Abstract
Wear debris in joint replacements has been suggested as a cause of associated tissue damaging inflammation. In this study, we examined whether solid Titanium micro-particles (mTi) of sufficient size to accumulate as wear debris could stimulate innate or adaptive immunity in vivo. mTi, administered in conjunction with OVA, promoted total and Ag-specific elevations in serum IgE and IgG1. Analysis of transferred transgenic OVA-specific naïve T cells further showed that mTi acted as an adjuvant to drive Ag-specific Th2 cell differentiation in vivo. Assessment of the innate response indicated that mTi induced rapid recruitment and differentiation of alternatively activated macrophages in vivo, through IL-4 and TLR-4 independent pathways. These studies suggest that solid micro-particles alone can act as adjuvants to induce potent innate and adaptive Th2-type immune responses and further suggest that wear debris in joint replacements may trigger Th2-type inflammatory properties.
Keywords: Adjuvant, TLR4, Titanium, Cytokines, Th2-type responses, IgE, IL-4, M2 macrophages
Introduction
Wear debris deposited in tissue adjacent to joint replacements containing Titanium are preferentially composed of relatively large titanium micro-particles (mTi). Previous studies have indicated that mTi particles, used as models for wear debris, may have inflammatory properties as increased TNF-α and IL-6 are observed when mTi are cultured in vitro with macrophages, raising the possibility that these particles may stimulate harmful inflammatory responses in vivo, potentially contributing to osteolysis and aseptic loosening (1, 2). It is possible, though, that these inflammatory effects of Ti particles may be stimulated by adherent lipopolysaccharide as some studies have suggested that LPS-free Ti is much less effective at stimulating innate immune cells in vitro (1, 3–5). It should be noted that these large inert mTi should not be confused with charged Ti dioxide nano particles, which have recently been suggested to be more biologically active and capable of eliciting elevations of serum IgE and IgG1 when administered with Ag (6), though whether this is associated with stimulation of an innate Th2-type immune response that may then promote naïve Th2 cell differentiation remains undetermined. Few studies have yet examined the effect on mTi on the immune response in vivo.
Helminth parasites and Alum have adjuvant properties that can induce potent Th2 cell differentiation and several helminth products have been identified that appear capable of inducing an innate Th2-type immune response that includes activation of dendritic cells, eosinophils and alternatively activated (M2) macrophages as well as other innate immune cell populations (7–9). LPS, particularly at low doses, has also been shown to promote Th2-type responses through TLR4-dependent mechanisms(10, 11). However, helminth parasites, and also Alum, appear capable of promoting Th2-type immune responses independently of TLR4-signaling (12–15) although many of the most potent isolated Th2-inducing helminth components, including c-lectins, appear to be TLR-4 dependent (7). It thus appears that intact, these relatively large multicellular parasites have distinct as yet undescribed properties that can elicit potent Th2-type responses in vivo.
In this article, we have examined whether micron-sized mTi particles may function as adjuvants to promote innate and adaptive immunity. Our findings show that total and Ag-specific serum IgG1 and IgE are markedly increased following administration of Ti particles with OVA peptide. Furthermore, mTi acts as an adjuvant in vivo that can promote naïve transgenic OVA-specific T cells to proliferate and differentiate into cytokine producing Th2 effector cells. Furthermore, rather than merely acting as a depot to stabilize and enhance OVA processing and presentation, mTi can directly stimulate a potent Th2-type innate immune response in vivo, characterized by development of M2 macrophages independently of IL-4. The development of both innate and adaptive Th2-type immunity occurred independently of endotoxin, as similar results were obtained in TLR-4 deficient mice. These findings suggest that solid microparticles are sufficient to induce potent Th2-type responses and further suggest that wear debris deposited in tissue surrounding titanium joint replacements may have potent Th2-type inflammatory effects.
Materials and methods
Mice
BALB/c mice were obtained from the Small Animal Division, National Cancer Institute (Fredrick, MD) and C3H/HeOuJ (wild), C3H/HeJ ((TLR-4−/−) mice were obtained from The Jackson Laboratory. Breeding pairs of BALB/c IL-4 deficient mice (IL-4−/−) and DO11.10 TCR Transgenic BALB/c mice were obtained from The Jackson Laboratory and were maintained and bred in a specific pathogen free facility during the experiments at the New Jersey Medical School-University of Medicine and Dentistry of New Jersey (Newark, NJ) research animal facility. All of the mice were maintained in a specific pathogen-free, virus free facility during the experiments. The studies have been reviewed and approved by Institutional Animal Care and Use Committee at New Jersey Medical School. The studies reported here conformed to the principle for laboratory animal research outlined by the Animal Welfare Act and the Department of Health, Education and Welfare (National Institutes of Health) guidelines for the experimental use of animals.
Administration of Ti particle, Alum/OVA and quantitation of total and OVA-specific IgE, IgG1 and IgG2a
Mice were inoculated intraperitoneally (ip) with either PBS alone; 50 μg of endotoxin free Ovalbumin (OVA) protein (OVA, grade V, Sigma-Aldrich, St Louis, MO) alone; 12.5 mg Ti particles + 50 μg OVA (OVA+TI); or 4mg Alum + 50 μg OVA (OVA +AL). All groups were subsequently challenged ip with OVA alone on day 7. Twenty-one days after the last immunization, sera were collected and total and OVA specific IgG1, IgG2a and IgE antibody determined by ELISA, as previously described (16).
OVA, Alum, and mTi preparations
Endo grade OVA (Hyglos GmbH, Germany), Imject Alum (Thermo Scientific, USA), and mTi (<20μm; Alfa Aesar, Ward Hill, MA, USA) and were used in all experiments described. Titanium particles were washed three times with absolute alcohol followed by 3 washes with sterile water and dried at 65°C under vacuum with a vaccum concentrator (Eppendorf vacufuge, Eppendorf, USA) and redissolved in sterile PBS. In additional experiments, the particles were further treated to remove residual endotoxin either by: 1) treating with 1% acetic acid and boiling for three hours (trt. A); or 2) autoclaving for 1 hour and incubating in a heated oven at 175 °C for 3 hours (trt. B), as previously described (17). Using the Endpoint Chromomeric LAL Assay, QCL-1000 (Lonza, Walkersville, MD, USA), the LPS levels of Ti particles treated with either method were found to be less than or equal to the negative control (≤ 0.01 EU/ml), while Ti particles treated with alcohol alone had detectable levels of LPS at approximately 0.38 EU/ml. Titanium particles were further characterized by low angle laser light scattering (LALLS), using previously described methods (18–20). The average size of the Ti particle was 0.38μm diameter (range 0.2–88μm) as determined using a number-based analysis (LALLS, Microtrac X-100, Bioengineering Solutions Inc).
