Abstract
Store-operated Ca2+ entry (SOCE) has recently been shown to be of physiological and pathological importance in the heart, particularly during cardiac hypertrophy. However, measuring changes in intracellular Ca2+ during SOCE is very difficult to study in adult primary cardiomyocytes. As a result there is a need for a stable and reliable in vitro model of SOCE which can be used to test cardiac drugs and investigate the role of SOCE in cardiac pathology. HL-1 cells are the only immortal cardiomyocyte cell line available that continuously divides and spontaneously contracts while maintaining phenotypic characteristics of the adult cardiomyocyte. To date the role of SOCE has not yet been investigated in the HL-1 cardiac cell line. We report for the first time that these cells express stromal interaction molecule 1 (STIM1) and the Ca2+ release-activated Ca2+ (CRAC) channel Orai1, which are essential components of the SOCE machinery. In addition, SOCE is tightly coupled to sarcoplasmic reticulum (SR)-Ca2+ release in HL-1 cells, and such response was not impaired in the presence of voltage dependent Ca2+ channels (L-type and T-type channels) or reverse mode Na+/ Ca2+ exchanger (NCX) inhibitors. We were able to abolish the SOCE response with known SOCE inhibitors (BTP-2 and SKF-96365) and by targeted knockdown of Orai1 with RNAi. In addition, knockdown of Orai1 resulted in lower baseline Ca2+ and an attenuated response to thapsigargin (TG) and caffeine, indicating that SOCE may play a role in Ca2+ homeostasis during unstressed conditions in cardiomyocytes. Currently, there is little knowledge about SOCE in cardiomyocytes, and the present results suggest that HL-1 cells will be of great utility in investigating the role of SOCE in the heart.
Keywords: Store-operated calcium entry, cardiomyocytes, HL-1 cells, Stim, Orai
Introduction
Store-operated calcium entry (SOCE) represents a pathway for calcium (Ca2+) entry that is activated by a release of endoplasmic/sarcoplasmic reticulum (ER/SR) Ca2+. Reduced ER/SR Ca2+ triggers the formation of subplasmalemmal clusters of the SR protein, stromal interacting molecule 1 (STIM1) [1; 2]. Thereby, STIM1 couples to the plasma membrane channel pore-forming subunit of the Ca2+ release-activated Ca2+ (CRAC) channel protein, Orai1 [1]. The interaction between STIM1 and Orai1 allows Ca2+ entry upon release of SR- Ca2+ [3; 4; 5].
While the role of SOCE has been shown to be important in non-excitable cell types, in striated muscle, particularly cardiac muscle, SOCE has not been extensively studied. Uehara et al. [6] were the first to show that SOCE was present in embryonic and neonatal myocytes. Later, Hunton et al. [7] clearly demonstrated that SOCE was also present in adult cardiomyocytes via Ca2+ imaging. More recently, the molecular machinery that mediates SOCE has been shown to contribute to both physiological and pathological cardiac hypertrophy [7; 8; 9; 10]. However, since the work of Hunton et al. [7], SOCE has not been demonstrated using Ca2+ imaging in adult cardiomyocytes.
While the use of adult primary myocytes is ideal, there are several problems with using them to measure SOCE. Primary myocytes can become overgrown by non-myocytes after a few days in culture, they do not divide after the neonatal period, genetic manipulation is difficult [11; 12], and they lose their phenotype in long-term culture. In all cases, testing adult primary myocytes in a typical fashion for SOCE (i.e. removing and replacing extracellular Ca2+) is complicated by the fact that myocytes tend to contract and die. In addition to these factors, it is necessary to use pharmacological inhibitors of contraction, 2,3-butanedione monoxime (BDM) or N-benzyl-p-toluenesulfonamide (BTS), which alter Ca2+ regulation [13; 14; 15]. The cumulative effect of these factors makes the study of SOCE in primary cardiomyocytes technically challenging and more difficult to interpret. To avoid these problems, groups studying SOCE have avoided Ca2+ imaging in adult primary cardiomyocytes. Although embryonic and neonatal primary cardiomyocytes are relatively easy to isolate, their utility is limited because they lack many of the characteristics of adult cardiomyocytes. Thus, HL-1 cells are a useful surrogate for studying SOCE because they display spontaneous electrophysiological and mechanical activity, cyclic Ca2+ movements [16; 17], maintain a differentiated cardiac phenotype, and are resilient to death from the Ca2+ paradox.
