Abstract
Purpose
To determine if transcutaneous electrical stimulation of somatic afferent nerves in the foot of cats can induce a post-stimulation increase in bladder capacity.
Materials and Methods
In α-chloralose anesthetized cats (N=12) electrical stimulation (5 Hz) was applied to the skin of the hind foot for two periods of 30 minutes via dual pad electrodes attached on the plantar and dorsal surfaces (combination 1-2) or at two sites on the plantar surface (combination 1-3). The post-stimulation effect was examined by performing repeated CMGs following 30 minute stimulation. In the control group (N=12) the isovolumetric contractions were allowed to continue during each 30 minute period without stimulation.
Results
Stimulation inhibited isovolumetric rhythmic bladder contractions. The bladder capacity was not increased after the first 30 minute foot stimulation via electrode combination 1-2, but was significantly increased 47.5±2.9% after the second 30 minute stimulation via electrode combination 1-3. After inducing the post-stimulation effect, the foot stimulation applied during CMGs via electrode combinations 1-2 or 1-3 elicited a further increase in bladder capacity (23.26±17.64% and 20.07±18.59% respectively).
Conclusions
This study shows that the transcutaneous plantar electrical stimulation of somatic afferent nerves in the foot can induce a post-stimulation increase in bladder capacity, suggesting that an intermittent stimulation pattern rather than a continuous stimulation might be effective in clinical applications to treat overactive bladder symptoms.
Keywords: bladder, foot, cat, electrical stimulation, transcutaneous
Introduction
Overactive bladder symptoms including detrusor overactivity, urinary frequency, urgency, and incontinence are often difficult to manage with medications.1 It is known that electrical stimulation of somatic afferent pathways in the pudendal nerve,2-5 posterior tibial nerve,6,7 or sacral spinal roots8,9 can inhibit bladder activity in both humans and animals, and is clinically effective in treating overactive bladder symptoms. However, sacral and pudendal neuromodulation is invasive and requires surgery to implant an electrical stimulator and an electrode (InterStim®, Medtronic Inc., Minneapolis, MN, USA).2,3,8,9 On the other hand percutaneous tibial nerve neuromodulation is minimally invasive only requiring the insertion of a needle electrode at the ankle to target the tibial nerve, but it requires frequent clinical visits. Following the initial treatment of 30 minute tibial nerve stimulation once per week for 12 weeks, a maintenance treatment of once every 2-3 weeks is usually required.7,10-16 Percutaneous tibial nerve neuromodulation also requires trained medical staff to insert the needle electrode close to the nerve. More recently a multicenter study17 has shown that transcutaneous tibial nerve neuromodulation using adhesive skin surface electrodes placed at the ankle is effective in the treatment of overactive bladder due to multiple sclerosis when the stimulation was applied 20 minutes per day by the patients at home. A non-invasive neuromodulation method easily manageable at home will be more accessible to overactive bladder patients.
Our previous study in cats18 demonstrated a non-invasive electrical stimulation method to inhibit bladder overacivity by activating somatic afferent nerves using electrodes attached to the skin surface of the foot. This stimulation method should be easily manageable by the patients due to its non-invasiveness. However, our previous study18 only revealed an acute inhibitory effect of foot stimulation on reflex bladder activity; and did not investigate whether foot stimulation can induce a long-lasting post-stimulation inhibition similar to the tibial nerve neuromodulation.7,1-16 Thus, if foot stimulation will be tested clinically it is not known whether it should be applied continuously or only applied for 30 minutes per week like the tibial nerve stimulation. To answer this question a pre-clinical study was undertaken to determine if foot stimulation can induce a prolonged post-stimulation increase in bladder capacity.
Material and methods
All protocols involving the use of animals in this study were approved by the Animal Care and Use Committee at the University of Pittsburgh.
