Abstract
Here we describe a new population of NK cells that reside in the normal, un-inflamed peritoneal cavity. Phenotypically, they share some similarities with the small population of CD49b negative, CD27 positive immature splenic NK cells, and liver NK cells but differ in their expression of CD62L, TRAIL and EOMES. Functionally, the peritoneal NK cells resemble the immature splenic NK cells in their production of IFN-γ, GM-CSF and TNF-α and in the killing of YAC-1 target cells. We also found that the peritoneum induces different behavior in mature and immature splenic NK cells. When transferred intravenously into RAGγcKO mice, both populations undergo homeostatic proliferation in the spleen, but only the immature splenic NK cells, are able to reach the peritoneum. When transferred directly into the peritoneum, the mature NK cells survive but do not divide, while the immature NK cells proliferate profusely. These data suggest that the peritoneum is not only home to a new subset of tissue resident NK cells but that it differentially regulates the migration and homeostatic proliferation of immature versus mature NK cells.
Keywords: NK cells, peritoneal cavity, tissue-specific NK cells, immature NK cells, trafficking of NK cells
Over the last decade, it has become clear that Natural Killer (NK) cells are not a homogeneous population, found mainly in bone marrow, spleen and blood, but that other tissues, such as thymus (1), lymph node (2), lung (3), liver (4) and uterus (5, 6), harbor resident NK cells with distinctive phenotypes and potentially different local functions. Here we asked whether the peritoneal cavity might be another such tissue.
In the spleen, different subpopulations of NK cells can be defined, based on their expression of surface markers such as CD49b (7), CD11b (4, 8), CD27 (9) and CD94 (10). Mature splenic NK cells are positive for CD11b (3) and CD49b (4, 7, 11), and about half are also positive for CD27. In addition, the spleen harbors a set of immature NK cells that are CD49b negative but CD27 positive. NK cells in other tissues vary in their expression of these markers (3, 4, 10).
The peritoneal cavity is known to be home to a specific type of macrophage (12, 13), a resident population of self-renewing B lymphocytes (14, 15), and a newly described population found in Fat-Associated-Lymphoid Clusters (FALC) (16); and these three populations play a critical role in innate immunity against helminth infection and in controlling peritoneal inflammation. Not much is known, however, about resident peritoneal NK (peNK) cells. A few studies have looked at NK cells after an inflammatory insult in the peritoneum, for example during peritonitis (17), or after i.p. injection of Vaccinia Virus (18), or i.p. placement of tumors (19) or grafts (20), etc.; but the rapid influx of mature splenic NK cells into the inflamed peritoneum makes the identification of any resident NK cells difficult in these conditions. Although the control panels in these studies show that a small number of NK cells also reside in the normal un-inflamed peritoneum, this population of NK cells has largely been ignored. Here, we show that, under steady-state conditions, the peritoneal cavities of both wild type and RAGKO mice harbor a distinctive population of NK cells that are phenotypically different from splenic and other tissue-resident NK cells, though they share some phenotypic and functional features with the small subset of immature NK cells in the spleen. When injected i.v., only this immature subset, and not the mature splenic NK cell population, is able to enter the peritoneum, even though both subsets engage in homeostatic proliferation in the spleen. When injected i.p., only the immature NK cells engage in homeostatic expansion while the mature NK cells survive in the peritoneum without dividing. Thus, it appears that the peritoneal cavity is a highly selective environment that not only contains its own specific populations of macrophages and B cells, but also contains a distinct resident NK cell population closely related to immature splenic NK cells.
Materials and Methods
Mice
Marilyn mice, which have been described previously (21), C57BL/10RAGKO (with either the CD45.2 or CD45.1 alleles), RAGγcKO, C57BL/6, B10.A, and Germ Free B10.A mice were from Taconic Farms. CXCR3KO mice and their control (C57Bl/6) mice were obtained from Jackson Laboratory. All mice, except Germ Free Mice, were housed in specific pathogen-free conditions. NIH is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Cell isolation and purification of NK cells
Cells from the peritoneal cavity were collected after three peritoneal washes with 5mL of ice-cold PBS (Invitrogen) containing 10U/mL heparin (American Pharmaceutical Partners Inc.). Before transfer into RAGγcKO mice, NK cells from spleens of RAGKO mice were enriched by negative selection using an NK cell isolation cocktail (Miltenyi). Purity of negatively selected NK cells, measured by FACS as the percentage of NK1.1+ cells, was 70% or greater. Whenever stated, NK cells were stained with antibodies against NK1.1, CD49b, and CD27, and different subpopulations sorted to a final purity greater than 96% using either a FACSAria or FACSVantage cell sorter (BD Biosciences).
Flow cytometry
Splenocytes, peritoneal exudate, liver cells or blood cells were incubated for 10 min at room temperature with a blocking mixture of 33% each mouse, rat, and hamster sera, plus 10ug/ml 2.4G2, followed by 20 min with the relevant antibodies (40 min when staining for chemokine receptors). We used antibodies against the following markers: NK1.1 (PK-136), CD27 (LG.7F9), CD43 (S7), CD49b (DX5), CD3ε (145-2C11), CD11b (M1/70), CD62L (MEL-14), CD4 (RM4-5), and Ly49C/I (5E6) from BD Pharmingen; CXCR3 (CXCR3-173), CD127 (A7R34), and TCRαβ (H57-597) from eBioscience; TRAIL (N2B2, Biolegend); NKp46 (goat polyclonal, R&D Systems). Intracellular staining for Granzyme B (Invitrogen), EOMES (Dan11mag, eBioscience), and Ki-67 (B56, BD Pharmingen) was performed using Fixation/Permeabilization reagents from eBioscience. To exclude dead cells, we stained them with 7-amino-actinomycin D (7-AAD) (BD Pharmingen). Data were acquired with a FACSCalibur (BD Biosciences) or a FACSCanto II (BD biosciences) and analyzed with CellQuest (BD Biosciences) or FlowJo (TreeStar Inc.) software.
CFSE labeling and adoptive transfer
Some RAGγcKO mice received unlabelled splenocytes from Marilyn mice i.p.. For cell division studies, we labeled column-enriched NK cells, or sorted CD49b+CD27- or CD49b-CD27+ NK cells, from RAGKO spleens (5 × 106 cells/m in 0.1% FCS-PBS) with 5μM CFSE (Invitrogen) for 8min at 37°C, washed them twice with 0.5% FCS-PBS, resuspended them in PBS, and transferred the indicated numbers i.p. or i.v. into RAGγcKO mice. Recipient splenocytes, and cells from peritoneal lavage were collected at the indicated time points, and cell division evaluated by FACS-based CFSE dilution profiles.
