Abstract
The objective of this study was to determine if in utero exposure to Bisphenol A (BPA) induced reproductive tract abnormalities in the adult male testis. Using the C57/Bl6 mouse, we examined sex-organ weights, anogenital distance (AGD), and testis histopathology in adult males exposed in utero via oral gavage to sesame oil, 50 μg/kg BPA, 1,000 μg/kg BPA, or 2 μg/kg diethylstilbestrol (DES) as a positive control from gestational days 10–16. No changes in sperm production or germ cell apoptosis were observed in adult testes following exposure to either chemical. Adult mRNA levels of genes associated with sexual maturation and differentiation, GATA4 and ID2, were significantly lower only in DES-exposed testes. In summary, the data indicate no gross alterations in spermatogenesis following in utero exposure to BPA or DES. At the molecular level, in utero exposure to DES, but not BPA, leads to decreased mRNA expression of genes associated with Sertoli cell differentiation.
Keywords: Endocrine disruptors, gene expression, hormones/cyclicity, molecular biology, postnatal evaluation, spermatogenesis
1. INTRODUCTION
Within the environment there are numerous chemicals that have hormone mimicking effects which interfere with the normal endocrine functions of humans and wildlife (Hotchkiss et al., 2008). These chemical compounds have been given the name endocrine disruptors. A growing number of these compounds have been reported to have estrogenic activity acting as estrogen agonists or antagonists within the human population. Animal studies have demonstrated that exposure of mice and rats to relatively high concentrations of certain chemicals with estrogenic activity during fetal or postnatal development lead to developmental abnormalities in the male reproductive system including cancer (Sharpe and Skakkebaek, 2008; Sonne et al., 2008). Therefore, one aim of the present study was to determine the effects on adult male reproductive parameters induced in the testis by in utero exposure to two estrogen disruptors of different potency, bisphenol A (BPA), a weak estrogenic compound, and diethylstilbestrol (DES), a potent estrogenic compound, during organogenesis of the male reproductive system. The second aim of the study was to determine if changes in gene expression associated with apoptosis, steroidogenesis, or testis maturation occurred in the adult male testis following in utero exposure to either of these estrogenic compounds.
BPA, a weak estrogenic phenolic compound, is a building block of polycarbonate plastic and epoxy resins that grosses six billion dollars a year for the companies that produce it in the United States (Borrell, 2010). BPA has generated increasing concern in the scientific and public health arenas over its potential to induce reproductive abnormalities similar to DES due to its similar structure and high production volume. BPA is found in plastic food containers, baby bottles, and the lining of canned goods to prevent corrosion. Unfortunately, one of the deleterious characteristics of BPA is its ability to leach from plastic, thus presenting a route for human exposure by ingestion. This suggests that humans are ubiquitously exposed to BPA, and measurable levels have been found in human breast milk, urine, serum, fetal and maternal plasma (Willhite et al., 2008). BPA at high doses has been shown to result in gene expression changes associated with apoptosis (Li et al., 2009) in the adult male, and with changes in ICaBP, IL4R, and progesterone receptor in the fetal testis (Naciff et al., 2005). Alterations in Sertoli cell number have also been found following intrauterine exposure to BPA (Wistuba et al., 2003).
In the 1940's, DES was developed as a chemotherapeutic agent for the treatment of metastatic prostate cancer (Huggins and Hodges, 1972) and was used as an off-label drug to prevent adverse pregnancy outcomes in women with a history of miscarriage (Dieckmann et al., 1953; Dutton, 1988). Since then, DES effects on the reproductive tract have been widely documented in both humans and experimental animals (Brouwers et al., 2006; Herbst et al., 1971; Klip et al., 2002; Newbold et al., 2009). Exposure to potent estrogenic agents like DES, in utero or postnatally, have resulted in induced male reproductive abnormalities including reduction in sperm count, cryptorchidism, and other reproductive tract abnormalities (Fielden et al., 2002; Rivas et al., 2002). Importantly, in utero exposure to DES can alter sex-specific genetic pathways governing early differentiation and cell proliferation of both the male and female gonads (Ikeda et al., 2008). Moreover, functional alterations in the Sertoli cell have been found following exposure to DES (Sharpe et al., 1998); and changes in gene expression in steroidogenesis and the INSL3 have also been observed (Zhang et al., 2009).
Understanding that the developing organism is uniquely sensitive to perturbations by chemical compounds with estrogenic activity, we designed the current study utilizing C57/BL6 mice to determine (1.) if in utero exposure during the major period of organogenesis (gestational days 10 through 16) to either BPA or DES resulted in male reproductive abnormalities in adulthood and (2.) if changes in gene expression were associated with defects in testis growth and/or maturation occurred in the adult mouse testis following in utero exposure to these estrogenic chemicals. We utilized oral gavage as our route of exposure because it is the main route through which humans are exposed to BPA, and also allows for first pass metabolism. Mice were exposed to BPA at either a moderate dose of 50 μg/kg, which corresponds to the Environmental Protection Agency's (EPA) acceptable daily intake, or a high dose of 1,000 μg/kg, or sesame oil control. We chose to expose an additional group of dams to 2 μg/kg DES as a positive control. We chose this dose because the estrogenic potency of DES has typically been 100 to1,000-fold times higher than that of BPA, and this dose of 2 μg/kg is 500 times lower than our high dose of BPA (vom Saal and Welshons, 2006). This dose of DES is also below the 5 μg/kg cutoff for a low dose of DES, as higher doses can result in opposite effects of lower doses (Richter et al., 2007). Our model utilized C57/Bl6 mice, an appropriate model system to study effects of xenoestrogen exposure in mammary glands (Wadia et al., 2007). We found that BPA failed at either dose to induce changes in gene expression in the adult testes of mice. Although no overt histopathological changes were observed following in utero exposure to either BPA or DES at the doses employed, at the molecular level in utero exposure to DES resulted in gene expression changes associated with the functional maturation of Sertoli cells. Taken together, while BPA displayed little effect on male reproductive development, DES disrupted the normal pattern of Sertoli cell differentiation.
