Abstract
Objective
To quantify the impact of TB co-infection on death and loss to follow-up (LTFU) 12 months after entry into an ART program.
Design
Prospective intervention study
Methods
From May 2007-2008, patients undergoing pre-ART training in Durban, South Africa were screened for pulmonary TB using mycobacterial culture. Subjects missing appointments for >3 months were phoned. Patients who could not be reached were considered LTFU. Deaths were ascertained by report from family members. We used the Kaplan-Meier method to estimate time to LTFU or death for 3 groups at enrollment: 1) newly-diagnosed with TB by sputum culture; 2) on TB treatment (i.e. previously-diagnosed); and 3) TB free. We evaluated the role of TB on mortality and LTFU using Cox proportional hazards models.
Results
Nine-hundred fifty-one HIV-infected subjects were enrolled; 59% were female and median baseline CD4 count was 90/μl (IQR 41-148/μl). One hundred forty-four (15%) were newly-diagnosed with TB by sputum culture; an additional 199 (21%) were already on TB treatment. By 12 months, 26% newly-diagnosed with TB at enrollment died or were LTFU, compared to 19% already on TB treatment, and 14% who were TB free (p=0.001). Controlling for age, sex, smoking, CD4, and opportunistic infection history, subjects newly-diagnosed with pulmonary TB were 76% more likely to die or be LTFU (HR 1.76, 95% CI 1.20-2.60) than those without TB.
Conclusions
HIV/TB co-infected individuals are more likely to die or be LTFU within 12 months of ART clinic entry in South Africa. These patients require intensive follow-up during ART initiation.
Keywords: HIV, Tuberculosis, South Africa, long-term outcomes, mortality
Introduction
Tuberculosis (TB) is the leading cause of death for sub-Saharan Africans infected with HIV.1 The substantial burden of TB at the time of enrollment into antiretroviral therapy (ART) clinics complicates health care delivery and leads to many deaths prior to, as well as in the early months following, ART initiation.2-4 Because of the deadly overlap of the HIV and TB epidemics, the World Health Organization (WHO) promotes intensified TB case finding for early case detection and integration of HIV and TB screening and treatment.5 The yield of intensified case finding depends on the screening strategy used, with as many as 19-25% of HIV-infected ART-eligible individuals found to have previously undiagnosed TB in South Africa when screened using mycobacterial culture.6, 7
The impact of tuberculosis on longer term mortality among HIV-infected people starting ART remains controversial. Some studies have found that individuals on TB treatment at the time of ART initiation do not suffer from increased mortality when controlling for CD4 count, body mass index, and other confounding factors,8, 9 while others have found a significant increase in mortality.2, 4 Most studies have relied on clinical signs and symptoms for the diagnosis of TB, which may underestimate both TB prevalence and incidence, particularly among HIV-infected individuals.6, 10, 11 Therefore, there are limited data available on the impact of an intensive TB screening strategy on mortality and loss to follow-up (LTFU). Our objective was to quantify the impact of HIV/TB co-infection on death and LTFU 12 months after culture-based screening at ART program entry.
Methods
Study setting
McCord Hospital is a state-aided, semi-private hospital in an urban setting in Durban, South Africa. The Sinikithemba HIV clinic at McCord Hospital has been treating patients with ART since 1999; in 2004, the clinic became a US President’s Emergency Plan for AIDS Relief (PEPFAR)-funded site and in 2007 received South African Department of Health support as an ART “rollout” site. The HIV clinic serves a predominantly African, Zulu-speaking population and has initiated over 8,000 patients on ART.12 During the study period, HIV-infected patients with CD4 count ≤200/μl or who met clinical criteria (WHO Stage 3 or 4) were given a date to commence ART literacy training. The clinic trains approximately 80-120 new patients per month,13 including educational sessions, clinical evaluation, and baseline laboratory work. Sinikithemba patients enrolled during the study period were treated with standard ART regimens as per contemporaneous South African guidelines.14 HIV-infected patients with TB who are Sinikithemba patients at McCord are offered weight-based, fixed-dose combination TB treatment on-site according to South African treatment guidelines.15 Patients already taking TB treatment from another clinic at the time of enrollment at Sinikithemba were encouraged to transfer their TB care to Sinikithemba.