Cytokine production and Killing assay
Sorted NK1.1+ cells (3-5×104/well in 96 well plates) from the spleen and peritoneal lavage of RAGKO mice were cultured for 24 hours with IL-2 (103 IU/mL) (PeproTech), and stimulated or not with PMA and Ionomycin (IO) (both from Calbiochem), or with 15,000 rads irradiated YAC-1 cells. Supernatants were collected and cytokines measured by RayBiotech, Inc. using the Quantibody Arrays QAM-INT-1 and QAM-INT-2, which detect the following cytokines: G-CSF, GM-CSF, IFNγ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-17, IL-21, IL-23, TNFα, IL-1rα, IL-2 Rα, IL-6 R, IL-11, IL-12p40, IL-17B, IL-17E, IL-17F, IL-20, IL-28. Cytotoxicity was measured using the JAM test (22) with YAC-1 cells as targets. The test was performed using sorted purified NK cells immediately ex vivo.
Results
Splenic NK cells survive and don’t leave (or are trapped in) the Peritoneal Cavity
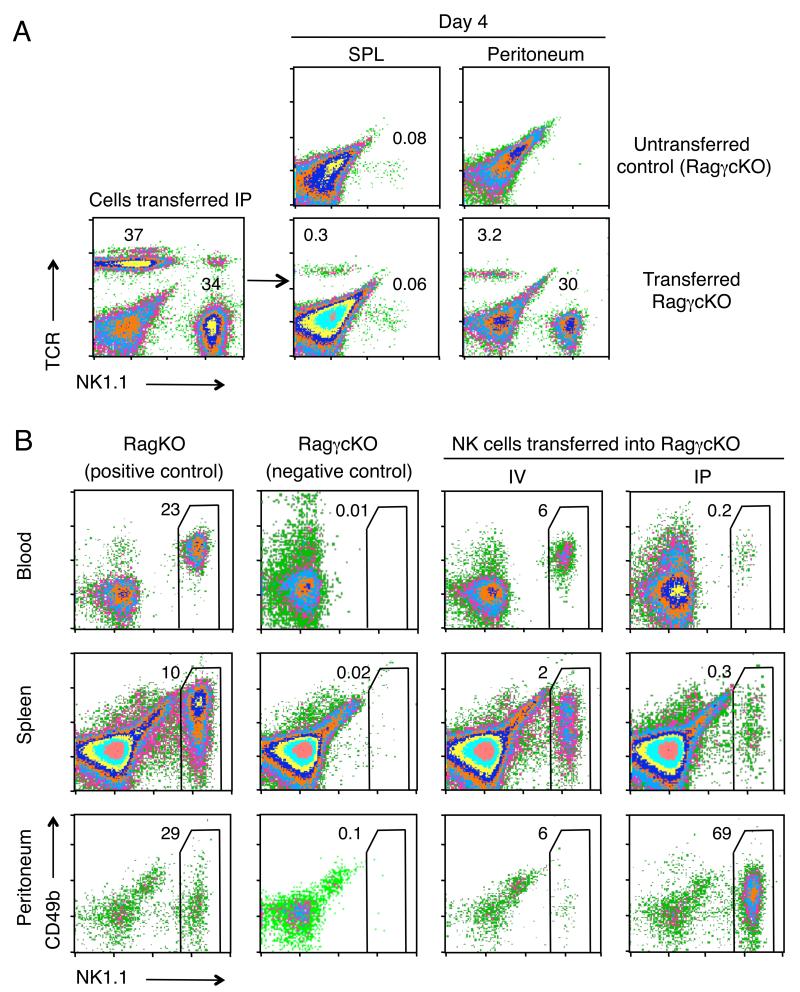
In the course of studies showing that CD4 T cells can clear tumors without the aid of CD8 T cells (23), we transferred spleen cells from the CD4 TCR Tg/RAGKO mouse, Marilyn, i.p into RAGγcKO recipients, and tracked the migration of the donor T cells. We found that they rapidly moved into the circulation and entered the tumors. Unlike the T cells, however, transferred NK cells did not leave the peritoneum and populate the periphery. At day four after transfer, we found a significant population of T cells in the spleen (0.3% of the spleen, equal to 1.4×104 cells, compared to 0% in untransferred controls; Fig. 1A), but no NK cells over the background, and the situation was reversed in the peritoneum, where there were eight times more NK cells than CD4 cells (8.1×104 versus 104 cells, respectively). This was a surprising result, as it is well established that NK cells are quite efficient at repopulating NK-cell deficient (RAGγcKO) mice, at least when given i.v. (24, 25). We therefore purified splenic NK cells, transferred them i.v. and i.p. into RAGγcKO mice, and found that the two routes of injection gave very different results. Although the i.v. route partially reconstituted NK cell numbers in the blood, spleen and peritoneum of the recipient mice (Fig. 1B), the i.p. route was extremely poor, as the majority of NK cells given i.p. remained trapped in the peritoneum 18 days after cell transfer. Thus there appears to be a block in peritoneal egress of NK cells that does not affect T cells.
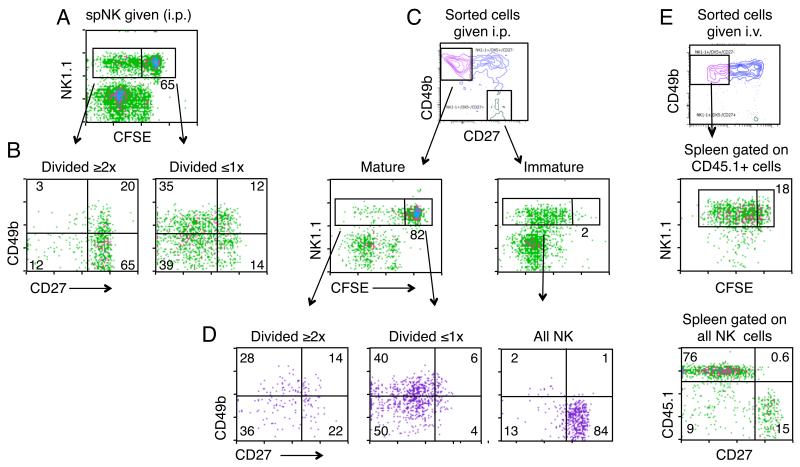
FIGURE 1.