Adoptive transfers of D011.10 TCR CD4+ T cell
Peripheral lymph nodes and spleen were harvested from WT DO11.10 TCR-transgenic mice, which express a TCR specific for chicken OVA peptide 323–339-I-Ad complexes and which is uniquely recognized by the KJ1-26 anti-clonotypic mAb (21). Single-cell suspensions were prepared, DO11.10 TCR transgenic CD4+ cells were isolated, CFSE-labelled, and adoptively transferred to age and sex matched BALB/c mice intravenously, as previously described(22). 30 μg OVA peptide alone or with either 12.5mg Ti particles or with 4 mg alum were injected ip into recipient mice 2 days after transfer. HPLC-purified OVA323–339 (OVA peptide) with the sequence ISQAVHAAHAEINEAGR-COOH was synthesized at the UMDNJ core facility.
Flow cytometry
Peritoneal exudate cell (PEC) suspensions were blocked with Fc Block (BD Pharmingen, San Jose, CA, USA) and then stained with various antibodies including: anti-LY6G FITC, anti-CD11cPE, anti-CD11b perCP-CY5.5, SiglecF PE, anti-c-kit APC, F4/80 APC (BD Pharmingen, San Jose, CA, USA) and CD206 FITC (ABD Serotec, Raleigh, NC, USA). For CFSE-labeled cells, anti-CD4 perCP (BD Harlingen, San Jose, CA, USA), and KJ1-26-PE (Caltag Laboratories, Burlingame, CA, USA) were used to phenotype DO11.10 T cells and cell cycle progression was monitored by measuring sequential reductions in CFSE fluorescence of KJ1-26+, CD4+ cells and the proliferation index was calculated using flow jo software (Tree Star, Inc., Ashland, OR, USA).
ELISPOT
Single-cell lymph node suspensions were prepared in RPMI 1640 containing 10% heat-inactivated FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine (all from Invitrogen, USA). Lymph node cells (0.5 × 106) were cultured with 10 μg/ml OVA peptide for 3 days on anti-IL-4 (Clone BVD4-1D11.2, BD Pharmingen, San Jose, CA, USA) coated plates/anti IFN-γ (BD Biosciences, USA) coated plates. ELISPOT was developed as previously described (16). After 72 hours the plates were washed several times with PBS followed by PBS with 0.5% Tween20 (PBST). Secondary biotinylated anti IL-4 Ab/anti IFN-g Ab (BD Biosciences, USA) was diluted in PBST + 5% FCS, added at 100 μl/well, and incubated overnight at 4°C. Plates were then washed, and a 1/2000 dilution of Streptavidin-alkaline phosphatase (Jackson Immuno-Research Laboratories, USA) was added. Plates were developed and results quantitated by counting the number of positive colonies under a microscope.
Sorting of CD4+T cells or DO11.10 T cells after immunization
For CD4+ T cell sorting, lymph node cell suspensions were prepared from DO11.10 mice inoculated mice, incubated with anti-CD4 micro beads (Miltenyi Biotec) and CD4+ T cells were purified by positive selection. The purities were >98%. For cell sorting of OVA-specific DO11.10 T cells, MLN cells were stained with anti-mouse DO11.10 TCR Biotin conjugate Ab (Caltag Laboratory), and then labeled with anti-biotin Ab beads (Miltenyi Biotec). Labeled cells were passed through MS columns (Miltenyi Biotec) according to the protocol provided by the manufacturer. The KJ1-26+ population was positively selected and assessed for purity using FACS analysis. The purities were 85–90% in all sorts. RNAs were extracted from sorted cells using RNAZOL (Invitrogen, USA).
Cytokine gene expression by RT-PCR
For RT-PCR, total RNA was extracted from PEC by using RNAZOL (Invitrogen, USA) and then reverse transcribed with Super Script II reverse transcriptase (Invitrogen, USA) as per the manufacturer’s protocol. Gene specific Taqman assay along with the taqman gene expression master mix (Applied biosystem) were used for amplification and detection of different genes in Applied Biosystems 7500 sequence detector. Gene expression fold changes of different mRNA of treated samples were quantified relative to the untreated by employing the 2 (−ΔΔct) relative quantification method using 18s RNA as the housekeeping gene, as previously described (23).
Statistical analysis
Data are presented as mean ± SEM, indicated by error bars. Statistical significance was determined by ANOVA followed by individual comparisons with Tukey test using Sigma plot version,11.0 (Systat Software, Inc., San Jose, CA, USA). Statistical difference at a level of p<0.05 between groups was considered significant.
Results
Titanium particles can enhance elevations of serum Igs in response to OVA protein immunization
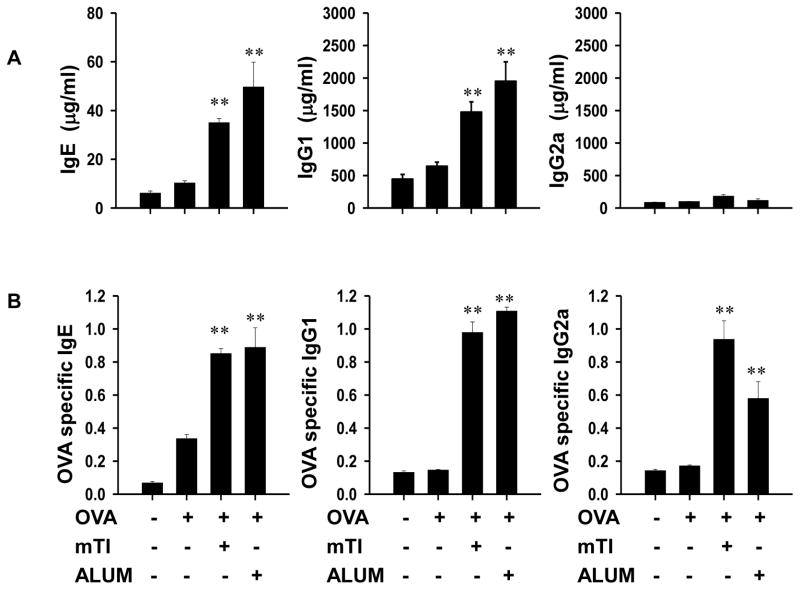
The effect of mTi particles, ranging from 0.2–88μm, on the development of the adaptive in vivo humoral immune response was first investigated. BALB/c female mice were initially inoculated intraperitoneally with 12.5mg mTi and 50μg OVA protein alone. Seven days after primary inoculation, all treatment groups were inoculated with OVA protein (50μg) alone and 21 days after secondary inoculation serum was collected and assayed for Igs. Alum, a well-studied adjuvant that can promote a potent Ag-specific Th2-type immune response (24, 25), was included for comparison. As shown in Fig. 1, administration of Alum or Ti in combination with OVA markedly enhanced serum elevations of total IgE and IgG1 compared to OVA alone, with negligible effects on IgG2a. Antigen-specific serum Ig levels were also examined using an OVA-specific ELISA assay. As also shown in Fig. 1, Ag-specific serum Ig levels were also elevated in mice inoculated with OVA + Ti compared to mice inoculated with OVA alone. Both total and Ag-specific serum Ig elevations were comparable in groups given either mTi or Alum in combination with OVA suggesting that mTi can function under these conditions as a potent adjuvant.