2. MATERIAL AND METHODS
2.1 Exposure paradigm and animal treatment
BPA (>99% purity) and DES (97.5% purity) were purchased from Sigma and dissolved in sesame seed oil (Sigma Aldrich, St. Louis, MO). Timed pregnant females purchased from Charles River (Wilmington, MA) were treated via oral gavage with either sesame oil as a vehicle control (N=12), 50μg BPA/kg (N=11), or 1,000μg BPA/kg (N=14) from gestational days 10 through 16. As a positive control, DES was administered at 2μg/kg (N=14). Doses were administered on a milligram per kilogram body weight basis and adjusted daily for weight changes. The weight of the dams during dosing and the length of gestation were recorded. Offspring were weaned at PND 21. Animals were housed in polycarbonate cages and given access to chow (Purina 5010) and water in a central pipe system ad libidum. The Brown University Institutional Animal Care and Use Committee approved all experimental animal protocols in compliance with National Institute of Health guidelines.
2.2 Body weights, testis weights, spermatid head counts, anogenital distance
The body and testis weights of F1 male mice were weighed and recorded at PND 56. Anogenital distance was also measured at PND 56 utilizing digital calipers (Control Company, Friendswood, Texas). Testes were detunicated, homogenized separately for 30 seconds in 1.5 ml saline merthiolate triton, and sperm heads were counted on TC-10 automated cell counter (Bio-Rad, Hercules, CA). This method resulted in similar sperm counts using a hemacytometer method as previously described (Rasoulpour et al., 2006).
2.3 Testis histopathology
The testes from adult male C57/BL6 mice exposed in utero with sesame oil vehicle, BPA, or DES were fixed in 10% neutral buffered formalin and paraffin embedded. Testis sections (7μm) were stained with hematoxylin and eosin. Images were captured using an Aperio Scan Scope (Aperio Technologies, Vista, CA) and images were analyzed using Aperio ImageScope v10.2 (Aperio Technologies). Testicular histopathological examination was conducted at the gross anatomy level to identify exposure-related effects such as retained spermatids, missing germ cell layers, multinucleated giant cells, or sloughing of spermatogenic cells into the lumen. Seminiferous tubule diameters were measured for each treatment group, and the average of at least 100 seminiferous tubule cross sections per animal and of at least five animals per treatment group were used for statistical analysis.
2.4 TUNEL staining and quantification
Germ cell apoptosis was detected in sections of paraffin-embedded tissue by the terminal deoxynucleotidyl transferase mediated dUTP nick end labeling (TUNEL) labeling method using the ApopTag kit (Chemicon, Temecula, CA). Tissue was counterstained with methyl green. Testis sections were viewed using a Nikon E800 microscope (Nikon, Melville, NY) using differential interference contrast microscopy for TUNEL quantification. The images were captured with Aperio Imagescope. TUNEL-positive germ cells were quantified in each tissue section by counting the number of TUNEL-positive cells in each essentially round seminiferous tubule. For each testis section, approximately 100–200 tubules were counted from at least six different mice. The incidence of apoptosis was then categorized into either of three groups, defined as none, one to three, or more than three TUNEL-positive germ cells per seminiferous tubule cross-section as previously described (Rasoulpour et al., 2006). In the control mouse testis, the percentage of seminiferous tubules with more than three TUNEL-positive cells is less than 10%, so that an increase in apoptosis is easily determined using this data presentation.
2.5 RNA isolation
The testes from C57/BL6 mice exposed in utero were detunicated, weighed, and homogenized in TriReagent (Sigma Aldrich) and further RNA isolation was performed according to the TriReagent manufacturer's protocols.
2.6 Quantitative RT-PCR
Total RNA (1 μg) was DNase (Promega, Madison, WI) treated and reverse-transcribed using the iScript cDNA Synthesis Kit (Bio-Rad) according to the manufacturer's protocols. cDNA templates were amplified with either self-designed primers from Invitrogen (Invitrogen, Madison, WI) or pre-optimized mouse-specific QuantiTect® primer assays (Qiagen, Valencia, CA) using iQ SYBR Green Supermix (Bio-Rad) on an iCycler iQ Multicolor Real-time PCR Detection System (Bio-Rad). Each sample was run in triplicate, and mRNA levels were analyzed relative to hypoxanthine phosphoribosyltransferase (HPRT). Log2-transformed relative expression ratios were calculated as described using the equation set forth by Pfaffl (Pfaffl, 2001). Primer pairs and their respective sequences where available are listed in Table 1.
Table 1.