NK cells are selectively trapped in the peritoneum and do not seed the periphery. A, Percentages of TCR+ and NK1.1+ cells found in the spleen and peritoneal cavity of RAGγcKO mice four days after i.p. transfer of 2×105 whole splenocytes (containing both CD4 and NK cells) from a Marilyn mouse (CD4 TCRTg RAGKO). Lower left plot shows an analysis of the donor cells, just before transfer. Middle and right columns show the cells recovered from the spleen and peritoneal cavity of the recipients. A RAGγcKO mouse that did not receive cells is shown as control (first row). Data represent two independent experiments, each with two mice per group. B, Percentages of NK1.1+ cells found in the blood, spleen and peritoneum of RAGγcKO mice 18 days after either i.p. or i.v. transfer of 2×106 NK cells enriched from RAGKO mouse spleens. Left two columns show the corresponding NK cell populations in the RagKO donors and the RagγcKO recipients before cell transfers. Right two columns show the populations in the transferred recipients. Data represent 3 independent experiments performed with a total of 15 mice. A-B, Percentages were calculated within the lymphocyte gate.
The Peritoneal Cavity harbors a specific subset of NK cells
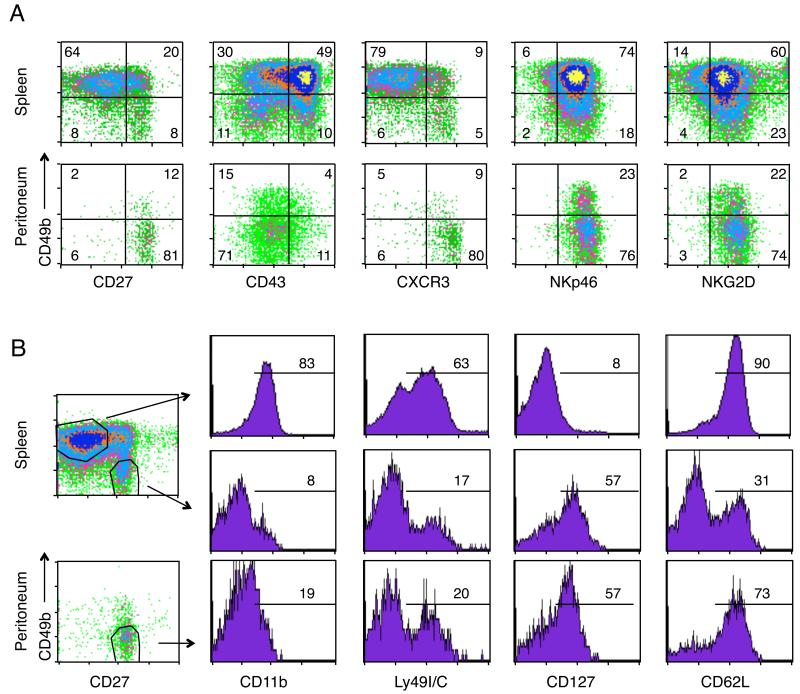
From the transfer studies, we noticed that the NK cells found in the peritoneal cavities of RAGKO mice, as well as the few NK cells that migrated to the peritoneum after iv transfer into the RAGγcKO mice, expressed very low levels of CD49b, whereas NK cells in the blood and spleen expressed high levels (Fig. 1B). This finding, together with the fact that the NK cells were unable to leave the peritoneum after i.p. transfer, suggested that the peritoneum might harbor a specific set of locally resident NK cells, different from spNK cells. To test this, we first compared the phenotypes of peNK versus spNK cells in both RAGKO and wild type mice, and found that the peritoneal NK cells did indeed have a distinct phenotype (Fig. 2A). Gating on NK1.1 positive cells in RAGKO mice (Fig. 2A), or on NK1.1+CD3-cells in normal B6 mice (Supplemental Fig. 1), we saw that most of the peNK cells expressed low levels of CD49b and CD43, and high levels of CD27 and CXCR3, a phenotype expressed by only 4-11% of the spNK cells. The only markers that both splenic and peritoneal NK cells expressed similarly were NK1.1, NKp46, and NKG2D, which are normally expressed by both mature and immature NK cells (7, 26). These differences suggest that, in steady state conditions, peNK cells may be a distinct population from spNK cells.
FIGURE 2.
The Peritoneal Cavity harbors a specific subset of NK cells. A. Flow cytometric analysis comparing splenic versus peritoneal NK cells for the following markers: CD49b, CD27, CD43 (115 kDa glycoform), CXCR3, NKp46, and NKG2D. Numbers represent the percentage of NK cells that fall within each quadrant. Data compiled from two different days. B, CD11b, Ly49C/I, CD127 and CD62L staining within the CD49b+/CD27-NK cells from spleen (upper row) and the CD49b negative/CD27+ NK cells from spleen or peritoneum (middle and lower row, respectively). Gates used are shown in the density plots. Numbers indicate the percentage of NK cells within that gate that were positive for each marker. CD11b staining done on a different day from the other markers. Density plots in A and B are gated on NK1.1+ cells in RAGKO mice. A-B, Each marker was analyzed in three to seven RAGKO mice.
However, a careful look at the phenotype of spNK cells showed that peNK cells looked very similar to the small subset of CD49b-CD27+ spNK cells previously identified as ‘immature’ NK cells. Both sets were CD49b negative, CD27+ and CXCR3R+ and lacked the expression of CD43 (Fig. 2A).
To further compare the two subsets, we assessed their expression of other markers that discriminate between mature and immature NK cells, such as CD11b (for which the immature CD49b-CD27+ spNK cells have previously been shown to be negative (3, 4)), Ly49I/C, CD62L, and CD127 (IL-7Rα). Peritoneal NK cells seemed to be similar to the immature spNK cells in the expression of most of these markers (expressing low levels of CD11b, and containing subsets expressing Ly49I/C (around 20%) and CD127 (57%)), but they differed in their expression of CD62L (Fig. 2B), for which the peNK cells resembled the mature splenic NK cells (Fig. 2B, upper panel). From these results we conclude that peNK cells are very similar to the ‘immature’ spNK, but differ in their expression of CD62L.