Figure 1. mTi administered with OVA antigen promotes serum IgE and IgG1 elevations.
Mice (8/treatment group) were administered OVA protein alone, OVA + mTi, or OVA + Alum, seven days later challenged with OVA alone, and 21 days after final inoculation serum was collected from each mouse and individually assayed for total and Ag-specific Igs. Both total serum IgE and IgG1 were markedly elevated, while slight increases in total serum IgG2a (Fig 1A) were observed. Similarly, OVA-specific IgE, IgG1, and IgG2a (Fig1B) were elevated. Data are expressed as the mean and s.e. of 8 individual mice within each treatment group and all experiments were repeated twice with similar results (**p<0.01).
Titanium can function as an effective adjuvant to promote naïve Ag-specific Th2 cell differentiation
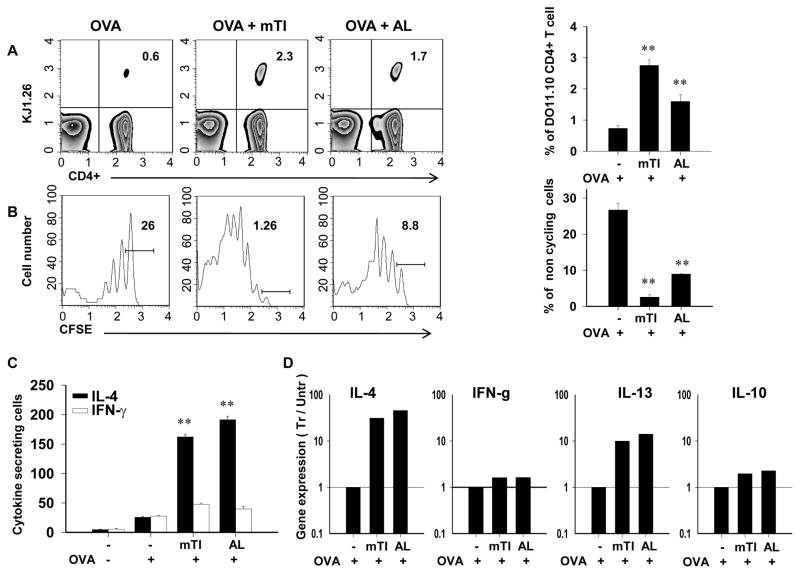
To examine whether mTi particles can function as an adjuvant to promote naïve T cell expansion and differentiation into effector T helper cells, CFSE labeled transgenic OVA-specific DO11.10 CD4+ cells were adoptively transferred to age-and sex-matched recipient BALB/c mice. Two days later, recipient mice were inoculated ip with OVA peptide(30 μg), OVA peptide(30 μg) + mTi (12.5 mg), or OVA peptide (30 μg)+ Alum(4mg) and seven days after inoculation mesenteric lymph nodes (MLNs) were collected and cell suspensions analyzed by flow cytometric analysis or OVA-specific ELISPOT. As shown in Fig. 2a, the percent of OVA-specific T cells (KJ-126+) was increased in mice inoculated with either OVA+ mTi or OVA + Alum, compared to mice inoculated with OVA alone, indicating that mTi as well as Alum could promote Ag-specific T cell expansion in vivo. CFSE analysis further showed pronounced cell cycle progression of OVA-specific T cells in mice administered OVA + mTi compared to mice administered OVA alone (Fig. 2b). An OVA-specific ELISPOT assay (23) was used to determine the number of IL-4 and IFN-γ-secreting cells following in vitro restimulation with OVA. As depicted in Fig. 2c, immunization with OVA + mTi or OVA + Alum resulted in pronounced increases in IL-4, but not IFN-γ. Sorted KJ- 126+ T cells showed pronounced IL-4, IL-10, and IL-13 gene expression in mice inoculated with OVA + Ti or OVA + Alum. Taken together, these studies indicate that Ti is an effective adjuvant that can promote naïve T cell differentiation to proliferating and cytokine producing Ag-specific Th2 cells.
Figure 2. In vivo Ag-specific T cell expansion, cell cycle progression, and IL-4 expression are enhanced by mTi administration.
