Abstract
Objectives
Subthalamic nucleus (STN) deep brain stimulation (DBS) is an effective intervention in advanced Parkinson's Disease (PD), but its efficacy and safety in early PD are unknown. Our team is conducting a randomized pilot trial investigating DBS in early PD. This report describes one participant who received bilateral STN-DBS.
Materials/Methods
Thirty subjects have been randomized to either optimal drug therapy (ODT) or DBS + ODT. Microelectrode recordings from the STN and substantia nigra (SN) are collected at implantation. The Unified Parkinson's Disease Rating Scale Motor Subscale (UPDRS-III) is administered in the ON and OFF states semi-annually and neuropsychological function and quality of life are assessed annually. We describe a 54-year-old man with a two-year history of PD who was randomized to DBS + ODT and followed for two years.
Results
The subject showed a lower STN to SN ratio of neuronal activity than advanced PD patients, and higher firing rate than non-PD patients. The subject's ON total UPDRS and UPDRS-III scores improved during the two-year follow-up, while his OFF UPDRS-III score and levodopa equivalent daily dose (LEDD) increased. Quality of life, verbal fluency and verbal learning improved. He did not experience any serious adverse events.
Conclusions
This report details the first successful application of bilateral STN DBS for early stage PD during a clinical trial.
Keywords: Parkinson's disease (PD), deep brain stimulation (DBS), early stage, Unified Parkinson's Disease Rating Scale-Motor Subscale (UPDRS-III)
Introduction
Parkinson's disease (PD) is a progressive and disabling neurodegenerative disorder affecting over one million Americans.1 The current standard of care, dopamine replacement with levodopa, improves the symptoms but to date, no pharmaceutical, biologic, procedure, or device has been proven to slow the relentless progression.2 Increasingly higher doses of anti-PD medications are needed for adequate symptom control, and the risk of developing motor complications of therapy reaches 50-75% within seven years of initiation;3 the development of disease-modifying interventions is therefore imperative. Recent animal reports have indicated that bilateral deep brain stimulation (DBS) of the subthalamic nucleus (STN) may slow the rate of nigral dopaminergic cell depletion when it is applied relatively early in the disease course.4-6 The influence of DBS on the progression of clinical symptoms of PD has not been measured in humans; to date, all trials have included patients with relatively advanced disease, without clinical progression as a stated endpoint.
Even without considering the possible influence on clinical progression, applying DBS in earlier stages of PD holds promise because, when applied in the advanced stages, it appears to provide better symptom control7 and quality of life8,9 than medications alone. Furthermore, after implantation, patients with DBS require 25-35% less medication,10 and when applied in the early stages, this could delay onset of medication-associated motor complications.
We are conducting a single-blind, parallel-group, randomized pilot assessment of DBS in 30 subjects with early stage PD to collect preliminary data necessary to plan a multi-center clinical trial. This illustrative case describes one of the first surgical subjects to complete the two-year follow-up of the trial.
Methods
Participants and Setting
The investigation was approved by the Vanderbilt Institutional Review Board. Participants are recruited from the Movement Disorders Clinic of Vanderbilt University Medical Center and through radio and print advertisements in Nashville and the surrounding area. Inclusion and exclusion criteria were designed to exclude patients with potential secondary parkinsonism or Parkinson-plus syndromes. Namely, eligible subjects are between the ages of 50 and 75 and have Hoehn & Yahr Stage II idiopathic PD that has responded to levodopa or dopamine agonist therapy for greater than six months (measured by a 30% or greater reduction in UPDRS-III score after 36-hour levodopa withdrawal followed by administration of 150% normal dose) but have been on therapy less than four years and are without motor fluctuations, dementia, or previous brain operation or injury.
Baseline and Follow-up Procedures
Subjects are admitted to the Vanderbilt General Clinical Research Center (GCRC) in the ON-medication state. On Day 1, subjects complete an 8-hour Hauser diary11 and are rated using the Unified Parkinson's Disease Rating Scale (UPDRS), including the videotaped Motor Subscale (UPDRS-III), by a neurologist with fellowship training in movement disorders. By 4:00 pm on Day 1, ON-medication testing is completed and subjects discontinue all antiparkinsonian medications; they are then rated daily for one week with the UPDRS-III. Subjects also undergo an annual battery of neuropsychological testing for safety evaluation and quality of life testing using the Parkinson's disease questionnaire (PDQ-39). On Day 8 of the subject's stay in the GCRC (after seven days without medication), subjects undergo another videotaped UPDRS-III assessment to document disease severity in the OFF state and provide more accurate preliminary insights into underlying disease progression. After this assessment is complete, subjects resume antiparkinsonian medications and are randomized to either optimal anti-parkinsonian drug therapy (ODT) or ODT plus deep brain stimulation of the bilateral subthalamic nucleus (DBS + ODT).
