Abstract
The role of erythropoietin (Epo) and Epo/Epo receptor (EpoR) signaling pathways for production of red blood cells are well established. However, little is known about Epo/EpoR signaling in non-hematopoietic cells. Recently, we demonstrated that Epo activates JAK/STAT signaling in hematopoietic stem cells (HSCs), leading to the production of bone morphogenetic protein 2 (BMP2) and bone formation and that Epo also directly activate mesenchymal cells to form osteoblasts in vitro. In this study, we investigated the effects of mTOR signaling on Epo-mediated osteoblastogenesis and osteoclastogenesis. We found that mTOR inhibition by rapamycin blocks Epo-dependent and -independent osteoblastic phenotypes in human bone marrow stromal cells (hBMSCs) and ST2 cells, respectively. Furthermore, we found that rapamycin inhibits Epo-dependent and -independent osteoclastogenesis in mouse bone marrow mononuclear cells and Raw264.7 cells. Finally, we demonstrated that Epo increases NFATc1 expression and decreases cathepsin K expression in an mTOR-independent manner, resulting in an increase of osteoclast numbers and a decrease in resorption activity. Taken together, these results strongly indicate that mTOR signaling plays an important role in Epo-mediated bone homeostasis.
Keywords: osteoblasts, HSCs, osteoclasts, erythropoietin, mTOR, rapamycin
INTRODUCTION
Erythropoietin (Epo) is a glycoprotein hormone that regulates red blood cell production in the bone marrow. Under hypoxic conditions, Epo is produced mainly by the adult kidney and secreted Epo binds to the Epo receptor (EpoR) expressed by erythroid progenitor cells in the marrow to stimulate proliferation, differentiation, and survival [Richmond et al., 2005; Szenajch et al., 2010]. Epo-mediated conformational changes to EpoR induces several signaling pathways, including Janus kinase 2/signal transducer and activator of transcription 3 or 5 (JAK2/STAT3 or JAK2/STAT5), mitogen activated protein kinase (MAPK), protein kinase C (PKC), and phosphatidylinositol 3 kinase/Akt (PI3K/Akt) [Richmond et al., 2005; Szenajch et al., 2010].
In the PI3K/Akt signaling pathway, activated Akt phosphorylates and decreases the ability of tuberous sclerosis complex 2 (TSC2) to inhibit the mammalian target of rapamycin (mTOR), resulting in the activation of mTOR [Inoki et al., 2002]. mTOR is the catalytic subunit of two distinct signaling complexes, mTOR complex 1 and 2 (mTORC1 and mTORC2) [Zoncu et al., 2011]. mTORC1 consists of mTOR, raptor, deptor, mLST8, and PRAS40 [Kim et al., 2002; Vander Haar et al., 2007; Zoncu et al., 2011]. mTORC1 activates ribosomal S6 kinase (S6K) and inactivates eukaryotic initiation factor 4E Binding protein 1 (4EBP1), and thus stimulates protein synthesis, cell growth, cell proliferation, and progression through the cell cycle [Hong et al., 2008; Kim et al., 2002; Nojima et al., 2003]. Rapamycin, a specific inhibitor of mTOR, directly binds to mTORC1 and inhibits mTORC1 activity [Kim et al., 2002]. In contrast, mTORC2, which contains mTOR, rictor, deptor, mLST8, and mSIN1, activates Akt and PKCα, promoting cell survival and cytoskeleton reorganization [Frias et al., 2006; Jacinto et al., 2004]. In addition, mTORC2 is insensitive to rapamycin, although prolonged treatment can inhibit mTORC2 in many cell types [Sarbassov et al., 2006].
While we previously established that Epo activates JAK/STAT signaling in hematopoietic stem cells (HSCs), resulting in the secretion of bone morphogenetic protein (BMP) and bone formation, Epo also had direct effects on bone marrow stromal cell differentiation into osteoblasts [Shiozawa et al., 2010]. Additionally, supra-physiologic stimulation with Epo increased the number of multinucleated osteoclasts from precursor populations without receptor activator of nuclear factor κB-ligand (RANKL), but failed to activate osteoclastic bone resorption [Shiozawa et al., 2010]. To elucidate which signaling pathway contributes to the direct effects of Epo on the differentiation of osteoblasts and osteoclasts, we explored the contribution of the mTOR pathway to Epo-induced osteogenesis since recent work has suggested that mTOR signaling is important for both osteoblastogenesis and osteoclastogenesis. Our results demonstrate that mTOR signaling plays an important role in both Epo-dependent and -independent bone homeostatic mechanisms.
MATERIALS AND METHODS
CELL CULTURES
Mouse bone marrow-derived stroma cell line ST2, human bone marrow stromal cells (hBMSCs, Lonza, Walkersville, MD), the mouse macrophage cell line RAW264.7, and mouse marrow mononuclear cells (mMMCs) were grown in alpha modified Eagles’s medium (α-MEM, Invitrogen, Carsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin/streptomycin.
