of TNF-α in the serum was measured by using a commercially enzyme-linked immunosorbent assay (ELISA) kit from BD Biosciences (San Diego, CA).
Statistical analysis
All data are expressed as means ± SEM and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls (SNK) method for multiple group analyses. The survival rate is estimated by Kaplan-Meier method and compared by the log-rank test. Differences in value were considered significant if P < 0.05.
RESULTS
Change of carnitine levels after I/R
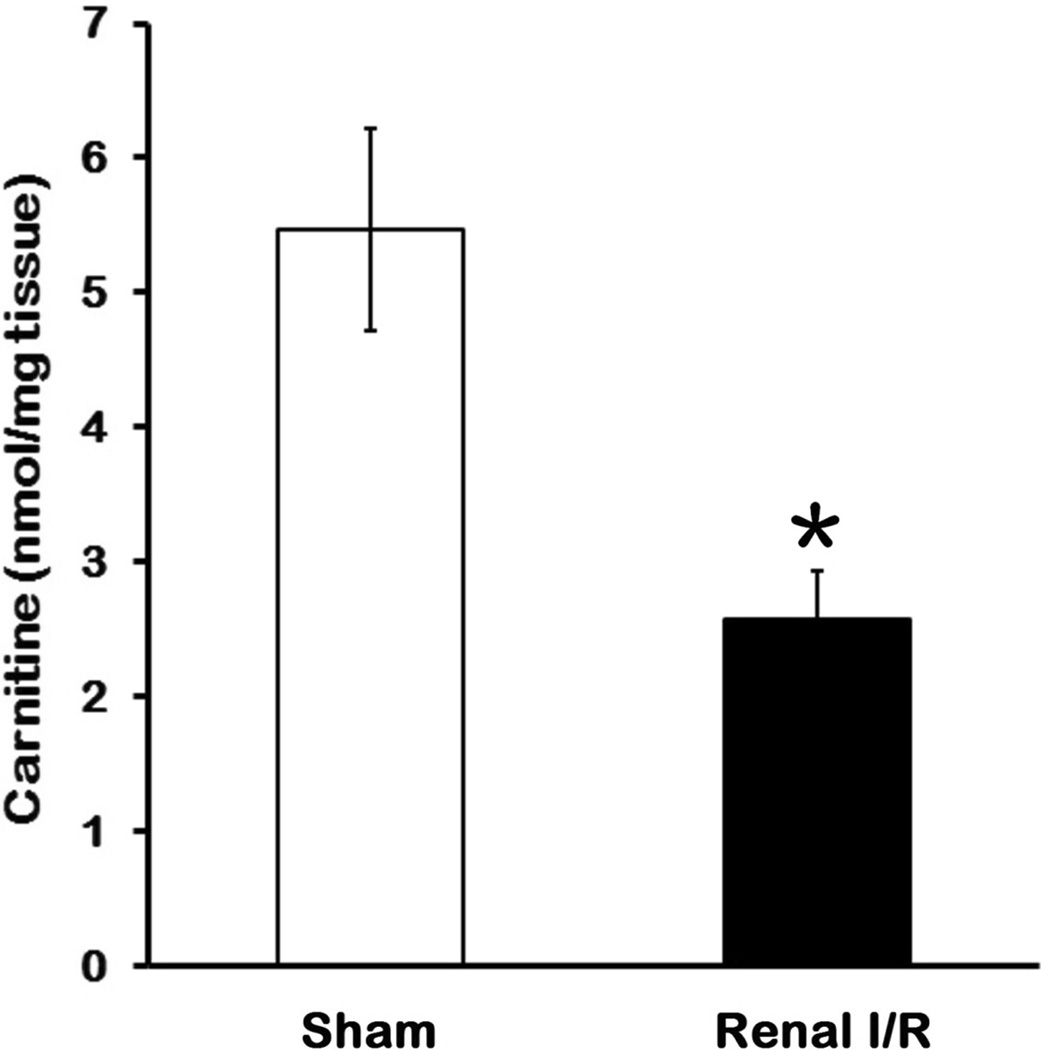
To determine whether carnitine played a role in renal I/R injury, we first determined the effect of I/R on carnitine levels in kidneys. As shown in Fig. 1, carnitine levels in kidney tissues after I/R decreased by 53% in comparison to the sham control 24 h after reperfusion (P < 0.05). The reduction of carnitine levels suggested that utilization of fatty acid for energy metabolism might be affected since carnitine is required for fatty acid transport to mitochondria. This result supported our rationale to replenish carnitine as one of the components for treating renal I/R injury.
Fig 1. Change in renal carnitine levels after I/R.
Kidney tissues were collected 24 h after reperfusion. Data are presented as means ± SEM (n = 5 per group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls method. *P < 0.05 vs. sham.