Experimental setup
The experiments were conducted in 24 normal cats (2.2 kg to 3.3 kg, 19 female and 5 male) under α-chloralose anesthesia (65 mg/kg, I.V. supplemented as necessary) after induction with isoflurane (2-3% in O2). Systemic blood pressure was monitored throughout the experiment via a catheter inserted in the right carotid artery. A tracheotomy was performed and a tube was inserted to keep the airway patent. A catheter for I.V. infusion was introduced into the right ulnar vein. The ureters were cut and drained externally. A double lumen catheter was inserted through the urethra into the bladder and secured by a ligature around the urethra. One lumen of the catheter was connected to a pump to infuse the bladder with saline at a rate of 0.5-2 ml/min and the other lumen was connected to a pressure transducer to measure the bladder pressure. After removing the fur, self-adhesive pad electrodes (Grass FE10ND, Astro-Medical Inc., Mentor, OH, USA; diameter 1 cm) were attached to the skin surface on the left hind foot (Fig. 1). Stimulation was applied via two electrode combinations, i.e. electrodes 1-2 and 1-3 as shown in Fig. 1. Electrode #1 served as the cathode. The peroneal nerve branches run on the dorsal surface of the foot, while the tibial nerve branches innervate the plantar surface.
Figure 1.
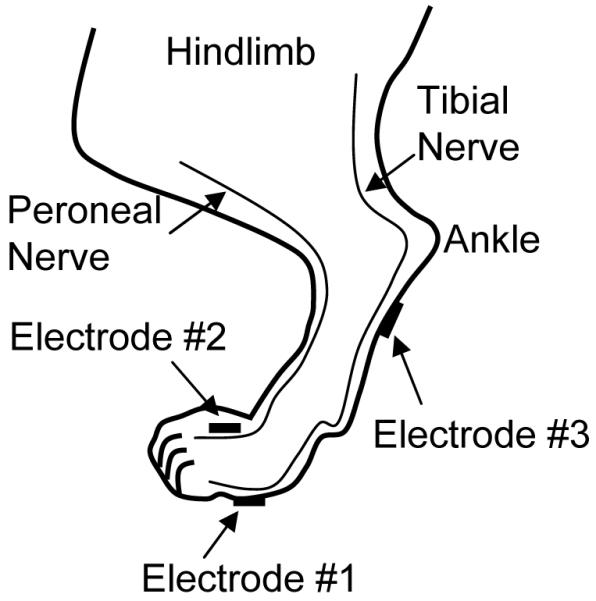
Electrode placement for electrical stimulation of the cat hind foot.
Stimulation protocol
A post-stimulation effect of continuous 30 minute foot stimulation was examined by performing repeated cystometrograms (CMGs). Initially two or three CMGs were preformed without stimulation to obtain the control bladder capacity and evaluate reproducibility. Then, two groups of experiments were conducted: 1) control group (12 cats) without stimulation; 2) treatment group (12 cats) with foot stimulation.
In the treatment group, the bladder volume was maintained at a volume slightly above the bladder capacity at the end of the last control CMG to induce isovolumetric rhythmic bladder contractions. Then, uniphasic rectangular pulses (5 Hz frequency, 0.2 ms pulse width) were delivered to the foot for 30 minutes via the electrode combination 1-2. The 5 Hz frequency was chosen based on our previous study of foot stimulation18 where 5 Hz was slightly better than 30 Hz. Also, 5 Hz will use less battery power than 30 Hz in practice. The 30 minute stimulation duration was based on the use of this duration in the clinical application of tibial nerve neuromodulation.7,10-16 The intensity threshold (T) to induce observable toe twitching was determined in each cat by a preliminary test just before applying the 30 minute stimulation. Then, stimulation intensity of 3-4 T was used throughout the experiment. After the first 30 minute stimulation, 5 CMGs were performed within a 1.5-2 hour period to examine the change of bladder capacity. At the end of the fifth CMG, the bladder volume was again maintained at a volume slightly above the bladder capacity to induce isovolumetric rhythmic contractions, during which the second 30 minute stimulation was applied via electrode combination 1-3 to inhibit the bladder contractions. The post-stimulation effect on bladder capacity induced by the second 30 minute stimulation was evaluated by another 5 CMGs repeated within 1.5-2 hours after the termination of the second stimulation treatment. After examining the post-stimulation effect, the foot stimulation (via electrode combination 1-2 or 1-3) was applied again during the CMG to determine if the bladder capacity could be further increased. The bladder was emptied after each CMG or 30 minute stimulation and a 5-10 min rest period was inserted after emptying the bladder to allow the distended detrusor to recover.