CD4+ T cells were purified from DO11.10 OVA specific TCR-transgenic mice and labeled with CFSE before transfer to BALB/c recipient mice. Two days later recipient mice were inoculated ip with OVA, OVA + mTi, or OVA + Alum. At day 7 after inoculation, draining mesenteric lymph nodes were removed and stained with anti-CD4-PerCP and KJ-126PE for subsequent analysis. A) T cell expansion was assessed in individual mice within each treatment group and a representative example and the mean and s.e. for four mice/treatment group is shown. B) Cell cycle progression was assessed through decreased CFSE fluorescence and the percent of noncycling cells shown for a representative example and the mean and s.e. for four mice/treatment group. C) MLN cell suspensions from individual mice from each treatment group were cultured with OVA to assess OVA-specific IL-4 and IFN-γ cells per million total cells in each treatment group; mean and s.e. for four mice/treatment group are shown. D) Cytokine gene expression was determined from sorted CD4+, KJ1-26+ MLN T cells (pooled from 5 mice/treatment group) administered OVA, OVA + Ti or OVA + Alum. All experiments were repeated twice with similar results (**p<0.01)
Ti induces a robust innate response characterized by M2 cell differentiation
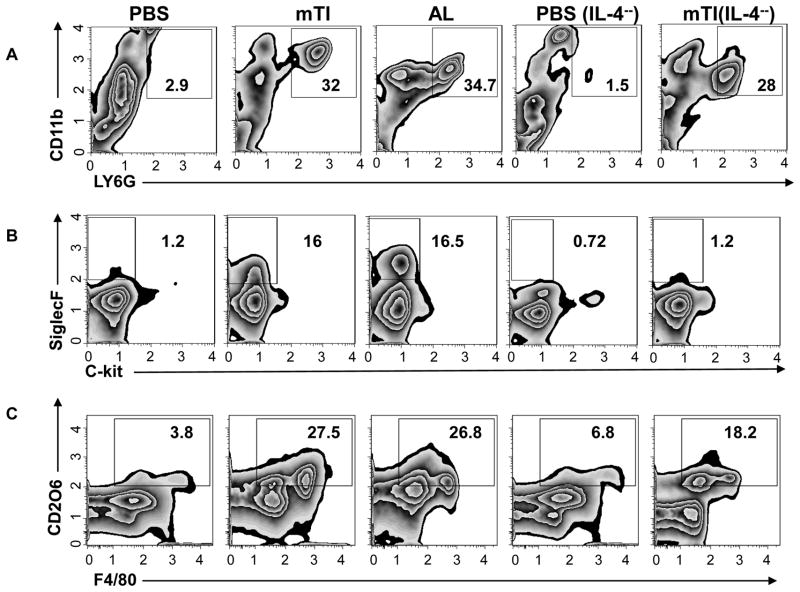
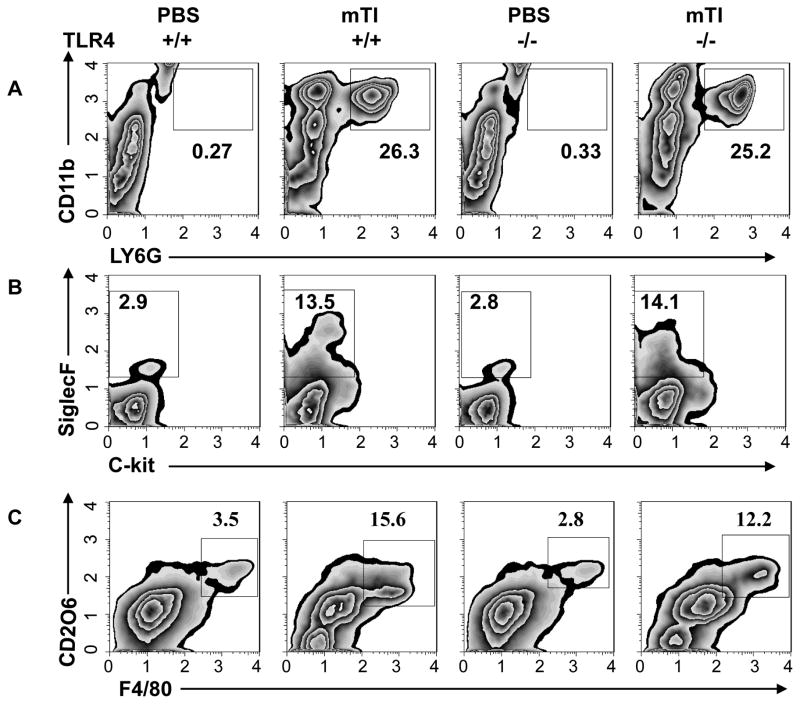
mTi may act as an adjuvant to stimulate Th2 cell differentiation by triggering an appropriate innate immune response or alternatively by enhancing antigenicity either through a depot effect, thereby increasing the time of Ag release, or by facilitating Ag uptake (6). Previous studies have suggested that mTi preparations similar to those used in this investigation are inert in biological systems (26). To investigate whether Ti may be triggering activation of innate immune cell populations characteristic of a Th2-type immune response, mice were inoculated ip with Ti alone and 48 hours later immune cell populations in the peritoneum were phenotyped using flow cytometric analysis. In addition, IL-4−/− BALB/c mice were inoculated with Ti to examine whether early production of IL-4 might be contributing to the development of a Ti-induced innate response. As shown in Fig. 3a, pronounced increases in neutrophils (Ly6G+, CD11b+) were observed following Ti inoculation in both WT and IL-4−/− mice, while eosinophils (SiglecF+, c-kit-) were increased more than 10X following Ti inoculation, consistent with a Th2-type innate response phenotype (Fig. 3b). Eosinophil increases were blocked in IL-4−/− mice, indicating that at this early time point after Ti inoculation IL-4 functions to promote eosinophil recruitment and expansion in the peritoneal cavity.
Figure 3. Ti triggers potent innate response in the absence of specific Ag characterized by both IL-4-dependent and IL-4-independent components.
Balb/c IL-4+/+ and IL-4−/− mice (5/treatment group) were inoculated ip with vehicle, Ti or Alum. Forty-eight hours later peritoneal exudate cells were collected and cell suspensions pooled from each treatment group. Pooled samples were stained for CD11b and Ly6G to detect neutrophils (A), c-kit and Siglec F to detect eosinophils (B), and F480 and CD206 to detect alternatively activated (M2) macrophages (C). This experiment was repeated two times with similar results.
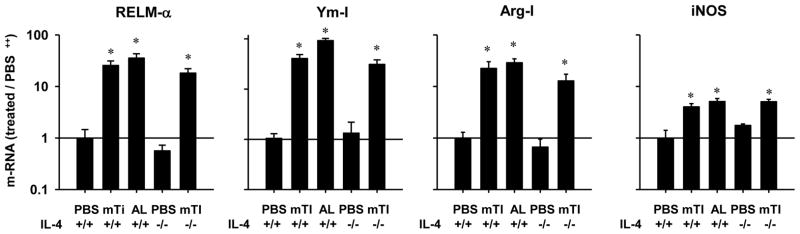
During a Th2-type innate immune response to helminthes macrophages characteristically differentiate to alternatively activated (M2) macrophages (27). To determine whether M2 macrophages were increased in Ti-inoculated mice, cell suspensions from the peritoneal cavity were dual stained for F480 and CD206, a characteristic M2 macrophage phenotype. As shown in Fig. 3c, the frequency of M2 macrophages was markedly increased in mTi -inoculated WT mice compared to untreated controls. Intriguingly, increased M2 macrophages were also observed in Ti-inoculated IL-4−/− mice, indicating that mTi induced M2 macrophage differentiation without IL-4 signaling. Gene expression analysis of characteristic M2 macrophage markers showed pronounced increases in RELM-α, Ym-1, and Arg-1 in peritoneal cells of mTi-inoculated mice compared to untreated controls; however, iNOS, an M1 macrophage marker, was elevated to a lesser degree (Fig. 4). Furthermore, these M2 markers were similarly increased in Ti-inoculated IL-4−/− mice.
Figure 4. Innate response to Ti triggers increases in characteristic markers of M2 macrophages independently of IL-4.
IL-4+/+ and IL-4−/− Balb/c mice (5/treatment group) were inoculated ip with vehicle, Ti or Alum. Forty-eight hours later peritoneal cells were collected and analyzed by quantitative fluorogenic PCR for expression of mRNA species characteristic of alternatively activated (M2) macrophages. The mean and s.e. for five mice/treatment group is shown for each marker. This experiment was repeated two times with similar results (*p<0.01).