Therefore, because of the increasing importance of SOCE in cardiac tissue and the difficulty in studying these Ca2+ currents in primary adult cardiomyocytes, we sought to test the hypothesis that the HL-1 cell line would exhibit SOCE. Furthermore, it is currently unknown what role SOCE plays in maintaining Ca2+ homeostasis at rest and thus we sought to determine if Orai1 was important in maintaining intracellular Ca2+ levels in unstressed cardiomyocytes.
Materials and Methods
Cell Culture
HL-1 cardiomyocytes were cultured in Claycomb media (Sigma-Aldrich) as previously described [18].
RT-PCR
Total RNA from HL-1 cells was extracted by use of the RNeasy Fibrous Tissue Kit (QIAGEN). Reverse transcriptase polymerase chain reaction (RT-PCR) was performed on mRNA isolated from cells following the protocol of the TaqMan RNA-to CT 1-step kit (ABI). STIM1 (ABI; Mm00486423_m1) and Orai1 (ABI; Mm00774349_m1) primer sets span an exon-exon junction and are expected to yield a product size of 140 and 150 bp, respectively. PCR products were run on a 1.8% ethidium bromide agarose gel and visualized by use of a commercially available UV system (GE).
Western Blot
Protein extraction, quantification, and subsequent Western blotting using STIM1 (BD Laboratories) and Orai1 (Millipore) antibodies were performed as previously described [18].
Calcium Imaging
Prior to experiments (48–72 h), HL-1 cells were washed twice with PBS and plated on 35 mm optical dishes (5.0×103). Calcium imaging using the Ca2+ indicator dye, fura-2-AM (Invitrogen) (2 μM) in 0.025% Pluronic F-127 was performed on attached populations of HL-1 cells as previously described [18; 19]. To test for SOCE, plates were perfused with 10 μM thapsigargin (TG) (AG Scientific) in HBSS (0 mM Ca2+) to trigger SR Ca2+ release. At the peak of the TG response, plates were perfused with caffeine (Caff; 10 mM) in HBSS (0 mM Ca2+) to test if the RyR-sensitive Ca2+ pool was depleted. SOCE was triggered upon reperfusion with HBSS (1.8 mM Ca2+) and myocytes were tested at the conclusion of the experiment with KCl (80 mM). Data was only included if the KCl response was greater than a 100% increase above the baseline fluorescence. In the next series, plates were pre-treated with 10 μM of SOCE inhibitors (BTP-2 or SKF-96365; EMD Chemicals) 10 min before application of TG and Caff. In the last series of experiments, cells were pre-treated for 10 min with 10 μM Verapamil (Sigma-Aldrich) (Verap) to block L-type Ca2+ channels, 10 μM Mibefradil (Sigma-Aldrich) (Mib) to block T-type Ca2+ channels, or 5 μM SN-6 (Tocris Biosciences) to block the reverse mode of the NCX prior to testing for SOCE. Mean data are presented as the peak increase in ratiometric fluorescence after treatment. All diluted drugs were carefully perfused to the plates at a rate of 0.3 ml/min. Six plates were tested per experimental treatment. Each plate represents the average ratiometeric changes from 10–15 cells. The averages from these 6 plates were used for statistical analysis.
Plasmid Construction
A plasmid expressing a small hairpin (sh)RNA probe targeting the mouse Orai1 mRNA (NM_175423) was produced by subcloning two complimentary oligonucleotide sequences into a custom-made pU6r-RFP vector containing a monomeric red fluorescence protein (RFP) expression cassette with its own constitutive promoter used to identify transfected cells [20]. The sequences of oligonucleotides used were:
Sense: 5’-GATCGTCCTGGCGCAAGCTCTACTTAATTCAAGAGATTAAGTAGAGCTTGCGCCAGGACTTTTTT-3
Antisense: 5’-AATTAAAAAAGTCCTGGCGCAAGCTCTACTTAATCTCTTGAATTAAGTAGAGCTTGCGCCAGGAC-3’
Transfection
HL-1 cells were plated on 35mm dishes (4×105) in 1 ml of Claycomb Medium (without norepinephrine and penicillin/streptomycin). Cells were transfected with lipofectamine according to the manufacturer’s protocol (Invitrogen).
