Abstract
Background
Following HIV diagnosis and linkage to care, achieving and sustaining viral load (VL) suppression has implications for patient outcomes and secondary HIV prevention. We evaluated factors associated with expeditious VL suppression and cumulative VL burden among patients establishing outpatient HIV care.
Methods
Patients initiating HIV medical care from January 2007-October 2010 at the University of Alabama at Birmingham and University of Washington were included. Multivariable Cox proportional hazards and linear regression models were used to evaluate factors associated with time to VL suppression (<50 copies/mL) and cumulative VL burden, respectively. Viremia copy-years (VCY), a novel area under the longitudinal VL curve measure, was used to estimate 2-year cumulative VL burden from clinic enrollment.
Results
Among 676 patients, 63% achieved VL<50 copies/mL in a median 308 days. In multivariable analysis, patients with more time-updated “no show” visits experienced delayed VL suppression (HR=0.83 per “no show” visit, 95%CI=0.76,0.91). In multivariable linear regression, visit non-adherence was independently associated with greater cumulative VL burden (log10VCY) during the first two years in care (Beta coefficient=0.11 per 10% visit non-adherence, 95%CI=0.04-0.17). Across increasing visit adherence categories, lower cumulative VL burden was observed (mean ± standard deviation log10 copy × years/mL); 0-79% adherence: 4.6 ± 0.8; 80-99% adherence: 4.3 ± 0.7; and 100% adherence: 4.1 ± 0.8 log10 copy × years/mL, respectively (P<0.01).
Conclusions
Higher rates of early retention in HIV care are associated with achieving VL suppression and lower cumulative VL burden. These findings are germane for a test and treat approach to HIV prevention.
Keywords: HIV, Viral load, Retention in care, Adherence, Engagement in care
Introduction
In recent years, a test and treat approach to HIV prevention has garnered considerable interest. Following HIV diagnosis (“test”), timely initiation and uninterrupted receipt of antiretroviral therapy (ART, “treat”), taken at a high level of adherence, can suppress plasma HIV viral load thereby significantly reducing the likelihood of transmission.1-3 Implicit to the success of a test and treat strategy is the need for prompt linkage and sustained retention in HIV medical care after diagnosis. Currently, barriers exist at each step of the treatment cascade from HIV testing and awareness of serostatus to viral load suppression that limit the effectiveness of test and treat in local communities and nationally.4,5 An estimated 21% of HIV-infected individuals in the United States are unaware of their HIV serostatus and 31% of newly diagnosed persons delay linkage to outpatient HIV medical care for 6 months or longer.6,7 Even with initial linkage into care, subsequent retention in HIV care is required for full access to treatment benefits. Early missed visits are common and have been associated with delayed initiation of ART in the short-term, and adverse clinical outcomes in the long-term.8,9 One-year attrition has been observed in upwards of 25-30% of patients following initial enrollment in outpatient HIV care, compromising the potential real world impact of treatment as prevention strategies.10,11
While numerous initiatives have focused on expanding HIV testing, and a brief case-management intervention has proven efficacious for linking newly diagnosed patients to care,11,12 limited evidence, both observational and interventional, exists for the period immediately following entry to care.9,10,13,14 The year following initial linkage to outpatient HIV medical care is a dynamic and formative time for patients adjusting to a life changing diagnosis. It is also a time of considerable vulnerability. Patients, many of whom have limited experience navigating the health care system and may not be fully prepared for a long-term commitment to taking prescribed medications, are called upon to attend frequently scheduled medical visits and initiate treatment with ART regimens that require high levels of sustained adherence to achieve success (e.g., viral suppression) and avoid harm (e.g., resistance mutations).9,10 Sub-optimal early retention in care represents a formidable obstacle to achieving HIV viral load suppression, which has important individual and public health implications, yet little is known about its impact on the ultimate goal of test and treat strategies, viral suppression.
Here, we address this gap in the literature through the careful evaluation of the effect of early retention in HIV care on viral load suppression. In addition to evaluating time to virologic suppression, we evaluate cumulative viral load burden over the first two years in care. Beyond any single time point, longitudinal plasma viremia has important implications for patient outcomes and for HIV transmission. We use a novel measure, viremia copy-years, an area under the curve estimate of cumulative plasma viral load exposure,15 to characterize the relation between early retention in care and longitudinal viral load burden. We hypothesized that early missed HIV care visits would be associated with delayed viral load suppression, and that patients demonstrating poorer retention in care as measured by visit adherence would have higher cumulative viremia copy-years over the first two years of HIV care.