Primer sequences for real-time RT-PCR analysis
| Gene | Primer Pair |
|---|---|
| WT1 | F: CCCATTTACTAACTGCCCTAGAAAG R: TCTTGCCTGGTGCCTTGC |
| GATA4 | F: GTGAAAGGACAAAGGTGATAGAC R: GTGGGTGATGAGGACAAGG |
| 3ß HSD | QuantiTect® primer assay |
| Inhibin B | F:GGCTGAGTGTTGGTTGTTC R:TGCGTGAGTCTGGCTATG |
| Cyp11a1 | QuantiTect® primer assay |
| FasL | F: GCAAATAGCCAACCCCAGTACAC R: GCCACCTTTCTTATACTTCACTCCAG |
| Fas | F: TGCACCCTGACCCAGAATAC R: GCCAGGAGAATCGCAGTAGAA |
| ID2 | F:ACTGTGATACCGTTATTTATGAGAGAC R: TCCAACTGTAGAAAGGGCACTG |
| StAR | QuantiTect® primer assay |
| HPRT | F:TTGCGCTCATCTTAGGCTTT R:CAGGCCAGACTTTGTTGGAT Or QuantiTect® primer assay |
| Cyp17a1 | QuantiTect® primer assay |
2.7 Serum isolation and hormone measurement
To measure serum testosterone, blood samples were collected by cardiac puncture. Serum was separated via centrifugation of clotted samples and stored at −80 °C for later analyses. Serum concentrations of testosterone were analyzed by the ELISA method. All samples were run in duplicate, and each n represented a different litter.
2.8 Statistical analysis
A one-way ANOVA followed by a Tukey HSD test was used to determine statistical significance for BPA treated groups using the sesame oil group as a reference. Given that DES is a different toxicant, a two-tailed student t-test was performed to determine statistical significance between sesame oil and DES treated groups. Litter averages were used for body weights, anogenital distance, and organ weights. Males from separate litters were used for other experiments. A p<0.05 was considered statistically significant. Statistics were calculated using R (www.r-project.org).
3. RESULTS
3.1 Assessment of litter outcomes and adult testicular parameters following in utero exposure to BPA or DES
To determine if maternal exposure to either BPA or DES resulted in differences in litter outcomes, we examined litter sizes and viability at birth and weaning. Litter size at birth, litter size at weaning, and percent viability of offspring remained consistent across the BPA treatment groups compared to sesame oil control (Table 2). In contrast, in utero exposure to DES resulted in a significant decrease in litter size at birth, and a strong trend for decreased litter size at weaning. These data indicate that BPA exposure had little effect on litter outcomes, while DES had negative effects. To characterize adult reproductive parameters of adult males previously exposed to BPA or DES in utero, we examined the body weight, testis weight, seminal vesicle weight, and AGD from BPA and DES exposed F1 males and compared them with adult F1 sesame oil control males. As table 3 illustrates, we found no significant differences in male body weight in any of the F1 exposed adult males relative to F1 control male body weights. No significant differences in the adult testis weights at both the gross and body-weight adjusted levels of F1 male mice exposed to either BPA or DES were observed when compared with that of sesame oil F1 control animals. Seminal vesical weights at both the gross and bodyweight adjusted levels were also not affected by either BPA or DES in utero exposure. However, in utero exposure to DES, but not BPA, resulted in a significant increase in anogenital distance at both the gross measurement and after adjustment for body weight.
Table 2.
Litter outcomes following exposure to sesame oil, 50 μg/kg BPA, 1,000 μg/kg BPA, or 2 μg/kg DES.
| Mouse Exposure Group | Number of Initial Litters | Litter Size at Birth (n) | Litter Size at Weaning (n) | Viability (%) |
|---|---|---|---|---|
| Sesame oil | 12 | 7.1 +/− 1.0 | 5.5 +/− 2.8 | 73.9 +/−35.7 |
| BPA 50 μg/kg | 11 | 8.2 +/− 1.0 | 5.5 +/− 3.5 | 66.8 +/− 42.0 |
| BPA 1,000 μg/kg | 14 | 6.2 +/− 2.5 | 4.0 +/− 3.2 | 63.5 +/− 47.8 |
| DES 2 μg/kg | 14 | 5.8 +/− 1.8* | 3.1 +/− 3.2# | 51.3 +/− 44.7 |
p=0.05
p<0.05
Table 3.