Electrophysiology
HL-1 cells were lightly trypsinized, suspended in HBSS, and aliquots placed in the well of a laminar perfusion chamber. Cells were superfused at 1 ml/min with bath buffer (in mM: 140 N-methyl-D-glucamine, 10 BaCl, 2 MgCl2, 10 glucose, 10 HEPES with pH adjusted to 7.4 using HCl). Micropipettes (3–5 MΩ resistance) were filled with pipette buffer (in mM: 140 CsCl, 1.0 MgCl2, 10 EGTA: ~6×10−15 M free Ca2+, and 10 HEPES) with pH adjusted to 7.2 using CsOH). Whole-cell patch clamping was performed as described previously [21].
Statistical Analysis
Statistical procedures were performed using Graphpad Prism 5.0. Data are presented as means ± SEM. Data were compared using either a paired t-test or one-way ANOVA where appropriate. Significance was set at the P = 0.05 level. When necessary the one-way ANOVA was followed up with post hoc testing using Dunnetts multiple comparison test.
Results
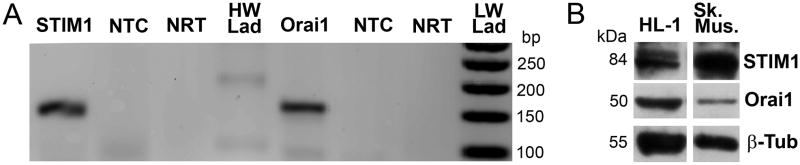
We first investigated the expression of two key components of SOCE in HL-1 cardiomyocytes. Fig. 1a demonstrates positive detection of both STIM1 and Orai1 mRNA (n=3) and Fig. 1b demonstrates positive detection of both STIM1 and Orai1 proteins from HL-1 cardiomyocyte protein extracts as well as from a positive control, adult mouse skeletal muscle (n=3).
Figure 1. STIM1 and Orai1 are expressed in HL-1 cardiomyocytes.
Panel A, RT-PCR was conducted using RNA isolated from HL-1 cardiomyocytes with the use of primers designed for mouse STIM1 and Orai1 (expected sizes, 140 and 150 bp respectively). There was no significant amplification in the no-template control (NTC) or the no-reverse transcriptase (NRT; Taq polymerase only) samples (HW Lad= 100 bp ladder; LW Lad= 50 bp ladder). Panel B, Western blot using STIM1, Orai1, and β-tubulin antibodies and total protein isolated from HL-1 cardiomyocytes as well as a positive control (skeletal muscle).
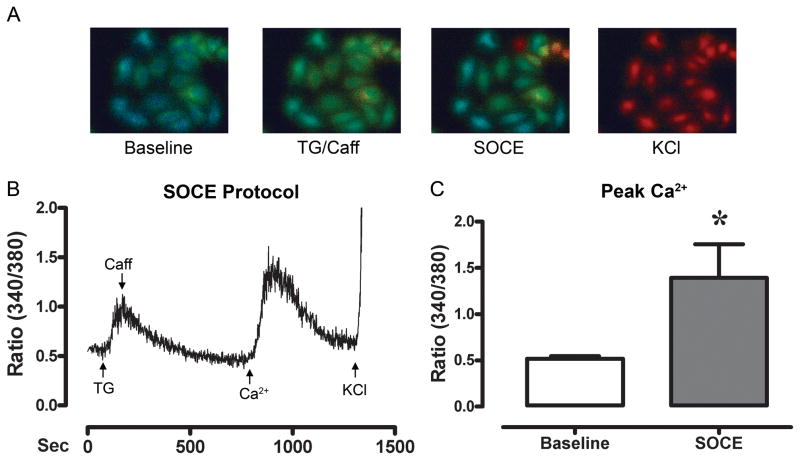
To test for the activation of SOCE, calcium imaging was conducted in the HL-1 cardiomyocytes (Fig. 2a). Fig. 2b shows a raw tracing of the ratiometric Ca2+ response of a HL-1 cell. Treatment of HL-1 cells with TG slowly elevated the peak cytosolic Ca2+ which then returned to baseline, indicating Ca2+-depletion. Reperfusion with Ca2+, elevated (p<0.05) the peak cytosolic Ca2+ above baseline suggesting that HL-1 cells may exhibit SOCE. Fig. 2c shows the average ratiometric Ca2+ level at baseline, and the peak SOCE response following Ca2+-depletion for all experiments (n=6).
Figure 2. SOCE is present and activated following SR-Ca2+ depletion in HL-1 cardiomyocytes.