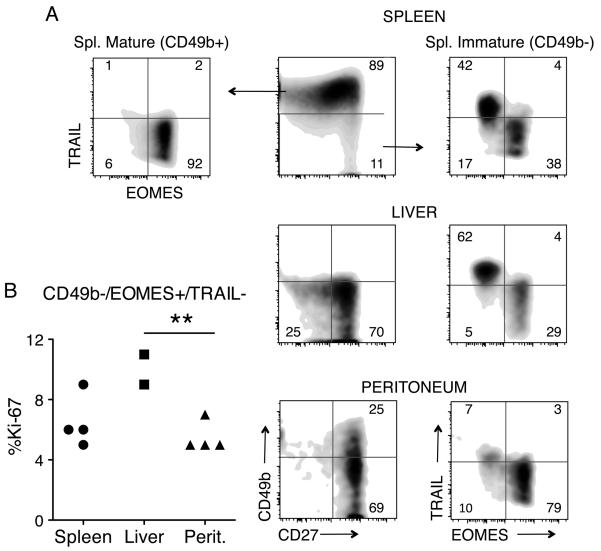
A population of liver-specific NK cells has been described that shares the CD49b-/CD27+ phenotype with the peNK and immature splenic NK cells (27) (Fig. 2A and 3A). The liver NK cells differ from the typical splenic NK cells in that they are TRAIL+, while most splenic NK cells are negative (27) and Fig. 3. Liver NK cells, however, are similar to neonatal splenic NK cells (27) and they have been suggested to be of a different origin from typical splenic mature CD49b+ NK cells (28). To see if peNK cells and liver NK cells are perhaps the same cells situated in different tissues, or if they are instead different subsets of NK cells, we stained liver and peNK cells with TRAIL, and with EOMES, a transcription factor that is typically expressed by splenic CD49b+ NK cells (29). We found that peNK cells are different from both liver NK cells and from immature splenic NK cells in TRAIL and EOMES expression (Fig. 3A). peNK cells and mature CD49b+ spNK cells were mostly EOMES+ and TRAIL negative, while liver NK cells and immature spNK cells were split into two groups, with about half the cells being TRAIL+ EOMES negative, and the other half being the opposite (Fig 3A). Thus, though the peNK cells resemble the immature splenic NK cells for CD49b, CD27 and a series of other markers, they differ from them and from the liver NK cells on TRAIL and EOMES expression. We also measured the cell division rate of the immature NK populations (defined as NK1.1+, CD49b-, EOMES+, TRAIL-) in different tissues under steady-state conditions and found that the immature liver TRAIL-NK population had approximately double the number of cells undergoing cell division than did the peritoneal NK cells. Peritoneal and spleen CD49b-/TRAIL-cells had similar cell division rate (Fig 3B).
FIGURE 3.
peNK cells differ from liver NK cells and immature spNK cells on EOMES and TRAIL expression. A, Flow cytometric analysis in RAGKO mice comparing gated NKp46+ cells from spleen, liver and peritoneum, for the expression of CD49b versus CD27 (middle column), and TRAIL versus EOMES (left and right columns). The spleen NK cells were further gated on mature CD49b+ (left) and immature CD49b- (right) populations. Numbers represent the percentage of NK cells that fall within each quadrant. Data compiled from three different experiments, with two mice in each one. B, Percentage of dividing cells (Ki-67+) within the immature NK cells (defined as NK1.1+/CD49b-/EOMES+/TRAIL-) found in spleen, liver, and peritoneum of RAGKO mice. Data compiled from two independent experiments. The difference in cell division was statistically different between liver and peritoneum (Student’s t test, p<0.01)
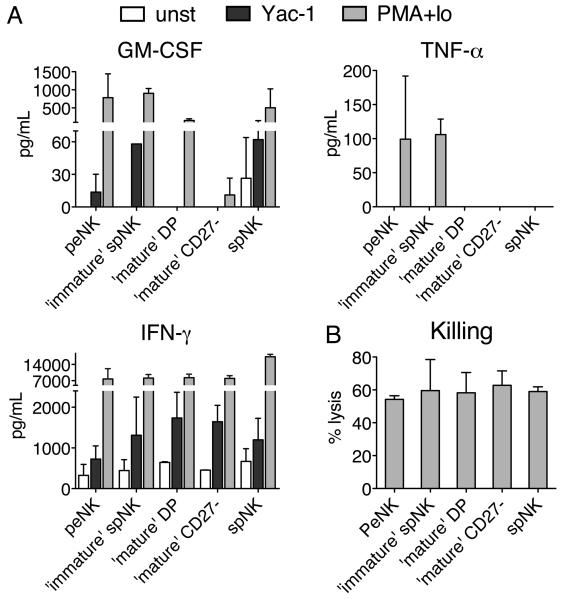
We next compared the functional capabilities of the peNK cells with those of the ‘mature’ and ‘immature’ sub-populations of spNK cells (sorted according to CD49b and CD27 expression), when cultured with a physiological stimulus like YAC-1 cells or with the stronger stimulus, PMA+IO. Twenty four hours after in vitro stimulation with YAC cells, all of the populations of NK cells produced IFN-γ, but only the peNK cells and the CD49b-/CD27+ ‘immature’ spNK cells produced GM-CSF, and none of the populations produced TNF-α (Fig. 4A). When stimulated with PMA+IO, all of the populations of NK cells produced higher amounts of IFN-γ and varying amounts of GM-CSF, while only the peNK cells and the CD49b-/CD27+ ‘immature’ spNK cells also made TNF-α. None of these NK cells produced any of the other thirty cytokines that we tested (see methods). peNK cells also had the ability to kill. In fact, all four subsets of NK cells showed similar killing activity against YAC-1 cells (Fig. 4B). It appears, therefore, that peNK cells functionally resemble immature CD49b-CD27+ spNK cells more than the other two populations of spNK cells.
FIGURE 4.
Peritoneal NK cells are functionally similar to immature splenic NK cells. A, Amount of GM-CSF, TNF-α and IFN-γ produced by sorted NK1.1+ peNK or spNK cells, or by the three different subpopulations of spNK cells [‘immature’ spNK (CD49b-/CD27+), mature DP (CD49b+/CD27+), and ‘mature’ CD27- (CD49b+/CD27-)] after 24h of culture in the presence of IL-2 (1,000 IU/mL) alone (unst, white bars), or with the addition of irradiated YAC-1 cells (black bars) or PMA+IO (grey bars). Only the cytokines IFN-γ, GM-CSF and TNF-α were reproducibly detected in the culture supernatant out of thirty cytokines measured (see materials and methods). Graphs represent the average and standard deviation measured in two to four independent experiments, using between 2.2 and 5×104 NK cells/well and normalized to 5×104 cells. B, Percentage of YAC-1 cells killed by the same populations of NK cells described in A, tested ex vivo, at an effector to target ratio of 3-5/1 (the first dilution at which the killing dropped off a plateau) using an 18hr. JAM Test. Graph represents the average and standard deviation measured in two to five independent experiments.