Effect of carnitine and AICAR on CPT1 activity and ATP levels after I/R
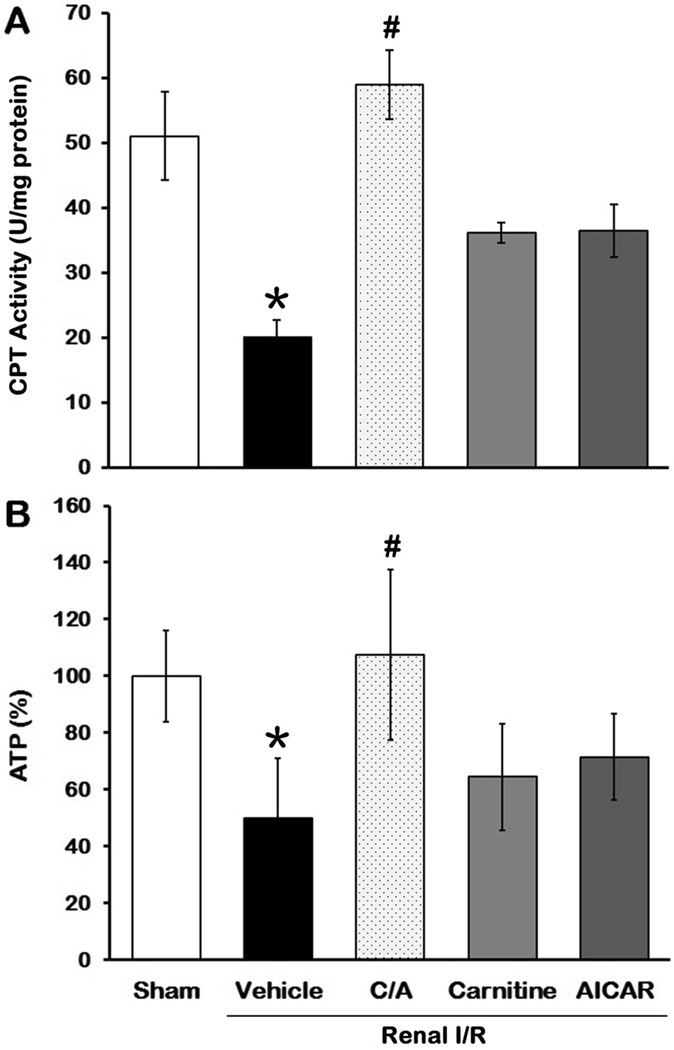
After observing a decrease of carnitine levels, we examined the effect of I/R and treatments on CPT1 activity. CPT1 activity in kidney tissues after I/R (the vehicle group) decreased 60% in comparison to the sham group 24 h after reperfusion (P < 0.05; Fig. 2A). CPT1 activity in the carnitine plus AICAR (C/A) group was restored to the levels comparable to the sham group, whereas it only partially recovered in the carnitine or AICAR groups (Fig. 2A).
Fig 2. Effect of carnitine and AICAR on CPT1 activity and ATP levels after I/R.
Kidney tissues were harvested 24 h after reperfusion to determine CPT1 activity (A) and ATP levels (B). Data are presented as means ± SEM (n = 5 per group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls method. C/A, carnitine plus AICAR. *P < 0.05 vs. sham; #P < 0.05 vs. vehicle.
CPT1 controls a crucial step to transport fatty acids to mitochondria for ATP generation. We then determined the ATP levels in kidneys 24 h after reperfusion. Similar to the CPT1 activity, the ATP levels in the vehicle group decreased 50.2% in comparison to the sham group (Fig. 2B). ATP levels in the C/A group were 216% higher than those in the vehicle group and similar to the sham group (Fig. 2B). ATP levels in the carnitine or AICAR groups increased 29.5% and 43.3%, respectively, in comparison to the vehicle group, but no statistical significance (Fig. 2B). Taken together, these results provided a link between regeneration of ATP and activation of CPT1 in kidneys by the combined treatment after I/R.
Effect of carnitine and AICAR on MDA levels after I/R
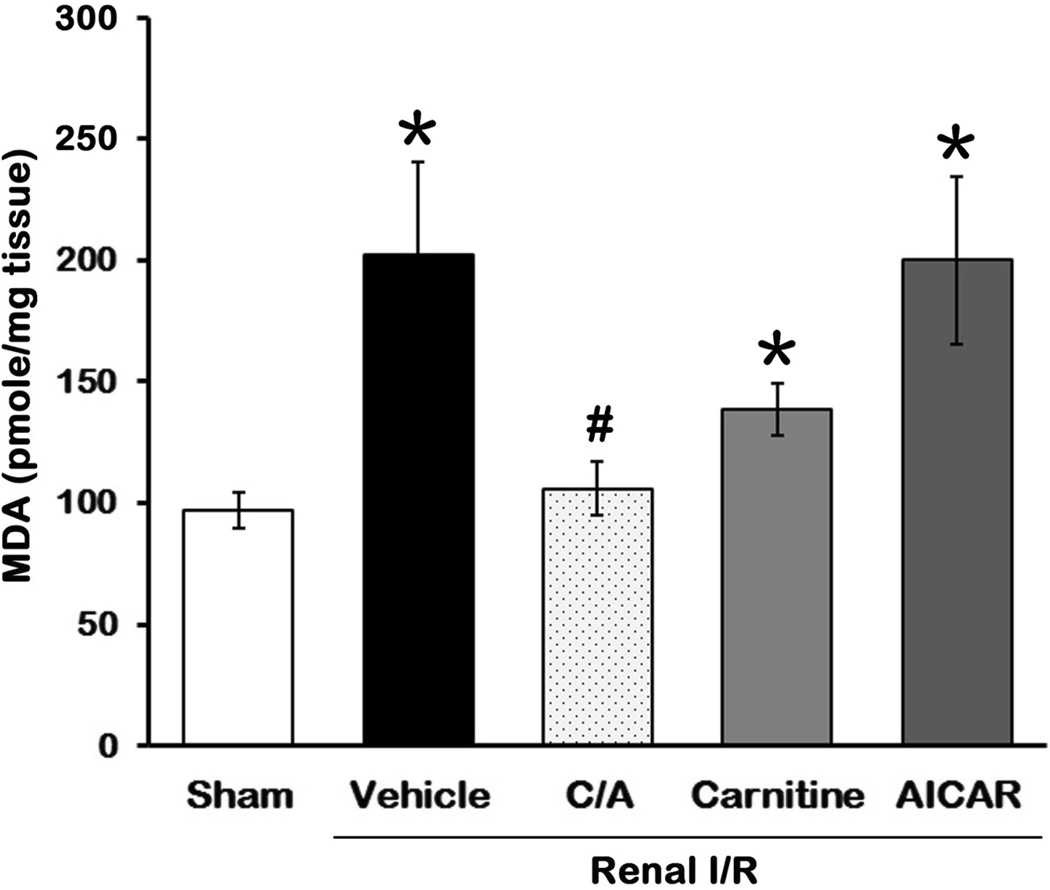
During I/R, free radicals will be generated to react with polyunsaturated fatty acids of the membranes leading to lipid peroxidation and causing cellular membrane damage. MDA formation is an indicator for the extent of lipid peroxidation in tissues (21, 22). MDA levels in the kidney tissues were 2.1-fold higher in the vehicle group than those in the sham group (P > 0.05; Fig. 3). Its level in the C/A group was significantly lower than those in the vehicle group and similar to the sham group (Fig. 3). Carnitine treatment reduced 30.8%, while AICAR treatment had no effect on MDA levels, compared to the vehicle group (Fig. 3).