Study sample
Patients eligible for this study included adults (≥18 years) commencing ART literacy training at Sinikithemba from May 2007-May 2008.6 Consecutive ambulatory HIV-infected patients were prospectively offered enrollment prior to physician or laboratory evaluation, regardless of signs or symptoms of active TB, during the ART training period. A small proportion (8%) of subjects transferred care to Sinikithemba and were already on ART at enrollment; they were excluded from further analysis.
The study was approved by the McCord Hospital Research Ethics Committee [Durban, South Africa] and the Partners Human Research Committee [Protocol 2007-P-000228, Boston, MA, USA].
Data collection
A trained research nurse enrolled patients undergoing ART literacy training and baseline laboratory investigations. The nurse administered a 12-item questionnaire, including demographic data, TB symptom history, as well as current and prior HIV and TB treatment. Patients were asked about recent cough of any duration, as well as other tuberculosis symptoms, including self-reported fever, night sweats, weight loss, dyspnea, or chest pain. All patients then expectorated a single sputum specimen spontaneously or with ultrasonic nebulization with single-use tubing if they were unable to generate a spontaneous specimen. All sputum samples were stained to assess for Acid-Fast Bacillus smear (AFB), and were processed for Mycobacterium tuberculosis culture (Middlebrook 7H11 solid agar medium and BACTEC mycobacterial growth indicator tube [MGIT] 960 culture; BD System) at the collaborative TB laboratory of the University of KwaZulu-Natal and the South African Medical Research Council in Durban. These procedures have been described in detail elsewhere.6 Sputum results became part of the patient medical record to inform clinical care.
Patient medical charts were reviewed 3 months following enrollment for each study participant to obtain additional data from the initial clinical evaluation, including a more extensive clinical history related to TB and other opportunistic infections, as well as any deaths reported to the clinic. Charts were reviewed again approximately 12 months after enrollment to evaluate whether patients were alive and in care, and to ascertain follow-up CD4 counts and HIV RNA. Subjects missing appointments for more than 3 months during the 12 months following enrollment were contacted by telephone by a Zulu/English speaking nurse. Deaths were ascertained at this point most commonly by report from patients’ family members or friends who answered the deceased person’s phone, as well as from the electronic medical record, for deaths which occurred in-hospital or which were reported to the HIV clinic. If the nurse was unable to reach the patient, or confirm that the patient was still alive, despite multiple phone call attempts, the subject was considered LTFU. If the subject was reached but did not return to clinic, the subject was also considered LTFU. The date last seen in clinic was recorded.
Statistical methods
The primary outcome was time to death or LTFU during the 12 months following enrollment; secondary analyses were conducted to investigate death and LTFU separately. Patients were classified into 3 groups based on TB status at enrollment: 1) newly-diagnosed with TB by sputum culture and not on TB treatment, 2) already on TB treatment for known/presumed active disease, initiated prior to study entry and 3) TB free, meaning a negative enrollment sputum culture and not on TB treatment. We used the Kaplan-Meier method to estimate the time to death or LTFU based on TB status at enrollment. We constructed Cox proportional hazards models for each comparison and present hazard ratios with 95% confidence intervals as effect measures. We assessed 12-month HIV RNA, change in CD4 count from baseline, and time on ART for subjects who were alive and in care at 12 months; we used the 6-month HIV RNA if the patient was alive and in care but did not have a 12-month value to approximate the 12-month data.