ALKALINE PHOSPHATASE AND ALIZARIN RED ASSAYS
hBMSCs and ST2 cells (5×104) were seeded onto 12-well culture plates (Falcon, Becton Dickinson Labware, Franklin Lakes, NJ) and cultured in 1 ml of medium at least 24 hours before the initiation of the experimental conditions. Cells were treated for 2 or 3 weeks with 20 U/ml rhEpo and/or 10 nM rapamycin (Cell Signaling, Danvers, MA) while cultured in osteogenic medium containing 50 μg/ml ascorbic acid (Sigma-Aldrich, St. Louis, MO), 10 mM β-glycerophosphate (Sigma-Aldrich), and 10 nM dexamethasone (Sigma-Aldrich). After 2 weeks, the cells and matrix were fixed with 4% paraformaldehyde and stained with Alkaline Phosphatase Substrate (Sigma-Aldrich). After 3 weeks, the cells were fixed with 4% paraformaldehyde and stained with 2% Alizarin red (Sigma-Aldrich). Quantification of the staining density was analyzed using Image J software (National Institutes of Health, USA).
TARTRATE-RESISTANT ACID PHOSPHATASE (TRAP) STAINING
Bone marrow cells were flushed from femurs, tibia, and humeri of 5-8 week old C57BL/6 (Jackson Laboratory, Bar Harbor, ME) with Dulbecco’s Phosphate-Buffered Saline (D-PBS, Invitrogen). Debris and cell aggregates were removed using a 40 μm-mesh nylon cell strainer (BD Biosciences, San Diego, CA). Mouse marrow mononuclear cells (mMMCs) were seeded onto 24-well plates. Cells were treated for 1 week with 20 U/ml rhEpo and/or 10 nM rapamycin while cultured in α-MEM containing 50 ng/ml recombinant human receptor activator of nuclear factor κB-ligand (rhRANKL, R&D Systems, Minneapolis, MN) and 50 ng/ml macrophage-colony stimulating factor (M-CSF, R&D Systems). RAW264.7 was also treated for 1 week with 20 U/ml rhEpo and/or 10 nM rapamycin while cultured in α-MEM in the presence and absence of 50 ng/ml rhRANKL. Medium was changed every 2 days. After 1 week, cells were stained by TRAP staining kit (Kamiya Biomedical Company, Seattle, WA) according to the manufacturer’s instructions. Numbers of TRAP+ multinucleated cells (three or more nuclei per cell) were analyzed.
IN VITRO BONE RESORPTION ASSAYS
Bone marrow cells and RAW264.7 cells were seeded on the BioCoat Osteologic Discs (BD Biosciences) in 24-well plates. Cells were treated for 1 week with 20 U/ml rhEpo and/or 10 nM rapamycin while cultured in α-MEM containing 50 ng/ml rhRANKL and/or 50 ng/ml M-CSF. Medium was changed every 2 days. After 1 week, bone resorption assays were performed according to the manufacturer’s instructions. The mean area of resorption from five randomly selected fields was analyzed by Image J software.
QUANTITATIVE RT-PCR
hBMSCs and ST2 cells were treated with/without 20 U/ml rhEpo for 24 hours. RAW264.7 cells were treated for 5 days with 20 U/ml rhEpo and/or 10 nM rapamycin while cultured in α-MEM containing 50 ng/ml rhRANKL. Total RNA was extracted from cells using the RNeasy mini kit (Qiagen, Valencia, CA) and converted into cDNA using First-Strand Synthesis Kit (Invitrogen). Quantitative PCR was carried out on ABI 7700 sequence detector (Applied Biosystems, Forster City, CA) using TaqMan Universal PCR Master Mix Kit (Applied Biosystems) according to the manufacturer’s instructions. TaqMan MGB probes (Applied Biosystems) were as follows: human EpoR (Hs00181092_m1), mouse EpoR (Mm00833882_m1), mouse NFATc1 (Mm00479445_m1), and mouse Cathepsin K (Mm00484036_m1). Human beta-actin (Hs99999903_m1) and mouse beta-actin (Mm00607939_s1) were used as the internal control for the normalization of target gene expression.
WESTERN BLOTS
Whole cell lysates were prepared from cells, separated on 10% SDS-polyacrylamide gel and transferred to PVDF membrane. The membranes were incubated with 5% milk for 1 hr and incubated with primary antibodies overnight at 4°C. Primary antibodies used were as follows: polyclonal anti-phospho-Akt (1:1000; Cell Signaling), polyclonal anti-phospho-S6 (Ser240/244) (1:4000; Cell Signaling), polyclonal anti-S6 (1:4000; Cell Signaling). Blots were incubated with peroxidase-coupled secondary antibodies (Promega, Madison, WI) for 1 hour, and protein expression was detected with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL). Membranes were reprobed with polyclonal anti-β-actin antibody (1:1000; Cell Signaling) to control for equal loading.
STATISTICAL ANALYSES
Results are presented as mean ± standard deviation of mean. Significance of the difference between two measurements was determined by unpaired Student’s t-test, and multiple comparisons were evaluated by the Newman-Keuls multiple comparison test. Values of p < 0.05 were considered significant.