Methods
Sample and Procedures
HIV-infected patients initiating outpatient HIV medical care at two academically affiliated HIV treatment centers at the University of Alabama at Birmingham (UAB 1917 Clinic) and the University of Washington (UW Harborview Clinic) between January 2007 and September 2010 were included in the overall study sample. Patients who had received outpatient HIV medical care at another facility prior to enrollment at the two study sites were excluded. To allow for a complete two-year observation period, only the subset of patients initiating care before September 2008 were included in analyses of cumulative viral load burden during the first two years following entry into outpatient HIV medical care. Data were captured through the Centers for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS), a nationally distributed HIV clinical cohort that has been described in detail previously.16 Briefly, every two months, sites transmit comprehensive and well-defined data elements captured from point-of-care electronic health record systems using standardized terminology and format. Systematic and rigorous processes for data verification and quality assurance are in place to generate a centralized high quality clinical database. This study and the CNICS protocol were approved by local institutional review boards at both study sites.
Outcome measures: Plasma viral load suppression following entry into outpatient HIV medical care
Plasma HIV viral load suppression following entry into care was evaluated using two distinct measures; time to suppression of plasma HIV RNA <50 copies/mL and cumulative viral load burden measured over the two years following the initial medical visit. Viremia copy-years, a time-varying measure of cumulative plasma HIV burden calculated using methods described in detail previously was used to evaluate longitudinal viral load burden.15 Briefly, the trapezoidal rule is used to approximate the integral representing the area under each patient's longitudinal viral load curve. Viral load burden for each segment (time interval between 2 consecutive viral load values) is calculated by multiplying the mean of the 2 values by the time interval. The copy × years/mL for each segment of a patient's viral load curve are summed to calculate viremia copy-years. Formally, viremia copy-years is the number of copies of HIV RNA per mL of plasma over time. For example, 10,000 copy-years of viremia equals having a viral load of 10,000 copies/mL for one year, or alternatively, a viral load of 1,000 copies/mL for 10 years. For the current study, viremia copy-years was calculated for each patient for two years starting from each patient's initial clinic visit date. Each patient's baseline viral load was assigned to the initial medical visit date and all viral load values over the subsequent 2 years (730 days) were used in the viremia copy-years calculation (Figure 1). Patients with a gap of >365 days between viral load measures (n=78) were excluded due to concerns about the reliability of calculating viral load segments separated by long periods of time. Plasma viral load values >1,000,000 copies/mL were administratively assigned a value of 1,000,000 copies/mL, and values below the limits of assay detection (49 copies/mL) were assigned a value of 24 copies/mL. Two-year viremia copy-years were calculated for all patients using natural, non-transformed plasma HIV viral load values. Subsequently, the 2-year viremia copy-year measure was log10 transformed for each patient. The 2-year log10 viremia copy-years measure was used for all analyses.
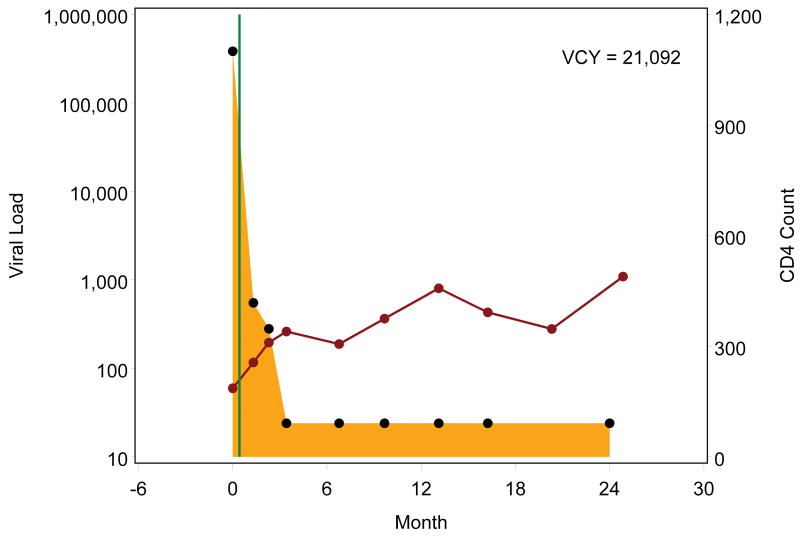
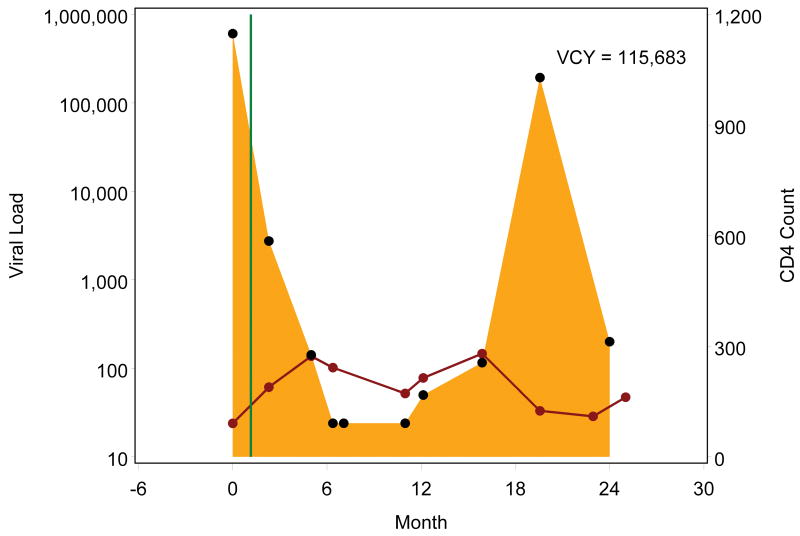
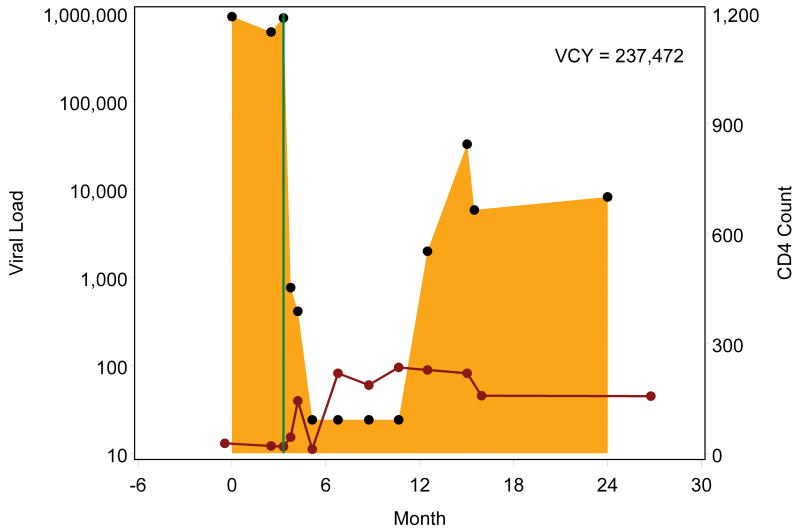
Figure 1.