Subjects who are randomized to DBS + ODT are implanted within two months of randomization. All subjects return to the GCRC and undergo an assessment identical to baseline (with the exception of neuropsychological testing performed annually) four additional times at six-month intervals for a total of two years of follow-up. Subjects randomized to DBS + ODT have their devices turned off in addition to forgoing antiparkinsonian medication at each follow-up to allow videotaped evaluation of motor function in both the fully ON (day 1; receiving both medication and stimulation) and fully OFF (day 8; after 7 days without medication and without stimulation) states. After collection of two year-follow-up data is complete in all subjects, these videotapes will be sent to a blinded reviewer who will view the videotapes and assign motor scores, thus adding a single-blinded component to the trial.
DBS Implantation and Programming
The DBS implantation procedure for the study is identical to the standard methodology utilized at Vanderbilt University by the surgeon (PEK) since 2002,12 and uses a rapid prototyped stereotactic system (WayPoint® Stereotactic system; FHC Inc; Bowdoin, ME). The surgery is performed in three steps, and is briefly reviewed as follows. The first stage is an outpatient procedure in which the patient undergoes high resolution CT and MRI imaging under anesthesia in addition to fiducial marker placement. Stereotactic trajectory planning and design of the rapid prototyped frame (microTargeting® platform, FHC, Inc.) is performed in this first step.
The patient returns within one week of the first procedure to undergo stereotactic mapping of the STN nucleus and bilateral placement of the DBS quadripolar leads (#3389; Medtronic Neurological, Inc; Minneapolis, MN). This second step is usually performed with the subject awake and minimally sedated to observe clinical efficacy and side effects to semi-micro-stimulation of the region surrounding the STN nucleus. In particular, once the stereotactic platform is applied and the burr hole created, micro-electrode recordings (MER) are first made, followed by semi-microstimulation of the region of interest. MER data are collected in ten-second epochs as multiple microelectrodes (impedance range typically 0.2-0.6 M ohm; Model MP1, FHC, Inc.) spaced 2 mm apart are simultaneously advanced in 0.5-1.0 mm increments along the optimal trajectory from 10 mm above to 5 mm below the planned STN target. Localization within STN and SN is determined by a neurophysiologist (CCK or MSR) based on increased background activity and high frequency, irregular firing.13,14 Microelectrode signals are band pass filtered (0.5 – 5 kHz), amplified, displayed, and digitally stored. Offline, individual neurons are isolated using automated cluster analysis of principal components (Spike Sorter 2, Plexon, Dallas, TX) and further refined by visual inspection. Single unit firing frequency is calculated with Neuroexplorer 4 (Nex Technologies, Littleton, MA). A custom MATLAB routine (MathWorks, Natick, MA) is used to calculate root mean square (RMS) amplitude. Finally, the ratio of STN/SN RMS activity, a measure of neuronal background activity which has the advantage of being independent of variables such as impedance, size, configuration of electrode and dynamic changes of neuronal firings, is also calculated.
After MER data are collected and an initial superior and anterior border of the STN identified, semi-microelectrode stimulation is performed using a 0.4mm × 1mm monopolar electrode (FHC, Inc.). Neurological changes resulting from stimulation are documented by a movement disorders neurologist (PDC) for numerous points of interest surrounding the STN region. The final position of the DBS lead is chosen based on analysis of MER data and optimal reduction in rigidity versus stimulation induced side-effects. A permanent DBS lead (Model #3389, Medtronic Neurological, Inc.; Minneapolis, MN) is then inserted and anchored to the skull such that the two middle contacts surround the intended final target. Final lead position is confirmed with postoperative CT imaging within 24 hours. This surgical method has been described by our team elsewhere,12,15 and results in clinical accuracy and electrode placement error rates that are similar to other centers using either traditional stereotactic frames or miniature platforms.
The third step of DBS implantation is performed as an outpatient procedure under general anesthesia within two weeks of the electrode implant. This procedure connects each DBS electrode to a single channel, internal pulse generator (SoletraTM, Medtronic Neurological, Inc.; Minneapolis, MN) located in the infraclavicular region ipsilateral to the lead. All trial participants received bilateral internal pulse generators, according to the protocol in place with the FDA for Investigational Device Exemption status in 2004 related to this study.
The device is initially turned off to allow adequate time for resolution of any intracranial air and impedance stabilization associated with healing. The implant is turned on at an initial programming visit four weeks after lead implantation. At this visit, stimulation mapping is performed to characterize the intensity threshold (in Volts) for both clinical benefit and side effects. Clinical benefit is assessed by the neurologist and patient perception of improvement in rigidity, bradykinesia and tremor. Stimulators are turned on in a monopolar configuration with the selected contact negative and case positive at a low initial setting just above the efficacy threshold (typically 0.8V for subjects in this study) with pulse width 60 μs and rate 130 Hz and left for approximately one month in that state. Following this visit, subjects visit the clinic as necessary (for no fewer than three sessions) for therapy optimization before the first follow-up visit, six months after the baseline visit. Subjects in either study arm may have medication and stimulation adjustments made as needed throughout the trial, and no specific guidelines are placed on individual patient treatment regimens with the exception of excluding other experimental therapies.