RESULTS
RAPAMYCIN INHIBITS EPO-DEPENDENT AND –INDEPENDENT OSTEOBLASTIC PHENOTYPE IN HUMAN BONE MARROW STROMAL CELLS AND ST2 CELLS
Recently, we found that Epo can regulate bone formation in vitro and in vivo [Shiozawa et al., 2010]. To determine if the mTOR signaling pathway is involved in Epo-mediated bone formation, we used rapamycin, a well known and specific inhibitor of mTOR. Epo induced differentiation and mineralization of human bone marrow stromal cells (hBMSCs) as determined by ALP staining (Fig. 1A) and Alizarin red staining (Fig. 1B). In contrast, Epo did not induce differentiation and mineralization of ST2 cells (Fig. 2A,B). However, rapamycin strongly blocked osteoblast differentiation in both cell types (Figs. 1A,B and 2A,B). Western blot analysis showed that Epo can activate Akt and mTOR signaling in both cells and that rapamycin can block Epo-mediated mTOR activation in both cells as demonstrated by the reduction in pAkt and pS6 levels (Figs. 1C and 2C). Epo receptor (EpoR) mRNA expression was examined by quantitative RT-PCR in hBMSCs and ST2 cells. EpoR mRNA expression was significantly increased in hBMSCs upon the Epo treatment (Fig. 1D). However, EpoR mRNA expression did not respond to Epo in ST2 cells (Fig. 2D). These data suggest that mTOR signaling is essential for Epo-dependent and -independent osteoblastic differentiation.
Fig. 1.
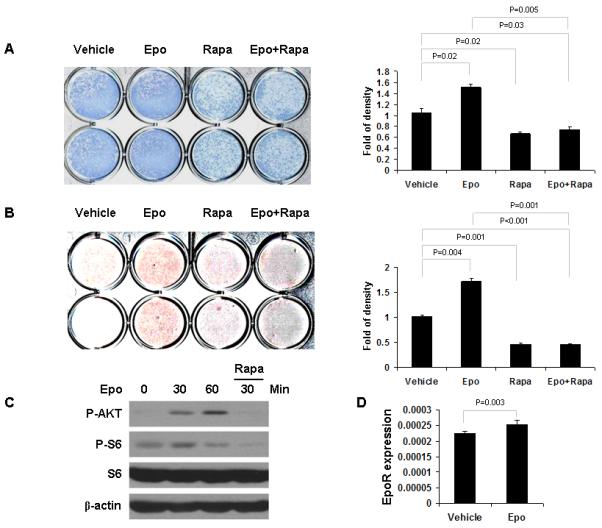
Rapamycin inhibits Epo-dependent osteoblast differentiation in hBMSC. hBMSC were cultured in differentiation inducing media containing Epo and/or rapamycin and assayed via alkaline phosphatase staining after 2 weeks (A) and Alizarin red staining after 3 weeks (B). Staining was quantified using Image J software and normalized to cell number. The experiments were performed twice. (C) Protein extracts from hBMSC cells were analyzed by Western blotting using antibodies against the indicated proteins. (D) hBMSC cells were treated for 24 hours with/without Epo. Expression of EpoR was quantified by qPCR. The results were normalized to β-actin. The results represent average values from triplicate assays and the experiments were performed twice.
Fig. 2.
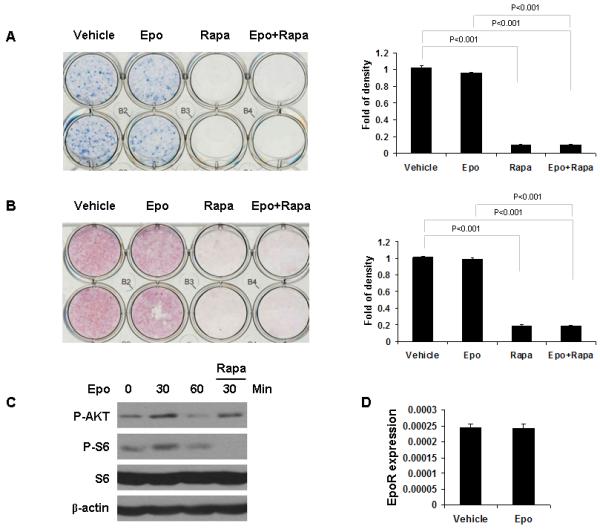
Rapamycin inhibits Epo-independent osteoblast differentiation in ST2 cells. ST2 cells were cultured in differentiation inducing media containing Epo and/or rapamycin and assayed via alkaline phosphatase staining after 2 weeks (A) and Alizarin red staining after 3 weeks (B). Staining was quantified using Image J software and normalized to cell number. The experiments were performed twice. (C) Protein extracts from ST2 cells were analyzed by Western blotting using antibodies against the indicated proteins. (D) ST2 cells were treated for 24 hours with/without Epo. Expression of EpoR was quantified by qPCR. The results were normalized to β-actin. The results represent average values from triplicate assays and the experiments were performed twice.
RAPAMYCIN INHIBITS EPO-DEPENDENT AND –INDEPENDENT OSTEOCLASTOGENESIS IN MOUSE MARROW MONONUCLEAR CELLS AND RAW264.7 CELLS
We previously reported that Epo induces osteoclast formation, but not osteoclast activity. To investigate the effect of rapamycin on EPO-mediated osteoclastogenesis, mouse marrow mononuclear cells (mMMCs) were treated for 1 week with or without receptor activator of nuclear factor κB-ligand (RANKL), Epo, and rapamycin in medium containing macrophage-colony stimulating factor (M-CSF). As expected, RANKL induced osteoclast formation (Fig. 3A,B) and Epo in combination with RANKL increased the number of osteoclasts (Fig. 3A,B), but decreased osteoclast activity (Fig. 3C,D). In contrast, mTOR inhibition by rapamycin robustly induced cell death (Fig. 3A) and blocked the induction of an osteoclastic phenotype in mouse bone marrow mononuclear cells (Fig. 3A-D). In RAW264.7 cells, rapamycin treatment did not affect cell death (Fig. 4A), but significantly decreased osteoclast formation by RANKL and/or Epo (Fig. 4A,B) and osteoclast activity on bone resorption (Fig. 4C,D). These data suggest that mTOR signaling is also important for Epo-dependent and -independent osteoclastic phenotype.