In the control group the procedures similar to those used in the treatment group were performed, but the foot stimulation was not applied during either the first or the second 30 minute treatment periods. Instead, the isovolumetric rhythmic bladder contractions were allowed to continue during each of these periods.
Data analysis
The bladder capacities were measured during the repeated CMG recordings and normalized to the measurement of the first control CMG in each animal. The normalized data from different animals are presented as mean ± SE. ANOVA and Student’s t-test were used to detect statistical significance (P<0.05).
Results
Post-stimulation inhibitory effect on reflex bladder activity
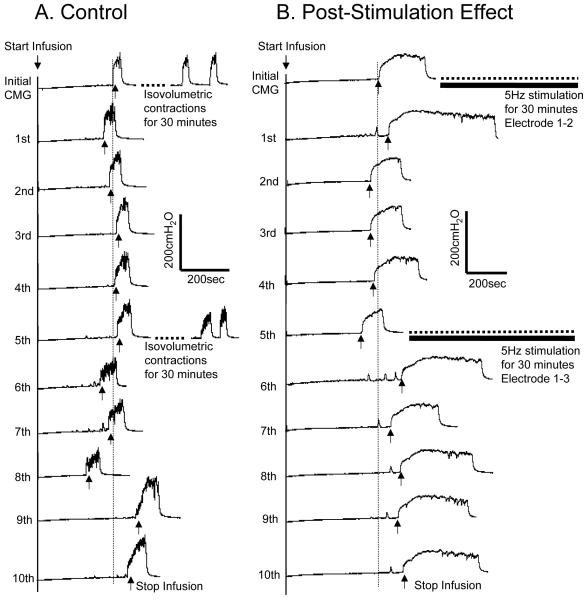
As shown in Fig.2 A the bladder capacity in control experiments remained relatively constant during repeated CMGs performed over 3-4 hours which included two 30 minute periods of bladder distension to induce rhythmic bladder contractions. In the treatment experiment the first 30 minute foot stimulation applied via electrode combination 1-2 inhibited the rhythmic bladder contractions, but the bladder capacity was not significantly increased during the 5 CMGs following the termination of the stimulation (Fig.2 B). However, the second 30 minute foot stimulation applied via electrode combination 1-3 elicited a post-stimulation increase in bladder capacity (Fig.2 B).
Fig.2.
Post-stimulation inhibitory effect of foot stimulation. (A) Control. After the initial and 5th CMG the bladder was maintained for 30 minutes in a distended condition and exhibited rhythmic contractions. (B) Post-stimulation effect. Five repeated CMGs (1st – 5th and 6th – 10th) were performed within 1.5-2 hours after each 30 minute period of stimulation. The vertical dotted line indicates the control bladder capacity. Infusion rate: 1 ml/min in A, but 2 ml/min in B. The horizontal black bar indicates the 30 minute foot stimulation. Stimulation: frequency 5 Hz, pulse width 0.2 ms. Stimulation intensity: 13.5 V (3T) for electrode 1-2, but 12V (3T) for electrode 1-3.