Mature and immature spNK cells respond differently to the peritoneal environment
In the transfer study shown in Figure 1B, we had seen that large numbers of splenic NK cells remained resident upon transfer directly into the peritoneum. To see if their persistence was due to cell division, or to simple survival, we transferred CFSE labeled splenic NK cells into the peritoneum of RAGγcKO mice, and assessed their phenotype 30 days later. Fig. 5A shows that a small proportion of the NK cells trapped in the peritoneum divided in the month that they were resident, but most of the cells remained quiescent.
FIGURE 5.
‘Mature’ CD49b+/CD27− spNK cells do not undergo homeostatic expansion in the peritoneum. A-B, One million CFSE labeled spNK cells from RAGKO mice were transferred i.p. into RagγcKO mice. 30 days later mice were killed and peritoneal cells stained with NK1.1, CD49b and CD27. A, CFSE dilution of the transferred NK1.1+ cells. B, CD49b versus CD27 staining within the NK1.1 cells that had divided either two or more times (left panel) or divided once at most (right panel). C-D, Same as in A-B except that we separately transferred either 3.5 × 104 sorted ‘mature’ CD27− spNK cells (NK1.1+ CD49b+ CD27−) (left panels) or ‘immature’ spNK cells (NK1.1+ CD49b− CD27+) (right panels) and analyzed eight days later. E, 7×105 sorted and CFSE labeled ‘mature’ CD27− spNK cells (NK1.1+ CD49b+ CD27−) carrying the congenic CD45.1 marker were transferred i.v. into CD45.2 RAGγcKO mice, and cells in the spleen were analyzed nine days later. CFSE dilution is shown on gated donor (CD45.1+) cells (right column, middle panel). CD27 versus CD45.1 expression is shown on gated NK1.1+ cells (lower panel). A-E, Numbers in CFSE plots indicate percentages of NK1.1+ cells that had not divided or divided just once. Numbers in quadrants represent the percentage of gated cells that fall within that quadrant. For each experiment, data represent a total of four mice in two independent experiments.
When we compared the phenotypes of the divided and undivided cells (Fig. 5B), we saw that those cells that had not divided, or divided just once, mostly continued to resemble spNK cells, (though their expression of CD49b was somewhat lower). In contrast, those that had divided two or more times displayed a CD49bneg/low, CD27+ phenotype similar to normal resident peNK cells and to ‘immature’ spNK cells, suggesting that they might have originated from the ‘immature’ spNK subset. To test this idea, we sorted spNK cells into two populations: ‘mature’ CD49b+/CD27neg or ‘immature’ (CD49b-/CD27+) spNK cells, labeled them with CFSE, transferred equal numbers i.p. into RAGγcKO mice, and analyzed their phenotype eight days later. We found that the two populations behaved very differently. Whereas the immature NK cells divided extensively, cell division was a rare event among the ‘mature’ CD27neg NK cells (Fig. 5C), suggesting that the peritoneal cavity preferentially allows expansion of the ‘immature’, but not the ‘mature’ spNK cells.
The local environment also had an effect on phenotype. The ‘immature’ spNK cells retained their peNK-like phenotype for the CD49b and CD27 markers, while the ‘mature’ CD49b+/CD27− NK cells down-regulated their expression of CD49b to become more like peNK cells, though they did not reach the complete CD49b-/CD27+ phenotype of the true peNK cell (Fig. 5D). Thus the peritoneal cavity seems to be a location wherein some NK populations (eg. immature NK cells) but not all, can undergo homeostatic expansion, and where even non-dividing mature NK cells are induced to change their phenotype.
There are two reasons why ‘mature’ CD49b+/CD27− spNK cells might not proliferate in the peritoneum. They might be fully differentiated end-stage cells that have little capacity to divide (11), or the peritoneum might not supply the right signals to support their division. To discriminate between these possibilities, we asked if they would divide if they reached the spleen instead of the peritoneum. We sorted ‘mature’ CD49b+/CD27− spNK cells, labeled them with CFSE, injected them i.v. into RAGγcKO mice and looked for homeostatic proliferation in the periphery nine days later. To distinguish any cells that have lost CFSE (because of multiple divisions) from the very few endogenous host NK cells found in the RAGγcKO recipients (Fig. 1A-B, RAGγcKO no transfer control), we used CD45.1 spNK cells and CD45.2 recipients. We found that, in striking contrast to their behavior in the peritoneum, the ‘mature’ CD49b+/CD27− spNK cells underwent extensive division in the spleen and did not increase their expression of CD27, compared to the few endogenous RAGγcKO NK cells, which were distinctly positive (Fig. 5E). Thus, it appears that the ‘mature’ CD49b+/CD27− spNK cells behaved differently in the two different sites; dividing in the spleen but not in the peritoneum.
‘Mature’ CD49b+/CD27− and ’immature’CD49b−/ CD27+ spNK cells differ in their ability to migrate to the Peritoneal Cavity under steady-state conditions
From Fig. 5B, we knew that the ‘mature’ CD49b+/CD27− spNK cells do not divide when placed directly into the peritoneum, suggesting that the peritoneum is not a supportive environment for this population. Yet, from Figure 1B, we knew that a few splenic NK cells given i.v. can populate the peritoneal cavity. Because these cells express the CD49bneg peritoneal phenotype, we surmised that they are more likely to be derived from immigrating immature spNK cells than from the mature population. To test this, we sorted ‘mature’ CD49b+/CD27− or ‘immature’ CD49b−/CD27+ spNK cells from CD45.1 congenic RAGKO mice, transferred them i.v. into RAGγcKO mice, and looked at the spleen and peritoneum eight days later. Figure 6 shows that sorted ‘mature’ CD49b+/CD27− spNK cells did not migrate into the peritoneum, though they did populate the spleen, where they maintained their phenotype. In contrast, the ‘immature’ CD49b− /CD27+ spNK cells migrated to both the spleen and the peritoneum. In the spleen most of them gained CD49b expression, and a few lost CD27, implying that they have the ability to reconstitute the splenic NK cell populations, as previously shown for the CD11b−/CD27+ spNK cells (9, 11). In the peritoneum, however, they remained CD49b−/CD27+, resembling the phenotype of the resident peNK cells. These data show that immature spNK cells have a selective ability to migrate to the peritoneum compared to the mature spNK cells and that, once there, the immature spNK cells don’t follow the same maturation process that they would have done in the periphery.