Fig. 3.
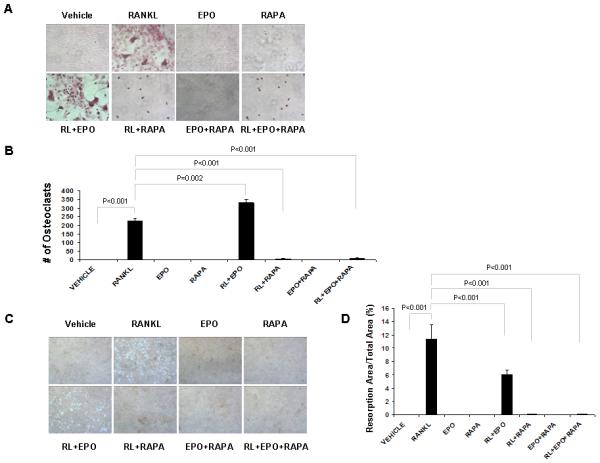
Rapamycin inhibits Epo-dependent and -independent osteoclastogenesis in mouse marrow mononuclear cells. Mouse marrow mononuclear cells (mMMCs) were treated with Epo and/or rapamycin while cultured in media with/without RANKL and M-CSF. After 1 week, TRAP staining was performed. (A) Representative micrographs of TRAP-positive osteoclast. Original magnification at 100x. (B) The numbers of TRAP-positive osteoclast were scored. The results represent average values from triplicate assays and the experiments were performed twice. (C) Representative micrographs of bone resorption. Original magnification at 100x. (D) Resorption area from five randomly selected fields was quantified using Image J software.
Fig. 4.
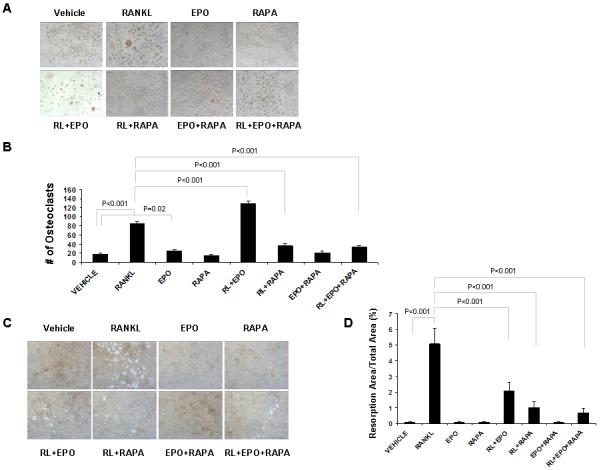
Rapamycin inhibits Epo-dependent and -independent osteoclastogenesis in Raw264.7 cells. Raw264.7 cells were treated with Epo and/or rapamycin while cultured in media with/without RANKL. After 1 week, TRAP staining was performed. (A) Representative micrographs of TRAP-positive osteoclast. Original magnification at 100x. (B) The numbers of TRAP-positive osteoclast were scored. The results represent average values from triplicate assays and the experiments were performed twice. (C) Representative micrographs of bone resorption. Original magnification at 100x. (D) Resorption area from five randomly selected fields was quantified using Image J software.
EPO INCREASES NFATc1 EXPRESSION AND DECREASES CATHEPSIN K EXPRESSION IN AN mTOR-INDEPENDENT MANNER
Because it is not known how Epo increases the number of osteoclasts yet fails to activate these cells, we investigated the down-stream target genes that Epo regulates during osteoclastogenesis. RAW264.7 cells were treated for 5 days with Epo and/or rapamycin in medium in the presence and absence of RANKL. Quantitative PCR revealed that both Epo and rapamycin treatments increased in NFATc1 mRNA which regulates osteoclast fusion in the presence of RANKL (Fig. 5A).
Fig. 5.
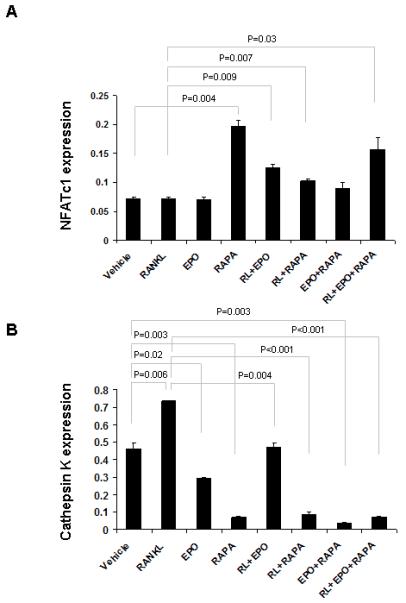
EPO and rapamycin increase NFATc1 expression and decrease cathepsin K expression. RAW264.7 cells were treated for 5 days with Epo and/or rapamycin while cultured in media with/without RANKL. Induction of the indicated genes was quantified by qPCR (A and B). The results were normalized to β-actin. The results represent average values from duplicate assays and the experiments were performed twice.