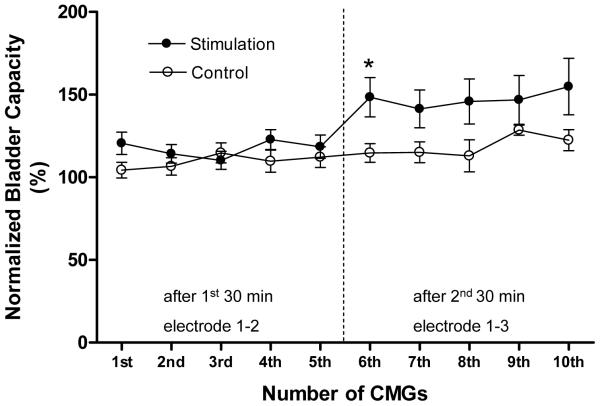
In summary (Fig.3 and Table 1) the bladder capacity did not change significantly (P>0.05, ANOVA) during the 10 repeated CMGs performed over a 3-4 hour period in the control animals. Furthermore, the bladder capacity measured during the five CMGs (1st – 5th) after the first 30 minute stimulation via electrode combination 1-2 did not significantly increase (P>0.05, ANOVA). However, the bladder capacity during the five CMGs (6th – 10th) after the second 30 minute stimulation via electrode combination 1-3 increased on average to 147.5±2.2% of the control capacity that was significantly (P<0.05, ANOVA) different from the capacity measured after the first 30 minute stimulation. The post-stimulation increase in bladder capacity induced by electrode combination 1-3 was not long-lasting; only the bladder capacity measured during the first CMG after the stimulation (i.e. the 6th CMG in Fig.3) was significantly (P<0.05, Student t-test) increased when compared to the control group.
Fig.3.
Post-stimulation inhibition induced by 30 minute foot stimulation. Stimulation: frequency 5 Hz, pulse width 0.2 ms, intensity 12-60 V (3-4 T). T – Intensity threshold for inducing toe movement. * indicates a statistically significant difference between the control and treatment groups. Vertical dotted line indicates the time of the second 30 minute stimulation. Control: N = 12. Stimulation: N = 12.
Table 1.
Bladder capacity (%) normalized to the first control CMG in both control and treatment groups.
| pre-30 minutes. |
CMGs after 1st 30 minutes | CMGs after 2nd 30 minutes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | 10th | ||
| control group |
101.7 ±2.2 |
104.3 ±4.8 |
106.6 ±5.2 |
114.7 ±6.0 |
109.7 ±6.7 |
112.1 ±6.3 |
114.7 ±5.7 |
115.1 ±6.3 |
113.0 ±9.6 |
128.5 ±3.0 |
122.5 ±6.4 |
| treatment group |
97.4 ±1.2 |
120.6 ±6.7 |
114.2 ±5.6 |
110.2 ±5.4 |
122.8 ±6.1 |
118.5 ±7.0 |
148.4 ±11.9 |
141.4 ±11.4 |
145.8 ±13.7 |
146.8 ±14.8 |
154.9 ±17.1 |
Additional acute inhibitory effect on reflex bladder activity
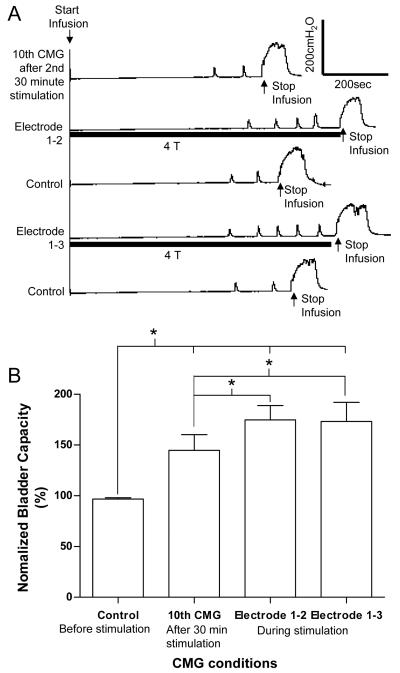
After the second 30 minute stimulation treatment, foot stimulation via electrode combinations 1-2 or 1-3 significantly (P<0.05, Student t-test) increased bladder capacity an additional 30.0±6.7% or 28.5±7.0%, respectively, when the stimulation was applied during the CMG (Fig.4).
Fig.4.
Acute inhibitory effect. (A) CMG traces. Infusion rate: 1 ml/min. Stimulation: frequency 5 Hz, pulse width 0.2 ms. Stimulation intensity: 50 V (4T) for electrode 1-2, but 30 V (4T) for electrode 1-3. (B) Summarized results (N = 10). Stimulation: frequency 5 Hz, pulse width 0.2 ms, intensity 3-4 T. * indicates a statistically significant difference.