To evaluate whether the impact of TB status on death or LTFU differed based on the degree of immune suppression at baseline, we performed a sensitivity analysis using a Cox model with TB status at enrollment stratified by baseline CD4 count (CD4 count >100/μl and CD4 count ≤100/μl). We tested for an interaction between baseline CD4 count and TB status in the model.
The analysis was conducted using SAS statistical software (version 9.2). Associations were examined at a p<0.05 significance level (two-sided test).
Results
Cohort characteristics
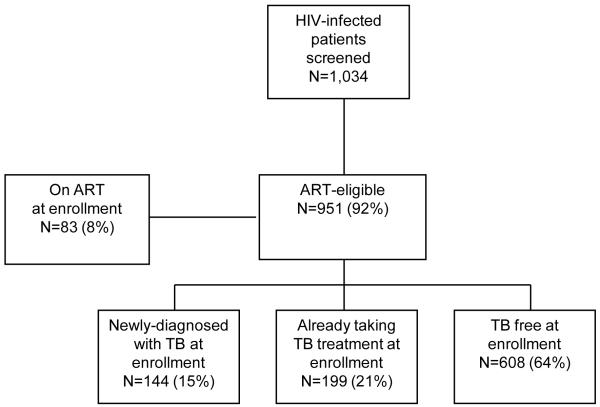
During the one-year study, 1,034 patients were screened and had complete TB culture results. This study sample represents approximately 90% of adult patients who underwent ART literacy training at Sinikithemba during the study period. Median CD4 count at the time of enrollment was 90/μl (IQR 41-148/μl). There were 167 patients (16%) who required nebulized sputum induction. Approximately one-quarter of the patients reported a prior history of TB; for those already on TB treatment at enrollment this prior episode predated the current episode. Of the 951 subjects not on ART at enrollment, 97% were Black African; 59% were female and the median age was 36 years (Table 1). One-hundred forty-four (15%) had culture evidence of undiagnosed active pulmonary TB. An additional 199 (21%) were already on TB treatment at enrollment. Six-hundred eight subjects (64%) were considered TB free: they had a negative sputum culture and were not on TB treatment at enrollment (Figure 1).
Table 1.
Baseline characteristics of patients initiating ART who were screened for TB with sputum cultures in Durban, South Africa, 2007-2008*
| Characteristic | Overall N=951 |
TB Sputum Culture Positive at Enrollment N=144 |
On TB Treatment at Enrollment N=199 |
TB-Free at Enrollment N=608 |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Female (N, %) | 560 (59) | 78 (54) | 121 (61) | 361 (59) |
| Age, in years, median (IQR) | 36 (31– 43) | 37 (31– 43) | 34 (30 – 41) | 37 (31– 43) |
| Unemployed (N, %) | 476 (50) | 68 (47) | 111 (56) | 297 (49) |
| Married (N, %) | 181 (19) | 24 (17) | 33 (17) | 124 (20) |
| HIV-related characteristics | ||||
| Baseline CD4 count/μl, median (IQR) | 90 (41–148) | 80 (41 – 125) | 65 (29 – 112) | 104 (49 – 160) |
| WHO Stage 3 or 4 (N, %) | 627 (66) | 98 (68) | 171 (86) | 358 (59) |
| History of OI (N, %) | 497 (52) | 69 (48) | 113 (57) | 315 (52) |
| Other baseline clinical characteristics | ||||
| Prior history of TB (N, %)† | 245 (26) | 28 (19) | 47 (24) | 170 (28) |
| Hospitalized in last 5 years (N, %) | 251 (26) | 26 (18) | 68 (34) | 157 (26) |
| Household member with history of TB (N, %) |
229 (24) | 33 (23) | 51 (26) | 145 (24) |
| Baseline hemoglobin, median (IQR) | 11.1 (9.5-12.6) | 10.2 (8.5 – 11.8) | 10.3 (9.2 – 11.8) | 11.6 (10.0 – 13.0) |
| Baseline weight (kg), median (IQR) | 61 ( 53 – 72) | 57 ( 51 – 65) | 60 ( 52 – 66) | 63 ( 55 – 75) |
| Current or past smoker (N, %) | 264 (28) | 47 (33) | 54 (27 ) | 163 (27) |
Excludes 84 patients who were screened but who were taking ART at study entry
Prior history indicates self-reported TB episode that precedes either current or new episode
TB: Tuberculosis; IQR: Inter-quartile range; WHO: World Health Organization; OI: Opportunistic infection; ART: antiretroviral therapy; kg: kilogram
Figure 1. Flow chart of study population, Sinikithemba HIV clinic, McCord Hospital, Durban, South Africa, 2007-2008.