Example viremia copy-year plots depicting cumulative viral load burden over the first two years following initial entry to outpatient HIV medical care. Each black point represents a plasma viral load value, presented on a log10 scale, with the shaded area representing the area under the viral load curve, which is estimated by the viremia copy-years (VCY) measure. The solid green line identifies the antiretroviral therapy (ART) start date, and each red point represents a CD4+ T lymphocyte value, connected by a solid red line. Figures 1a and 1b depict patients who started ART shortly after entering care at roughly the same viral load level who both achieved viral load suppression (<50 copies/mL) within 6 months of ART start. In contrast to patient 1a, patient 1b had viral load rebound after several suppressed viral load measurements resulting in accumulation of greater viremia copy years. Figures 1c though 1e depict patients who started ART after accruing several viral load measurements. Patients 1c and 1d rapidly achieved viral load suppression (<50 copies/mL), with the former patient having sustained suppression whereas the latter had viral load rebound around month 12, after several suppressed values, with subsequent sustained detectable viremia resulting in greater viremia copy-years. Patient 1e achieved viral load suppression (<50 copies/mL) on one occasion, roughly 6 months following ART start, but had subsequent rebound, leading to considerably greater viremia copy-years than patients 1c and 1d, who similarly achieved cross-sectional viral load suppression within 6 months of ART start.
Principal exposure of interest: Early retention in care
Early retention in care was measured as a time-varying count of “no show” visits for time to viral load suppression analyses (<50 copies/mL), and as visit adherence, the proportion of scheduled visits that were attended, for the evaluation of two-year viremia copy-years.17 Different measures were selected to best capture short- and long-term retention in care in accordance with the outcome measures and analytic plan. During shorter observation periods fewer visits are available analytically, as observed for the time to viral load suppression analyses (median=5 visits, interquartile range=3-9 visits), guiding our a priori selection of the “no show” count measure for these analyses. Additionally, the “no show” count measure functions well as a time-varying covariate in survival analyses.17 During the two year observation period for the cumulative viremia copy years analyses, more HIV medical visits were scheduled (median=10 visits, interquartile range=8-16 visits), allowing a sufficient denominator to draw meaningful inference from the visit adherence measure. The visit adherence measure allows for more detailed evaluation of exposure-response relationships between retention in care and two year viral load burden across a wider range (proportion bound by 0,1) relative to the “no show” count measure, and may be preferable for longer observation periods.17 Only scheduled visits with a primary HIV medical care provider were included. In accordance with prior studies,17-20 visits cancelled in advance by the patient and visits cancelled by the clinic and/or medical provider were excluded. In separate analyses, visit adherence was evaluated as a continuous measure and as a categorical variable; 0-79%, 80-99% and 100% adherence, which roughly represented tertiles of the study sample.
Additional Covariates
Additional measures selected a priori included age, sex, race/ethnicity, health insurance, baseline CD4+ T lymphocyte count and baseline plasma HIV viral load. Baseline plasma HIV viral load and CD4+ T lymphocyte count measurements were the value on the date nearest the initial medical visit date within a window of -180 to +7 days; median ± interquartile range of -3 (-23, 0) and -5 (-23, 0) days, respectively, from initial visit date.