Conversely, both Epo and rapamycin were able to decrease the expression of cathepsin K which is involved in osteoclast-mediated bone resorption (Fig. 5B). These data suggest that Epo increases the number of osteoclasts by up-regulation of NFATc1 while at the same time decreases their activity by down-regulation of cathepsin K in an mTOR-independent manner. Taken together, these results strongly indicate that mTOR signaling plays an important role in Epo-dependent and -independent bone formation.
DISCUSSION
Emerging evidence demonstrates that mTOR signaling plays an important role in bone formation [Niziolek et al., 2009; Phornphutkul et al., 2009; Sanchez and He, 2009]. Here, we investigated the role of mTOR signaling in Epo-mediated differentiation of osteoblasts and osteoclasts. We found that Epo directly induces the differentiation of human bone marrow stromal cells (hBMSCs) into osteoblasts and that mTOR inhibition by rapamycin blocks osteoblast differentiation. These results suggest that mTOR signaling pathway is necessary for Epo-mediated osteoblastic differentiation of hBMSCs. However, Epo was unable to induce differentiation of the mouse bone marrow-derived stroma cell line ST2. Interestingly, rapamycin blocks Akt phosphorylation induced by Epo in hBMSC, but has little effect on ST2 cells, although rapamycin totally blocks Epo-mediated S6 phosphorylation in both cells (Fig. 1C and 2C). These findings indicate that ST2 cells may be more dependent on rapamycin-insensitive mTORC2, rather than rapamycin-sensitive mTORC1. Moreover, we found that Epo increases the expression of Epo receptor (EpoR) in hBMSC but fails to induce EpoR in ST2 cells, although both cell types express EpoR at the same level (Figs. 1D and 2D). Nevertheless, rapamycin blocks the osteoblastic differentiation of ST2 cells, suggesting that the mTOR pathway is also necessary for Epo-independent osteoblastic differentiation of ST2.
We also found that Epo in combination with RANKL increases the number of osteoclasts generated from mouse marrow mononuclear cells (mMMCs) and from RAW264.7 cells, and that rapamycin inhibits Epo-mediated osteoclast formation in both cell types. However, Epo inhibits the activity of osteoclasts by down-regulation of cathepsin K in an mTOR-independent manner. In osteoblastogenesis, mTOR signaling may affect the proliferation and differentiation of osteoblasts. However, the effect of rapamycin on osteoblast differentiation remains controversial. Rapamycin promotes the osteoblastic differentiation of human embryonic stem cells by blocking the mTOR pathway and stimulating the BMP/Smad pathway [Lee et al., 2010]. Moreover, NVP-BEZ235, a dual pan class I PI3 kinase and mTOR inhibitor, also promotes osteogenic differentiation in human mesenchymal stromal cells [Martin et al., 2010]. In mouse bone marrow-derived stromal cell line ST2, rapamycin increases osteoprotegerin (OPG)/osteoclastogenesis inhibitory factor, which is a soluble decoy receptor for osteoclast differentiation factor as well as receptor activation of NF-κB ligand (RANKL) [Mogi and Kondo, 2009]. In contrast, rapamycin inhibits osteoblast proliferation and differentiation in MC3T3-E1 cells and primary mouse bone marrow stromal cells [Singha et al., 2008]. In rat bone marrow stromal cells, rapamycin inhibits the effect of osteogenic differentiation induced by dexamethasone [Isomoto et al., 2007]. In fetal rat calvaria cells, the inhibition of p70S6 kinase by rapamycin blocks osteogenic protein-1 induction of alkaline phosphatase activity [Shoba and Lee, 2003].
In osteoclastogenesis, mTOR signaling regulates survival and differentiation of osteoclasts. Glantschnig et al. reported that Rapamycin blocks M-CSF- and RANKL-dependent osteoclast survival, resulting in apoptosis [Glantschnig et al., 2003]. mTOR knock-down using siRNA gene silencing also induces apoptosis through increase of Bim, a proapoptotic Bcl-2 family member and completely blocked osteoclast formation [Sugatani and Hruska, 2005]. Furthermore, mTOR regulates osteoclast differentiation by modulating the C/EBPβ isoform ratio and MafB expression [Smink et al., 2009].
The effect of Epo on non-hematopoietic tissues remains controversial. Recent work demonstrated that EpoR may be expressed by a number of tumor cell lines and as a result the use of Epo in the tumor setting has been questioned [Acs et al., 2001; Henke et al., 2006; Jelkmann et al., 2008]. However, the expression of EpoR in tumor tissues has been heavily dependent on the detection methods and which reagents are used [Jelkmann et al., 2008]. Epo receptors have also been found in several non-hematopoietic tissues including neural, reproductive and cardiac systems [Arcasoy, 2010]. Epo also plays a protective role in a number of tissues and situations [Bianchi et al., 2004; Calvillo et al., 2003; Gorio et al., 2002; Grasso et al., 2004]. As such, EpoR expression has been shown to induce proliferation of muscle, intestine and cardiomyocytes [Brines and Cerami, 2005; Calvillo et al., 2003; Celik et al., 2002]. In fact, several types of vascular endothelial cells have been shown to express EpoR [Anagnostou et al., 1990; Yamaji et al., 1996] and Epo is able to stimulate angiogenesis [Anagnostou et al., 1990]. EpoR expression in neuronal cells [Masuda et al., 1993] and brain capillary endothelial cells [Yamaji et al., 1996] suggests that Epo plays a role in neuroprotection.