Male reproductive parameters in adult C57/Bl6 male mice following in utero exposure to sesame oil, 50 μg/kg BPA, 1,000 μg/kg BPA, or 2 μg/kg DES
| Mouse Exposure Group (N=litters examined) | Age (days) | Body Weight (g) | Combined Testis Weight (mg) | Adjusted Combined Testis Weight (mg/g) | Seminal Vesicle Weight (mg/g) | Adjusted Seminal Vesicle Weight (mg/g) | AGD (mm) | Adjusted AGD (mm/g) |
|---|---|---|---|---|---|---|---|---|
| Sesame oil N=8 | 56 | 23.5 +/− 1.2 | 154.6 +/− 23.5 | 6.8 +/− 0.9 | 72.5 +/− 13.5 | 3.2 +/− 0.5 | 10.9 +/− 1.3 | 0.5 +/− 0.1 |
| BPA 50 μg/kg N=8 | 56 | 23.0 +/− 1.4 | 164.5 +/− 19.6 | 7.2 +/− 0.9 | 73.1 +/− 13.3 | 3.2 +/− 0.5 | 11.1 +/− 0.5 | 0.5 +/− 0.1 |
| BPA 1,000 μg/kg N=10 | 56 | 23.2 +/− 1.0 | 167.8 +/− 8.3 | 7.2 +/− 0.5 | 76.6 +/− 9.6 | 3.3 +/− 0.3 | 10.8 +/− 0.4 | 0.5 +/− 0.1 |
| DES 2 μg/kg N=6 | 56 | 22.5 +/ 1.1 | 143.8 +/− 41.1 | 6.6 +/− 2.0 | 76.9 +/− 13.3 | 3.5 +/− 0.6 | 12.6 +/− 0.6** | 0.6 +/− 0.0** |
p<0.01
3.2 No gross alteration in the seminiferous tubules or sperm production of adult F1 males following in utero exposure to BPA or DES
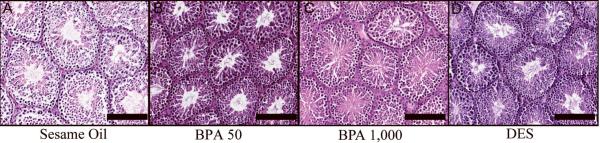
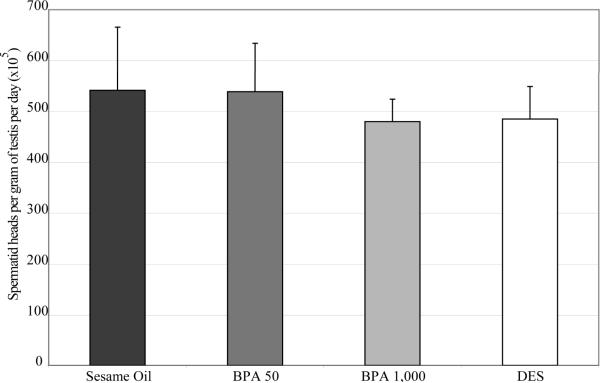
To determine if spermatogenesis was altered in the adult F1 males, we examined cross sections of testis seminiferous tubules. Gross testis histopathology was not altered in BPA (Figure 1b and 1c) or DES (Figure 1d) exposure groups, and no differences were detected in seminiferous tubule diameters (Table 4). In addition to testis weights and histopathology, the measurement of spermatid heads at PND 56 (Figure 2) did not reveal significant differences between any of the F1 exposure groups or sesame oil control animals, suggesting a full complement of germ cells in the testis.
Figure 1. Testis histopathology.
H&E sections of sesame oil (a), BPA 50 μg/kg (b), BPA 1,000 μg/kg (c), and DES 2 μg/kg (d) treated testis. No observable histopathological changes were detected in any treatment group. Scale bars indicate 1,000 μm.
Table 4.
Seminiferous tubule diameters in adult C57/Bl6 male mice following in utero exposure to sesame oil, 50 μg/kg BPA, 1,000 μg/kg BPA, or 2 μg/kg DES
| Mouse Exposure Group | Tubule Diameter (μm) |
|---|---|
| Sesame oil N=5 | 144.82 +/− 9.76 |
| BPA 50 μg/kg N=6 | 155.53 +/− 19.35 |
| BPA 1,000 μg/kg N=6 | 148.54 +/− 20.88 |
| DES 2 μg/kg N=6 | 137.87 +/− 3.59 |
Figure 2. Spermatid head counts of adult C57/Bl6 male mice following in utero exposure to sesame oil, 50 μg/kg BPA, 1,000 μg/kg BPA, or 2 μg/kg DES.
Spermatid heads were counted using a TC-10 automated cell counter (Bio Rad). No statistical significance was found amongst any treatment groups compared to sesame oil control. Data represented as mean + standard deviation. Statistical analyses were conducted using one-way ANOVA followed by a Tukey HSD test for BPA and a two-tailed t-test for DES. n=6–7 samples per treatment group, with each n representing a different litter.
3.3 No increase in germ cell apoptosis or Fas and FasL mRNA levels in the adult testis following in utero exposure to BPA or DES
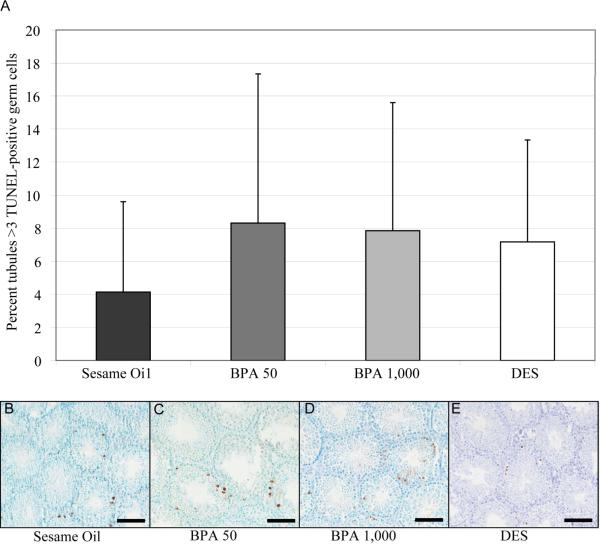
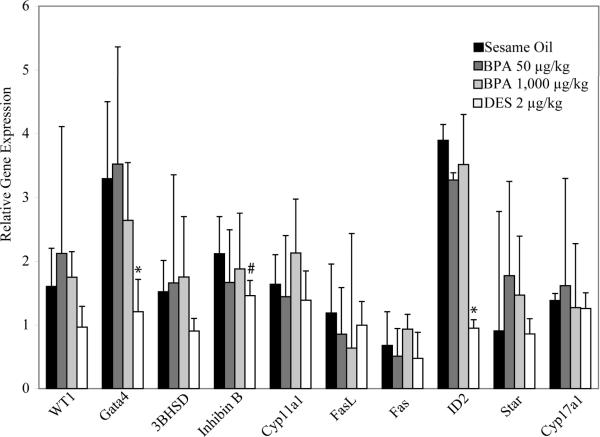
Exposure to high doses of BPA in adult male mice ranging from 160 mg/kg to 960 mg/kg body weight has been shown to lead to increased germ cell apoptosis in the testis with concomitant increases in Fas and FasL gene expression (Li et al., 2009). Therefore, to determine the potential consequences of in utero exposure to BPA and DES in the adult testis, we examined the level of germ cell apoptosis in F1 exposed and F1 control seminiferous tubules at PND 56. Apoptosis was assessed by counting seminiferous tubules with more than three apoptotic germ cells per cross section. At the doses examined, we found no significant differences in germ cell apoptosis in the F1 in utero exposed groups relative to control animals (Figure 3). Fas and FasL mRNA expression also remained similar in the F1 sesame oil control group compared to either BPA or DES at all doses examined (Figure 4).