Illustrative Case
All 30 subjects have enrolled in the study and been randomized (15 ODT, 15 DBS + ODT). They are 27 men and 3 women aged 60 ± 6.6 years (mean ± standard deviation) who have been on antiparkinsonian medication an average of 2.2 ± 1.2 years at randomization.
The subject described in this report is a white right-handed male, aged 54 years with Hoehn & Yahr Stage II PD, who was diagnosed with PD at age 52 and had been a stable responder to antiparkinsonian medications for almost two years without development of motor fluctuations. Other medical history included atrial fibrillation and cranial nerve IV palsy. The subject's UPDRS-III score after 36 hours OFF medication was 12, which improved to 8 one hour following levodopa challenge (33.3% reduction). Results of the baseline evaluation are described in Table 1. At completion of the baseline visit, the subject was randomized to DBS + ODT.
Table 1. Outcome measures collected at baseline and follow-up.
| Measure: | Baseline: | Six-Month Follow-up: | 12-Month Follow-up: | 18-Month Follow-up: | 24-Month Follow-up: |
|---|---|---|---|---|---|
| UPDRS (Parts I-IV) (ON†) | 38 | 32 | 27 | 22 | 20 |
| UPDRS-III (ON) | 13 | 14 | 8 | 6 | 9 |
| UPDRS-III lateralized27 scores (ON) (R/L) | 6 / 4 | 3 / 7 | 2 / 5 | 2 / 4 | 3 / 6 |
| UPDRS-III (OFF‡) | 20 | 23 | 19 | 25 | 24 |
| UPDRS-III lateralized27 scores (OFF) (R/L) | 9 / 6 | 10 / 7 | 8 / 8 | 10 / 11 | 12 / 8 |
| % time ON with dyskinesias | 0 | 0 | 0 | 0 | 0 |
| Anti-PD medications (levodopa equivalents)28,29 | 225 | 133 | 133 | 436 | 361 |
| IPG amplitude setting§ (R/L) | N/A | 1.5 / 1.5 | 1.8 / 1.8 | 1.8 / 1.8 | 1.8 / 1.8 |
| PDQ-39 | 8.91 | UTD* | 2.34 |
ON refers to ON stimulation/ON medication (collected on day 1 of inpatient evaluation)
OFF refers to OFF stimulation/OFF medication (collected on day 8 of inpatient evaluation, after 7-day washout period)
§All other stimulation parameters remained constant throughout the two-year follow-up period: monopolar configuration with the case set to positive and the active contact set to negative (Left: contact 2; Right: contact 1) at a pulse width of 60 μs and frequency of 130 Hz.
Unable to do: one question was left unanswered by the subject. The scoring instructions for the PDQ-39 instrument state that if one item is left unanswered, the overall index cannot be calculated.
The target identified using preoperative image acquisition and trajectory planning software is described in Table 2. In the operating room, four microrecording electrodes were passed simultaneously through the left brain targeting STN; all of these leads pierced the STN. During the initial pass on the right side, only one of the four microelectrodes pierced the STN. Offset was required and the array was passed once more for a total of eight passes, five of which pierced the STN. Overall, average firing rate in the STN was 38.0 ± 5.3 Hz, and background amplitude was 9.1 ± 0.6 mV. The root mean square of STN/SN activity was 1.27 on the left side and 0.97 on the right (Figures 1 and 2); the higher overall background activity in the left STN was accompanied by more severe right-sided parkinsonism at baseline evaluation in both the ON (right: 6; left: 4) and OFF (right: 9; left: 6) states (Table 1). High frequency test stimulation produced significant reduction in rigidity and bradykinesia at low amplitudes, with paresthesias and involuntary muscle spasms noted at higher amplitudes. The final target position is described in Table 2. Related post-operative adverse events were nausea, vomiting, and pain, and these resolved without sequelae.
Table 2.
Location in standard AC-PC coordinates of (1) planned target; (2) final lead position (centered with two contacts above and two contacts below target); and (3) therapeutic contact used.
| Left | Right | |||||
|---|---|---|---|---|---|---|
| Anterior | Lateral | Superior | Anterior | Lateral | Superior | |
| Planned Target: | -3.9 | 10.9 | -4.6 | -3.7 | 11.9 | -4.2 |
| Final Lead Position: | -1.7 | 11.0 | -4.3 | -2.6 | 12.8 | -3.9 |
| Active Contact: | 2 | 1 | ||||
| Active Contact Position: | -1.4 | 11.3 | -3.4 | -3.0 | 12.5 | -4.7 |
Figure 1.