Fig 3. Effect of carnitine and AICAR on MDA levels after I/R.
Kidney tissues were harvested 24h after reperfusion for measuring MDA levels. Data are presented as means ± SEM (n = 5 per group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls method. C/A, carnitine plus AICAR. *P < 0.05 vs. sham; #P < 0.05 vs. vehicle.
Effect of carnitine and AICAR on TNF-α release after I/R
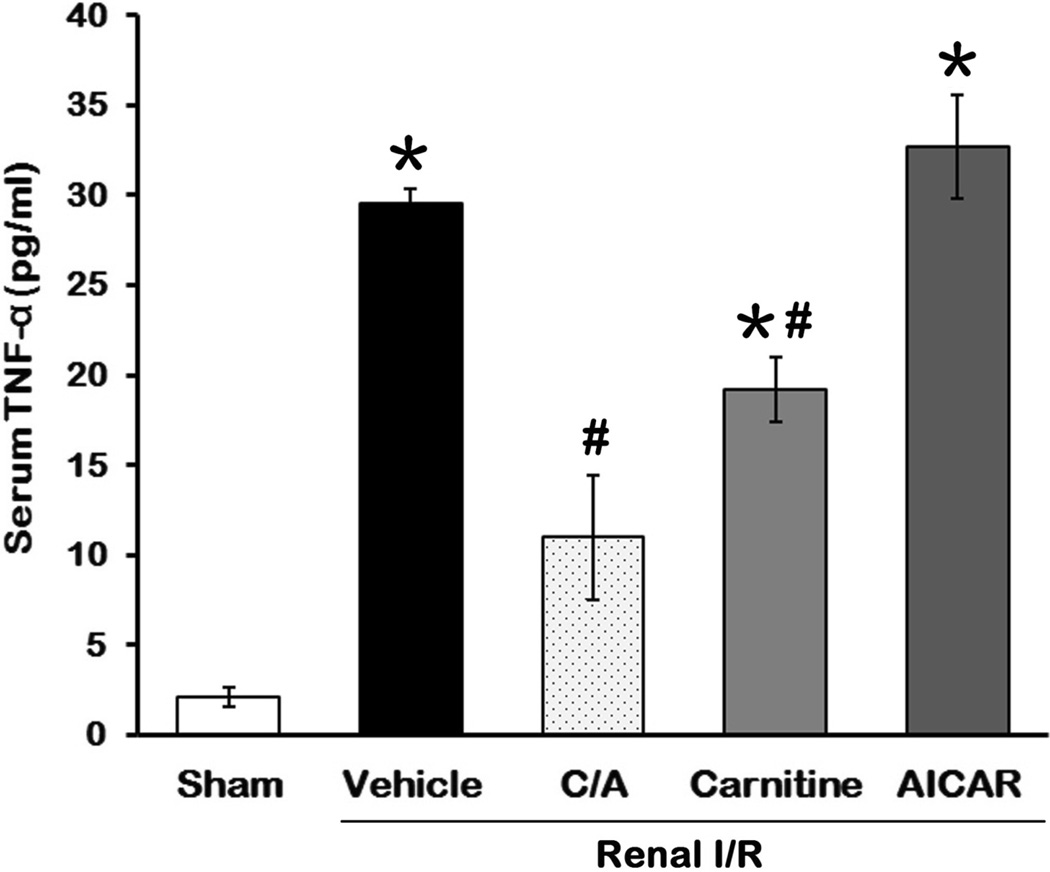
Inflammation is one of the major factors in contribution to the renal damage after I/R (4). To monitor the effect of the treatments on systemic inflammation after I/R, we measured the levels of proinflammatory cytokine TNF-α in serum 24 h after reperfusion. Serum TNF-α levels in the vehicle group were increased 12.9-fold in comparison to the sham group (Fig 4). Treatment with C/A and carnitine resulted in a 63.9% and 35.0% reduction, respectively, compared to the vehicle group (P < 0.05; Fig. 4). AICAR had no significant effect on TNF-α levels induced by I/R (Fig. 4).
Fig 4. Effect of carnitine and AICAR on TNF-α release after I/R.
Serum samples were collected 24 h after reperfusion for measuring TNF-α. Data are presented as means ± SEM (n = 5 per group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls method. C/A, carnitine plus AICAR. *P < 0.05 vs. sham; #P < 0.05 vs. vehicle.