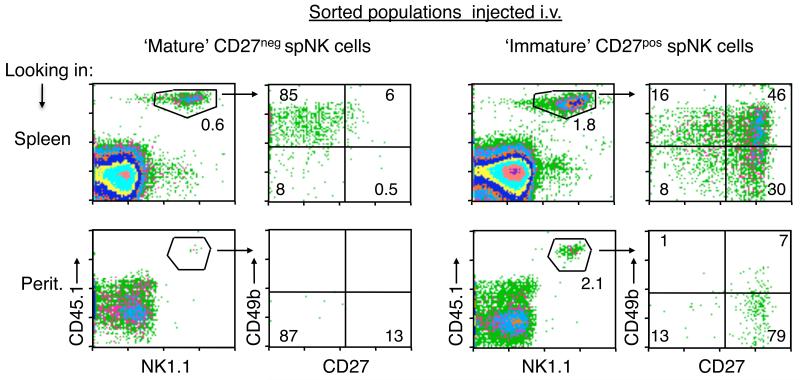
FIGURE 6.
‘Immature’ CD49b−/CD27+ spNK cells but not ‘mature’ CD49b+/CD27− spNK cells repopulate the peritoneal cavity from the periphery under steady-state conditions. 4.5 × 105 sorted ‘mature’ CD27− spNK cells (NK1.1+ CD49b+ CD27−) (left panels) or ‘immature’ CD27+ spNK cells (NK1.1+ CD49b− CD27+) (right panels) carrying the congenic CD45.1 marker were transferred i.v. into CD45.2 RAGγcKO mice. Eight days later, cells from the spleen (upper row) and peritoneum (lower row) were collected and the percentage of CD45.1+/NK1.1+ cells calculated, as well as their expression of CD49b and CD27. Numbers in each quadrant represent the percentage of gated cells that fall within that quadrant. Data represent 2 or more independent experiments with two mice per each group.
It is currently unknown by which mechanism NK cells migrate from the periphery to tissues under steady-state conditions (30). Immature spNK cells and peNK cells are both positive for the G-coupled chemokine receptor CXCR3 (3) and (Fig. 2A). And as Prlic et al (18) have shown that G-coupled receptors are important for NK cell migration into the peritoneum after Vaccinia Virus infection, we hypothesized that the selective migration of immature, rather than mature, NK cells into the peritoneum might be due to their expression of CXCR3 (Fig. 2A). To test this, we first asked if the CXCR3KO mice might lack peNK cells, but found no difference in the number or phenotype of peNK cells with respect to WT mice (Supplemental Fig. 2). We also transferred sorted spleen NK cells from wild type or CXCR3KO mice i.v. into RAGγcKO mice and again found no difference in the number or phenotype of the NK cells that migrated to the peritoneum (Supplemental Fig. 3). These data show that CXCR3 expression in the immature spleen NK cells is not necessary for their migration and retention in the peritoneum, at least under steady state conditions.
Because CD62L is involved in the migration of NK cells to the LN (31), and because peNK cells are largely CD62L positive, this molecule might also have had a role on migration and/or retention of the immature NK cells into the peritoneum. However, 15 days after i.v. transfer of whole spleen NK cells into RAGγcKO mice, we noticed that most of the NK cells found in the peritoneum remained CD62L negative, even though almost half of the NK cells found in the spleens of the same mice were CD62L+ (Supplemental Fig. 3). This is the characteristic phenotype of the ‘immature’ CD49b− /CD27+ spNK cells, suggesting that CD62L expression on NK cells is not required for migration into the peritoneal cavity, nor for their persistence over a period of about two weeks.
We next asked whether the presence of commensal bacteria was necessary for the migration and/or development of peNK cells. To examine this possibility, we looked at the peritoneal lavage and spleens of germ free mice and found no differences, either in the ‘immature’ CD449b−/CD27+ spNK cells or in the peNK cells, between the germ free and the conventionally raised specific pathogen free mice (Supplemental Fig. 4). This shows that the presence of the peNK cell population is not dependent on endogenous microflora.
Discussion
Here we show that the peritoneal cavity is home to a previously uncharacterized resident population of NK cells. At first sight, the lack of CD49b expression together with the expression of CD27, suggested that peritoneal NK cells were related to immature splenic NK cells and/or liver NK cells. Further phenotypic analysis using TRAIL (a characteristic marker of liver NK cells) and the transcription factor EOMES (characteristic of mature splenic NK cells), showed that the immature (CD49b−/CD27+) splenic NK cells actually consist of two subsets, one subset is TRAIL+/EOMES−, while the other is TRAIL−/EOMES+, similar to mature (CD49b+/CD27−) splenic NK cells. We found that liver NK cells share their phenotype with the former, while peritoneal NK cells share their phenotype with the latter (TRAIL−/EOMES+). Thus, although sharing some phenotypic markers with liver NK cells, the peNK cells seem to be a different population of tissue resident NK cells that might be closely related to approximately half of the immature (CD49b−/CD27+) splenic NK cells. The peNK cells were functionally active as they produced cytokines (IFN-γ, GM-CSF and TNF-α) and also killed YAC-1 target cells.
Interestingly, the peritoneal cavity not only has its own characteristic NK cell population but it also influences NK cell behavior, in that it supports the immigration and homeostatic proliferation of the ‘immature’ splenic NK cells, but not of ‘mature’ splenic CD49b+/CD27− NK cells, suggesting that the peritoneal cavity can specifically regulate the entry and division of its resident NK cells. Mature CD49b+/CD27− NK cells did not proliferate when placed directly in the peritoneum although they did not disappear, as we were able to find the undivided population even one month after transfer. The same cells, when injected i.v., followed homeostatic expansion in the spleen. This type of selective regulation is not only seen under steady state conditions, as in our study here, but also in inflammatory states. For example, Prlic et al (18) transferred RAGKO spleen NK cells i.v. with or without an intraperitoneal injection of Vaccinia Virus, and found that large numbers of NK cells entered the peritoneum under the inflammatory conditions, but that they did not divide there as much as those that had migrated to the spleen. Further, in our experiments, ‘mature’ splenic NK cells changed their phenotype when placed directly into the peritoneum, losing some of their markers (CD49b) and approaching a more peritoneal phenotype, though they didn’t divide and never quite attained the fully peritoneal phenotype. Our data, paired with the previous studies, suggest that the peritoneum differentially regulates different NK-cell subsets, both at the level of migration and of proliferation.