A schematic of HIV-infected participants undergoing ART literacy training at the HIV clinic who were screened, enrolled, eligible to initiate ART, and diagnosed with pulmonary TB by sputum culture.
ART: antiretroviral therapy; TB: Tuberculosis
Twelve-month outcomes
Mortality and LTFU
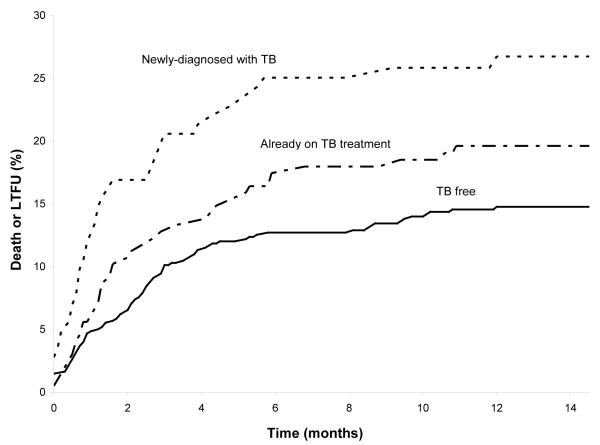
By 12 months, 26% (n=37) of those newly-diagnosed with pulmonary TB at enrollment had died or were LTFU, compared to 19% (n=38) of those who were on TB treatment at study entry, and 14% (n=86) of those who were TB free (p = 0.001). The incidence rate (per 100 person-years) for the combined outcome of death or LTFU was 33 (95% CI 22-43) for those newly-diagnosed with pulmonary TB at enrollment, 22 (95% CI 15-29) for those on TB treatment at study entry, and 16 (95% CI 13-19) for those who were TB free. The majority of patients who reached the primary outcome did so within 6 months of commencing ART literacy training (Figure 2).
Figure 2. Kaplan-Meier curve of the combined endpoints of death or LTFU.
The 3 groups of patients are classified based on TB status at enrollment: 1) newly-diagnosed with TB by sputum culture, 2) already taking TB treatment, and 3) TB free.
LTFU: Lost to follow-up
We considered death and LTFU separately in secondary analyses. For death alone, 15% (n=22) of those newly-diagnosed with TB died, compared to 10% (n=20) on TB treatment at study entry, and 8% (n=50) who were TB free (p = 0.018). For LTFU alone, 10% (n=15) of those newly-diagnosed with TB were LTFU, compared to 9% (n=18) on TB treatment at study entry and 6% (n=36) who were TB free (p = 0.062).
HIV RNA and CD4 cell count
For those who started ART during the study period, time spent on ART was slightly shorter for those newly-diagnosed with TB (median 10.6 months, IQR 7.3-11.9 months), compared to those on TB treatment at study entry (median 11.3 months, IQR 9.9-12.0), and TB free (median 11.5 months, IQR 10.3-12.0 months, p = 0.004). Among patients alive and in care at 12 months, the proportion of patients with suppressed viral load, defined as HIV RNA <50 copies/ml, was similar by baseline TB status (81% among those newly-diagnosed with TB, 84% among those on TB treatment at study entry, and 85% among TB free subjects). TB status at study entry hindered median increase in CD4 count from baseline, with a median increase of 98 cells/μl (IQR 42-171) for those newly-diagnosed with TB, 142 cells/μl (IQR 84-215) among those on TB treatment at study entry, and 109 cells/μl (IQR 47-194) among TB free subjects (p = 0.012).