Effect of carnitine and AICAR on renal morphology after I/R
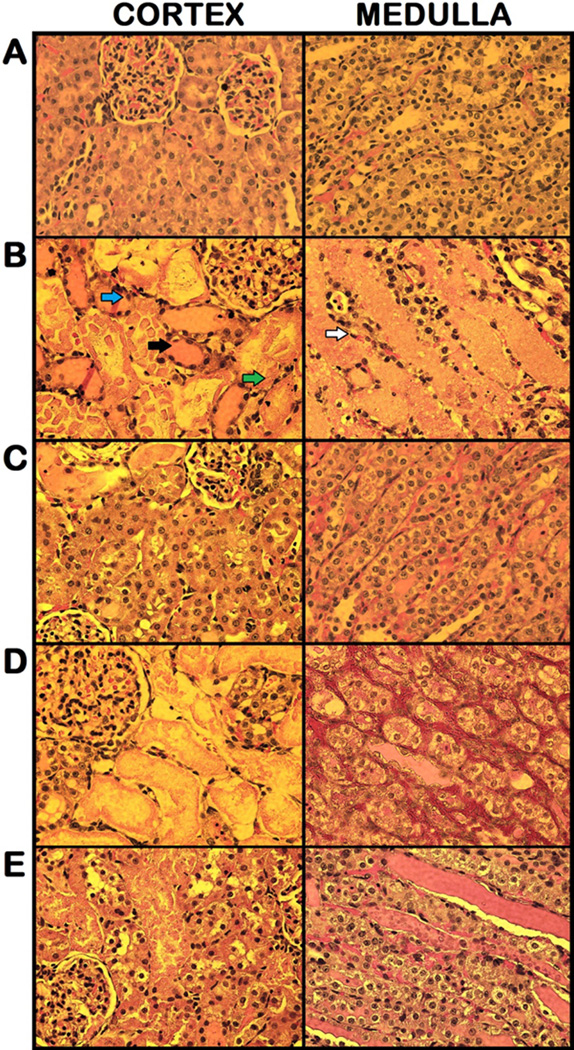
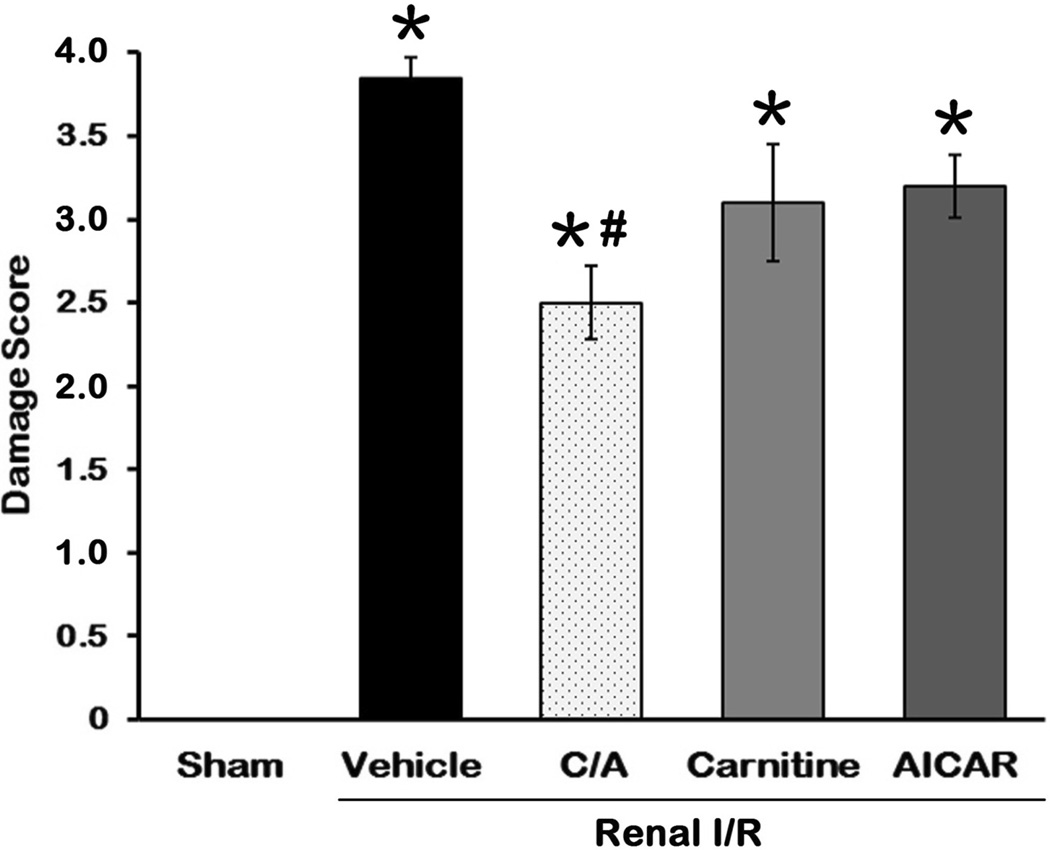
To evaluate the efficacy of the treatments on alleviating the renal damage after I/R, we examined the morphological features of the kidneys by histological staining 24 h after reperfusion. The right renal cortex and medulla in the vehicle group (Fig. 5B) were severely damaged, showing large areas of tubular dilation, necrosis, formation of proteinaceous casts, and loss of tubular brush borders in comparison to the sham group (Fig. 5A). Treatments with C/A (Fig. 5C), carnitine (Fig. 5D), and AICAR (Fig. 5E) exhibited some degrees of improvement in renal morphology. In renal I/R injury, the cells located in the outer medulla are more serve damage than cells located at other areas (23). Thus, the damage levels in the outer medulla and corticomedullary junction were examined by histology and graded in a semiquantitative manner. Semiquantitative scoring of the renal damage levels by histological examination in each group is indicated in Fig. 6. The score of the vehicle-treated rats was 3.7 ± 0.1, closely to the maximum score. Only treatment with C/A had a significant reduction in damage score of 2.5 ± 0.2, compared to the vehicle group.
Fig 5. Effect of carnitine and AICAR on renal morphology after IR.
Histological findings of the right kidneys in the sham (A), vehicle (B), carnitine plus AICAR (C), carnitine (D), and AICAR (E) treated rats. Kidneys were harvested 24 h after reperfusion, processed and stained with hematoxylin and eosin. Representative photomicrograghs from renal cortex (left) and renal medulla (right) are shown. Compared with the sham group (essentially normal histology), examination of kidneys obtained from the vehicle group demonstrated a significant degree of renal injury with proteinaceous casts (black arrow), flattened epithelium (white arrow), interstitial inflammation (green arrow) and loss of tubular brush borders (blue arrow). The integrity of renal morphology in the carnitine plus AICAR, carnitine, and AICAR treated rats was lay between the sham and vehicle groups. Original magnification 200×.
Fig 6. Histological score of renal I/R injury and treatments.