Our findings also suggest that experiments based on adoptive transfer of NK cells into NK-empty mice should carefully choose the NK marker used for the sorting or purification of the NK cells, as well as the route of injection. The latter is usually not a problem as long as researchers keep using the intravenous route. However, some of the purification protocols being used today are based on DX5, which recognizes CD49b on NK cells. Transfer of CD49b+ NK cells might not fully reconstitute all populations of tissue resident NK cells: for example in liver (as shown by Takeda et al. when transferring TRAIL− mature spNK cells) or the peritoneal cavity of the host mice. This could have important consequences for the experimental results. The use of a common marker for both mature and immature splenic NK cells (such as NK1.1 or NKp46) for NK-cell purification before the transfer would be allow for a more complete reconstitution of tissue resident NK cells. In addition, there are likely to be tissue-resident NK cells in tissues that have not yet been characterized. The results of NK-cell transfer experiments should take into account that these cells may be missing.
Peritoneal regulation of NK cell populations might have consequences for peritoneal related pathologies. For example, NK cells found in the peritoneum of patients with endometriosis have been found to have lower levels of lytic activity than those of control patients (32). Although this is an inflammatory condition, our finding of a phenotypically new subset of NK cells in the peritoneum suggests that a similar population may also exist in humans. It has been suggested that human peritoneal NK cells help to clear endometrial bodies during the normal phases of menstruation, and the low lytic function may explain why endometrial bodies produced during the menstruation cycle are not cleared from the peritoneal cavity (33). It is possible, therefore, that the human peritoneum also harbors a unique population of NK cells, and that the understanding of their origin and function could lead to better prevention or treatment of endometriosis.
To assess the factors that govern the selective migration of immature NK cells into the peritoneum, we started with the chemokine receptor, CXCR3. Both ‘immature’ CD49b− /CD27+ spleen NK cells and peNK cells express this chemokine receptor, while most of the ‘mature’ CD49b+/CD27− splenic NK cells do not. Further, CXCR3 and its ligand CXCL9, have been identified as one of the factors that increase the recruitment of T and B cells into the peritoneal cavity of mice lacking Mertk (34). We therefore analyzed the peritoneal populations in CXCR3KO mice, and tested the homing ability of CXCR3KO NK cells. These studies showed that expression of CXCR3 is not necessary for the recruitment or the persistence of NK cells in the peritoneum under steady state conditions, although other chemokine receptors might have possible compensatory effects. In addition, the CXCR3KO NK cells that migrated to the peritoneum were negative for CD62L, suggesting that L-selectin is also not necessary. Other authors have found that the production of CXCL13 by specific cells of the peritoneal cavity (such as peritoneal macrophages and omentum cells) is responsible for the recruitment of B1 B cells into the peritoneum through ligation to its receptor CXCR5, which is expressed by peritoneal B1 B cells (35). However, neither the ‘immature’ CD49b−/CD27+ spNK cells nor the peNK cells express CXCR5 (not shown), thus it is not likely to be a major recruiting factor. To test the role of other potential chemokine receptors, we analyzed the splenic and peritoneal NK cell populations of CX3CR1KO, CXCR6KO, and CCR1KO mice. None of these mice differed from WT mice in their populations of immature spleen NK cells and peritoneal NK cells (not shown), showing that none of these receptors have a prominent role in NK cell migration into the peritoneum under steady state conditions. Finally, the presence of the resident peritoneal NK cells does not depend on the presence of microflora, as germ free mice also harbor the same peritoneal NK cell populations as conventional mice, suggesting that the signals involved in the migration and differentiation of the resident peritoneal NK cell populations are intrinsic to the tissue.
To assess a role for S1P receptors on the differential egress of CD4 cells and NK cells from the peritoneum, we treated Marilyn splenocytes (containing both CD4 and NK cells) with their inhibitor, FTY720, injected them i.p. and assessed their ability to exit from the peritoneum. Both FTY720-treated and untreated cells behaved in the same way. The CD4 T cells migrated to the spleen while the NK cells did not (data not shown). The possibility still remains that the S1P receptor family is involved in the trapping of the NK cells in the peritoneum since others have suggested that migration of T and NK cells are regulated by different mechanisms (26). NK cells use mainly the S1P5 receptor and some of the effects triggered by this receptor are not inhibited by FTY720 (26).
In summary, our data reveal a new population of peritoneal NK cells, suggesting that the peritoneal cavity is home to at least three specific subsets of leukocytes: the B1 B cells (14, 15), the newly described Fat-Associated-Lymphoid Clusters FALC cells (16) and the new population of peritoneal NK cells described here. The next task will be to understand the possible relationship between these populations, as well as their role in regulating the health of the peritoneal tissue. Further, it is known that the peritoneal B1 B cells are a self-renewing population that can also contribute to B cell populations in the spleen. Perhaps the peNK cells serve a similar role as a reservoir for the ‘immature’ CD27+ spNK cells.
Supplementary Material
Acknowledgments
We thank the NIAID flow cytometry core facility for its help in the sorting experiments, Xiangdong Liu for technical assistance, Dr. Nevil Singh for valuable discussions and sharing of reagents, and Drs. Giorgio Trinchieri, Jianhua Yu, and Ron Schwartz for critically reading the manuscript.
This work was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. RG was partially funded by the NIAID Office of Training and Diversity’s program to recruit populations underrepresented in biomedical research.
Abbreviations used in this paper
- peNK
peritoneal NK
- spNK
splenic NK
- IO
Ionomycin
Footnotes
Disclosures
The authors declare no competing financial interests.
R.G. current address: Center for Neural Science, New York University, New York, NY 10003.