Statistical Analyses
Descriptive statistics including means, standard deviations, medians, interquartile range, counts and percents were calculated for all study variables as appropriate. Survival methods including Kaplan-Meier plots and Cox proportional hazards models were used to evaluate factors associated with time to first viral load suppression (<50 copies/mL). ANOVA, linear regression, and ANCOVA were used to evaluate factors associated with two-year log10 viremia copy-years. All adjusted models controlled for covariates as outlined in the previous section. Sensitivity analyses included patients with a baseline CD4+T lymphocyte count <350 cells/μL in accordance with the recommended threshold for ART initiation during the majority of the study period. Additional sensitivity analyses replaced the visit adherence measure with the “no show” count measure in the evaluation of two-year viremia copy-years. All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC).
Results
Among 676 patients the mean ± standard deviation age at entry into care was 36 ± 11 years, 44% were non-white males, 13% were non-white females, 38% were white males, and 36% were uninsured (Table 1). The mean log10 baseline viral load was 4.56 ± 0.96 log10 copies/mL, and 33% and 43% of patients had baseline CD4+ T lymphocyte counts <200 cells/μL and >350 cells/μL, respectively. Twenty-five percent of patients had 2 or more no show visits, 79% initiated ART (median=35 days from initial visit, interquartile range: 14-105 days), and 63% of the overall sample achieved viral load suppression (<50 copies/mL) in a median 308 days (Kaplan-Meier estimate, interquartile range: 268-343 days) from entry into care. In adjusted Cox proportional hazards analysis, patients with private insurance (HR=1.37 vs. uninsured; 95% CI=1.08, 1.73) and lower baseline CD4+ T lymphocyte counts (<200 cells/μL: HR=3.74; 95%CI=2.83, 4.95, and 200-350 cells/μL: HR=2.95; 95%CI=2.26, 3.86 vs. >350 cells/μL) at entry into care experienced shorter times to viral load suppression below 50 copies/mL (Table 2). Patients with higher baseline viral loads (HR=0.97 per additional 50,000 copies/mL; 95%CI=0.96, 0.99) and with more no show clinic visits (HR=0.83 per additional no show visit; 95%CI=0.76, 0.92) experienced significantly longer time to viral load suppression.
Table 1.
Characteristics of 676 HIV-infected patients initiating outpatient HIV medical care at the University of Alabama at Birmingham (UAB) 1917 and University of Washington (UW) Harborview Clinics, January 2007 – September 2010.
| Characteristic | Overall sample (N=676) | Patients with 2-years follow-up (N=258) |
|---|---|---|
| Age (years) | 35.8 ± 10.8 | 37.1 ± 10.3 |
|
| ||
| Sex × Race/ethnicity | ||
| Non-white Female | 88 (13.3%) | 31 (12.2%) |
| Non-white Male | 289 (43.7%) | 109 (42.9%) |
| White Female | 31 (4.7%) | 15 (5.9%) |
| White Male | 253 (38.3%) | 99 (39.0%) |
|
| ||
| Health insurance | ||
| Private | 245 (37.6%) | 96 (39.8%) |
| Public | 174 (26.7%) | 62 (25.7%) |
| Uninsured | 232 (35.6%) | 83 (34.4%) |
|
| ||
| Site | ||
| UAB 1917 Clinic | 417 (61.7%) | 147 (57.0%) |
| UW Harborview Clinic | 259 (38.3%) | 111 (43.0%) |
|
| ||
| Baseline plasma HIV RNA (log10 c/mL) | 4.56 (0.97) | 4.72 (0.94) |
|
| ||
| Baseline CD4+ T lymphocyte count (cells/μL) | 337 ± 255 | 334 ± 250 |
| <200 cells/μL | 215 (32.8%) | 81 (32.4%) |
| 200-350 cells/μL | 158 (24.1%) | 65 (26.0%) |
| >350 cells/μL | 282 (43.1%) | 104 (41.6%) |
|
| ||
| “No show” visits | ||
| Zero | 363 (53.7%) | 80 (31.0%) |
| One | 144 (21.3%) | 53 (20.5%) |
| ≥ Two | 169 (25.0%) | 125 (48.4%) |
|
| ||
| Initiate antiretroviral therapy | 536 (79.3%) | 224 (86.8%) |
|
| ||
| Achieve plasma HIV RNA <50 c/mL | 425 (62.9%) | 208 (80.6%) |
|
| ||
| 2-year visit adherence | N/A | 0.84 ± 0.16 |
| 0-79% | 83 (32.2%) | |
| 80-99% | 95 (36.8%) | |
| 100% | 80 (31.0%) | |
|
| ||
| 2-year viremia copy years (log10 copy × years/mL) | N/A | 4.31 ± 0.76 |
Data are presented as mean ± standard deviation or frequency (column percent).
Baseline plasma HIV RNA and CD4+ T lymphocyte count measurements were the value on the date nearest the initial clinic visit date within a window of -180 to +7 days; median ± interquartile range of -3 (-23, 0) and -5 (-23, 0) days, respectively, from initial visit date.