Panel A, ratiometric fluorescent images of HL-1 cardiomyocytes starting at baseline (perfusion with 0 mM Ca2+), at the peak ratiometric response to TG (10 μM) and Caff (10 mM), at the peak of SOCE following reperfusion with Ca2+ (1.8 mM), and at the peak KCl (80 mM) response. Panel B, a raw tracing of the ratiometric fluorescent measurements of a HL-1 cell during testing for SOCE. Treatment of cells with TG (10 μM, in 0mM Ca2+) increased intracellular Ca2+. Following a return to baseline, the cells were perfused with Ca2+ (1.8 mM), resulting in SOCE. Panel C, shows the average change in ratiometric fluorescence following SR-depletion and subsequent reperfusion with Ca2+ to initiate SOCE (n=6 experiments). * indicates a significant increase.
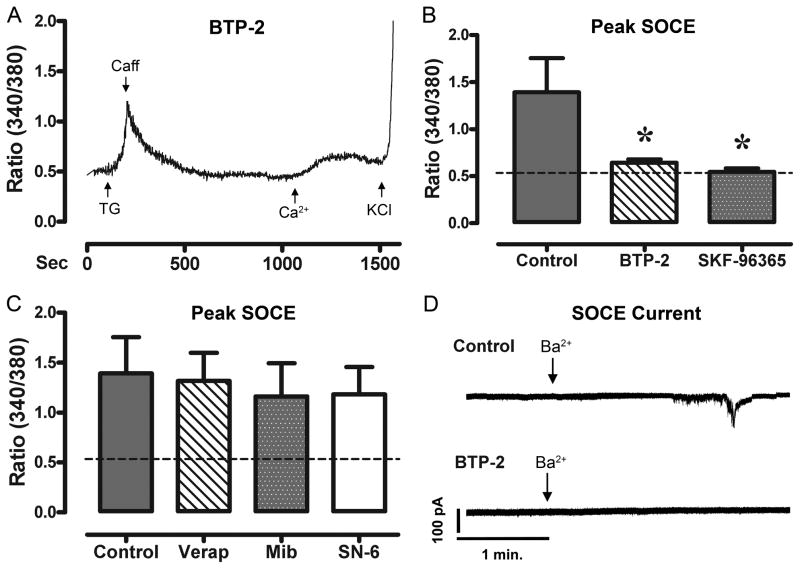
To determine if the Ca2+ entry seen following Ca2+-depletion was regulated via SOCE channels, we pre-treated HL-1 cardiomyocytes with known inhibitors of SOCE. Fig. 3a shows a raw tracing of the ratiometric Ca2+ response of a HL-1 cell that was pre-treated with BTP-2. BTP-2 and SKF-96365 prevented a SOCE response (p<0.01) following Ca2+-depletion when compared to control. Fig. 3b shows the average ratiometric Ca2+ level during SOCE in untreated HL-1 cells, as well as HL-1 cells pre-treated with BTP-2 and SKF-96365 (n=6/treatment). The dotted line represents the average baseline ratiometric Ca2+ level for these cells. Treatment of HL-1 cardiomyocytes with BTP-2 and SKF-96365 had no effect on the TG response, eliminating the possibility of reduced Ca2+ entry due to reduced Ca2+-release. Further, to ensure that this Ca2+ entry did not arise from voltage sensitive Ca2+ channels or NCX, we conducted concurrent experiments pre-treating cells with Verap (L-type inhibitor), Mib (T-type inhibitor), and SN-6 (NCX inhibitor). None of these drugs reduced Ca2+ entry following Ca2+-depletion, as compared to control (Fig. 3c). Fig. 3d shows a raw trace of a HL-1 cell during a whole cell patch clamp experiment. HL-1 cells were pretreated with TG 10 min prior to patching. Cells were voltage-clamped at −100 mV to inactivate L-type and T-type Ca2+ channels in a Na+ and Ca2+ free bath buffer. Perfusion with extracellular Ba2+ (Ca2+ surrogate) caused an inward current (8.70 ± 2.8 pA/pF, n=7 cells) carried by Ba2+, which was undetectable following pre-incubation with BTP-2.
Figure 3. SOCE is effectively blocked by selective SOCE inhibitors.