Combined outcome of death or LTFU at 12 months: Cox proportional hazards model
Subjects newly-diagnosed with pulmonary TB at enrollment were more likely to have died or be LTFU at 12 months, controlling for baseline CD4 count, gender, age, smoking status, and history of opportunistic infections (Table 2). The adjusted hazard ratio for death or LTFU for patients newly-diagnosed with TB was 1.76 (95% CI 1.20, 2.60) compared to subjects who were TB free at study entry. For subjects already on TB treatment at study entry, there was a trend toward an increased risk of death or LTFU, but this did not reach statistical significance (HR 1.24, 95% CI 0.84, 1.82). The increased hazard of death for men in the univariate analysis (HR 1.32, 95% CI 0.97, 1.80) was not seen in the multivariate model (HR 0.99, 95% CI 0.68, 1.45), as a result of CD4 count and smoking being added to the model.
Table 2.
Cox proportional hazards model for the combined outcome of death or LTFU at 12 months based on TB status at baseline enrollment among HIV-infected adults undergoing ART literacy training in Durban, South Africa
| Death or LTFU |
||||
|---|---|---|---|---|
| Univariate HR (95% CI) |
P value | Multivariate HR (95% CI) |
P value | |
| TB status at enrollment | 0.0018 | 0.0165 | ||
| Newly-diagnosed by sputum culture | 2.00 (1.36, 2.93) | 1.76 (1.20, 2.60) | ||
| Already taking TB treatment | 1.37 (0.93, 2.00) | 1.24 (0.84, 1.82) | ||
| TB free | 1.00 | 1.00 | ||
| Baseline CD4 count | 0.0002 | 0.0001 | ||
| CD4 ≤100/μl | 1.92 (1.37, 2.70) | 2.00 (1.41, 2.85) | ||
| CD4 >100/μl | 1.00 | 1.00 | ||
| Smoking status | 0.0340 | 0.1447 | ||
| Current or past | 1.42 (1.03, 1.96) | 1.34 (0.91, 1.98) | ||
| Never | 1.00 | 1.00 | ||
| Age | 1.01 (0.99, 1.03) | 0.2034 | 1.01 (0.99, 1.03) | 0.1996 |
| OI history | 0.0220 | 0.0037 | ||
| No | 1.44 (1.05, 1.96) | 1.60 (1.16, 2.19) | ||
| Yes | 1.00 | 1.00 | ||
| Gender | 0.0812 | 0.9718 | ||
| Male | 1.32 (0.97, 1.80) | 0.99 (0.68, 1.45) | ||
| Female | 1.00 | 1.00 | ||
HR: hazard ratio; CI: confidence interval
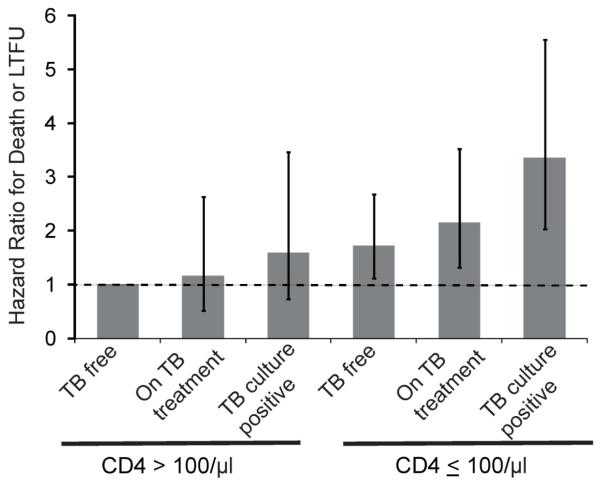
Formal testing did not reveal an interaction between CD4 count and TB status. However, a sensitivity analysis of baseline TB status, stratified by CD4 count ≤100/μl and >100/μl and adjusted for other factors, found that subjects newly-diagnosed with TB with CD4 ≤100/μl were at the highest risk of death or being LTFU compared to TB free subjects with CD4 >100/μl (HR 3.51, 95% CI 2.11, 5.83). Confidence intervals for the groups showed substantial overlap, with the highest risk in patients with low CD4 counts (compared to the TB free subjects with CD4 >100/μl reference group, Figure 3).