“No show” visits: for the overall sample this represents a count with patients censored at first of plasma HIV RNA <50 c/mL or administrative censoring; for the patients with 2-years follow-up (n=258) this represents a count of no show visits during the first 2 years from clinic entry.
Missing data for overall sample and patients with 2 years follow-up: race/ethnicity (n=8, 4), health insurance (n=25, 17), baseline CD4+ T lymphocyte count (n=21, 8), respectively.
Table 2.
Factors associated with more expeditious suppression of plasma HIV RNA (<50 c/mL) among 676 HIV-infected patients initiating outpatient HIV medical care at the UAB 1917 and UW Harborview Clinics, January 2007 – October 2010.
| Characteristic | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|
| Age (per 10 years) | 1.01 (0.92, 1.10) | 0.93 (0.84, 1.02) |
|
| ||
| Sex × Race/ethnicity | ||
| Non-white Female | 0.82 (0.58, 1.14) | 0.76 (0.53, 1.09) |
| Non-white Male | 1.24 (1.01, 1.53)* | 1.10 (0.88, 1.37) |
| White Female | 0.73 (0.44, 1.20) | 0.91 (0.55, 1.52) |
| White Male | 1.0 | 1.0 |
|
| ||
| Health insurance | ||
| Private | 1.28 (1.02, 1.61)* | 1.37 (1.08, 1.73)** |
| Public | 1.09 (0.85, 1.40) | 1.17 (0.88, 1.56) |
| Uninsured | 1.0 | 1.0 |
|
| ||
| Baseline plasma HIV RNA (per 50,000 c/mL) | 1.01 (1.00, 1.03) | 0.97 (0.96, 0.99)** |
|
| ||
| Baseline CD4+ T lymphocyte count (cells/μL) | ||
| <200 cells/μL | 2.97 (2.35, 3.76)** | 3.74 (2.83, 4.95)** |
| 200-350 cells/μL | 2.73 (2.12, 3.50)** | 2.95 (2.26, 3.86)** |
| >350 cells/μL | 1.0 | 1.0 |
|
| ||
| “No show” visits (per additional “no show”) | 0.91 (0.83, 0.98)* | 0.83 (0.76, 0.92)** |
Hazard ratio: HR, 95% Confidence interval: 95% CI.
P<0.05,
P<0.01
Unadjusted and adjusted Cox proportional hazards models. Adjusted model controls for variables included in the table and study site.
“No show” = count of no show visits as a time-varying covariate
Two hundred fifty-eight patients with 2 years of follow-up from clinic entry were included in the evaluation of cumulative viral load burden measured by viremia copy-years, as a function of early retention in care measured by visit adherence. In general, socio-demographic characteristics of these patients were comparable to the overall sample (Table 1). Over the first 2 years in outpatient HIV medical care, nearly half of patients (48%) had 2 or more no show clinic visits. The mean ± standard deviation visit adherence was 0.84 ± 0.16, and the following visit adherence categories were observed among patients: 0-79% adherence in 83 patients (32%), 80-99% adherence in 95 patients (37%), and 100% adherence in 80 patients (31%). Eighty-seven percent of patients started ART during the 2-year period, with 81% achieving a viral load <50 copies/mL on at least one occasion. The mean ± standard deviation 2-year log10 viremia copy years was 4.3 ± 0.8 copy × years/mL. Across increasing visit adherence categories, lower 2-year log10 viremia copy-years (mean ± standard deviation log10 copy × years/mL) was observed: 0-79% adherence: 4.6 ± 0.8 (β=0.47; 95%CI=0.24, 0.70), 80-99% adherence: 4.3 ± 0.7 (β=0.18; 95%CI=-0.04, 0.40), and 100% adherence: 4.1 ± 0.8 log10 copy × years/mL (referent), respectively (Table 3). In multivariable linear regression, lower visit adherence (β=-0.11 per 10% visit adherence; 95%CI=-0.17, -0.04) was associated with greater 2-year viremia copy-years.
Table 3.
Factors associated with 2 year viremia copy-years, an area under the curve estimate of cumulative plasma HIV RNA exposure, among 258 HIV-infected patients initiating outpatient HIV medical care at the UAB 1917 and UW Harborview Clinics, January 2007 – October 2008.