Panel A, shows a raw tracing of the ratiometric fluorescent measurements of a HL-1 cell pretreated and continually perfused with BTP-2 (10 μM). Treatment of cells with TG (10 μM, in 0 mM Ca2+) created a large accumulation of Ca2+ in the cells. Following a return to baseline, the cells were perfused with Ca2+ (1.8 mM), and showed minimal Ca2+ entry, until stimulated with KCl. Panel B, shows the average change in ratiometric Ca2+ entry following SR-stimulation and reperfusion with Ca2+ (n=6 experiments) in HL-1 cells pre-treated with BTP-2 and SKF-96365. Panel C, shows the average ratiometric fluorescence following SR-stimulation and subsequent reperfusion with Ca2+ (n=6 experiments) in HL-1 cells pre-treated with Verap, Mib, and SN-6. Panel D, shows a raw tracing from a whole cell patch clamp experiment with HL-1 cardiomyocytes. Cells were voltage clamped at -100 mv and then perfused with Ba2+ (arrow; 1.8 mM). We were able to detect a small inward Ba2+ current (n=5 experiments), which was completely eliminated by pre-incubation of BTP-2 (n=5 experiments). Dashed lines represent the average baseline Ca2+ levels of these cells. * indicates significant reduction from control.
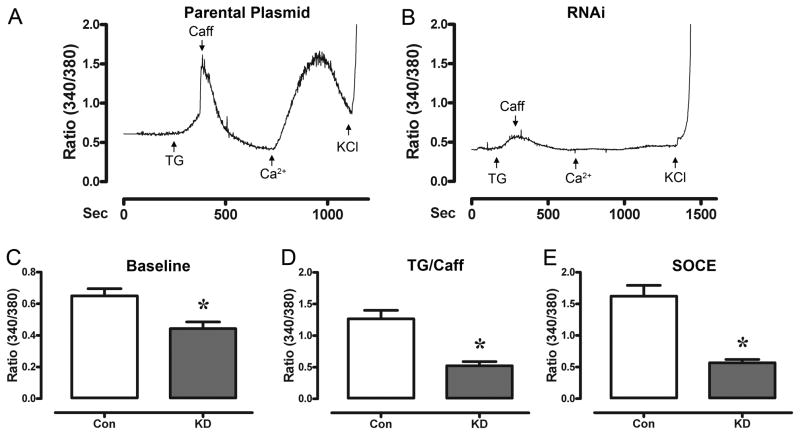
To confirm our pharmacological and electrophysiological data showing the inhibition of SOCE in HL-1 cells, we used RNA interference (RNAi) to suppress the expression of Orai1. RT-PCR experiments using RNA from all cells treated for targeted knockdown of Orai1 demonstrated cells collectively reduced (p<0.05) Orai1 mRNA by 45% (0.55 ± 0.01) 48 hr following transfection when compared to the parental plasmid (1.00 ± 0.1) (n=2 plates/treatment). We also investigated the effect of Orai1 knockdown on STIM1 expression 48 hr following transfection and found that neither the parental plasmid nor the targeted Orai1 plasmid had any effect on the expression of STIM1 (p=0.56). Only transfected cells (identified by the expression of red fluorescent protein) were used for Ca2+ imaging 96 hr following transfection. Fig 4A presents a raw tracing of a HL-1 cell treated with the parental plasmid. The transfection procedure itself did not impair normal Ca2+ handling of HL-1 cardiomyocytes as the baseline ratiometric Ca2+ levels (0.64 ± 0.04), as well as the SOCE responses (1.62 ± 0.17) of the parental plasmid were not statistically different from HL-1 cells that did not undergo transfection (baseline: 0.52 ± 0.03 and SOCE: 1.40 ± 0.36). Fig 4B presents a raw tracing of the same SOCE experiment performed in a HL-1 cell targeted for Orai1 knockdown. We investigated the effect of Orai1 knockdown on resting Ca2+. Baseline Ca2+ levels in the Orai1 knockdown cells were lower (p<0.01) than parental plasmid controls (Fig 4C). The average TG/Caff response from HL-1 cells treated with Orai1 plasmid were also lower (P<0.01) than parental plasmid controls (Fig 4D). Furthermore, the average SOCE response of HL-1 cardiomyocytes targeted for Orai1 knockdown was inhibited (p<0.01) when compared to cells transfected with the parental plasmid (Fig 4E).
Figure 4. SOCE is abolished following Orai1 knockdown (KD).