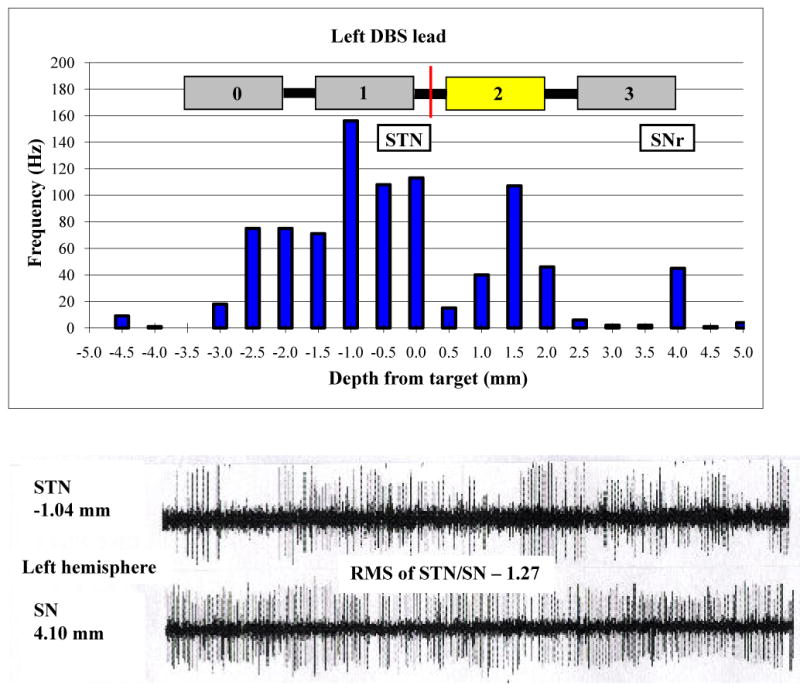
Upper panel: Microelectrode recordings in the left STN and SNr (frequency of neuronal firing vs. distance above or below target) along the optimal track and the final lead implant position. The midpoint of the lead is represented by the red line. Lower panel: Neuronal firing patterns at -1.0 mm (STN) and 4.0 mm (SN) along the same track.
Figure 2.
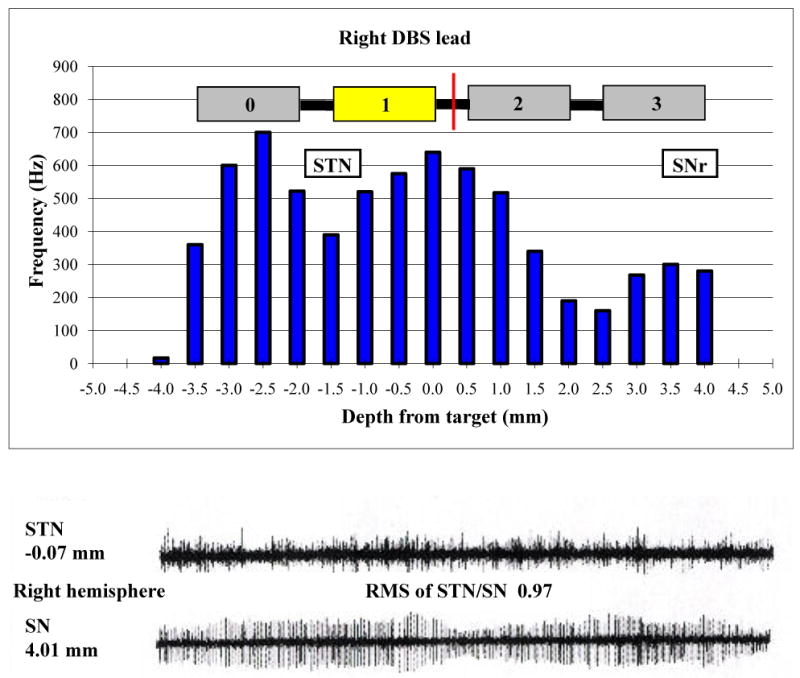
Upper panel: Microelectrode recordings in the right STN and SNr (frequency of neuronal firing vs. distance above or below target) along the optimal track and the final lead implant position. The midpoint of the lead is represented by the red line. Lower panel: Neuronal firing patterns at -0.1 mm (STN) and 4.0 mm (SN) along the same track.