The extent damage of right renal in the outer medulla and corticomedullary junction was graded with a modified schema as described in Materials and Methods. The score represents the percentage of morphologic alterations as follows: 0, none; 1+, < 10%; 2+, 10–25%; 3+, 26–75%; and 4+, > 75%. Data are presented as means ± SEM (n = 5 per group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls method. C/A, carnitine plus AICAR. *P < 0.05 vs. sham; #P < 0.05 vs. vehicle.
Effect of carnitine and AICAR on renal function and injury after I/R
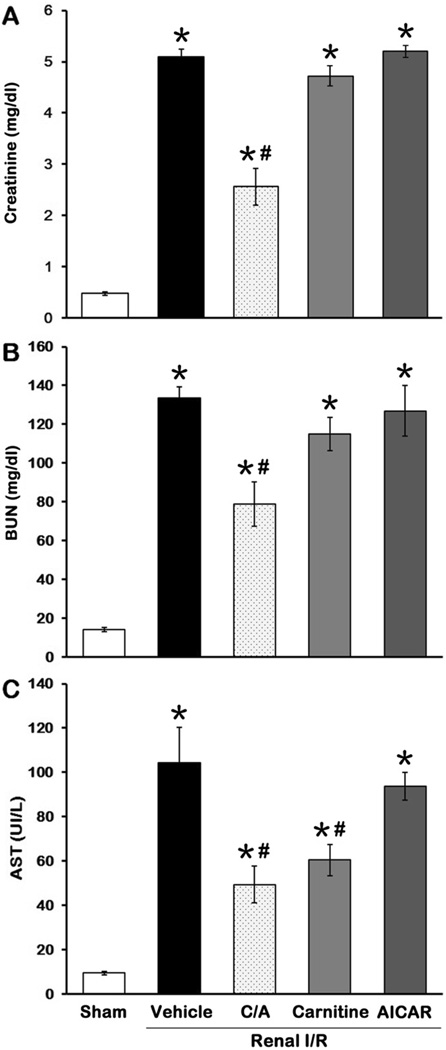
The levels of several serum markers used as indicators of renal function and injury, including creatinine, BUN and AST (24), were measured to evaluate the effect of treatment. As expected, the levels of these markers were significantly increased in the vehicle group in comparison to the sham group (Fig. 7), indicating a severe degree of renal dysfunction after I/R in our model. C/A treatment showed a 49.8%, 40.9% and 52.7% decrease in creatinine, BUN and AST levels, respectively, in comparison to the vehicle group (P < 0.05; Fig. 7). Carnitine treatment only decreased AST levels with significance, while AICAR treatment had no effect on reducing the levels of these markers after I/R (Fig. 7).
Fig 7. Effect of carnitine and AICAR on renal function and injury after I/R.
Serum samples were collected 24 h after reperfusion for measuring creatinine (A), BUN (B), and AST (C). Data are presented as means ± SEM (n = 5 per group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls method. C/A, carnitine plus AICAR. *P < 0.05 vs. sham; #P < 0.05 vs. vehicle.
Effect of carnitine and AICAR on survival after I/R
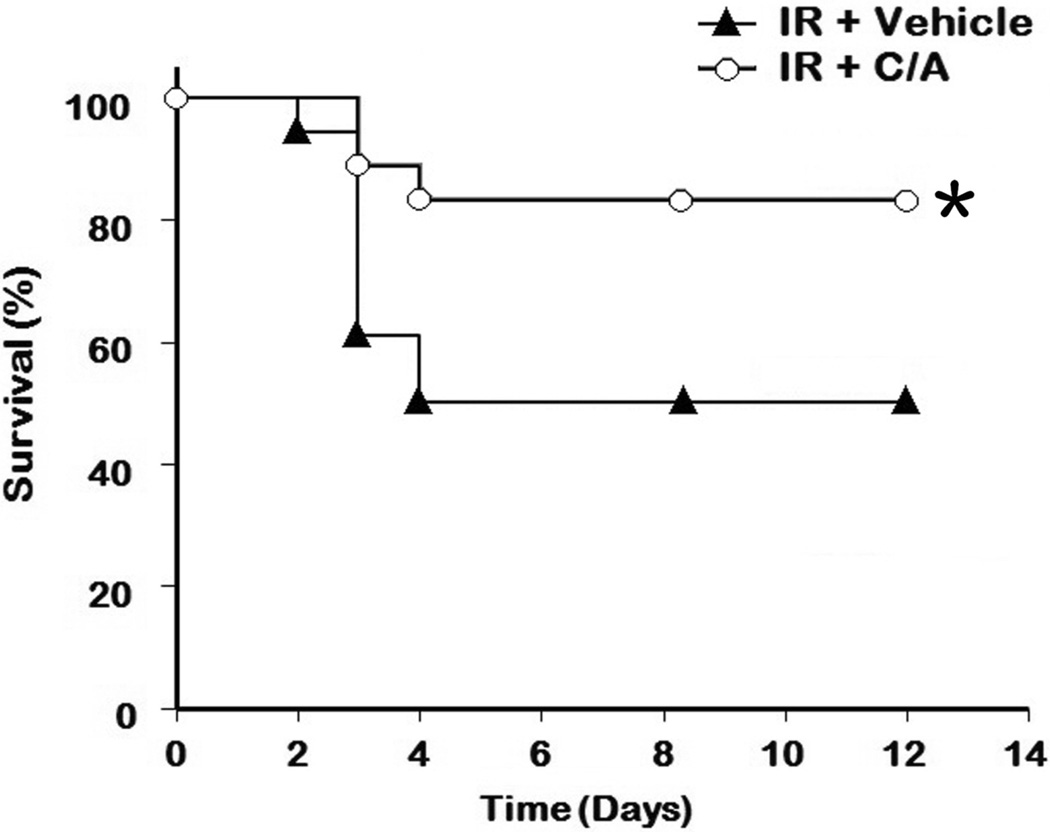
To further evaluate the long-term benefit of the treatment on renal I/R injury, we conducted a 12-day survival study. Since C/A treatment was superior to the individual treatments on all measurements, we then only compared the survival rate between the C/A and vehicle groups. As shown in Fig. 8, the survival rate of the C/A group improved significantly in comparison to the vehicle group (3 animals out of 18 died in the C/A group vs. 9 out of 18 died in the vehicle group, P=0.032).