Panel A, shows a raw tracing of the ratiometric fluorescent measurements of a HL-1 cell transfected with the parental plasmid (48 hr) during testing for SOCE. Treatment of cells with TG (10 μM, in 0mM Ca2+) created a large accumulation of Ca2+in the cells. Following a return to baseline, the cells were perfused with Ca2+ (1.8 mM), resulting in SOCE. Panel B, a raw tracing of the ratiometric fluorescent measurements of a HL-1 cell targeted for Orai1 KD (48 hr) during testing for SOCE. Note that SOCE is inhibited. Panel C, shows the average ratiometric fluorescence at baseline. HL-1 cells targeted for Orai1 knockdown had lower baseline levels of Ca2+ (n=6 experiments) when compared to control (parental plasmid). Panel D, shows the average ratiometric fluorescence following treatment with TG and Caff. The basal Ca2+ levels and SR-stimulated release of Ca2+ were significantly inhibited in HL-1 cardiomyoctes targeted for Orai1 KD. Panel E, shows the average ratiometric fluorescence following treatment with TG and reperfusion with Ca2+ in HL-1 cells targeted for Orai1 knockdown. n=6 experiments; * indicates significant reduction from control (parental plasmid).
Discussion
This is the first study to examine the role of SOCE in the HL-1 cardiomyocyte cell line and to investigate the effects of Orai1 expression on baseline Ca2+ levels. In the past few years, there has been a focus on the role of SOCE in excitable tissues, particularly skeletal [22; 23] and cardiac muscle [7; 9; 24; 25]. In the cardiomyocyte, intracellular Ca2+ functions as an important signal to regulate muscle contraction, apoptosis, proteolysis and hypertrophy. Recently, the molecular machinery of SOCE and the CRAC current has been elucidated with the discovery of STIM1 [26] and Orai1 [10]. Using RT-PCR and western blotting we found that both of these essential components of the molecular machinery are present in HL-1 cells. Our results are in agreement with previous research which has shown that STIM1 and Orai1 channels are present in primary cardiomyocytes [8; 9; 10; 27].
HL-1 cells showed significant intracellular Ca2+ accumulation following TG treatment and subsequent extracellular Ca2+ entry following perfusion with Ca2+. We verified that the Ca2+ refilling was truly an SOCE current through pharmacological blockade and genetic inhibition of Orai1. Furthermore, SOCE was specifically responsible for the Ca2+ current as blockade of L-Type channels, T-type channels and the NCX inhibitors did not attenuate the SOCE current. These results are in agreement with previous research in testing SOCE in adult cardiomyocytes [7]. To extend our findings beyond Ca2+ imaging, a similar experiment was performed with whole cell patch clamping. After treatment with TG, perfusion with extracellular Ba2+ (Ca2+ surrogate) caused an inward current carried by Ba2+, which was eliminated by pre-incubation with BTP-2. This provides further support that HL-1 cells exhibit SOCE. These results agree with the limited reports of SOCE in patch-clamped primary neonatal cardiomyocytes [24; 28].
While SOCE has been shown to play a role in cardiac hypertrophy [8; 9; 10; 28], no studies have determined if SOCE chronically regulates resting levels of intracellular Ca2+. Interestingly, cells identified with Orai1 knockdown had substantially reduced baseline Ca2+ levels, as well as a reduced response to TG/Caff. These effects on basal SR-Ca2+ stores were not noted in the parental plasmid (control) or cells acutely treated (10min) with SKF-96365 and BTP-2, suggesting that they are directly linked with chronically impaired SOCE. These data suggest that SOCE channel Orai1 helps maintain the resting levels of SR-Ca2+ in unstressed cardiomyocytes. In cardiomyocytes the fluctuations in free cytosolic Ca2+ serve as a potent regulator of signal transduction, physiological growth and cardiac contractility. Currently it is thought that SOCE may not play an important role in cardiac function under normal physiological conditions [24]. A similar dogma downplaying the importance of SOCE was previously held in skeletal muscle physiology. Now SOCE is considered as an important regulator of muscle contractility and fatigability of skeletal muscle [20]. Our data begin to challenge this dogma and we suggest that SOCE, particularly with regard to Orai1, plays an important role in maintaining intracellular Ca2+ in cardiac cells on a beat to beat basis.
In summary, we believe that HL-1 cells are an ideal model to circumvent most of the difficulties encountered while studying SOCE in primary cardiomyocytes. HL-1 cells have been used as a model system for cellular signaling, electrophysiology, Ca2+ handling [17], and hypertrophy [29; 30]. Using this model, we show for the first time that HL-1 cardiomyocytes exhibit SOCE, and that SOCE (via Orai1) is involved in maintaining baseline Ca2+. Given the emerging role of SOCE in mediating cardiac function during health and disease, we feel HL-1 cells may serve as an important model to help examine the role of SOCE in cardiomyocytes.
Highlights.
SOCE is of physiological and pathological importance in the heart.
Investigators need a model to investigate [Ca2+]i during SOCE in the heart.