Figure 3. Germ cell apoptosis in adult C57/Bl6 male mice following in utero exposure to sesame oil, 50 μg/kg BPA, 1,000 μg/kg BPA, or 2 μg/kg DES.
The apoptotic incidence in seminiferous tubules was quantified by using TUNEL staining (a). The percent of TUNEL-positive tubules with greater than 3 apoptotic cells is represented as mean + standard deviation. No significant differences in apoptotic incidence were found for BPA or DES treated testes. Statistical analyses were conducted using one-way ANOVA followed by a Tukey HSD test for BPA and a two-tailed t-test for DES. n=6 per treatment group, with each n representing a different litter. Representative images of TUNEL staining in the testis for sesame oil (b), BPA 50 μg/kg (c), BPA 1,000 μg/kg (d), and DES 2 μg/kg (e). Scale bars indicate 1,000 μm.
Figure 4. Gene expression of adult C57/Bl6 male mice following in utero exposure to sesame oil, 50 μg/kg BPA, 1,000 μg/kg BPA, or 2 μg/kg DES.
Quantitative RT-PCR was conducted on a panel of genes associated with steroidogenesis, apoptosis, or differentiation of the testis. Statistical analyses were conducted using one-way ANOVA followed by a Tukey HSD test for BPA and a two-tailed t-test for DES. Data represented as mean + standard deviation. n=3–4 per treatment group, with each n representing a different litter. Asterisk (*) indicates significance compared with sesame oil control (p<0.05). # indicates p<0.1.
3.4 In utero exposure to DES, but not BPA, alters the mRNA levels of GATA4 and ID2in the adult testis
In the adult testis, mRNA levels of genes associated with sexual differentiation and maturation of the testis, GATA4 and ID2, were significantly lower in the F1 adult male DES exposure group; and there was a trend for decreased expression of inhibin B (p=0.07) a marker of Sertoli cell function suggesting that in utero exposure to DES alters gene expression in the testis (Figure 4). In contrast to the gene changes observed following in utero exposure to DES, no changes in gene expression in the panel of genes examined (Table 1) were observed in the adult testes of F1 male mice exposed in utero to BPA.
3.5 In utero exposure to BPA or DES does not affect testosterone levels in adult male serum
Given that DES altered a number of genes related to sexual maturation in the testis, we decided to investigate the levels of testosterone in serum from BPA and DES exposed F1 males. We found no significant changes in testosterone from either exposure group (Table 5).
Table 5.
Serum testosterone levels in adult C57/Bl6 male mice following in utero exposure to sesame oil, 50 μg/kg BPA, 1,000 μg/kg BPA, or 2 μg/kg DES
| Mouse Exposure Group | Serum Testosterone (ng/ml) |
|---|---|
| Sesame oil N=5 | 0.16 +/− 0.09 |
| BPA 50 μg/kg N=5 | 0.18 +/− 0.15 |
| BPA 1,000 μg/kg N=4 | 0.12 +/− 0.08 |
| DES 2 μg/kg N=4 | 0.08 +/− 0.05 |
4. DISCUSSION
Although BPA has been extensively studied, questions remain about whether BPA causes male reproductive abnormalities in rodents and/or humans (Goodman et al., 2009; Howdeshell et al., 2008; Maffini et al., 2006; Ryan et al., 2010; Vandenberg et al., 2009; Willhite et al., 2008). Differences in experimental outcomes which have been attributed to differences in species and/or strains of animals used, routes of administration of chemical, and critical window(s) of exposure (Myers et al., 2009; Vandenberg et al., 2009). The developing fetus and children are of particular concern for toxicant exposure due to their small size and vulnerability. Human exposure to BPA is primarily through the oral route, and based on this, we chose to study the major period of organogenesis in the C57/Bl6 mouse (gestation days 10–16) with the dam receiving the dosage via oral gavage. The oral gavage route allows for the BPA to enter first pass metabolism in the dam, thereby resulting in less bioavailability to the fetus rather than through intraperitoneal or subcutaneous injections of the same dosage. Mice were housed in polycarbonate cages, which previously work has indicated could potentially expose animals to additional BPA, with the highest exposure in very old cages (Howdeshell et al., 2003). Our animals were housed in new polycarbonate cages with metal wire food trays and a centralized metal pipe system for water, and we believe that exposure to BPA through leaching was kept at a minimum.