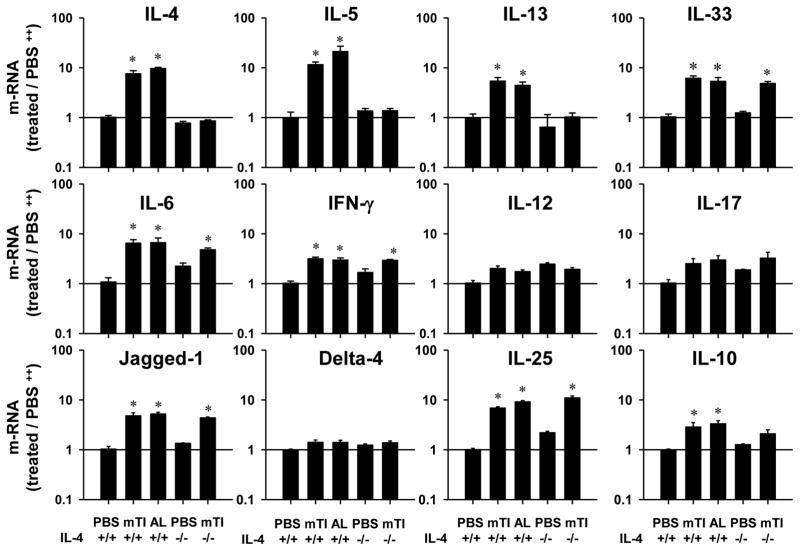
Cytokines and notch ligands typically elevated during Th1-type or Th2-type responses were also examined at 48 hours after mTi inoculation. As shown in Fig. 5, IL-4, IL-5, and IL-13 were elevated in mTi -inoculated WT mice, but blocked in Ti-inoculated IL-4−/− mice, while Jagged-1, IL-25, IL-6 and IL-33 were increased in both Ti-inoculated WT and IL-4−/− mice. In contrast, Delta-4, IL-12 and IFN-γ showed at most modest elevations. Alum, a well-known Th2 adjuvant, was used for comparison; in most cases mTi showed a comparable ability to activate the Th2-type innate immune response.
Figure 5. Ti induced pronounced increases in Th2 cytokines as early as 48 hours after inoculation.
IL-4+/+ and IL-4−/− Balb/c mice (5/treatment group) were inoculated ip with vehicle, Ti or Alum. Forty-eight hours later peritoneal cells were collected and analyzed by quantitative fluorogenic PCR for expression of mRNA species characteristic of Th2-, Th1-, TH17-type cytokines and notch ligands. The mean and s.e. for five mice/treatment group is shown for each marker. This experiment was repeated two times with similar results (*p<0.01).
Adjuvant functions of Ti are TLR4-independent
The presence of lipopolysaccharide (LPS), which signals through TLR4, is ubiquitous and can sometimes confound results during adjuvant studies. Although we used commercially available endotoxin-free mTi particles, previous findings have shown that under some circumstances even low levels of LPS can trigger a Th2-type immune response through TLR4 signaling (10), raising the possibility that some of the adjuvant functions observed with mTi particles may be due to associated LPS. As described in the methods, two previously published methods were used to effectively remove residual LPS from the mTi particles. Treatment with either method triggered a similar activation of innate cell populations, including neutrophils, eosinophils, and M2 macrophages, as compared to untreated mTi (Fig. S1). In additional experiments mice were immunized with treated mTi + OVA and 21 days later marked increases in serum IgE and IgG1 were detected (FigS2).
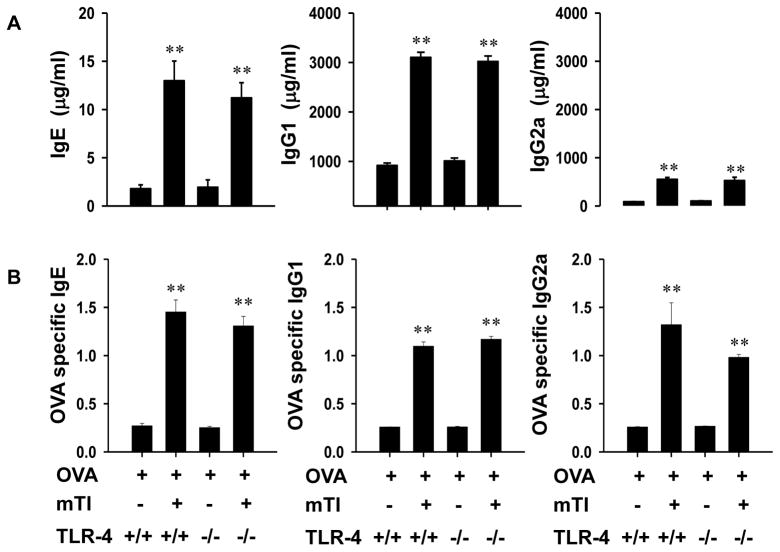
To further exclude the possibility that LPS-induced TLR4-signaling was contributing to the adjuvant function of mTi particles, TLR4-sufficient (C3H/OuJ) and TLR4-deficient (C3H/HeJ) mice were inoculated with mTi + OVA or OVA alone and seven days later given a secondary inoculation with OVA alone. In both mTi-inoculated TLR-4 deficient and TLR-4 sufficient mice, total serum IgE and IgG1, but not IgG2a, were markedly elevated 21 days after inoculation.
The innate immune response was also examined in TLR4-deficient mice at 48 hours after inoculation. As shown in Fig. 7, after Ti-inoculation pronounced and similar increases in neutrophils, eosinophils, and M2 macrophages were observed in TLR4-deficient and -sufficient mice. In both strains, the phenotype was similar to that observed in Ti-inoculated BALB/c mice, indicating that this response is sufficiently robust to be reproducible in different background murine strains. We also examined the innate immune response in Ti-inoculated BL/6 MYD-88−/ − mice, which lack this important TLR signaling pathway, and found similar increases in neutrophils, eosinophils, and M2 macrophages (data not shown) Taken together, these results indicate that potential low-dose LPS contamination is not playing an important role in affecting the innate or adaptive Th2-type immune response following mTi inoculation.
Figure 7. TLR4 deficiency does not impair development of Ti-induced Th2-type innate immune response.
TLR4 deficient C3H/HeJ and control C3H/OuJ mice were inoculated ip with Ti or vehicle. Forty-eight hours later peritoneal exudate cells were collected and cell suspensions pooled from each treatment group. Pooled samples were stained for CD11b and Ly6g to detect neutrophils (A), c-kit and Siglec F to detect eosinophils (B), and F480 and CD206 to detect alternatively activated (M2) macrophages (C). This experiment was repeated two times with similar results.