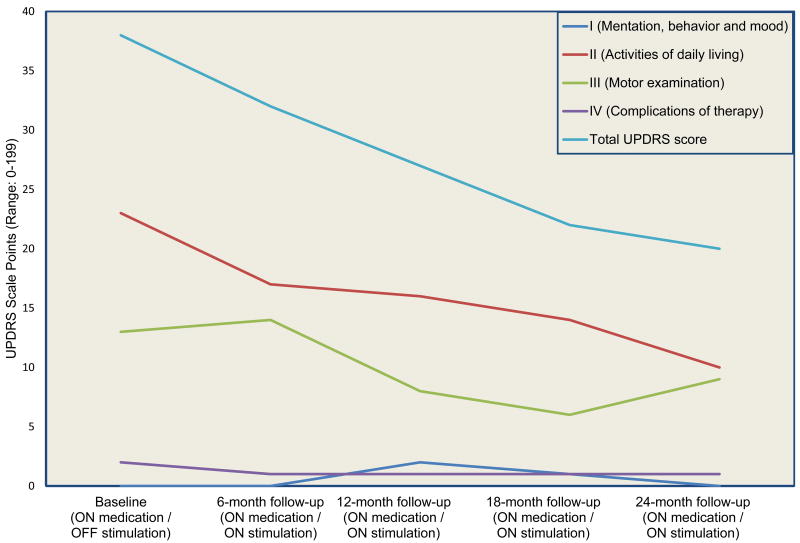
All study visits were completed and outcome measures collected within the specified time frames with the exception of the baseline visit, which was repeated to comply with the time frame specified in the study protocol. Table 1 lists the primary measures collected at every endpoint (Baseline, 6-month, 12-month, 18-month, and 24-month follow-ups). The subject's total UPDRS score in the ON state decreased by 18 points over the follow-up period, primarily due to an improved activities of daily living rating (subscale II) and improved motor ability rating (subscale III), which remained lower than baseline scores by 13 and 4 points, respectively, two years after implantation (Figure 3). The decrease in the ON UPDRS-III score was a result of improved posture, facial expression, and leg agility. These clinical improvements were achieved by increases in both medication dose and stimulation amplitude (Table 1). The subject did not experience dyskinesias or wearing-off phenomena at any point in the study.
Figure 3.
Change in Unified Parkinson's disease rating scale (UPDRS) total score and subscale scores in a patient with early stage Parkinson's disease treated with B-STN DBS for two years.
Table 3 lists the results of neuropsychological testing at baseline and 12- and 24-month follow-ups. Overall, the subject showed no significant decrease in any aspect of neuropsychological functioning. Significant improvements were seen in verbal fluency (FAS), as well as word naming and color naming on the Stroop Test. In addition, verbal memory (Word List Learning) showed an improvement, which became more pronounced with time.
Table 3. Neuropsychological testing results at baseline and follow-up.
| Measure: | Baseline: | 12-Month Follow-up: | 24-Month Follow-up: |
|---|---|---|---|
| Wisconsin Card Sorting Test | |||
| Categories Achieved | 6/6* | 6/6 | 6/6 |
| Perseverative Errors | 13 | 11 | 10 |
| Total Errors | 13 | 12 | 11 |
| Stroop Word/Color Test | |||
| Word | 6 | 10 | 9 |
| Color | 8 | 13 | 13 |
| Color-Word | 14 | 11 | 15 |
| Verbal Fluency Test | |||
| Phonemic (FAS) | 8 | 13 | 13 |
| Categorical (Animals) | 11 | 13 | 10 |
| Paced Auditory Serial Addition Test Heaton Version | |||
| 2.4 second | 12 | 13 | 12 |
| 2.0 second | 14 | 14 | 14 |
| 1.6 second | 11 | 14 | 14 |
| 1.2 second | 14 | 13 | 12 |
| WAIS-III Digit Span | |||
| Score | 14 | 13 | 15 |
| Wechsler Memory Scale-III | |||
| Faces I | 19 | 18 | 19 |
| Faces II | 18 | 18 | 19 |
| Word List I | 9 | 13 | 16 |
| Word List II | 10 | 12 | 16 |
| Boston Naming Test | |||
| Score | 15 | 16 | 16 |
| Benton Judgment of Line Orientation Test | |||
| Score | 12 | 15 | 15 |
Except where indicated otherwise, all test results are scaled scores, with M = 10 and SD = 3. An increase in scaled score of at least 4 to 5 points was used as the minimum in judging significant improvement between baseline and follow-up tests.
The subject experienced one device-related adverse event. Eighteen months after implantation the pulse generator began to turn off unexpectedly, at which time the subject would experience worsening of PD symptoms. The generator was replaced and post-explant analysis by the device maker revealed failure of an electrical connection within the pulse generator. The subject has experienced no other complications since replacement. Other adverse events experienced over the course that were possibly related to study participation were dizziness and insomnia. Adverse events unrelated to study participation were transient weight gain, sleep apnea, frequent urination, and back pain.