Figure 3. Sensitivity analysis showing Cox proportional hazards model for the combined outcome of death or LTFU at 12 months based on TB status and stratified by baseline CD4 count among HIV-infected adults in Durban, South Africa.
The three TB groups: 1) newly-diagnosed with TB by sputum culture, 2) already taking TB treatment for known/presumed active disease prior to enrollment, and 3) TB free, meaning a negative enrollment sputum culture and not on TB treatment, are further stratified based on CD4 count ≥100/μl and CD4 <100/μl. Analysis is adjusted for smoking status, age, opportunistic infection history, and gender. Vertical lines represent 95% CI around hazard ratios.
Discussion
We evaluated the impact of pulmonary TB diagnosed through intensive TB screening during pre-ART literacy training on patient outcomes at 12 months in Durban, South Africa. Intensive TB screening revealed not only the enormous burden of TB among ART-eligible patients in this high-volume HIV clinic,6 but also the detrimental impact of TB diagnosed prior to ART initiation. By 12 months, 26% of those newly-diagnosed with pulmonary TB had died or were LTFU, with most of these events occurring in the first 6 months after clinic enrollment. Subjects newly-diagnosed with TB were 76% more likely to die or be LTFU compared to patients who were TB free at enrollment, even when controlling for baseline CD4 count, smoking status, and history of opportunistic infections. The magnitude of this effect was similar to the impact of a CD4 count ≤100/μl, which had a hazard ratio of 2.00 for death or LTFU, compared to CD4 count >100/μl.
We also analyzed death and LTFU as separate outcomes. There was a significant relationship between TB status and risk of death, with 15% of those newly-diagnosed with TB being dead at follow-up compared to about half of that in those without TB. This is similar to what was seen in a clinical cohort in Cape Town, South Africa, where the impact of newly-diagnosed TB just prior to ART or early during ART initiation was examined using active community-based follow-up of deaths and LTFU.2 We found a trend toward TB status correlating with LTFU; however, we did not have the power in this study to show a significant relationship.
We found a smaller impact of being on TB treatment already at the time of entry to the ART clinic on patient outcomes, which was not significant when adjusting for other important baseline factors. Some patients already on TB treatment at study entry may have continued to obtain TB treatment outside of the HIV clinic; the logistical and clinical barriers due to this lack of integration of HIV and TB care may have made patients already on TB treatment more likely to be lost to follow-up. Studies have shown mixed results regarding the impact of being on TB treatment at the time of ART initiation. In a rural Ugandan cohort, mortality was higher among patients already receiving TB treatment at the time of ART initiation, or during the first year of follow-up, compared to subjects without TB.4 However, another South African study of a clinical cohort in Johannesburg found that the nearly two-fold increased risk of death in an unadjusted analysis of patients on TB treatment at ART initiation was not found when adjusted for other factors, such as low CD4 count, body mass index, and hemoglobin.9 An important difference is that in that study the cohort consisted only of patients who had actually started ART; in the current study, subjects were ART-eligible, but could have died or been LTFU prior to ART initiation because of TB. The current study employs a more intensive approach to TB screening than the prior two studies, and therefore we expect to have a more accurate ascertainment of TB disease at the time of clinic entry.