Discussion
Our findings indicate that a micron sized Ti particle can act as an adjuvant to promote naïve antigen-specific CD4 T cells to expand and differentiate into cytokine producing T helper 2 effector cells in vivo. Our findings further show that rather than simply acting as a depot or through general enhancement of Ag presentation, mTi particles elicit a potent innate Th2-type immune response associated with IL-4-independent M2 macrophage activation. This effect has implications for titanium implants suggesting that associated wear debris may contribute to harmful Th2-type inflammation.
Previous studies have suggested that wear debris, present in periprosthetic tissues and ranging in size from 0.5 to 100 μms, may contribute to osteolysis and aseptic loosening of total joint prostheses (2, 28, 29). We demonstrated that the commercial mTi preparation used had a very similar size range, from 0.2 to 88uMs. In vitro cultures with similar preparations of mTi particles can stimulate macrophages to secrete inflammatory cytokines, raising the possibility that mTi may also trigger an inflammatory response in vivo. However, such inflammatory responses may in many cases result from endotoxin adhering to the mTi, as mTi with adherent LPS preferentially induces inflammatory cytokines, including IL-1, IL-6 and TNF-α(3, 5). Our studies indicate that the very different Th2-type immune response induced by mTi in vivo is LPS independent as Ti-inoculated TLR4-deficient mice supported potent innate and adaptive Th2-type responses and as effective removal of endotoxin did not impair induction of the innate or adaptive Th2-type responses.
Our observation that total and Ag-specific serum IgG1 and IgE were elevated following administration of mTi with specific Ag suggested that mTi could act as an adjuvant to drive a potent humoral response and raised the possibility that mTi could promote Th2 cell differentiation. Previous studies have shown that Ti dioxide (TiO2) nano-particles can enhance allergy-associated inflammation in the lung; however, the small nanoparticle size along with associated charge have been thought to be important characteristics in driving this response (6). Our studies indicate that micron sized particles can also be potent adjuvants. This is particularly relevant to inflammation of joint replacements as wear debris that deposits and accumulates in surrounding tissue is primarily in the micron size range (28, 29). Through adoptive transfer of CFSE-labelled OVA-specific DO11.10 T cells, we were able to demonstrate that mTi particles increased cell cycle progression and expansion of Ag-specific T cells and promoted their differentiation into IL-4 secreting Th2 cells in vivo. In the same experiment the adjuvant effect of mTi on Th2 cell differentiation was similar to that observed with Alum. Alum is a well-documented potent Th2 adjuvant (12, 30) and the only vaccine adjuvant currently approved by FDA (31). Our inclusion of Alum in the described experiments allowed us to examine the relative strength of mTi as an adjuvant. Our findings indicate that mTi is as potent as Alum in driving both Ag-specific Th2 cell expansion and differentiation in vivo and also in promoting serum IgG1 and IgE elevations.
The mechanisms through which adjuvants drive Th2-type responses remain unclear. Traditionally, Alum was thought to work through a “depot” effect stabilizing adherent Ag and thereby prolonging exposure of the Ag to the immune system (32, 33). Alternatively, Alum has also been shown to trigger a strong innate immune response resulting in activation of the nod-like receptor protein 3 (NLRP3) inflammasome, resulting in caspase-1-mediated conversion of members of the IL-1 family to active cytokines (34, 35). More recent studies, however, suggest that blockade of this innate inflammasome response does not affect alum-mediated stimulation of the Th2-type immune response (30, 36–39). Most recently PGE2 has been implicated in promoting IgE production (39) while other studies suggest Alum may affect Ag presentation by directly perturbing dendritic cell membranes(40).
Our studies indicate that alum and mTi particles both trigger a potent Th2 type innate response previously associated primarily with helminth infection. Intriguingly, components of this innate response varied in their IL-4 dependence, as eosinophil recruitment was blocked in response to both alum and Ti particles in IL-4 deficient mice, while M2 cell activation remained intact. The IL-4 independent increases in IL-33, IL-25, and Jagged-1, all of which are associated with initiation of Th2-type responses (41, 42), indicate a robust Th2-type innate response even in the absence of IL-4 signaling. The overall innate Th2-type response induced by both Alum and Ti particles were remarkably similar suggesting the involvement of similar signaling pathways. M2 cells have been shown to develop in response to a number of stimuli including: IL-4, adenosine, colony stimulating factor (CSF), IL-10, and helminths (27, 43–46). Previous studies have shown macrophage (47, 48) infiltration at the periprosthetic site of osteolysis. Intriguingly, recent studies have also shown increased gene expression of M2 markers in synovial-like tissue in late-stage osteolysis patients and also elevations in chemokines potentially involved in recruitment of osteoclast precursor cells, while elevations in TNF-α and other Th1-type inflammatory markers were not detected (49). It should be noted that these studies with patients examined sites of chronic inflammation whereas our studies examined the acute response that may include infiltration by cell populations, such as neutrophils, that may only transiently infiltrate the site of osteolysis. Also, our study likely resulted in a more systemic response, including increases in serum Igs, compared to the more local response associated with peri-implant inflammation.
Helminthes can induce Th2 cell differentiation in the absence of IL-4, although subsequently IL-4 is required for Th2 cell expansion (23, 50, 51). Our findings with Ti and Alum suggest that at least M2 cell differentiation is IL-4-independent. Helminth products that trigger Th2-type responses include both glycans and phosphorycholine-containing proteins (e.g. ES62), both of which can induce Th2-type responses through TLR4-dependent pathways (7, 52, 53). Our results show that mTi, as well as Alum, are TLR4-independent. Interestingly, the Th2-type response induced by many live helminthes is also TLR4-independent (7, 14, 15), suggesting that both mTi, alum, and helminthes can all induce polarized Th2 cell differentiation in vivo in the absence of TLR-4-signaling. Cell interactions associated with relatively large solid structures in tissue, such as frustrated phagocytosis or cell and tissue damage (8, 40, 54), may be an important mechanism and common pathway through which an innate response is stimulated that ultimately contributes to Th2 cell differentiation. It will thus be of interest to examine whether a Th2-type response is associated with other implant materials, particularly since inflammatory infiltrates are observed with non metal on metal (MOM) as well as MOM implants (55).
These studies thus indicate that mTi particles can act as potent adjuvants to stimulate an innate Th2-type in vivo immune response, characterized by M2 cell activation, and subsequently the development of Ag-specific Th2 cells and associated serum IgE and IgG1 elevations. Their similarity to wear debris in prosthetic implants raises the possibility that this type of inflammation may occur shortly after joint replacement surgery. The characteristic lack of pathogen associated molecular patterns and the relatively large size of this adjuvant raises the possibility that some Th2 adjuvants, unlike many Th1 adjuvants, may largely function through stimulating as yet unidentified endogenous danger signals resulting from tissue insult.