Discussion
The application of DBS in early stage PD holds great potential, including reduction in medication dose, delay of motor fluctuations and alteration of clinical progression. Even if these postulated benefits are confirmed, patients and physicians must still weigh them against the risk of DBS therapy. The safety profile of the surgical implantation procedure and stimulation in advanced PD is well known, and includes cognitive decline, affective changes, and intracranial hemorrhage causing permanent disability or death. In addition, it is currently unclear whether administration of stimulation in subjects with early stage PD would cause some unexpected effect, such as the runaway dyskinesias in subjects during the fetal nigral dopaminergic cell trials of the 1990s.16 No such unexpected adverse events occurred in this case, as the adverse events experienced by this single subject were all mild and have been reported in advanced PD patients implanted with DBS.
In advanced PD, STN hyperactivity serves as a landmark for neurophysiological mapping and lead implantation, but it was not clear at study inception whether early PD patients would exhibit the same hyperactivity. The subject's average STN firing rate (38.1 ± 5.3 Hz) was comparable to previously published rates in advanced PD (33 – 42 Hz),13,17,18 and higher than essential tremor patients (19.3 Hz),14 indicating higher frequency is associated with presence of disease. The overall activity in the STN (9.1 ± 0.6 mV) was lower than previously published data from advanced PD patients (140 mV),18 with lower STN/SN ratios (1.27 on the left and 0.97 on the right) than the average ratio of 2.4 in advanced PD.19 Furthermore, the higher STN/SN ratio in the left hemisphere correlated with more severe symptoms on the right side. The higher ratio could reflect STN hyperactivity (as a reaction to neuronal loss in the SN) or decreased SN activity from the neuronal loss.
Total UPDRS scores typically worsen by 8-14 points per year in patients with early stage PD,19-20 while the subject in this case had an improvement of 9 points per year. Similarly, previously published rates of motor decline (measured by the UPDRS-III) range from 1.9-6.7 points per year,21,22 and our subject declined by only two points per year in the OFF state and improved in the ON state. This observation may be consistent with the hypothesis that DBS in early stage PD provides a disease-modifying effect but may also be due to optimized treatment. The subject's anti-PD medication increased from 225 to 361 mg of levodopa equivalents (60%) over the two-year period, a substantial increase but one that is within the range of previously published rates.23
Quality of life measures have gained attention in recent years, and patients with advanced PD treated with DBS experience better quality of life than patients treated with medication alone.7,9 This subject's PDQ-39 and UPDRS-II (ADL subscale) scores both improved after implantation, suggesting that in this single case, the procedure had a positive impact on quality of life and function. Perhaps most importantly for patients with early stage disease, who must weigh the known and potential adverse effects of DBS with the therapeutic effect, clinical improvement occurred without significant adverse events. This is contradictory to some reports indicating higher rates of disability-causing adverse events and neuropsychological consequences of DBS compared to medication alone. Interestingly, adverse cognitive outcomes often involve aspects of frontal-executive functioning, including verbal fluency.24,25 This subject displayed improvement in both verbal fluency and verbal learning. Further study is necessary to investigate the potential influence of DBS on neuropsychological function when it is applied in the early stages of the disease.
The pilot trial from which this case is taken represents a first step in exploring the use of DBS in early stage PD, but there are several limitations, the most obvious of which is the single-blind design. In the case described, neither the physician rater nor the subject were blinded to treatment assignment or duration of follow-up. When designing the study, we acknowledged that positive findings from a double-blind trial would be ideal, but ultimately failed to conceive of a method of blinding patients that was both effective and ethically defensible. The current design, which includes a single-blinded videotaped component, represents our best attempt to balance these considerations. Another limitation is the possibility that one or more participants may actually have atypical or secondary parkinsonism rather than true idiopathic PD. This chance is unavoidable for any clinical trial in early PD because an objective biomarker to diagnose or measure progression does not currently exist. However, our inclusion and exclusion criteria are designed to exclude patients with prominent atypical features (most specifically in the requirement of a six-month stable medication response documented by challenge test)26 and any revised diagnoses, if made, will be considered in the final analysis.
The most important result of this single case was that stimulation did not cause unforeseen worsening of any aspect of motor or cognitive function. In fact, the total UPDRS, a measure of the overall burden of parkinsonism, actually improved over the two-year period, with the most dramatic decreases seen in the motor function and ADL subscales. No conclusions can be made from these data given the extreme variability of natural progression between patients as well as the substantial increases in both stimulation and medical therapy, but further consideration of a role for DBS in disease modification may be warranted. If substantiated by results from the pilot trial, these findings provide a rationale and practical guidance for conducting a multicenter clinical trial to determine whether DBS is safe, efficacious, and positively affects the progression of early stage PD.
Acknowledgments
Funding: The clinical trial from which this case is presented is supported by Vanderbilt CTSA grant 1 UL1 RR024975 from the National Center for Research Resources, National Institutes of Health, by a research grant from Medtronic, Inc., and by gifts from private donors. The investigators were free to independently design and conduct the research, were responsible for all data analysis and interpretation, and independently wrote and reviewed the manuscript. Dr. Charles, Ms. Gill and Ms. Allen had complete access to the study data. We would like to thank all contributing authors for their editorial support and advice during the preparation of this manuscript.