The C57/Bl6 mouse is a more aggressive mouse, and as a result we observed high levels of infanticide following birth in all exposure groups. This behavior is normally observed in this strain, and resulted in relatively low rates of viability in all the exposure groups, but no observable difference in litter viability was found between BPA, DES, or sesame oil exposed litters (Table 2) We did however observe differences in litter size at birth and litter size at weaning for the DES-treatment group, indicating potential maternal toxicity or higher levels of infanticide following stress from DES exposure. It should be noted that very occasionally entire litters were eaten, which is why the F1 litter numbers are less than the original number of dams as described in the materials and methods (section 2.1). While litter culling was not performed, we instead chose to use litter averages for male reproductive outcomes, and randomly selected 1 male offspring per litter for molecular experiments so each n would represent a different litter.
Similar to previously published results (Howdeshell et al., 2008; Ryan et al., 2010), we observed few significant differences in body weight, testis weight, or seminal vesicle weight following in utero exposure to BPA. Histological analyses revealed little apparent difference between either BPA or DES-exposed F1 seminiferous tubules when compared to sesame oil controls. Moreover, we found no significant increases in germ cell apoptosis or in the genes, Fas and FasL, which have previously been reported to be up-regulated in the rodent testis following exposure to extremely high doses of BPA (Li et al., 2009; Ryan et al., 2010). However, DES, but not BPA, exposure resulted in a significant increase in anogenital distance. As DES is a potent estrogen, high doses will result in anti-androgenic effects in males. For example, it has been reported that prenatal exposure to high levels of DES (200 μg/kg/day) results in a significant decrease in anogenital distance (Palanza et al., 2001). However, we did not find any differences in serum testosterone levels in any of the exposure groups compared to sesame oil controls, indicating normal testosterone synthesis. While there is a trend for decreased levels of serum testosterone in the DES treated males compared to sesame oil controls, this did not reach statistical significance (p=0.14). This is similar to a prior study which indicated that BPA and the positive control ethinyl estradiol did not alter serum hormone levels in rats exposed during gestation and lactation (Howdeshell et al., 2008). Based on these results we propose that at lower doses of DES, there is a potentially agonistic effect rather than an antagonist effect on androgen receptor signaling.
At the molecular level, high levels of estrogens have been shown to affect gene expression in the testis early in life (Saunders et al., 1997). Therefore, we examined a panel of genes associated with steroidogenesis, germ cell apoptosis, and Sertoli cell maturation which have been shown by other laboratories to be altered by endocrine disrupting chemicals including BPA (Li et al., 2009; Naciff et al., 2005) and DES (Li et al., 2009; Naciff et al., 2005; Saunders et al., 1997). We found that DES reduced the expression of two transcription factors, GATA4 and ID2, in the testes of adult F1 males. The transcription factor GATA4 is expressed in Sertoli cells, steroidogenic Leydig cells, and other testicular somatic cells, and GATA4, along with its cofactor FOG2, is necessary for proper Sry expression and all subsequent steps in testicular organogenesis, including testis cord formation and differentiation of both Sertoli and fetal Leydig cells (Bielinska et al., 2007). Interestingly a recent study in which GATA4 was conditionally deleted in Sertoli cells in the testis of mice resulted in Sertoli cell vacuolization, impaired spermatogenesis, increased permeability of the blood-testis barrier, and infertility (Kyronlahti et al., 2011). ID2, similar to GATA4, is a transcription factor and is associated with the inhibition of differentiation of various cell types (Chaudhary et al., 2005). ID2 is most abundant in Sertoli cell nuclei, but also detectable in pachytene and diplotene spermatocytes (Sablitzky et al., 1998). Inhibin B is associated with disorders of sexual and gonadal differentiation (Barrionuevo et al., 2009; Toulis et al., 2010). Collectively, the gene expression changes in our DES exposed mice may indicate future problems with fertility in the DES-exposed males, and future studies are warranted. The data indicate no gross spermatogenic changes following in utero exposure to DES or BPA at the doses employed by adulthood. However, at the molecular level, in utero exposure to DES results in gene expression changes in the adult testis associated with alterations in Sertoli cell function.
In summary, we found that moderate (50 μg/kg) to high exposure (1,000 μg/kg) to BPA in utero does not lead to gross changes in adult body weight, testis weight, AGD, spermatogenesis, sperm production, or specific changes in gene expression associated with steroidogenesis, apoptosis, or Sertoli cell maturation in the F1 generation of adult C57/BL6 mice. However, in utero exposure to DES resulted in increased AGD in F1 males, and gene expression changes associated with an altered Sertoli cell phenotype. Overall these results indicate that at the doses employed via oral gavage, BPA does not affect young adult male reproductive health in regards to the testis. The use of the C57/Bl6 mouse in this study may be useful for future studies using transgenic mouse models to study gene-environment interactions with BPA. Future studies in our laboratory will focus on other target organs as well as additional lower doses as BPA may elicit its effects differently in other organ systems.
ACKNOWLEDGEMENTS
The authors would like to thank Lucy Boekelheide for her technical assistance in animal work.
This research was supported by ES015704 from the NIEHS/NIH to M.H. J.L. was supported by 2T32E5007272 from the NIH.