Fig 8. Effect of carnitine and AICAR on survival after I/R.
Rats (n=18 per group) were performed renal I/R as described in Materials and Methods, followed by infusion intravenously with saline (vehicle) or carnitine plus AICAR (C/A). The survival rate was analyzed by Kaplan-Meier survival analysis and compared by the log-rank test. *P < 0.05 vs. vehicle.
DISCUSSION
Renal I/R injury is usually manifested as ARF. It is an important aggravator after major surgeries, septic shock, and renal transplantation, and leads to high mortality. In this study, we demonstrated that the administration of carnitine and AICAR together at the reperfusion stage significantly reduced renal damage and improved renal function after I/R. These improvements were associated with an enhancement of renal energy metabolism, reduction of MDA in the kidneys and decrease of serum TNF-α levels. Furthermore, the combined treatment significantly increased the survival rate over the untreated group after I/R. However, treatment with individual drugs did not show significant benefits on the alleviation of the renal I/R injury in most of the measurements. Although carnitine or AICAR has been reported to provide a protective effect on renal I/R injury, these agents are administrated 10 to 15 min before renal ischemia in these studies (14, 17). Furthermore, the linkage of the effect of these agents on cellular activity to the improvement of renal function is not fully addressed.
Another significance of this result is that the beneficial effects shown by the combined treatment can be seen when administered post-ischemia. The timing of this treatment can be considered therapeutic intervention rather than a preventive one. Various pharmacological agents that target to distinct deteriorated mechanisms induced by I/R have been shown effectively in ameliorating renal injury; however, majority of these studies administer these agents by preventive approach (i.e., prior to the induction of ischemia). Delay of intervention is one of the factors for the failure of these agents for treating ARF in clinical trials, since the biomarkers to predict or detect ARF early are not clinically available for early intervention (25). Therefore, developing therapeutic agents administered after I/R, as demonstrated in this study, will be a better strategy to meet current clinical needs. We have administered the treatment right after ischemia which is still at early stage of developing I/R injury. At this time point, the onset of renal ischemic injury may not be diagnosed yet. Whether the delay administration of the combined treatment at the late reperfusion stage can still alleviate the renal injury needs to further investigate.
The drugs, carnitine and AICAR, used in this study are considered safe and well tolerated. Carnitine in humans is derived from diet and de novo biosynthesis using lysine and methionine. The main dietary sources of carnitine are red meat, fish, and dairy products which can supply 2 to 12 µmols/day/kg of body weight, whereas 1–2 µmols of carnitine is endogenously synthesized (26). According to the Physicians’ Desk Reference, carnitine (carnitor) can be applied 50 mg/kg/day intravenous bolus or infusion to maximum 300 mg/kg/day. We have administered 250 mg/kg intravenously one time to the rat in this study. AICAR (acadesine) has been used to conduct a Phase III study for prevention of reperfusion injury in coronary-artery bypass graft surgery (NCT00872001). However, the trial was terminated due to a low probability of meeting the primary efficacy goal. In addition, a Phase I/IIa study for safety and tolerability open label dose escalation study of acadesine in B-CLL patients had been completed (NCT00559624). No Grade 3 or 4 adverse events were reported in this trial.
Energy depletion is the first event occurred during ischemia, followed by a series of deteriorated cascades at the reperfusion stage. Effective restoration of the depleted energy should be critical to protect cells for further damage. In exploring the renal energy metabolism, we identified that carnitine, a compound required for the process of fatty acid oxidation, reduced to 50% in kidneys after I/R. CPT1 is a mitochondrial membrane enzyme that utilizes carnitine to transport fatty acids to mitochondria (27). We also observed that I/R caused down-regulation of CPT1 activity. These results suggest that reduction of carnitine levels and CPT1 activity may be attributed to dysregulation of energy metabolism in kidneys after I/R. However, supplement of carnitine alone to animals with renal I/R injury did not significantly restore CPT1 activity. AMPK is an upstream regulator of CPT1 through the control of ACC and MCD to lower malonyl-CoA levels (11). With the combination of carnitine and AICAR, a pharmacological activator of AMPK, the CPT1 activity in kidneys completely recovered from the I/R injury. Similar to carnitine, administration of AICAR alone had only slight effect on recovery of CPT1 activity. One of the major functions of CPT1 is to assist the process of fatty acid oxidization. Correspondingly, the pattern of the ATP levels in each group was almost identical to that of the CPT1 activity. We further do the linear regression analysis between the mean values of the CPT1 activity and ATP levels in the five treatment groups. The R value is 0.9638.
Free radicals, particularly reactive oxygen species (ROS) formed during ischemia and subsequent reperfusion, cause tissue injury by their chemical modification of lipids, proteins, nucleotides, and carbohydrates. MDA formation is an indicator of lipid peroxidation, but also reflects the generation of free radicals. Consistent with other studies (28), we also observed that carnitine could function as an antioxidant and reduce the MDA levels induced by I/R. Intriguingly, the combined treatment further decreased MDA levels to those of the sham group. It has been indicated that the damage to the components of the mitochondrial electron transfer chain is the main source of ROS production during ischemia (29, 30). In the combined treatment, the CPT1 activity and ATP production were restored to the sham levels reflecting a preserved mitochondrial functioning. Thus, combination of carnitine and AICAR may provide an additional protective effect on mitochondrial stability after reperfusion, resulting in reducing ROS levels and consequent lowering lipid peroxidation. In this study, we focused on the assessment of the effects of the treatments on renal ischemia and reperfusion damages as a whole. We did not elucidate which stage (ischemia vs. reperfusion) has more profound effects on reducing energy metabolism and increasing lipid peroxidation by further measuring the CPTI activity, ATP and MDA levels right after ischemia.