In bone the effects are also controversial. In rodent models, bleeding stimulates bone formation in addition to hematopoietic activities [Bab and Einhorn, 1993; Brager et al., 2000; Foldes et al., 1989; Lucas et al., 1997]. However, most of the investigation in this area has focused on what how bone tissue adapts to states of hematopoietic stress where expanded hematopoietic activities have been known to be associated with the conversion of fatty marrow to hematopoietic marrow at the expense of bone. Likewise, osteoporosis is a common finding in ß-thalassemia, and bone mineral densities are improved in transfused individuals [Yazigi et al., 2002]. In contrast, Domracheva et al. reported that an acute change in altitude, and by inference Epo, may increase MSC numbers in patients with cytolytic syndromes [Domracheva et al., 1985]. They noted that fibroblastic colony-formation was 2 to 4 times increased after altitude change of 3,200 m [Domracheva et al., 1985].
The effects of hormones are often organ and/or dose-dependent. Parathyroid hormone (PTH) for example, initially was identified on the basis of its ability to stimulate bone resorption. However, more recent findings have moved PTH into the clinic as an anabolic bone agent even though it appears to signal via the same receptor [Bashutski et al., 2010]. Thus it is very likely that Epo may also have both anabolic and catabolic actions in bone depending on the models used, dosing and skeletal site examined. As a case in point, Singbrant et al. recently demonstrated an interaction between erythropoiesis and skeletal homeostasis based on the activation of osteoclasts but did not find direct effects on osteoblasts, their precursors, or hematopoietic stem cells [Singbrant et al., 2011]. Moreover, it was demonstrated that the expression of EpoR is limited to cells of the erythoroid lineage using lineage-tracing methods [Singbrant et al., 2011]. These findings suggest that osteoclasts or their immediate precursors are of erythroid origin, yet there is little support in the literature nor are osteoclasts depleted in aplastic anemia [Flanagan, 1990]. In contrast, we have showed that HSCs express low levels of EpoR using antibodies and quantitative RT-PCR, and HSCs respond to Epo by inducing BMP expression via JAK/STAT signaling [Shiozawa et al., 2010]. We also found that supraphysiologic doses of Epo stimulated bone formation in the vertebral spines of neonatal and young mice, while modestly increasing the hematocrit (4,500-6,000 U/kg, 3 times/week for 4 weeks) [Shiozawa et al., 2010]. Consistent with our results, a separate group has demonstrated that Epo improves fracture healing in long bones [Lee et al., 1991].
In summary, the current work further elucidates the signaling pathway that contributes to the direct effects of Epo on the differentiation of osteoblasts and osteoclasts. We demonstrate that the mTOR pathway is intimately involved in Epo-induced osteogenesis and substantiates recent work that shows that mTOR signaling is important for both osteoblastogenesis and osteoclastogenesis. Our results suggest that mTOR signaling is important for Epo-dependent and -independent bone formation (Fig. 6).
Fig. 6.
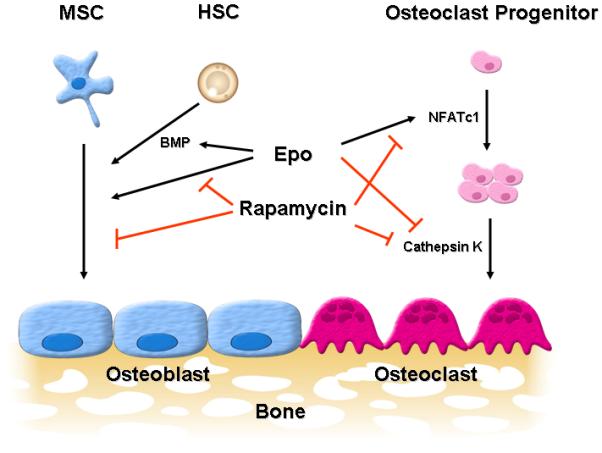
Model for the role of mTOR signaling in Epo-mediated bone formation. Epo directly or indirectly (by inducing BMP expression from hematopoietic stem cells (HSCs)) induces osteoblast differentiation of mesenchymal stem cells (MSCs) and mTOR inhibition by rapamycin blocks Epo-dependent and -independent osteoblast differentiation. Epo also increases the number of osteoclasts by up-regulation of NFATc1 while at the same time decreases their activity by down-regulation of cathepsin K in an mTOR-independent manner. Taken together, these results strongly indicate that mTOR signaling plays an important role in Epo-dependent and -independent bone formation.
ACKNOWLEDGMENTS
This study was supported by grants from the Department of Defense (PC073952) and National Institutes of health (DK082481, DE020721, 1RC1DE020721, CA141426 and CA093900).