Abbreviations
- BPA
Bisphenol A
- DES
Diethylstilbestrol
- AGD
Anogenital Distance
REFERENCES
- Barrionuevo F, Georg I, Scherthan H, Lecureuil C, Guillou F, Wegner M, Scherer G. Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8. Dev Biol. 2009;327(2):301–312. doi: 10.1016/j.ydbio.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Bielinska M, Seehra A, Toppari J, Heikinheimo M, Wilson DB. GATA-4 is required for sex steroidogenic cell development in the fetal mouse. Dev Dynam. 2007;236(1):203–213. doi: 10.1002/dvdy.21004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell B. Toxicology: The big test for bisphenol A. Nature. 2010;464(7292):1122–1124. doi: 10.1038/4641122a. [DOI] [PubMed] [Google Scholar]
- Brouwers MM, Feitz WFJ, Roelofs LAJ, Kiemeney LALM, de Gier RPE, Roeleveld N. Hypospadias: a transgenerational effect of diethylstilbestrol? Hum Reprod. 2006;21(3):666–669. doi: 10.1093/humrep/dei398. [DOI] [PubMed] [Google Scholar]
- Chaudhary J, Sadler-Riggleman I, Ague JM, Skinner MK. The helix-loop-helix inhibitor of differentiation (ID) proteins induce post-mitotic terminally differentiated sertoli cells to re-enter the cell cycle and proliferate. Biol Reprod. 2005;72(5):1205–1217. doi: 10.1095/biolreprod.104.035717. [DOI] [PubMed] [Google Scholar]
- Dieckmann WJ, Davis ME, Rynkiewicz LM, Pottinger RE. Does the administration of diethylstilbestrol during pregnancy have therapeutic value? Am J Obstet Gynecol. 1953;66(5):1062–1081. doi: 10.1016/s0002-9378(16)38617-3. [DOI] [PubMed] [Google Scholar]
- Dutton DB. Worse than the disease: pitfalls of medical progress. Cambridge University Press; Cambridge: 1988. [Google Scholar]
- Fielden MR, Halgren RG, Fong CJ, Staub C, Johnson L, Chou K, Zacharewski TR. Gestational and lactational exposure of male mice to diethylstilbestrol causes long-term effects on the testis, sperm fertilizing ability in vitro, and testicular gene expression. Endocrinology. 2002;143(8):3044–3059. doi: 10.1210/endo.143.8.8968. [DOI] [PubMed] [Google Scholar]
- Goodman JE, Witorsch RJ, McConnell EE, Sipes IG, Slayton TM, Yu CJ, Franz AM, Rhomberg LR. Weight-of-evidence evaluation of reproductive and developmental effects of low doses of bisphenol A. Crit Rev Toxicol. 2009;39(1):1–75. doi: 10.3109/10408440903279946. [DOI] [PubMed] [Google Scholar]
- Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284(15):878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Furr J, Lambright CR, Wilson VS, Ryan BC, Gray LE., Jr. Gestational and lactational exposure to ethinyl estradiol, but not bisphenol A, decreases androgen-dependent reproductive organ weights and epididymal sperm abundance in the male long evans hooded rat. Toxicol Sci. 2008;102(2):371–382. doi: 10.1093/toxsci/kfm306. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Peterman PH, Judy BM, Taylor JA, Orazio CE, Ruhlen RL, vom Saal FS, Welshons WV. Bisphenol A is released from used polycarbonate animal cages into water at room temperature. Environ Health Persp. 2003;111(9):1180–1187. doi: 10.1289/ehp.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22(4):232–240. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Tanaka H, Esaki M. Effects of gestational diethylstilbestrol treatment on male and female gonads during early embryonic development. Endocrinology. 2008;149(8):3970–3979. doi: 10.1210/en.2007-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klip H, Verloop J, van Gool JD, Koster ME, Burger CW, van Leeuwen FE. Hypospadias in sons of women exposed to diethylstilbestrol in utero: a cohort study. Lancet. 2002;359(9312):1102–1107. doi: 10.1016/S0140-6736(02)08152-7. [DOI] [PubMed] [Google Scholar]
- Kyronlahti A, Euler R, Bielinska M, Schoeller EL, Moley KH, Toppari J, Heikinheimo M, Wilson DB. GATA4 regulates Sertoli cell function and fertility in adult male mice. Mol Cell Endocrinol. 2011;333(1):85–95. doi: 10.1016/j.mce.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YJ, Song TB, Cai YY, Zhou JS, Song X, Zhao X, Wu XL. Bisphenol A exposure induces apoptosis and upregulation of Fas/FasL and caspase-3 expression in the testes of mice. Toxicol Sci. 2009;108(2):427–436. doi: 10.1093/toxsci/kfp024. [DOI] [PubMed] [Google Scholar]
- Maffini MV, Rubin BS, Sonnenschein C, Soto AM. Endocrine disruptors and reproductive health: the case of bisphenol-A. Mol Cell Endocrinol. 2006:254–255. 179–186. doi: 10.1016/j.mce.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Myers JP, Zoeller RT, vom Saal FS. A Clash of Old and New Scientific Concepts in Toxicity, with Important Implications for Public Health. Environ Health Persp. 2009;117(11):1652–1655. doi: 10.1289/ehp.0900887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naciff JM, Hess KA, Overmann GJ, Torontali SM, Carr GJ, Tiesman JP, Foertsch LM, Richardson BD, Martinez JE, Daston GP. Gene expression changes induced in the testis by transplacental exposure to high and low doses of 17 alpha-ethynyl estradiol, genistein, or bisphenol A. Toxicol Sci. 2005;86(2):396–416. doi: 10.1093/toxsci/kfi198. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Jefferson WN, Padilla-Banks E. Prenatal Exposure to Bisphenol A at Environmentally Relevant Doses Adversely Affects the Murine Female Reproductive Tract Later in Life. Environ Health Persp. 2009;117(6):879–885. doi: 10.1289/ehp.0800045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanza P, Parmigiani S, vom Saal FS. Effects of prenatal exposure to low doses of diethylstilbestrol, o,p ' DDT, and methoxychlor on postnatal growth and neurobehavioral development in male and female mice. Horm Behav. 2001;40(2):252–265. doi: 10.1006/hbeh.2001.1697. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9) doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasoulpour T, DiPalma K, Kolvek B, Hixon M. Akt1 suppresses radiation-induced germ cell apoptosis in vivo. Endocrinology. 2006;147(9):4213–4221. doi: 10.1210/en.2006-0174. [DOI] [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FSV. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24(2):199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas A, Fisher JS, McKinnell C, Atanassova N, Sharpe RM. Induction of reproductive tract developmental abnormalities in the male rat by lowering androgen production or action in combination with a low dose of diethylstilbestrol: Evidence for importance of the androgen-estrogen balance. Endocrinology. 2002;143(12):4797–4808. doi: 10.1210/en.2002-220531. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Hotchkiss AK, Crofton KM, Gray LE., Jr. In utero and lactational exposure to bisphenol A, in contrast to ethinyl estradiol, does not alter sexually dimorphic behavior, puberty, fertility, and anatomy of female LE rats. Toxicol Sci. 2010;114(1):133–148. doi: 10.1093/toxsci/kfp266. [DOI] [PubMed] [Google Scholar]
- Sablitzky F, Moore A, Bromley M, Deed RW, Newton JS, Norton JD. Stage- and subcellular-specific expression of Id proteins in male germ and Sertoli cells implicates distinctive regulatory roles for Id proteins during meiosis, spermatogenesis, and Sertoli cell function. Cell Growth Differ. 1998;9(12):1015–1024. [PubMed] [Google Scholar]
- Saunders PT, Majdic G, Parte P, Millar MR, Fisher JS, Turner KJ, Sharpe RM. Fetal and perinatal influence of xenoestrogens on testis gene expression. Adv Exp Med Biol. 1997;424:99–110. doi: 10.1007/978-1-4615-5913-9_19. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Atanassova N, McKinnell C, Parte P, Turner KJ, Fisher JS, Kerr JB, Groome NP, Macpherson S, Millar MR, Saunders PT. Abnormalities in functional development of the Sertoli cells in rats treated neonatally with diethylstilbestrol: a possible role for estrogens in Sertoli cell development. Biol Reprod. 1998;59(5):1084–1094. doi: 10.1095/biolreprod59.5.1084. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Skakkebaek NE. Testicular dysgenesis syndrome: mechanistic insights and potential new downstream effects. Fertil Steril. 2008;89(2 Suppl):e33–38. doi: 10.1016/j.fertnstert.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Sonne SB, Kristensen DM, Novotny GW, Olesen IA, Nielsen JE, Skakkebaek NE, Rajpert-De Meyts E, Leffers H. Testicular dysgenesis syndrome and the origin of carcinoma in situ testis. Int J Androl. 2008;31(2):275–287. doi: 10.1111/j.1365-2605.2007.00855.x. [DOI] [PubMed] [Google Scholar]
- Toulis KA, Iliadou PK, Venetis CA, Tsametis C, Tarlatzis BC, Papadimas I, Goulis DG. Inhibin B and anti-Mullerian hormone as markers of persistent spermatogenesis in men with non-obstructive azoospermia: a meta-analysis of diagnostic accuracy studies. Hum Reprod Update. 2010;16(6):713–724. doi: 10.1093/humupd/dmq024. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30(1):75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Welshons WV. Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environmental Research. 2006;100(1):50–76. doi: 10.1016/j.envres.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Wadia PR, Vandenberg LN, Schaeberle CM, Rubin BS, Sonnenschein C, Soto AM. Perinatal bisphenol A exposure increases estrogen sensitivity of the mammary gland in diverse mouse strains. Environ Health Persp. 2007;115(4):592–598. doi: 10.1289/ehp.9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willhite CC, Ball GL, McLellan CJ. Derivation of a bisphenol A oral reference dose (RfD) and drinking-water equivalent concentration. J Toxicol Environ Health B Crit Rev. 2008;11(2):69–146. doi: 10.1080/10937400701724303. [DOI] [PubMed] [Google Scholar]
- Wistuba J, Brinkworth MH, Schlatt S, Chahoud I, Nieschlag E. Intrauterine bisphenol A exposure leads to stimulatory effects on Sertoli cell number in rats. Environ Res. 2003;91(2):95–103. doi: 10.1016/s0013-9351(02)00019-1. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zheng XM, Hubert J, Zheng H, Yang ZW, Li SW. Prenatal exposure to diaethylstilbestrol in the rat inhibits transabdominal testicular descent with involvement of the INSL3/LGR8 system and HOXA10. Chin Med J (Engl) 2009;122(8):967–971. [PubMed] [Google Scholar]