A subtle affect of TB on patient outcomes was seen among those who survived and were in care at follow-up. Rates of virologic suppression did not differ by TB status for patients who were alive and in care at 12 months, but these rates did not account for patients who had died or were LTFU. We saw a modest negative impact of TB on median increase in CD4 count on ART. Subjects newly-diagnosed with TB started ART slightly later and therefore spent a shorter time on ART during the 12 months of follow-up, which could have influenced their rates of virologic suppression and relative CD4 count increase; however, data are also emerging suggesting that TB itself may influence CD4 reconstitution. A Ugandan study noted that incident TB cases during the first year of ART were associated with a blunting of CD4 count increase over two years of follow-up.16 The potential for a long-lasting immunosuppressive effect of TB may have implications for morbidity and mortality for patients initiating ART in high HIV/TB prevalence settings.
This study has several limitations. We are likely to have underestimated the overall prevalence of TB because of the focus on screening for pulmonary disease alone and the use of a single sputum culture for pulmonary TB diagnosis.6 We may have also underestimated actual mortality, in that some patients classified as LTFU may have been misclassified;17, 18 therefore our primary outcome was either death or LTFU at 12 months. Though we enrolled 90% of eligible patients, the data on study outcomes for the remaining 10% were not available, precluding formal comparison between these two groups with respect to death and LTFU. We did not have statistical power to find an interaction between CD4 count and TB status, which may exist and affect patient outcomes. Other clinical factors not collected in our study, such as body mass, nutritional status, and drug toxicities, have also been associated with morbidity and mortality in the setting of TB in other studies. We could not assess whether these factors may have influenced patient outcomes in the current study.8, 9, 19, 20
This study points to the massive unrecognized TB burden and its impact on long-term outcomes for people infected with HIV who are being evaluated for ART. Even in the setting of an intensive TB screening program where mycobacterial culture was available to all patients, a new diagnosis of TB in the setting of pre-ART training predicted mortality and LTFU at 12 months nearly as strongly as baseline CD4 count. Comprehensive screening for TB must occur as early as possible in the care of people infected with HIV, ideally at the same time as their HIV diagnosis. People co-infected with HIV/TB require prompt initiation of therapy for both diseases as well as more frequent clinical follow-up. Integration of HIV and TB disease management,5, 21 contact with case workers or patient navigators, and the use of incentives are all possible interventions that may promote better engagement and retention in care. Interventions to decrease LTFU among HIV/TB co-infected patients are urgently needed to improve outcomes for these patients who have entered the health care system but remain at substantially increased risk of death.
Acknowledgements
We would like to thank the patients, clinicians, and monitoring and evaluation department of the McCord Hospital Sinikithemba HIV Clinic as well as the staff of the collaborative TB laboratory for their participation. We also appreciate the technical assistance offered by Sarah Lorenzana, Alexis Sypek, and Alison Erlwanger.
Source Funding This work was supported in part by: the National Institute of Allergy and Infectious Disease: K23 AI 068458 (IVB); a PEPFAR supplement to R01 AI058736 (KAF); K24 AI062476 (KAF); the Harvard University Center for AIDS Research P30 AI060354; the National Institute of Mental Health: R01 MH090326 (IVB); R01 MH073445 (RPW); the National Institute of Arthritis and Musculoskeletal and Skin Diseases: K24AR057827 (EL); the Doris Duke Charitable Foundation, Clinical Scientist Development Award (RPW) and Operations Research on AIDS Care and Treatment in Africa (KAF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Presented in part at the 18th International AIDS Conference, July 18-23, 2010, Vienna, Austria
Conflicts of Interest There are no conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization [Accessed 17 February 2011];Global tuberculosis control : a short update to the 2009 report. Available at http://www.who.int/tb/publications/global_report/2009/update/tbu_9.pdf.
- 2.Lawn SD, et al. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20(12):1605–1612. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 3.Lawn SD, et al. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19(18):2141–8. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 4.Moore D, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007;21(6):713–9. doi: 10.1097/QAD.0b013e328013f632. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization [Accessed 17 February 2011];The WHO Three ‘I’s meeting: Report of a joint WHO HIV/AIDS and TB Department meeting. 2008 Available at: http://www.who.int/hiv/pub/meetingreports/WHO_3Is_meeting_report.pdf.