REFERENCES
- Acs G, Acs P, Beckwith SM, Pitts RL, Clements E, Wong K, Verma A. Erythropoietin and erythropoietin receptor expression in human cancer. Cancer Res. 2001;61:3561–5. [PubMed] [Google Scholar]
- Anagnostou A, Lee ES, Kessimian N, Levinson R, Steiner M. Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proc Natl Acad Sci U S A. 1990;87:5978–82. doi: 10.1073/pnas.87.15.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcasoy MO. Non-erythroid effects of erythropoietin. Haematologica. 2010;95:1803–5. doi: 10.3324/haematol.2010.030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bab IA, Einhorn TA. Regulatory role of osteogenic growth polypeptides in bone formation and hemopoiesis. Crit Rev Eukaryot Gene Expr. 1993;3:31–46. [PubMed] [Google Scholar]
- Bashutski JD, Eber RM, Kinney JS, Benavides E, Maitra S, Braun TM, Giannobile WV, McCauley LK. Teriparatide and osseous regeneration in the oral cavity. N Engl J Med. 2010;363:2396–405. doi: 10.1056/NEJMoa1005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi R, Buyukakilli B, Brines M, Savino C, Cavaletti G, Oggioni N, Lauria G, Borgna M, Lombardi R, Cimen B, Comelekoglu U, Kanik A, Tataroglu C, Cerami A, Ghezzi P. Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proc Natl Acad Sci U S A. 2004;101:823–8. doi: 10.1073/pnas.0307823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager MA, Patterson MJ, Connolly JF, Nevo Z. Osteogenic growth peptide normally stimulated by blood loss and marrow ablation has local and systemic effects on fracture healing in rats. J Orthop Res. 2000;18:133–9. doi: 10.1002/jor.1100180119. [DOI] [PubMed] [Google Scholar]
- Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6:484–94. doi: 10.1038/nrn1687. [DOI] [PubMed] [Google Scholar]
- Calvillo L, Latini R, Kajstura J, Leri A, Anversa P, Ghezzi P, Salio M, Cerami A, Brines M. Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. Proc Natl Acad Sci U S A. 2003;100:4802–6. doi: 10.1073/pnas.0630444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik M, Gokmen N, Erbayraktar S, Akhisaroglu M, Konakc S, Ulukus C, Genc S, Genc K, Sagiroglu E, Cerami A, Brines M. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci U S A. 2002;99:2258–63. doi: 10.1073/pnas.042693799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domracheva EV, Popova OI, Raimzhanov AR. [Effect of high mountain altitude on the growth of bone marrow fibroblasts in patients with cytolytic syndromes] Ter Arkh. 1985;57:40–2. [PubMed] [Google Scholar]
- Flanagan AM. The osteoclast, which derives from a haemopoietic stem cell, is not depleted in aplastic anaemia. J Pathol. 1990;162:261–3. doi: 10.1002/path.1711620313. [DOI] [PubMed] [Google Scholar]
- Foldes J, Naparstek E, Statter M, Menczel J, Bab I. Osteogenic response to marrow aspiration: increased serum osteocalcin and alkaline phosphatase in human bone marrow donors. J Bone Miner Res. 1989;4:643–6. doi: 10.1002/jbmr.5650040424. [DOI] [PubMed] [Google Scholar]
- Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA, Sabatini DM. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865–70. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Glantschnig H, Fisher JE, Wesolowski G, Rodan GA, Reszka AA. M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003;10:1165–77. doi: 10.1038/sj.cdd.4401285. [DOI] [PubMed] [Google Scholar]
- Gorio A, Gokmen N, Erbayraktar S, Yilmaz O, Madaschi L, Cichetti C, Di Giulio AM, Vardar E, Cerami A, Brines M. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc Natl Acad Sci U S A. 2002;99:9450–5. doi: 10.1073/pnas.142287899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso G, Sfacteria A, Cerami A, Brines M. Erythropoietin as a tissue-protective cytokine in brain injury: what do we know and where do we go? Neuroscientist. 2004;10:93–8. doi: 10.1177/1073858403259187. [DOI] [PubMed] [Google Scholar]
- Henke M, Mattern D, Pepe M, Bezay C, Weissenberger C, Werner M, Pajonk F. Do erythropoietin receptors on cancer cells explain unexpected clinical findings? J Clin Oncol. 2006;24:4708–13. doi: 10.1200/JCO.2006.06.2737. [DOI] [PubMed] [Google Scholar]
- Hong F, Larrea MD, Doughty C, Kwiatkowski DJ, Squillace R, Slingerland JM. mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell. 2008;30:701–11. doi: 10.1016/j.molcel.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–57. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Isomoto S, Hattori K, Ohgushi H, Nakajima H, Tanaka Y, Takakura Y. Rapamycin as an inhibitor of osteogenic differentiation in bone marrow-derived mesenchymal stem cells. J Orthop Sci. 2007;12:83–8. doi: 10.1007/s00776-006-1079-9. [DOI] [PubMed] [Google Scholar]
- Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–8. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- Jelkmann W, Bohlius J, Hallek M, Sytkowski AJ. The erythropoietin receptor in normal and cancer tissues. Crit Rev Oncol Hematol. 2008;67:39–61. doi: 10.1016/j.critrevonc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–75. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- Lee KW, Yook JY, Son MY, Kim MJ, Koo DB, Han YM, Cho YS. Rapamycin promotes the osteoblastic differentiation of human embryonic stem cells by blocking the mTOR pathway and stimulating the BMP/Smad pathway. Stem Cells Dev. 2010;19:557–68. doi: 10.1089/scd.2009.0147. [DOI] [PubMed] [Google Scholar]