Supplementary Material
Figure 6. TLR4 deficient mice administered OVA + Ti show elevations in serum IgG1 and IgE.
TLR4 deficient C3H/HeJ and control C3H/HeOuJ mice were administered OVA protein alone or OVA + Ti, seven days later challenged with OVA alone, and 21 days after final inoculation serum was collected from each mouse and individually assayed for total and Ag-specific Igs. Both total serum IgE (A) and IgG1 (B) were markedly elevated, while slight increases in total serum Ig2a (C) were observed. Similarly, OVA-specific IgE (D), IgG1 (E), and IgG2a (F) were elevated. Data are expressed as the mean and s.e. of 5 individual mice within each treatment group treated compared to the untreated (OVA) group). This experiment was repeated two times with similar results (**p<0.01).
Acknowledgments
We thank Ariel Millman for technical assistance with maintaining mice and genotyping.
This study was partly supported by National Institutes of Health (NIH) grant 5R01AI66188.
Abreeviations
- AL
Alum
- mTi
Titanium micro-particles
Footnotes
The authors have no competing financial interests
References
- 1.Bi Y, Seabold JM, Kaar SG, Ragab AA, Goldberg VM, Anderson JM, Greenfield EM. Adherent endotoxin on orthopedic wear particles stimulates cytokine production and osteoclast differentiation. J Bone Miner Res. 2001;16:2082–2091. doi: 10.1359/jbmr.2001.16.11.2082. [DOI] [PubMed] [Google Scholar]
- 2.Goldring SR, Schiller AL, Roelke M, Rourke CM, O’Neil DA, Harris WH. The synovial-like membrane at the bone-cement interface in loose total hip replacements and its proposed role in bone lysis. J Bone Joint Surg Am. 1983;65:575–584. [PubMed] [Google Scholar]
- 3.Greenfield EM, Beidelschies MA, Tatro JM, Goldberg VM, Hise AG. Bacterial Pathogen-associated Molecular Patterns Stimulate Biological Activity of Orthopaedic Wear Particles by Activating Cognate Toll-like Receptors. Journal of Biological Chemistry. 2010;285:32378–32384. doi: 10.1074/jbc.M110.136895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Islam AS, Beidelschies MA, Huml A, Greenfield EM. Titanium particles activate toll-like receptor 4 independently of lipid rafts in RAW264.7 murine macrophages. J Orthop Res. 2011;29:211–217. doi: 10.1002/jor.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bi Y, Collier TO, Goldberg VM, Anderson JM, Greenfield EM. Adherent endotoxin mediates biological responses of titanium particles without stimulating their phagocytosis. J Orthop Res. 2002;20:696–703. doi: 10.1016/S0736-0266(01)00176-0. [DOI] [PubMed] [Google Scholar]
- 6.Larsen ST, Roursgaard M, Jensen KA, Nielsen GD. Nano titanium dioxide particles promote allergic sensitization and lung inflammation in mice. Basic Clin Pharmacol Toxicol. 2010;106:114–117. doi: 10.1111/j.1742-7843.2009.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carvalho L, Sun J, Kane C, Marshall F, Krawczyk C, Pearce EJ. Review series on helminths, immune modulation and the hygiene hypothesis: mechanisms underlying helminth modulation of dendritic cell function. Immunology. 2009;126:28–34. doi: 10.1111/j.1365-2567.2008.03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anthony RM, Rutitzky LI, Urban JF, Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maizels RM, Pearce EJ, Artis D, Yazdanbakhsh M, Wynn TA. Regulation of pathogenesis and immunity in helminth infections. J Exp Med. 2009;206:2059–2066. doi: 10.1084/jem.20091903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piggott DA, Eisenbarth SC, Xu L, Constant SL, Huleatt JW, Herrick CA, Bottomly K. MyD88-dependent induction of allergic Th2 responses to intranasal antigen. J Clin Invest. 2005;115:459–467. doi: 10.1172/JCI22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 13.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helmby H, Grencis RK. Essential role for TLR4 and MyD88 in the development of chronic intestinal nematode infection. Eur J Immunol. 2003;33:2974–2979. doi: 10.1002/eji.200324264. [DOI] [PubMed] [Google Scholar]
- 15.Kerepesi LA, Hess JA, Leon O, Nolan TJ, Schad GA, Abraham D. Toll-like receptor 4 (TLR4) is required for protective immunity to larval Strongyloides stercoralis in mice. Microbes Infect. 2007;9:28–34. doi: 10.1016/j.micinf.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Liu Z, Liu Q, Pesce J, Whitmire J, Ekkens MJ, Foster A, VanNoy J, Sharpe AH, Urban JF, Jr, Gause WC. Nippostrongylus brasiliensis can induce B7-independent antigen-specific development of IL-4-producing T cells from naive CD4 T cells in vivo. J Immunol. 2002;169:6959–6968. doi: 10.4049/jimmunol.169.12.6959. [DOI] [PubMed] [Google Scholar]
- 17.Cho DR, Shanbhag AS, Hong CY, Baran GR, Goldring SR. The role of adsorbed endotoxin in particle-induced stimulation of cytokine release. J Orthop Res. 2002;20:704–713. doi: 10.1016/S0736-0266(01)00179-6. [DOI] [PubMed] [Google Scholar]
- 18.Smith RA, Hallab NJ. In vitro macrophage response to polyethylene and polycarbonate-urethane particles. Journal of biomedical materials research Part A. 2010;93:347–355. doi: 10.1002/jbm.a.32529. [DOI] [PubMed] [Google Scholar]
- 19.Brown T, Bao QB, Agrawal CM, Hallab NJ. An In Vitro Assessment of Wear Particulate Generated from NUBAC, A PEEK on PEEK Articulating Nucleus Replacement Device. Methodology and Results from a Series of Wear Tests Using Different Motion Profiles, Test Frequencies and Environmental Conditions. Spine. 2011 doi: 10.1097/BRS.0b013e31821ac8a0. [DOI] [PubMed] [Google Scholar]
- 20.Kanaji A, Caicedo MS, Virdi AS, Sumner DR, Hallab NJ, Sena K. Co-Cr-Mo alloy particles induce tumor necrosis factor alpha production in MLO-Y4 osteocytes: a role for osteocytes in particle-induced inflammation. Bone. 2009;45:528–533. doi: 10.1016/j.bone.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haskins K, Kubo R, White J, Pigeon M, Kappler J, Marrack P. The major histocompatibility complex-restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J Exp Med. 1983;157:1149–1169. doi: 10.1084/jem.157.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Q, Liu Z, Rozo CT, Hamed HA, Alem F, Urban JF, Jr, Gause WC. The role of B cells in the development of CD4 effector T cells during a polarized Th2 immune response. J Immunol. 2007;179:3821–3830. doi: 10.4049/jimmunol.179.6.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z, Liu Q, Hamed H, Anthony RM, Foster A, Finkelman FD, Urban JF, Jr, Gause WC. IL-2 and autocrine IL-4 drive the in vivo development of antigen-specific Th2 T cells elicited by nematode parasites. J Immunol. 2005;174:2242–2249. doi: 10.4049/jimmunol.174.4.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brewer JM, Conacher M, Hunter CA, Mohrs M, Brombacher F, Alexander J. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4-or IL-13-mediated signaling. J Immunol. 1999;163:6448–6454. [PubMed] [Google Scholar]