Discussion
This study revealed in cats that activation of somatic afferent nerves in the foot using skin surface electrodes could induce an acute inhibition of reflex bladder activity (Fig.4) and a post-stimulation increase in bladder capacity (Fig.2 and Fig.3). Although the acute inhibition was equally effective with both electrode combinations (Fig.1 and Fig.4), the post-stimulation effect could only be induced by electrode combination 1-3 (Fig.1 and Fig.3). The significant post-stimulation effect was only observed for the duration of one CMG (i.e. about 10-15 minutes, Fig.3).
This study indicates that clinical application of foot stimulation to treat overactive bladder symptoms might require a very different treatment plan than tibial nerve stimulation which in cats elicits a prolonged (>2 hours) post-stimulation increase in bladder capacity.19 Foot stimulation might have to be applied continuously rather than 30 minutes per week like the tibial nerve stimulation,7,10-16 since the post-stimulation effect of foot stimulation might be short-lasting (Fig.3). However, continuous stimulation might not be practical because stimulation induced foot movement might interfere with patient activity. Therefore, a small electrical stimulator that is either embedded in the shoe or carried in the pocket might be required for the patients to turn on the stimulation at convenient times during the day. On the other hand, a continuous stimulation might not be necessary if electrodes are attached to the plantar surface of the foot (i.e., electrodes 1-3 in Fig.1) since a short-lasting post-stimulation effect could be induced (Fig.3). Therefore, intermittent stimulation of the foot might be sufficient to inhibit bladder activity. However, the optimal stimulation duration and interval will still need to be determined in clinical trials.
The inhibitory mechanisms underlying foot and tibial nerve stimulation might be very different. There are two major nerves (peroneal and tibial) innervating the foot.20 The peroneal nerve branches run on the dorsal surface of the foot, while the tibial nerve branches innervate the plantar surface. Based on the different electrode locations, the electrode combination 1-3 (Fig.1) should activate primarily the tibial nerve branches while the electrode combination 1-2 (Fig.1) should activate nerve branches from both tibial and peroneal nerves. The post-stimulation effect could only be observed with electrode combination 1-3 but not 1-2 (Fig.3) indicating that activation of peroneal nerve might not contribute to the induction of the post-stimulation effect, or might even interfere with the effect. The shorter post-stimulation effect (Fig.3) of foot stimulation (electrode combination 1-3) compared to the effect of tibial nerve stimulation (>2 hours)19 might be related to the fact that foot stimulation only activated a portion of the tibial nerve branches, thereby generating a weak effect. However, foot stimulation (both electrode combinations, Fig.4) and tibial nerve stimulation19 induced acute inhibition of similar magnitude indicating that acute inhibition may not be linked with the post-stimulation effect. Our results in this study are also consistent with previous studies in both cat19,21 and monkey22 showing that electrical stimulation of either peroneal or tibial nerve can acutely inhibit reflex bladder activity.
Since the electrode combination 1-2 was always used first, it might have influenced or contributed to the post-stimulation effect induced by the electrode combination 1-3 during the second 30 minute stimulation. This possibility can only be clarified by additional experiments using the electrode combination 1-3 first. However, the post-stimulation effect of foot stimulation is short-lasting (Fig.3), suggesting that repeated foot stimulation has to be used in clinical trials. Furthermore, since different electrode combinations are equally effective in inducing acute inhibition (Fig.4) they should probably both be tested in clinical trials. Therefore, the potential influence of applying electrode combination 1-2 first should be elucidated and included in the clinical tests.