- 6.Bassett IV, et al. Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa. Clin Infect Dis. 51(7):823–9. doi: 10.1086/656282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawn SD, et al. Tuberculosis during the first year of antiretroviral therapy in a South African cohort using an intensive pretreatment screening strategy. AIDS. 24(9):1323–8. doi: 10.1097/QAD.0b013e3283390dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stringer JS, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296(7):782–93. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 9.Westreich D, et al. Effect of pulmonary tuberculosis on mortality in patients receiving HAART. AIDS. 2009;23(6):707–15. doi: 10.1097/QAD.0b013e328325d115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawn SD, et al. Urine lipoarabinomannan assay for tuberculosis screening before antiretroviral therapy diagnostic yield and association with immune reconstitution disease. AIDS. 2009;23(14):1875–80. doi: 10.1097/qad.0b013e32832e05c8. [DOI] [PubMed] [Google Scholar]
- 11.Shah S, et al. Intensified tuberculosis case finding among HIV-Infected persons from a voluntary counseling and testing center in Addis Ababa, Ethiopia. J Acquir Immune Defic Syndr. 2009;50(5):537–45. doi: 10.1097/QAI.0b013e318196761c. [DOI] [PubMed] [Google Scholar]
- 12.Ojikutu BO, et al. Predictors of mortality in patients initiating antiretroviral therapy in Durban, South Africa. S Afr Med J. 2008;98(3):204–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Bassett IV, et al. Loss to care and death before antiretroviral therapy in Durban, South Africa. J Acquir Immune Defic Syndr. 2009;51(2):135–9. doi: 10.1097/qai.0b013e3181a44ef2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. [Accessed 17 February 2011];Operational Plan for Comprehensive HIV and AIDS Care, Management and Treatment for South Africa. 2003 Available at: http://www.info.gov.za/issues/hiv/careplan.htm.
- 15.Republic of South Africa Department of Health [Accessed 17 January 2011];The South African national tuberculosis control programme practical guidelines. 2004 Available at: http://www.kznhealth.gov.za/chrp/documents/Guidelines/Guidelines%20National/Tuberculosis/SA%20TB%20Guidelines%202004.pdf.
- 16.Hermans SM, et al. Incident tuberculosis during antiretroviral therapy contributes to suboptimal immune reconstitution in a large urban HIV clinic in sub-Saharan Africa. PLoS ONE. 5(5):e10527. doi: 10.1371/journal.pone.0010527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brinkhof MWG, et al. Adjusting Mortality for Loss to Follow-Up: Analysis of Five ART Programmes in Sub-Saharan Africa. PLoS ONE. 5(11):e14149. doi: 10.1371/journal.pone.0014149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geng EH, et al. Sampling-Based Approach to Determining Outcomes of Patients Lost to Follow-Up in Antiretroviral Therapy Scale-Up Programs in Africa. JAMA: The Journal of the American Medical Association. 2008;300(5):506–507. doi: 10.1001/jama.300.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.L’Homme R F, et al. Clinical experience with the combined use of lopinavir/ritonavir and rifampicin. AIDS. 2009;23(7):863–5. doi: 10.1097/QAD.0b013e328329148e. [DOI] [PubMed] [Google Scholar]
- 20.McIlleron H, et al. Complications of antiretroviral therapy in patients with tuberculosis: drug interactions, toxicity, and immune reconstitution inflammatory syndrome. J Infect Dis. 2007;196(Suppl 1):S63–75. doi: 10.1086/518655. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization [Accessed 29 September 2011];Interim policy on collaborative HIV/TB activities. Available at: http://whqlibdoc.who.int/hq/2004/who_htm_tb_2004.330.pdf.