- 25.Lambrecht BN, Kool M, Willart MA, Hammad H. Mechanism of action of clinically approved adjuvants. Curr Opin Immunol. 2009;21:23–29. doi: 10.1016/j.coi.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 26.de Haar C, Hassing I, Bol M, Bleumink R, Pieters R. Ultrafine but not fine particulate matter causes airway inflammation and allergic airway sensitization to co-administered antigen in mice. Clin Exp Allergy. 2006;36:1469–1479. doi: 10.1111/j.1365-2222.2006.02586.x. [DOI] [PubMed] [Google Scholar]
- 27.Anthony RM, Urban JF, Jr, Alem F, Hamed HA, Rozo CT, Boucher JL, Van Rooijen N, Gause WC. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margevicius KJ, Bauer TW, McMahon JT, Brown SA, Merritt K. Isolation and characterization of debris in membranes around total joint prostheses. J Bone Joint Surg Am. 1994;76:1664–1675. doi: 10.2106/00004623-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Elfick AP, Green SM, Krikler S, Unsworth A. The nature and dissemination of UHMWPE wear debris retrieved from periprosthetic tissue of THR. Journal of biomedical materials research Part A. 2003;65:95–108. doi: 10.1002/jbm.a.10455. [DOI] [PubMed] [Google Scholar]
- 30.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nature Reviews Immunology. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schubert C. Boosting our best shot. Nat Med. 2009;15:984–988. doi: 10.1038/nm0909-984. [DOI] [PubMed] [Google Scholar]
- 32.Morefield G, Sokolovska A, Jiang D, Hogenesch H, Robinson J, Hem S. Role of aluminum-containing adjuvants in antigen internalization by dendritic cells in vitro. Vaccine. 2005;23:1588–1595. doi: 10.1016/j.vaccine.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 33.Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Current Opinion in Immunology. 2010;22:411–416. doi: 10.1016/j.coi.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Nookala S, Re F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J Immunol. 2007;178:5271–5276. doi: 10.4049/jimmunol.178.8.5271. [DOI] [PubMed] [Google Scholar]
- 36.McKee AS, Munks MW, MacLeod MKL, Fleenor CJ, Van Rooijen N, Kappler JW, Marrack P. Alum Induces InnateImmune Responses through Macrophage and Mast Cell Sensors, But These Sensors Are Not Required for Alum to Act As an Adjuvant for Specific Immunity. The Journal of Immunology. 2009;183:4403–4414. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franchi L, Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38:2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kool M, Soullie T, van Nimwegen M, Willart MAM, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. Journal of Experimental Medicine. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuroda E, Ishii KJ, Uematsu S, Ohata K, Coban C, Akira S, Aritake K, Urade Y, Morimoto Y. Silica crystals and aluminum salts regulate the production of prostaglandin in macrophages via NALP3 inflammasome-independent mechanisms. Immunity. 2011;34:514–526. doi: 10.1016/j.immuni.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 40.Flach TL, Ng G, Hari A, Desrosiers MD, Zhang P, Ward SM, Seamone ME, Vilaysane A, Mucsi AD, Fong Y, et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nature medicine. 2011;17:479–487. doi: 10.1038/nm.2306. [DOI] [PubMed] [Google Scholar]
- 41.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 42.Saenz SA, Noti M, Artis D. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol. 2010;31:407–413. doi: 10.1016/j.it.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Kreider T, Anthony RM, Urban JF, Jr, Gause WC. Alternatively activated macrophages in helminth infections. Curr Opin Immunol. 2007;19:448–453. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature Reviews Immunology. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80:1298–1307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hasko G, Pacher P, Deitch EA, Vizi ES. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol Ther. 2007;113:264–275. doi: 10.1016/j.pharmthera.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Looney RJ, Schwarz EM, Boyd A, O’Keefe RJ. Periprosthetic osteolysis: an immunologist’s update. Current opinion in rheumatology. 2006;18:80–87. doi: 10.1097/01.bor.0000198004.88568.96. [DOI] [PubMed] [Google Scholar]
- 48.Bullough PG, DiCarlo EF, Hansraj KK, Neves MC. Pathologic studies of total joint replacement. The Orthopedic clinics of North America. 1988;19:611–625. [PubMed] [Google Scholar]
- 49.Koulouvaris P, Ly K, Ivashkiv LB, Bostrom MP, Nestor BJ, Sculco TP, Purdue PE. Expression profiling reveals alternative macrophage activation and impaired osteogenesis in periprosthetic osteolysis. J Orthop Res. 2008;26:106–116. doi: 10.1002/jor.20486. [DOI] [PubMed] [Google Scholar]
- 50.Zhu J, Cote-Sierra J, Guo L, Paul WE. Stat5 activation plays a critical role in Th2 differentiation. Immunity. 2003;19:739–748. doi: 10.1016/s1074-7613(03)00292-9. [DOI] [PubMed] [Google Scholar]
- 51.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goodridge HS, Marshall FA, Else KJ, Houston KM, Egan C, Al-Riyami L, Liew FY, Harnett W, Harnett MM. Immunomodulation via novel use of TLR4 by the filarial nematode phosphorylcholine-containing secreted product, ES-62. J Immunol. 2005;174:284–293. doi: 10.4049/jimmunol.174.1.284. [DOI] [PubMed] [Google Scholar]
- 53.Harn DA, McDonald J, Atochina O, Da’dara AA. Modulation of host immune responses by helminth glycans. Immunological reviews. 2009;230:247–257. doi: 10.1111/j.1600-065X.2009.00799.x. [DOI] [PubMed] [Google Scholar]
- 54.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fujishiro T, Moojen DJ, Kobayashi N, Dhert WJ, Bauer TW. Perivascular and diffuse lymphocytic inflammation are not specific for failed metal-on-metal hip implants. Clinical orthopaedics and related research. 2011;469:1127–1133. doi: 10.1007/s11999-010-1649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.