A long-lasting post-stimulation modulatory effect on bladder reflexes of electrical stimulation of somatic afferent nerves has also been reported previously in both rats and cats.23,24 After 5 minutes of anogenital stimulation in rats23 the increase in bladder capacity was maintained for about 40 minutes. In cats24 the pelvic efferent nerve activity induced by electrical stimulation of the pelvic afferent or the urethral branch of the pudendal afferent nerve was significantly enhanced for more than an hour after a 5 minute stimulation of the respective nerve. It has been speculated that these post-stimulation effects could be due to a modulation of excitatory synapses in the central micturition reflex pathway. A prolonged decrease in synaptic efficacy of excitatory synapses, designated as long-term depression, has been observed in the hippocampus after intense activation of an inhibitory input to the target cells.25 More electrophysiological studies are warranted to further investigate the mechanisms of the post-stimulation inhibitory effect on reflex bladder activity induced by activation of somatic afferent nerves.
Stimulation intensity of 3-4 times threshold for inducing toe twitching was used in this study, which probably only activated the large A afferent fibers rather than the small C fibers that could induce painful sensation in clinical application. The diameters of large A afferent fibers (10-20 μm) are 20-50 fold larger than the small C-fibers (0.2-1 μm). Thus stimulation intensities higher than 20 times the motor threshold would be needed in order to activate the noxious C-fibers and induce painful sensation. Electrical stimulation of the foot using skin surface electrodes is non-invasive and should be easily manageable by the patients. Therefore, foot stimulation to treat overactive bladder symptoms will be a very promising treatment option if it is proven to be clinically effective.
Conclusions
Electrical stimulation of somatic afferent nerves in the foot using skin surface electrodes might be an effective treatment for overactive bladder symptoms. A clinical trial could be easily conducted due to the non-invasiveness of this stimulation method. Based on the results of the present study an intermittent stimulation pattern with on and off time at intervals of 10-15 minutes might be used in a clinical test.
Acknowledgement
This study is supported by the National Institutes of Health under grants DK068566, DK090006, and DK077783 and by the Christopher and Dana Reeve Foundation.
References
- 1.Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: Basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004;56:581. doi: 10.1124/pr.56.4.4. [DOI] [PubMed] [Google Scholar]
- 2.Peters KM, Feber KM, Bennett RC. Sacral versus pudendal nerve stimulation for voiding dysfunction: a prospective, single-blinded, randomized, crossover trial. Neurourol Urodyn. 2005;24:643. doi: 10.1002/nau.20174. [DOI] [PubMed] [Google Scholar]
- 3.Peters KM, Feber KM, Bennett RC. A prospective, single-blind, randomized crossover trial of sacral vs pudendal nerve stimulation for interstitial cystitis. BJU Int. 2007;100:835. doi: 10.1111/j.1464-410X.2007.07082.x. [DOI] [PubMed] [Google Scholar]
- 4.Tai C, Smerin SE, de Groat WC, et al. Pudendal-to-bladder reflex in chronic spinal cord injured cat. Exp Neurol. 2006;197:225. doi: 10.1016/j.expneurol.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Tai C, Shen B, Wang J, et al. Inhibitory and excitatory perigenital-to-bladder spinal reflexes in the cat. Am J Physiol Renal Physiol. 2008;294:591. doi: 10.1152/ajprenal.00443.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGuire EJ, Zhang SC, Horwinski ER, et al. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol. 1983;129:78. doi: 10.1016/s0022-5347(17)51928-x. [DOI] [PubMed] [Google Scholar]