| Characteristic | 2-year viremia copy years (Least Squares Mean ± 95% CI log10 copy × years/mL) | Unadjusted Beta-coefficient (β) (95% CI) | Adjusted Beta-coefficient (β) (95% CI) |
|---|---|---|---|
| Age (per 10 years) | N/A | -0.02 (-0.11, 0.07) | 0.04 (-0.06, 0.13) |
|
| |||
| Sex × Race/ethnicity | |||
| Non-white Female | 4.01 (3.74, 4.27) | -0.33 (-0.64, -0.02)* | -0.06 (-0.37, 0.25) |
| Non-white Male | 4.37 (4.22, 4.51) | 0.03 (-0.18, 0.24) | 0.13 (-0.08, 0.34) |
| White Female | 4.36 (3.97, 4.74) | 0.02 (-0.39, 0.43) | 0.10 (-0.29, 0.49) |
| White Male | 4.34 (4.19, 4.49) | Referent | Referent |
|
| |||
| Health insurance | |||
| Private | 4.13 (3.98, 4.27) | -0.23 (-.044, -0.01)* | -0.05 (-0.27, 0.17) |
| Public | 4.51 (4.33, 4.69) | 0.16 (-0.90, 0.40) | -0.08 (-0.33, 0.18) |
| Uninsured | 4.35 (4.20, 4.51) | Referent | Referent |
|
| |||
| Baseline CD4+ T lymphocyte count (cells/μL) | |||
| <200 cells/μL | 4.30 (4.13, 4.46) | -0.03 (-0.25, 0.19) | -0.02 (-0.24, 0.21) |
| 200-350 cells/μL | 4.23 (4.04, 4.41) | -0.10 (-0.34, 0.13) | -0.16 (-0.40, 0.08) |
| >350 cells/μL | 4.33 (4.18, 4.48) | Referent | Referent |
|
| |||
| Visit adherence (β per 10%) | -0.11 (-0.17, -0.06)** | -0.11 (-0.17, -0.04)** | |
| 0-79% | 4.56 (4.40, 4.72) | 0.47 (0.24, 0.70)** | |
| 80-99% | 4.27 (4.12, 4.42) | 0.18 (-0.04, 0.40) | |
| 100% | 4.09 (3.93, 4.26) | Referent | |
95% Confidence interval: 95% CI.
P<0.05,
P<0.01
Unadjusted and adjusted linear regression models. Adjusted model controls for variables included in the table and study site with visit adherence as a continuous variable (β-coefficient per additional 10% visit adherence).
Sensitivity analyses restricted to patients with baseline CD4+ T lymphocyte counts <350 cells/μL on clinic entry (n=372) yielded comparable findings to primary analyses. Ninety-seven percent of patients (n=361) started ART with 76% (n=283) achieving a viral load <50 copies/mL in a median 192 days (Kaplan-Meier estimate, interquartile range: 175-211 days) from entry to care. In adjusted Cox proportional hazards analysis, patients with more no show clinic visits (HR=0.81 per additional no show visit; 95%CI=0.72, 0.92) experienced significantly delayed time to viral load suppression. As observed in primary analyses, visit adherence demonstrated an inverse relationship with 2-year log10 viremia copy-years in multivariable linear regression (β=-0.14 per 10% visit adherence; 95%CI=-0.19, -0.08). Finally, when replacing the visit adherence measure of early retention in care with the “no show” count measure, a comparable relationship was observed with greater 2-year log10 viremia copy-years among patients with more missed visits (β=0.11 per “no show” visit; 95%CI=0.08, 0.14).
Discussion
Early retention in HIV care was associated with time to viral load suppression (<50 copies/mL) and two-year cumulative viral load burden among patients newly initiating outpatient HIV medical care. Each “no show” clinic visit conveyed a 17% increased risk of delayed viral load suppression. Significantly greater viremia copy-years, an estimate of cumulative HIV burden, were accumulated among patients with poorer visit adherence over the first 2-years in care (4.6 ± 0.8 vs. 4.1 ± 0.8 log10 copy × years/mL for 0-79% vs. 100% visit adherence). These findings have implications for patient outcomes as recent studies have identified increased risk of deleterious clinical events among patients experiencing greater cumulative viral load burden over time.21,22
Beyond individual health consequences, these findings have implications for HIV prevention efforts, particularly in the context of a test and treat approach to HIV prevention.1,2,5 Failure to achieve and sustain viral load suppression poses increased risk of HIV transmissibility.3,23 In the current study we identified poor early retention in HIV care as a barrier to timely viral load suppression and a factor associated with greater cumulative viral load burden over the first two years following entry into care. A recent study by Metsch and colleagues identified reductions in sexual risk transmission behaviors among recently diagnosed patients with better early retention in HIV care relative to patients who were poorly retained.24 Taken together, these two studies highlight the vital role of early retention in HIV care as it relates to sexual risk behavior and viral load suppression – critical elements in secondary HIV transmission, making early retention in care an important target for prevention interventions.
Previously, we identified significant, independent associations between viremia copy-years and clinical outcomes among HIV-infected patients. Among Multicenter AIDS Cohort Study (MACS) HIV-uninfected participants, who seroconverted, viremia copy-years was associated with AIDS-defining clinical events and mortality in the pre-combination ART era (1984-96).15 Among CNICS patients initiating modern ART regimens (NNRTI or PI/r-based regimens) in recent years (2000-08), viremia copy-years was independently associated with all-cause mortality when controlling for traditional, cross-sectional viral load measures and time-updated CD4+ T lymphocyte count.25
Here, we extend the use of this novel, area under the curve estimate of cumulative viral load burden by evaluating viremia copy-years as an outcome variable for the first time. Beyond single, cross-sectional measures of viral load suppression, we posit longitudinal measures of cumulative viremia may better predict individual risk of HIV transmission and may augment the evaluation of treatment as prevention interventions, the success of which is predicated on not merely achieving, but subsequently sustaining viral load suppression. Moreover, we suggest viremia copy-years should be explored as a population-level indicator of transmission risk compared to single-measure indicators such as community viral load or the cross-sectional proportion of patients suppressed at the most recent measure or other single time point.