In the early phase of renal I/R, TNF-α levels increase and stimulate the expression of other cytokines and chemokines for further promoting inflammation, which leads to tissue damage (31, 32). We observed that the combined treatment and carnitine significantly suppressed the production of TNF-α induced by I/R, which was corresponding to their inhibitions on MDA formation. The link between generation of free radical and stimulation of inflammatory responses has been well established (33). In this study, we did not examine the role of neutrophil in contributing to renal I/R injury. Over infiltration of neutrophil to the injured sites will increase hydrogen peroxide levels and result in tissue damage. Whether stimulation of renal cell recovery from energy depletion by the combined treatment can prevent neutrophil infiltration needs to be investigated. Although both the combined treatment and carnitine showed an attenuation of inflammatory responses, only the combined treatment had a significant improvement of the renal function after I/R. These results indicate that in addition to down-regulated secretion of proinflammatory cytokines, enhancement of energy metabolism is another crucial factor in rescuing the renal damage after I/R.
Surprisingly, in our study AICAR did not inhibit the levels of TNF-α induced by I/R, since the capability of AICAR in blocking inflammation has been demonstrated in other disease models. For example, AICAR can inhibit the expression of proinflammatory cytokines, including TNF-α, induced by lipopolysaccharide in several immune cells (34, 35). In animal models, pre-treatment with AICAR can attenuate inflammatory responses in dextran sulfate sodium-induced acute and chronic colitis, and lipopolysaccharide-induced acute lung injury (36, 37). In this study, AICAR was applied after ischemic insult, where renal cells were already under high stressed status. Thus, the discrepancy between our observation and other studies may be attributed to the standing of the cell integrity in responding to AICAR.
In summary, infusion of carnitine combined with AICAR into ischemic-damaged kidneys provides a protective effect on further renal damage induced by the reperfusion in an animal model. The overall benefit of the combined treatment is illustrated by a significant increase of survival rate over the untreated group after renal I/R. Moreover, our findings suggest that CPT1 may be a target for pharmacological interventions to advance energy production. Finally, the results of this study endorse that stimulating energy metabolism can be a therapeutic strategy for treating renal I/R injury and improve outcomes of patients with delayed graft function following kidney transplantation, acute renal failure after major surgery and trauma, or septic shock.
ACKNOWLEDGMENTS
The authors thank Dr. Morris Edelman for evaluating the histology of the renal tissues and Dr. Asha Jacob for critically reviewing this manuscript.
This study was supported by National Institutes of Health grants, R01HL076179, R01GM057468, and R01GM053008 (PW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Financial Interests and Potential Conflicts of Interest. All authors reported no financial interests or potential conflicts of interest.
This work was awarded The Shock Society New Investigator Award (first place) at the 34th Annual Conference on Shock, Norfolk, Virginia, June 11–14, 2011.
REFERENCES
- 1.Liangos O, Wald R, O'Bell JW, Price L, Pereira BJ, Jaber BL. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1(1):43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 3.Cecka JM, Cho YW, Terasaki PI. Analyses of the UNOS Scientific Renal Transplant Registry at three years--early events affecting transplant success. Transplantation. 1992;53(1):59–64. doi: 10.1097/00007890-199201000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14(8):2199–2210. doi: 10.1097/01.asn.0000079785.13922.f6. [DOI] [PubMed] [Google Scholar]
- 5.Portilla D. Energy metabolism and cytotoxicity. Semin Nephrol. 2003;23(5):432–438. doi: 10.1016/s0270-9295(03)00088-3. [DOI] [PubMed] [Google Scholar]
- 6.Lehninger AL, Nelson DL, Cox MM. Lehninger principles of biochemistry. 5th ed. New York: W.H. Freeman; 2008. [Google Scholar]
- 7.Bonnefont JP, Djouadi F, Prip-Buus C, Gobin S, Munnich A, Bastin J. Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol Aspect Med. 2004;25(5–6):495–520. doi: 10.1016/j.mam.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Steiber A, Kerner J, Hoppel CL. Carnitine: a nutritional, biosynthetic, and functional perspective. Mol Aspects Med. 2004;25(5–6):455–473. doi: 10.1016/j.mam.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S, Black SM. Carnitine Homeostasis, Mitochondrial Function, and Cardiovascular Disease. Drug Discov Today Dis Mech. 2009;6(1–4):e31–e39. doi: 10.1016/j.ddmec.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. 1997;244(1):1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 11.Hopkins TA, Dyck JR, Lopaschuk GD. AMP-activated protein kinase regulation of fatty acid oxidation in the ischaemic heart. Biochem Soc Trans. 2003;31(Pt 1):207–212. doi: 10.1042/bst0310207. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan JE, Brocklehurst KJ, Marley AE, Carey F, Carling D, Beri RK. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 1994;353(1):33–36. doi: 10.1016/0014-5793(94)01006-4. [DOI] [PubMed] [Google Scholar]
- 13.Shah KG, Rajan D, Jacob A, Wu R, Krishnasastry K, Nicastro J, Molmenti EP, Coppa GF, Wang P. Attenuation of renal ischemia and reperfusion injury by human adrenomedullin and its binding protein. J Surg Res. 2010;163(1):110–117. doi: 10.1016/j.jss.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorur S, Bagdatoglu OT, Polat G. Protective effect of L-carnitine on renal ischaemia-reperfusion injury in the rat. Cell Biochem Funct. 2005;23(3):151–155. doi: 10.1002/cbf.1159. [DOI] [PubMed] [Google Scholar]