HL-1 cardiomyocytes express Orai1/Stim1 and exhibit SOCE.
SOCE plays a role in maintaining resting Ca2+ homeostasis via Orai1.
Acknowledgments
This research was supported by grants from the American Heart Association to MW (11SDG5330016), MB (0535555N), XZ (10SDG2630086), and JA (0735053N). NW was supported by NIH/NIAMS (AR054793). WC was supported by grants from NIH (HL076498) and the Joe W. and Dorothy Brown Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malli R, Naghdi S, Romanin C, Graier WF. Cytosolic Ca2+ prevents the subplasmalemmal clustering of STIM1: an intrinsic mechanism to avoid Ca2+ overload. J Cell Sci. 2008;121:3133–9. doi: 10.1242/jcs.034496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mullins FM, Park CY, Dolmetsch RE, Lewis RS. STIM1 and calmodulin interact with Orai1 to induce Ca2+-dependent inactivation of CRAC channels. Proc Natl Acad Sci U S A. 2009;106:15495–500. doi: 10.1073/pnas.0906781106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–5. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 5.Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–3. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uehara A, Yasukochi M, Imanaga I, Nishi M, Takeshima H. Store-operated Ca2+ entry uncoupled with ryanodine receptor and junctional membrane complex in heart muscle cells. Cell Calcium. 2002;31:89–96. doi: 10.1054/ceca.2001.0257. [DOI] [PubMed] [Google Scholar]
- 7.Hunton DL, Zou L, Pang Y, Marchase RB. Adult rat cardiomyocytes exhibit capacitative calcium entry. Am J Physiol Heart Circ Physiol. 2004;286:H1124–32. doi: 10.1152/ajpheart.00162.2003. [DOI] [PubMed] [Google Scholar]
- 8.Hulot JS, Fauconnier J, Ramanujam D, Chaanine A, Aubart F, Sassi Y, Merkle S, Cazorla O, Ouille A, Dupuis M, Hadri L, Jeong D, Muhlstedt S, Schmitt J, Braun A, Benard L, Saliba Y, Laggerbauer B, Nieswandt B, Lacampagne A, Hajjar RJ, Lompre AM, Engelhardt S. Critical role for stromal interaction molecule 1 in cardiac hypertrophy. Circulation. 2011;124:796–805. doi: 10.1161/CIRCULATIONAHA.111.031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohba T, Watanabe H, Murakami M, Sato T, Ono K, Ito H. Essential role of STIM1 in the development of cardiomyocyte hypertrophy. Biochem Biophys Res Commun. 2009;389:172–6. doi: 10.1016/j.bbrc.2009.08.117. [DOI] [PubMed] [Google Scholar]
- 10.Voelkers M, Salz M, Herzog N, Frank D, Dolatabadi N, Frey N, Gude N, Friedrich O, Koch WJ, Katus HA, Sussman MA, Most P. Orai1 and Stim1 regulate normal and hypertrophic growth in cardiomyocytes. J Mol Cell Cardiol. 2010;48:1329–34. doi: 10.1016/j.yjmcc.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claycomb WC. Long-term culture and characterization of the adult ventricular and atrial cardiac muscle cell. Basic Res Cardiol. 1985;80(Suppl 2):171–4. [PubMed] [Google Scholar]
- 12.Claycomb WC, Lanson N., Jr Isolation and culture of the terminally differentiated adult mammalian ventricular cardiac muscle cell. In Vitro. 1984;20:647–51. doi: 10.1007/BF02619615. [DOI] [PubMed] [Google Scholar]
- 13.Fryer MW, Gage PW, Neering IR, Dulhunty AF, Lamb GD. Paralysis of skeletal muscle by butanedione monoxime, a chemical phosphatase. Pflugers Arch. 1988;411:76–9. doi: 10.1007/BF00581649. [DOI] [PubMed] [Google Scholar]
- 14.Lang RJ, Paul RJ. Effects of 2,3-butanedione monoxime on whole-cell Ca2+ channel currents in single cells of the guinea-pig taenia caeci. J Physiol. 1991;433:1–24. doi: 10.1113/jphysiol.1991.sp018411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung A, Dantzig JA, Hollingworth S, Baylor SM, Goldman YE, Mitchison TJ, Straight AF. A small-molecule inhibitor of skeletal muscle myosin II. Nat Cell Biol. 2002;4:83–8. doi: 10.1038/ncb734. [DOI] [PubMed] [Google Scholar]