Our study has limitations. As with all observational studies, we can identify associations but not ascribe causality because our findings are subject to possible uncontrolled confounding. Patients had variable number and timing of viral load measures, reflecting the realities of HIV clinical care. This may impact the precision of the viremia copy-years estimate of cumulative HIV burden. Patients with >365 days between viral load measures were excluded to address this limitation. While conducted at 2 sites in distinct regions of the US, study findings may not be generalizable to other regions or settings. Future studies are planned to evaluate the generalizabilty of these findings to the larger, nationally distributed CNICS cohort (8 sites). In this initial analysis we did not formally evaluate the timing of ART receipt, ART persistence (or durability) and ART adherence in multivariable analyses as these factors are on the causal pathway between early retention in care and viral load suppression.4,10 Here we sought to first establish the relationship between early retention in outpatient HIV care and viral load outcomes. However, in exploratory analyses, the relationship between the number of “no show” visits and VL suppression persisted after controlling for initiation of ART as a dichotomous time-varying covariate (data not shown). Future studies using methods of causal inference (e.g., structural equation models or marginal structural models) are planned to evaluate the intricacies of the dynamic relationships among variables on the causal pathway from entry to HIV care to longitudinal viral load suppression, including early retention in care, initial ART receipt, ART persistency and ART adherence. Finally, we did not systematically capture substance abuse, mental illness and other psychosocial factors at care initiation such that we could not evaluate the role of these important and prevalent factors on early retention in care and viral load suppression.
In conclusion, we identified significant associations between early retention in care and viral load suppression among patients initiating outpatient HIV medical care. Beyond delayed time to viral load suppression (<50 copies/mL) among patients with more “no show” visits, we demonstrated the importance of early visit adherence as it relates to cumulative HIV burden, measured by viremia copy-years. Longitudinal measures of cumulative viral load burden, like viremia copy-years, may significantly contribute to the evaluation of test and treat HIV prevention interventions, the success of which are predicated on both rapidly achieving, and also longitudinally sustaining viral load suppression.
Acknowledgments
We thank the patients, providers, clinical and research personnel at the UAB 1917 Clinic and the UW Harborview Clinic as well as the CNICS administrative and data management teams. We thank Stephen Cole, Sonia Napravnik, Joseph Eron and Bryan Lau for their collaboration in developing the viremia copy-years measure.
Funding support: 1R21AI087360-01, 3K23MH082641-02S1, 1R24AI067039-04, P30AI27767, 5K23MH090923-02
Footnotes
Presented in part at the 6th International Conference on HIV Treatment and Prevention Adherence; May 22-24, 2011, Miami, FL
Financial Disclosure: This study was supported by grants 1R21AI087360-01, 3K23MH082641-02S1, 1R24AI067039-04, P30AI27767, 5K23MH090923-02 from the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
Conflicts of Interest: MJM has received consulting fees (advisory board) from Bristol-Myers Squibb, Gilead Sciences and Merck Foundation, and grant support from Bristol-Myers Squibb, Pfizer, Inc, Tibotec Therapeutics,a nd Definicare, LLC. JHW has received consulting fees from Bristol-Myers Squibb and Gilead Sciences, and grant support from Bristol-Myers Squibb, Pfizer, Inc, Tibotec Therapeutics and Definicare LLC. MSS has received consulting fees from Ardea Biosciences, Avexa, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck, Monogram Biosciences, Pain Therapeutics, Panacos, Pfizer, Progenics, Roche Laboratories, Tibotec, Tobira Therapeutics, and Vicro and research support from Achillion Pharmaceuticals, Avexa, Boehringer-Ingelheim, GlaxoSmithKline, Merck, Panacos, Pfizer, Progenics, Theratechnologies and Tibotec. All remaining authors have no conflicts of interest to declare.
Citations
- 1.Dieffenbach CW, Fauci AS. Universal voluntary testing and treatment for prevention of HIV transmission. Jama. 2009 Jun 10;301(22):2380–2382. doi: 10.1001/jama.2009.828. [DOI] [PubMed] [Google Scholar]
- 2.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009 Jan 3;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 3.Initiation of Antiretroviral Treatment Protects Uninfected Sexual Partners from HIV Infection (HPTN Study 052) [Last accessed: May 17, 2011]; Press release: Thursday, 12 May 2011, 11 am EST. Available at: http://www.hptn.org/web%20documents/PressReleases/HPTN052PressReleaseFINAL5_12_118am.pdf.