- 15.Hosgorler FU, Atila K, Terzi C, Akhisaroglu ST, Oktay G, Kupelioglu A, Ergor G, Saydam S. Carnitine protects the intestine against reperfusion injury in rats. J Surg Res. 2010;159(1):603–610. doi: 10.1016/j.jss.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 16.Fabian TC, Fabian MJ, Yockey JM, Proctor KG. Acadesine and lipopolysaccharide-evoked pulmonary dysfunction after resuscitation from traumatic shock. Surgery. 1996;119(3):302–315. doi: 10.1016/s0039-6060(96)80117-6. [DOI] [PubMed] [Google Scholar]
- 17.Lee SJ, Chang YK, Na KR, Lee KW, Suh KS, Kim SY, Chang YS, Shin YT, Bang BK. The preconditioning with AICAR protects against subsequent renal ischemia reperfusion injury. Korean J Nephrol. 2009;28(2):96–102. [Google Scholar]
- 18.Wong AK, Howie J, Petrie JR, Lang CC. AMP-activated protein kinase pathway: a potential therapeutic target in cardiometabolic disease. Clin Sci (Lond) 2009;116(8):607–620. doi: 10.1042/CS20080066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bieber LL, Abraham T, Helmrath T. A rapid spectrophotometric assay for carnitine palmitoyltransferase. Anal Biochem. 1972;50(2):509–518. doi: 10.1016/0003-2697(72)90061-9. [DOI] [PubMed] [Google Scholar]
- 20.Kelly KJ, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97(4):1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong D, Browne R. The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. Adv Exp Med Biol. 1994;366:43–58. doi: 10.1007/978-1-4615-1833-4_4. [DOI] [PubMed] [Google Scholar]
- 22.Yagi K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol Biol. 1998;108:101–106. doi: 10.1385/0-89603-472-0:101. [DOI] [PubMed] [Google Scholar]
- 23.Gobe G, Willgoss D, Hogg N, Schoch E, Endre Z. Cell survival or death in renal tubular epithelium after ischemia-reperfusion injury. Kidney Int. 1999;56(4):1299–1304. doi: 10.1046/j.1523-1755.1999.00701.x. [DOI] [PubMed] [Google Scholar]
- 24.Chatterjee PK, Patel NS, Sivarajah A, Kvale EO, Dugo L, Cuzzocrea S, Brown PA, Stewart KN, Mota-Filipe H, Britti D, Yaqoob MM, Thiemermann C. GW274150, a potent and highly selective inhibitor of iNOS, reduces experimental renal ischemia/reperfusion injury. Kidney Int. 2003;63(3):853–865. doi: 10.1046/j.1523-1755.2003.00802.x. [DOI] [PubMed] [Google Scholar]
- 25.Kunzendorf U, Haase M, Rolver L, Haase-Fielitz A. Novel aspects of pharmacological therapies for acute renal failure. Drugs. 2010;70(9):1099–1114. doi: 10.2165/11535890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Sahajwalla CG, Helton ED, Purich ED, Hoppel CL, Cabana BE. Multiple-dose pharmacokinetics and bioequivalence of L-carnitine 330-mg tablet versus 1-g chewable tablet versus enteral solution in healthy adult male volunteers. J Pharm Sci. 1995;84(5):627–633. doi: 10.1002/jps.2600840520. [DOI] [PubMed] [Google Scholar]
- 27.Bremer J. The role of carnitine in intracellular metabolism. J Clin Chem Clin Biochem. 1990;28(5):297–301. [PubMed] [Google Scholar]
- 28.Mister M, Noris M, Szymczuk J, Azzollini N, Aiello S, Abbate M, Trochimowicz L, Gagliardini E, Arduini A, Perico N, Remuzzi G. Propionyl-L-carnitine prevents renal function deterioration due to ischemia/reperfusion. Kidney Int. 2002;61(3):1064–1078. doi: 10.1046/j.1523-1755.2002.00212.x. [DOI] [PubMed] [Google Scholar]
- 29.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1(6):401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Krauss S, Zhang CY, Lowell BB. The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol. 2005;6(3):248–261. doi: 10.1038/nrm1592. [DOI] [PubMed] [Google Scholar]
- 31.Donnahoo KK, Shames BD, Harken AH, Meldrum DR. Review article: the role of tumor necrosis factor in renal ischemia-reperfusion injury. J Urol. 1999;162(1):196–203. doi: 10.1097/00005392-199907000-00068. [DOI] [PubMed] [Google Scholar]
- 32.Ysebaert DK, De Greef KE, Vercauteren SR, Ghielli M, Verpooten GA, Eyskens EJ, De Broe ME. Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrol Dial Transplant. 2000;15(10):1562–1574. doi: 10.1093/ndt/15.10.1562. [DOI] [PubMed] [Google Scholar]
- 33.Goligorsky MS. Immune system in renal injury and repair: burning the candle from both ends? Pharmacol Res. 2008;58(2):122–128. doi: 10.1016/j.phrs.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giri S, Nath N, Smith B, Viollet B, Singh AK, Singh I. 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase. J Neurosci. 2004;24(2):479–487. doi: 10.1523/JNEUROSCI.4288-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jhun BS, Jin Q, Oh YT, Kim SS, Kong Y, Cho YH, Ha J, Baik HH, Kang I. 5-Aminoimidazole-4-carboxamide riboside suppresses lipopolysaccharide-induced TNF-alpha production through inhibition of phosphatidylinositol 3-kinase/Akt activation in RAW 264.7 murine macrophages. Biochem Biophys Res Commun. 2004;318(2):372–380. doi: 10.1016/j.bbrc.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 36.Bai A, Yong M, Ma AG, Ma Y, Weiss CR, Guan Q, Bernstein CN, Peng Z. Novel anti-inflammatory action of 5-aminoimidazole-4-carboxamide ribonucleoside with protective effect in dextran sulfate sodium-induced acute and chronic colitis. J Pharmacol Exp Ther. 2010;333(3):717–725. doi: 10.1124/jpet.109.164954. [DOI] [PubMed] [Google Scholar]
- 37.Zhao X, Zmijewski JW, Lorne E, Liu G, Park YJ, Tsuruta Y, Abraham E. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295(3):L497–L504. doi: 10.1152/ajplung.90210.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]