References
- 1.Vosshenrich CA, Garcia-Ojeda ME, Samson-Villeger SI, Pasqualetto V, Enault L, Richard-Le Goff O, Corcuff E, Guy-Grand D, Rocha B, Cumano A, Rogge L, Ezine S, Di Santo JP. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol. 2006;7:1217–1224. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- 2.Veinotte LL, Halim TY, Takei F. Unique subset of natural killer cells develops from progenitors in lymph node. Blood. 2008;111:4201–4208. doi: 10.1182/blood-2007-04-087577. [DOI] [PubMed] [Google Scholar]
- 3.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 4.Kim S, Iizuka K, Kang HS, Dokun A, French AR, Greco S, Yokoyama WM. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3:523–528. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 5.Croy BA, van den Heuvel MJ, Borzychowski AM, Tayade C. Uterine natural killer cells: a specialized differentiation regulated by ovarian hormones. Immunol Rev. 2006;214:161–185. doi: 10.1111/j.1600-065X.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 6.Yadi H, Burke S, Madeja Z, Hemberger M, Moffett A, Colucci F. Unique receptor repertoire in mouse uterine NK cells. J Immunol. 2008;181:6140–6147. doi: 10.4049/jimmunol.181.9.6140. [DOI] [PubMed] [Google Scholar]
- 7.Di Santo JP. Natural killer cell developmental pathways: a question of balance. Annu Rev Immunol. 2006;24:257–286. doi: 10.1146/annurev.immunol.24.021605.090700. [DOI] [PubMed] [Google Scholar]
- 8.Vosshenrich CA, Samson-Villeger SI, Di Santo JP. Distinguishing features of developing natural killer cells. Curr Opin Immunol. 2005;17:151–158. doi: 10.1016/j.coi.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunol Rev. 2006;214:47–55. doi: 10.1111/j.1600-065X.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 10.Yu J, Wei M, Mao H, Zhang J, Hughes T, Mitsui T, Park IK, Hwang C, Liu S, Marcucci G, Trotta R, Benson DM, Jr., Caligiuri MA. CD94 defines phenotypically and functionally distinct mouse NK cell subsets. J Immunol. 2009;183:4968–4974. doi: 10.4049/jimmunol.0900907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–5496. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 12.Cailhier JF, Partolina M, Vuthoori S, Wu S, Ko K, Watson S, Savill J, Hughes J, Lang RA. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J Immunol. 2005;174:2336–2342. doi: 10.4049/jimmunol.174.4.2336. [DOI] [PubMed] [Google Scholar]
- 13.Liu G, Xia XP, Gong SL, Zhao Y. The macrophage heterogeneity: difference between mouse peritoneal exudate and splenic F4/80+ macrophages. J Cell Physiol. 2006;209:341–352. doi: 10.1002/jcp.20732. [DOI] [PubMed] [Google Scholar]
- 14.Hardy RR, Hayakawa K. CD5 B cells, a fetal B cell lineage. Adv Immunol. 1994;55:297–339. doi: 10.1016/s0065-2776(08)60512-x. [DOI] [PubMed] [Google Scholar]
- 15.Paciorkowski N, Porte P, Shultz LD, Rajan TV. B1 B lymphocytes play a critical role in host protection against lymphatic filarial parasites. J Exp Med. 2000;191:731–736. doi: 10.1084/jem.191.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 17.Godshall CJ, Scott MJ, Burch PT, Peyton JC, Cheadle WG. Natural killer cells participate in bacterial clearance during septic peritonitis through interactions with macrophages. Shock. 2003;19:144–149. doi: 10.1097/00024382-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Prlic M, Gibbs J, Jameson SC. Characteristics of NK cell migration early after vaccinia infection. J Immunol. 2005;175:2152–2157. doi: 10.4049/jimmunol.175.4.2152. [DOI] [PubMed] [Google Scholar]
- 19.Smyth MJ, Kelly JM, Baxter AG, Korner H, Sedgwick JD. An essential role for tumor necrosis factor in natural killer cell-mediated tumor rejection in the peritoneum. J Exp Med. 1998;188:1611–1619. doi: 10.1084/jem.188.9.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin ML, Zhan Y, Nutt SL, Brady J, Wojtasiak M, Brooks AG, Lew AM. NK cells promote peritoneal xenograft rejection through an IFN-gamma-dependent mechanism. Xenotransplantation. 2006;13:536–546. doi: 10.1111/j.1399-3089.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 21.Lantz O, Grandjean I, Matzinger P, Di Santo JP. Gamma chain required for naive CD4+ T cell survival but not for antigen proliferation. Nat Immunol. 2000;1:54–58. doi: 10.1038/76917. [DOI] [PubMed] [Google Scholar]
- 22.Usharauli D, Perez-Diez A, Matzinger P. The JAM Test and its daughter P-JAM: simple tests of DNA fragmentation to measure cell death and stasis. Nat Protoc. 2006;1:672–682. doi: 10.1038/nprot.2006.107. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Diez A, Joncker NT, Choi K, Chan WF, Anderson CC, Lantz O, Matzinger P. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 2007;109:5346–5354. doi: 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. J Exp Med. 2003;197:967–976. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 26.Walzer T, Blery M, Chaix J, Fuseri N, Chasson L, Robbins SH, Jaeger S, Andre P, Gauthier L, Daniel L, Chemin K, Morel Y, Dalod M, Imbert J, Pierres M, Moretta A, Romagne F, Vivier E. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci U S A. 2007;104:3384–3389. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda K, Cretney E, Hayakawa Y, Ota T, Akiba H, Ogasawara K, Yagita H, Kinoshita K, Okumura K, Smyth MJ. TRAIL identifies immature natural killer cells in newborn mice and adult mouse liver. Blood. 2005;105:2082–2089. doi: 10.1182/blood-2004-08-3262. [DOI] [PubMed] [Google Scholar]
- 28.Andrews DM, Smyth MJ. A potential role for RAG-1 in NK cell development revealed by analysis of NK cells during ontogeny. Immunol Cell Biol. 88:107–116. doi: 10.1038/icb.2009.94. [DOI] [PubMed] [Google Scholar]
- 29.Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LH. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 30.Gregoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 32.Oosterlynck DJ, Meuleman C, Waer M, Vandeputte M, Koninckx PR. The natural killer activity of peritoneal fluid lymphocytes is decreased in women with endometriosis. Fertil Steril. 1992;58:290–295. doi: 10.1016/s0015-0282(16)55224-8. [DOI] [PubMed] [Google Scholar]
- 33.Sikora J, Mielczarek-Palacz A, Kondera-Anasz Z. Role of Natural Killer Cell Activity in the Pathogenesis of Endometriosis. Curr Med Chem. 2011;18:200–208. doi: 10.2174/092986711794088416. [DOI] [PubMed] [Google Scholar]
- 34.Williams JC, Wagner NJ, Earp HS, Vilen BJ, Matsushima GK. Increased hematopoietic cells in the mertk−/− mouse peritoneal cavity: a result of augmented migration. J Immunol. 184:6637–6648. doi: 10.4049/jimmunol.0902784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16:67–76. doi: 10.1016/s1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.