- Lee MY, Fukunaga R, Lee TJ, Lottsfeldt JL, Nagata S. Bone modulation in sustained hematopoietic stimulation in mice. Blood. 1991;77:2135–41. [PubMed] [Google Scholar]
- Lucas TS, Bab IA, Lian JB, Stein GS, Jazrawi L, Majeska RJ, Attar-Namdar M, Einhorn TA. Stimulation of systemic bone formation induced by experimental blood loss. Clin Orthop Relat Res. 1997:267–75. doi: 10.1097/00003086-199707000-00034. [DOI] [PubMed] [Google Scholar]
- Martin SK, Fitter S, Bong LF, Drew JJ, Gronthos S, Shepherd PR, Zannettino AC. NVP-BEZ235, a dual pan class I PI3 kinase and mTOR inhibitor, promotes osteogenic differentiation in human mesenchymal stromal cells. J Bone Miner Res. 2010;25:2126–37. doi: 10.1002/jbmr.114. [DOI] [PubMed] [Google Scholar]
- Masuda S, Nagao M, Takahata K, Konishi Y, Gallyas F, Jr., Tabira T, Sasaki R. Functional erythropoietin receptor of the cells with neural characteristics. Comparison with receptor properties of erythroid cells. J Biol Chem. 1993;268:11208–16. [PubMed] [Google Scholar]
- Mogi M, Kondo A. Down-regulation of mTOR leads to up-regulation of osteoprotegerin in bone marrow cells. Biochem Biophys Res Commun. 2009;384:82–6. doi: 10.1016/j.bbrc.2009.04.084. [DOI] [PubMed] [Google Scholar]
- Niziolek PJ, Murthy S, Ellis SN, Sukhija KB, Hornberger TA, Turner CH, Robling AG. Rapamycin impairs trabecular bone acquisition from high-dose but not low-dose intermittent parathyroid hormone treatment. J Cell Physiol. 2009;221:579–85. doi: 10.1002/jcp.21887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima H, Tokunaga C, Eguchi S, Oshiro N, Hidayat S, Yoshino K, Hara K, Tanaka N, Avruch J, Yonezawa K. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J Biol Chem. 2003;278:15461–4. doi: 10.1074/jbc.C200665200. [DOI] [PubMed] [Google Scholar]
- Phornphutkul C, Lee M, Voigt C, Wu KY, Ehrlich MG, Gruppuso PA, Chen Q. The effect of rapamycin on bone growth in rabbits. J Orthop Res. 2009;27:1157–61. doi: 10.1002/jor.20894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 2005;15:146–55. doi: 10.1016/j.tcb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Sanchez CP, He YZ. Bone growth during rapamycin therapy in young rats. BMC Pediatr. 2009;9:3. doi: 10.1186/1471-2431-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Shiozawa Y, Jung Y, Ziegler AM, Pedersen EA, Wang J, Wang Z, Song J, Lee CH, Sud S, Pienta KJ, Krebsbach PH, Taichman RS. Erythropoietin couples hematopoiesis with bone formation. PLoS One. 2010;5:e10853. doi: 10.1371/journal.pone.0010853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoba LN, Lee JC. Inhibition of phosphatidylinositol 3-kinase and p70S6 kinase blocks osteogenic protein-1 induction of alkaline phosphatase activity in fetal rat calvaria cells. J Cell Biochem. 2003;88:1247–55. doi: 10.1002/jcb.10474. [DOI] [PubMed] [Google Scholar]
- Singbrant S, Russell MR, Jovic T, Liddicoat B, Izon DJ, Purton LE, Sims NA, Martin TJ, Sankaran VG, Walkley CR. Erythropoietin couples erythropoiesis, B-lymphopoiesis, and bone homeostasis within the bone marrow microenvironment. Blood. 2011;117:5631–42. doi: 10.1182/blood-2010-11-320564. [DOI] [PubMed] [Google Scholar]
- Singha UK, Jiang Y, Yu S, Luo M, Lu Y, Zhang J, Xiao G. Rapamycin inhibits osteoblast proliferation and differentiation in MC3T3-E1 cells and primary mouse bone marrow stromal cells. J Cell Biochem. 2008;103:434–46. doi: 10.1002/jcb.21411. [DOI] [PubMed] [Google Scholar]
- Smink JJ, Begay V, Schoenmaker T, Sterneck E, de Vries TJ, Leutz A. Transcription factor C/EBPbeta isoform ratio regulates osteoclastogenesis through MafB. EMBO J. 2009;28:1769–81. doi: 10.1038/emboj.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugatani T, Hruska KA. Akt1/Akt2 and mammalian target of rapamycin/Bim play critical roles in osteoclast differentiation and survival, respectively, whereas Akt is dispensable for cell survival in isolated osteoclast precursors. J Biol Chem. 2005;280:3583–9. doi: 10.1074/jbc.M410480200. [DOI] [PubMed] [Google Scholar]
- Szenajch J, Wcislo G, Jeong JY, Szczylik C, Feldman L. The role of erythropoietin and its receptor in growth, survival and therapeutic response of human tumor cells From clinic to bench - a critical review. Biochim Biophys Acta. 2010;1806:82–95. doi: 10.1016/j.bbcan.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–23. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- Yamaji R, Okada T, Moriya M, Naito M, Tsuruo T, Miyatake K, Nakano Y. Brain capillary endothelial cells express two forms of erythropoietin receptor mRNA. Eur J Biochem. 1996;239:494–500. doi: 10.1111/j.1432-1033.1996.0494u.x. [DOI] [PubMed] [Google Scholar]
- Yazigi A, Maalouf G, Inati-Khoriati A, Tamim H, Saab C. Bone mineral density in beta - thalassemic Lebanese children. J Musculoskelet Neuronal Interact. 2002;2:463–8. [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]