- 7.Peters KM, MacDiarmid SA, Wooldridge LS, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol. 2009;182:1055. doi: 10.1016/j.juro.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 8.Nakib K, Siegel S. Neuromodulation versus neurotoxin for the treatment of refractory detrusor overactivity: for neuromodulation. Nat Clin Pract Urol. 2008;5:118. doi: 10.1038/ncpuro1033. [DOI] [PubMed] [Google Scholar]
- 9.Sutherland SE, Lavers A, Carlson A, et al. Sacral nerve stimulation for voiding dysfunction: one institution’s 11-year experience. Neurourol Urodyn. 2007;26:19. doi: 10.1002/nau.20345. [DOI] [PubMed] [Google Scholar]
- 10.van Balken MR. Percutaneous tibial nerve stimulation: the Urgent PC® device. Expert Rev Med Dev. 2007;4:693. doi: 10.1586/17434440.4.5.693. [DOI] [PubMed] [Google Scholar]
- 11.Agro EF, Campagna A, Sciobica F, et al. Posterior tibial nerve stimulation: is the once-a-week protocol the best option? Minerva Urol Nefrol. 2005;57:119. [PubMed] [Google Scholar]
- 12.Amarenco G, Ismael SS, Even-Schneider A, et al. Urodynamic effect of acute transcutaneous posterior tibial nerve stimulation in overactive bladder. J Urol. 2003;169:2210. doi: 10.1097/01.ju.0000067446.17576.bd. [DOI] [PubMed] [Google Scholar]
- 13.Andrews BJ, Reynard JM. Transcutaneous posterior tibial nerve stimulation for treatment of detrusor hyperreflexia in spinal cord injury. J Urol. 2003;170:926. doi: 10.1097/01.ju.0000080377.71804.fd. [DOI] [PubMed] [Google Scholar]
- 14.Pal FVD, van Balken MR, Heesakkers JPFA, et al. Percutaneous tibial nerve stimulation in the treatment of refractory overactive bladder syndrome: is maintenance treatment necessary? BJU Int. 2006;97:547. doi: 10.1111/j.1464-410X.2006.06055.x. [DOI] [PubMed] [Google Scholar]
- 15.Pal FVD, van Balken MR, Heesakkers JPFA, et al. Implant-drive tibial nerve stimulation in the treatment of refractory overactive bladder syndrome: 12-month follow-up. Neuromodulation. 2006;9:163. doi: 10.1111/j.1525-1403.2006.00056.x. [DOI] [PubMed] [Google Scholar]
- 16.Peters KM, Carrico DJ, Perez-Marrero RA, et al. Randomized trial of percutaneous tibial nerve stimulation versus sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol. 2010;183:1438. doi: 10.1016/j.juro.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 17.de Seze M, Raibaut P, Gallien P, et al. Transcutaneous posterior tibial nerve stimulation for treatment of the overactive bladder syndrome in multiple sclerosis: results from a multicenter prospective study. Neurourol Urodyn. 2011;30:306. doi: 10.1002/nau.20958. [DOI] [PubMed] [Google Scholar]
- 18.Tai C, Shen B, Chen ML, et al. Suppression of bladder overactivity by activation of somatic afferent nerves in the foot. BJU Int. 2010;107:303. doi: 10.1111/j.1464-410X.2010.09358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tai C, Shen B, Chen ML, et al. Prolonged Post-stimulation inhibition of bladder activity induced by tibial nerve stimulation in cats. Am J Physiol - Renal Physiol. 2011;300:F385. doi: 10.1152/ajprenal.00526.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reighard J, Jennings HS. Anatomy of the cat. ed.3 Holt, Rinehart and Winston; New York: 1935. [Google Scholar]
- 21.Sato A, Sato Y, Schmidt R. Reflex bladder activity induced by electrical stimulation of hind limb somatic afferents in the cat. J Auto Nerv Sys. 1980;1:229. doi: 10.1016/0165-1838(80)90019-3. [DOI] [PubMed] [Google Scholar]
- 22.McGuire E, Morrissey S, Zhang S, et al. Control of reflex detrusor activity in normal and spinal injured nonhuman primates. J Urol. 1983;129:197. doi: 10.1016/s0022-5347(17)51982-5. [DOI] [PubMed] [Google Scholar]
- 23.Jiang CH, Lindström S. Prolonged increase in micturition threshold volume by anogenital afferent stimulation in the rat. British Journal of Urology. 1998;82:398. doi: 10.1046/j.1464-410x.1998.00682.x. [DOI] [PubMed] [Google Scholar]
- 24.Jiang CH, Lindstrom S. Prolonged enhancement of the micturition reflex in the cat by repetitive stimulation of bladder afferents. J Physiol. 1999;517:599. doi: 10.1111/j.1469-7793.1999.0599t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]