- 4.Mugavero MJ, Norton WE, Saag MS. Health care system and policy factors influencing engagement in HIV medical care: piecing together the fragments of a fractured health care delivery system. Clin Infect Dis. 2011 Jan 15;52 2:S238–246. doi: 10.1093/cid/ciq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011 Mar;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campsmith ML, Rhodes PH, Hall HI, Green TA. Undiagnosed HIV prevalence among adults and adolescents in the United States at the end of 2006. J Acquir Immune Defic Syndr. 2010 Apr;53(5):619–624. doi: 10.1097/QAI.0b013e3181bf1c45. [DOI] [PubMed] [Google Scholar]
- 7.Marks G, Gardner LI, Craw J, Crepaz N. Entry and retention in medical care among HIV-diagnosed persons: a meta-analysis. Aids. Nov 13;24(17):2665–2678. doi: 10.1097/QAD.0b013e32833f4b1b. [DOI] [PubMed] [Google Scholar]
- 8.Giordano TP, White AC, Jr, Sajja P, et al. Factors associated with the use of highly active antiretroviral therapy in patients newly entering care in an urban clinic. J Acquir Immune Defic Syndr. 2003 Apr 1;32(4):399–405. doi: 10.1097/00126334-200304010-00009. [DOI] [PubMed] [Google Scholar]
- 9.Mugavero MJ, Lin HY, Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009 Jan 15;48(2):248–256. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulett KB, Willig JH, Lin HY, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDS. 2009 Jan;23(1):41–49. doi: 10.1089/apc.2008.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. Aids. 2005 Mar 4;19(4):423–431. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- 12.Craw JA, Gardner LI, Marks G, et al. Brief strengths-based case management promotes entry into HIV medical care: results of the antiretroviral treatment access study-II. J Acquir Immune Defic Syndr. 2008 Apr 15;47(5):597–606. doi: 10.1097/QAI.0b013e3181684c51. [DOI] [PubMed] [Google Scholar]
- 13.Olatosi BA, Probst JC, Stoskopf CH, Martin AB, Duffus WA. Patterns of engagement in care by HIV-infected adults: South Carolina, 2004-2006. Aids. 2009 Mar 27;23(6):725–730. doi: 10.1097/QAD.0b013e328326f546. [DOI] [PubMed] [Google Scholar]
- 14.Torian LV, Wiewel EW. Continuity of HIV-related medical care, New York City, 2005-2009: Do patients who initiate care stay in care? AIDS Patient Care STDS. 2011 Feb;25(2):79–88. doi: 10.1089/apc.2010.0151. [DOI] [PubMed] [Google Scholar]
- 15.Cole SR, Napravnik S, Mugavero MJ, Lau B, Eron JJ, Jr, Saag MS. Copy-years viremia as a measure of cumulative human immunodeficiency virus viral burden. Am J Epidemiol. 2010 Jan 15;171(2):198–205. doi: 10.1093/aje/kwp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol. 2008 Oct;37(5):948–955. doi: 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mugavero MJ, Davila JA, Nevin CR, Giordano TP. From access to engagement: measuring retention in outpatient HIV clinical care. AIDS Patient Care STDS. 2010 Oct;24(10):607–613. doi: 10.1089/apc.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keruly JC, Conviser R, Moore RD. Association of medical insurance and other factors with receipt of antiretroviral therapy. Am J Public Health. 2002 May;92(5):852–857. doi: 10.2105/ajph.92.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melnikow J, Kiefe C. Patient compliance and medical research: issues in methodology. J Gen Intern Med. 1994 Feb;9(2):96–105. doi: 10.1007/BF02600211. [DOI] [PubMed] [Google Scholar]
- 20.Robbins GK, Johnson KL, Chang Y, et al. Predicting virologic failure in an HIV clinic. Clin Infect Dis. Mar 1;50(5):779–786. doi: 10.1086/650537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole SR, Napravnik S, Mugavero MJ, Lau B, Eron JJ, Jr, Saag MS. Copy-years viremia as a measure of cumulative human immunodeficiency virus viral burden. Am J Epidemiol. Jan 15;171(2):198–205. doi: 10.1093/aje/kwp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marconi VC, Grandits G, Okulicz JF, et al. Cumulative Viral Load and Virologic Decay Patterns after Antiretroviral Therapy in HIV-Infected Subjects Influence CD4 Recovery and AIDS. PLoS One. 2011;6(5):e17956. doi: 10.1371/journal.pone.0017956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000 Mar 30;342(13):921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 24.Metsch LR, Pereyra M, Messinger S, et al. HIV transmission risk behaviors among HIV-infected persons who are successfully linked to care. Clin Infect Dis. 2008 Aug 15;47(4):577–584. doi: 10.1086/590153. [DOI] [PubMed] [Google Scholar]
- 25.Mugavero M, Napravnik S, Cole S, et al. CFAR Network of Integrated Clinical Systems. Viremia Copy-Years Predicts Mortality among Treatment-naïve HIV-infected Patients Initiating Antiretroviral Therapy. Clin Infect Dis. doi: 10.1093/cid/cir526. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]