Footnotes
Research Location: All research activities were carried out at Vanderbilt University Medical Center in Nashville, TN, USA.
Authorship Statement: Dr. P. David Charles designed and conducted the study, including patient recruitment, data collection, and data analysis with the assistance of Ms. Chandler E. Gill and Ms. Laura A. Allen. Dr. Michael G. Tramontana also assisted with the data collection and analysis. Ms. Gill and Ms. Allen prepared he manuscript draft with important intellectual input and revision from Drs. Peter E. Konrad, Thomas L. Davis, Michael G. Tramontana, C. Chris Kao, Michael S. Remple, Courtney H. Harrison and P. David Charles as well as from Mr. Mark Bliton and Mr. Stuart G. Finder. Dr. Charles, Ms. Gill and Ms. Allen had complete access to the study data. All authors approved the final manuscript.
Conflict of Interest: The clinical trial from which this case is reported is funded by Medtronic, Inc., by Vanderbilt CTSA grant 1 UL1 RR024975 from the NCRR-NIH, and by private donations. Medtronic representatives did not take part in data collection, management, analysis, or interpretation of the data or in preparation, review, or approval of the manuscript. Drs. Charles, Davis, Konrad and Kao have received personal compensation from Medtronic in the past. Vanderbilt University has received grants from Medtronic in excess of $10,000to support research led by Drs. Charles and Konrad. Ms. Gill, Ms. Allen, Dr. Harrison, Dr. Finder, Dr. Tramontana, Dr. Remple and Dr. Bliton have no conflicts to disclose.
References
- 1.Shagam JY. Unlocking the secrets of Parkinson disease. Radiol Technol. 2008;79:227–30. [PubMed] [Google Scholar]
- 2.Schapira AH, Olanow CW. Neuroprotection in Parkinson disease: mysteries, myths, and misconceptions. JAMA. 2004;291:358–64. doi: 10.1001/jama.291.3.358. [DOI] [PubMed] [Google Scholar]
- 3.Hermanowicz N. Drug therapy for Parkinson's disease. Semin Neurol. 2007;27:97–105. doi: 10.1055/s-2007-971177. [DOI] [PubMed] [Google Scholar]
- 4.Wallace BA, Ashkan K, Heise CE, Foote KD, Torres N, Mitrofanis J, et al. Survival of midbrain dopaminergic cells after lesion or deep brain stimulation of the subthalamic nucleus in MPTP-treated monkeys. Brain. 2007;130:2129–45. doi: 10.1093/brain/awm137. [DOI] [PubMed] [Google Scholar]
- 5.Temel Y, Visser-Vandewalle V, Kaplan S, Kozan R, Daemen MA, Blokland A, et al. Protection of nigral cell death by bilateral subthalamic nucleus stimulation. Brain Res. 2006;1120:100–5. doi: 10.1016/j.brainres.2006.08.082. [DOI] [PubMed] [Google Scholar]
- 6.Spieles-Engemann AL, Behbehani MM, Collier TJ, Wohlgenant SL, Steece-Collier K, Paumier K, et al. Stimulation of the rat subthalamic nucleus is neuroprotective following significant nigral dopamine neuron loss. Neurobiol Dis. 2010;39:105–115. doi: 10.1016/j.nbd.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Jr, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schupbach WM, Maltete D, Houeto JL, du Montcel ST, Mallet L, Welter ML, et al. Neurosurgery at an earlier stage of Parkinson disease: a randomized, controlled trial. Neurology. 2007;68:267–71. doi: 10.1212/01.wnl.0000250253.03919.fb. [DOI] [PubMed] [Google Scholar]
- 9.Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 10.Charles PD, Padaliya BB, Newman WJ, Gill CE, Covington CD, Fang JY, et al. Deep brain stimulation of the subthalamic nucleus reduces antiparkinsonian medication costs. Parkinsonism Relat Disord. 2004;10:475–9. doi: 10.1016/j.parkreldis.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Hauser RA, Friedlander J, Zesiewicz TA, Adler CH, Seeberger LC, O'Brien CF, et al. A home diary to assess functional status in patients with Parkinson's disease with motor fluctuations and dyskinesia. Clin Neuropharmacol. 2000;23:75–81. doi: 10.1097/00002826-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Konrad PE, Neimat JS, Yu H, Kao CC, Remple MS, D'Haese PF, Dawant BM. Customized, miniature rapid-prototype stereotactic frames for use in deep brain stimulator surgery: initial clinical methodology and experience from 263 patients from 2002 to 2008. Stereotact Funct Neurosurg. 2011;89:34–41. doi: 10.1159/000322276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benazzouz A, Breit S, Koudsie A, Pollak P, Krack P, Benabid AL. Intraoperative microrecordings of the subthalamic nucleus in Parkinson's disease. Mov Disord. 2002;17 3:S145–149. doi: 10.1002/mds.10156. [DOI] [PubMed] [Google Scholar]