- 16.Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A. 1998;95:2979–84. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White SM, Constantin PE, Claycomb WC. Cardiac physiology at the cellular level: use of cultured HL-1 cardiomyocytes for studies of cardiac muscle cell structure and function. Am J Physiol Heart Circ Physiol. 2004;286:H823–9. doi: 10.1152/ajpheart.00986.2003. [DOI] [PubMed] [Google Scholar]
- 18.Wacker MJ, Kosloski LM, Gilbert WJ, Touchberry CD, Moore DS, Kelly JK, Brotto M, Orr JA. Inhibition of thromboxane A2-induced arrhythmias and intracellular calcium changes in cardiac myocytes by blockade of the inositol trisphosphate pathway. J Pharmacol Exp Ther. 2009;331:917–24. doi: 10.1124/jpet.109.157677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Touchberry CD, Bales IK, Stone JK, Rohrberg TJ, Parelkar NK, Nguyen T, Fuentes O, Liu X, Qu CK, Andresen JJ, Valdivia HH, Brotto M, Wacker MJ. Phosphatidylinositol 3,5-bisphosphate (PI(3,5)P2) potentiates cardiac contractility via activation of the ryanodine receptor. J Biol Chem. 2010 doi: 10.1074/jbc.M110.179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao X, Min CK, Ko JK, Parness J, Kim do H, Weisleder N, Ma J. Increased store-operated Ca2+ entry in skeletal muscle with reduced calsequestrin-1 expression. Biophys J. 2010;99:1556–64. doi: 10.1016/j.bpj.2010.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parelkar NK, Silswal N, Jansen K, Vaughn J, Bryan RM, Jr, Andresen J. 2,2,2-trichloroethanol activates a nonclassical potassium channel in cerebrovascular smooth muscle and dilates the middle cerebral artery. J Pharmacol Exp Ther. 2010;332:803–10. doi: 10.1124/jpet.109.162313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin DM, Muallem S. Skeletal muscle dressed in SOCs. Nat Cell Biol. 2008;10:639–41. doi: 10.1038/ncb0608-639. [DOI] [PubMed] [Google Scholar]
- 23.Zhao X, Weisleder N, Thornton A, Oppong Y, Campbell R, Ma J, Brotto M. Compromised store-operated Ca2+ entry in aged skeletal muscle. Aging Cell. 2008;7:561–8. doi: 10.1111/j.1474-9726.2008.00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, van Breemen C, Kuo KH, Hove-Madsen L, Tibbits GF. Store-operated Ca2+ entry modulates sarcoplasmic reticulum Ca2+ loading in neonatal rabbit cardiac ventricular myocytes. Am J Physiol Cell Physiol. 2006;290:C1572–82. doi: 10.1152/ajpcell.00226.2005. [DOI] [PubMed] [Google Scholar]
- 25.Ohba T, Watanabe H, Murakami M, Takahashi Y, Iino K, Kuromitsu S, Mori Y, Ono K, Iijima T, Ito H. Upregulation of TRPC1 in the development of cardiac hypertrophy. J Mol Cell Cardiol. 2007;42:498–507. doi: 10.1016/j.yjmcc.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–45. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-hora M, Neems DS, Hogan PG, Rao A. Biochemical and functional characterization of Orai proteins. J Biol Chem. 2007;282:16232–43. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- 28.Hunton DL, Lucchesi PA, Pang Y, Cheng X, Dell'Italia LJ, Marchase RB. Capacitative calcium entry contributes to nuclear factor of activated T-cells nuclear translocation and hypertrophy in cardiomyocytes. J Biol Chem. 2002;277:14266–73. doi: 10.1074/jbc.M107167200. [DOI] [PubMed] [Google Scholar]
- 29.Brunt KR, Tsuji MR, Lai JH, Kinobe RT, Durante W, Claycomb WC, Ward CA, Melo LG. Heme oxygenase-1 inhibits pro-oxidant induced hypertrophy in HL-1 cardiomyocytes. Exp Biol Med (Maywood) 2009;234:582–94. doi: 10.3181/0810-RM-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandrasekar B, Mummidi S, Claycomb WC, Mestril R, Nemer M. Interleukin-18 is a pro-hypertrophic cytokine that acts through a phosphatidylinositol 3-kinase-phosphoinositide-dependent kinase-1-Akt-GATA4 signaling pathway in cardiomyocytes. J Biol Chem. 2005;280:4553–67. doi: 10.1074/jbc.M411787200. [DOI] [PubMed] [Google Scholar]