- 14.Starr PA, Christine CW, Theodosopoulos PV, Lindsey N, Byrd D, Mosley A, Marks WJ. Implantation of deep brain stimulators into the subthalamic nucleus: technical approach and magnetic resonance imaging-verified lead locations. J Neurosurg. 2002;97:370–387. doi: 10.3171/jns.2002.97.2.0370. [DOI] [PubMed] [Google Scholar]
- 15.Fitzpatrick JM, Konrad PE, Nickele C, Cetinkaya E, Kao C. Accuracy of customized miniature stereotactic platforms. Stereotact Funct Neurosurg. 2005;83:25–31. doi: 10.1159/000085023. [DOI] [PubMed] [Google Scholar]
- 16.Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, et al. A double blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol. 2003;54(3):403–14. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 17.Steigerwald F, Potter M, Herzog J, Pinsker M, Kopper F, Mehdorn H, et al. Neuronal activity of the human subthalamic nucleus in the parkinsonian and nonparkinsonian state. J Neurophysiol. 2008;100:2515–2524. doi: 10.1152/jn.90574.2008. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Oroz MC, Rodriguez M, Guridi J, Mewes K, Chockkman V, Vitek J, DeLong MR, Obeso JA. The subthalamic nucleus in Parkinson's disease: somatotopic organization and physiological characteristics. Brain. 2001;124:1777–1790. doi: 10.1093/brain/124.9.1777. [DOI] [PubMed] [Google Scholar]
- 19.Effect of lazabemide on the progression of disability in early Parkinson's disease. The Parkinson Study Group. Ann Neurol. 1996;40:99–107. doi: 10.1002/ana.410400116. [DOI] [PubMed] [Google Scholar]
- 20.Effects of tocopherol and deprenyl on the progression of disability in early Parkinson's disease. The Parkinson Study Group. N Engl J Med. 1993;328:176–83. doi: 10.1056/NEJM199301213280305. [DOI] [PubMed] [Google Scholar]
- 21.Louis ED, Tang MX, Cote L, Alfaro B, Mejia H, Marder K. Progression of parkinsonian signs in Parkinson disease. Arch Neurol. 1999;56:334–7. doi: 10.1001/archneur.56.3.334. [DOI] [PubMed] [Google Scholar]
- 22.Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med. 2004;351:2498–508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 23.Poewe WH, Deuschl G, Gordin A, Kultalahti ER, Leinonen M Celomen Study Group. Efficacy and safety of entacapone in Parkinson's disease patients with suboptimal levodopa response: a 6-month randomized placebo-controlled double-blind study in Germany and Austria (Celomen study) Acta Neurol Scand. 2002;105:245–55. doi: 10.1034/j.1600-0404.2002.1o174.x. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs DM, Marder K, Cote LJ, Sano M, Stern Y, Mayeux R. Neuropsychological characteristics of preclinical dementia in Parkinson's disease. Neurology. 1995;45:1691–6. doi: 10.1212/wnl.45.9.1691. [DOI] [PubMed] [Google Scholar]
- 25.Funkiewiez A, Ardouin C, Caputo E, Krack P, Fraix V, Klinger H, et al. Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2004;75:834–9. doi: 10.1136/jnnp.2002.009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suchowersky O, Reich S, Perlmutter J, Zesiewicz T, Gronseth G, Weiner WJ Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: diagnosis and prognosis of new onset Parkinson disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66:968–977. doi: 10.1212/01.wnl.0000215437.80053.d0. [DOI] [PubMed] [Google Scholar]
- 27.Espay AJ, Li JY, Johnston L, Chen R, Lang AE. Mirror movements in parkinsonism – evaluation of a new clinical sign. J Neurol Neurosurg Psychiatry. 2005;76:1355–1359. doi: 10.1136/jnnp.2005.062950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeWitt PA, Boroojerdi B, MacMahon D, Patton J, Jankovic J. Overnight switch from oral dopaminergic agonists to transdermal rotigotine patch in subjects with Parkinson disease. Clin Neuropharmacol. 2007;30:256–65. doi: 10.1097/wnf.0b013e318154c7c4. [DOI] [PubMed] [Google Scholar]
- 29.Vingerhoets FJ, Villemure JG, Temperli P, Pollo C, Pralong E, Ghika J. Subthalamic DBS replaces levodopa in Parkinson's disease: two-year follow-up. Neurology. 2002;58:396–401. doi: 10.1212/wnl.58.3.396. [DOI] [PubMed] [Google Scholar]